Abstract
Mammalian cells have developed an elaborate network of immunoproteins that serve to identify and combat viral pathogens. Interferon-stimulated gene 15 (ISG15) is a 15.2 kDa tandem ubiquitin-like protein (UBL) that is used by specific E1–E2–E3 ubiquitin cascade enzymes to interfere with the activity of viral proteins. Recent biochemical studies have demonstrated how the E3 ligase HECT and RCC1-containing protein 5 (HERC5) regulates ISG15 signaling in response to hepatitis C (HCV), influenza-A (IAV), human immunodeficiency virus (HIV), SARS-CoV-2 and other viral infections. Taken together, the potent antiviral activity displayed by HERC5 and ISG15 make them promising drug targets for the development of novel antiviral therapeutics that can augment the host antiviral response. In this review, we examine the emerging role of ISG15 in antiviral immunity with a particular focus on how HERC5 orchestrates the specific and timely ISGylation of viral proteins in response to infection.
1. Introduction
Viral replication is a highly coordinated process that relies heavily on the host cellular machinery and can disrupt critical cellular activities leading to disease and/or death [1,2,3,4]. Viruses exert immense evolutionary pressure on their hosts, driving the development of complex cellular immune responses that work to identify, combat and eliminate infectious pathogens. For example, various mammalian tissues contain immune cells that use pattern recognition receptors (PPRs), including toll-like receptors (TLRs), NOD-like receptors (NLRs) and RIG-1-like receptors (RLRs), to screen for pathogen-associated molecular patterns (PAMPs) in the cytosol and extracellular matrix [5,6]. When cells detect a viral pathogen, specific PRRs become activated that signal for mitochondrial antiviral signaling (MAVS) proteins to upregulate interferon-α/β (IFN-α/β) cytokine production [2,7,8,9]. Cells then secrete IFN-α/β cytokines into the extracellular matrix to alert naive cells of an impending infection [10,11].
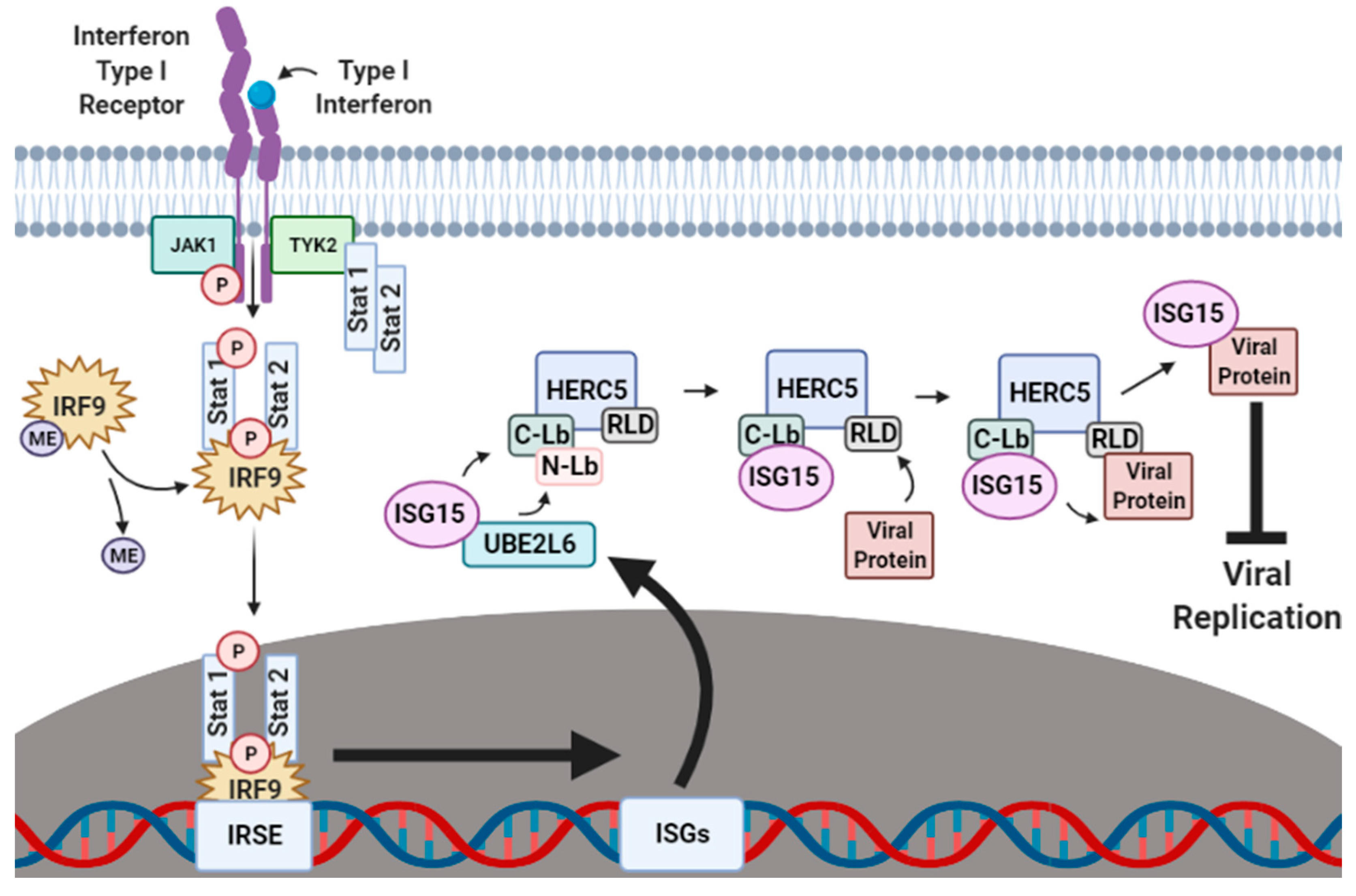
The production of IFN-α/β is one of the first lines of cellular defense against viral pathogens. IFN-α/β activates the Janus kinase-signal transducer and activation of transcription (JAK–STAT) signaling pathway (Figure 1). When IFN-α/β binds to an IFN-specific receptor on the cellular surface, a conformational change occurs that exposes sites on the cytoplasmic part of the receptor that become phosphorylated by Janus kinase 1 (JAK1) and tyrosine-protein kinase 2 (TYK2). This phosphorylation attracts the nuclear transcriptional regulators signal transducer and activator of transcription proteins 1 and 2 (STAT1 and STAT2) to the cellular membrane where they are also phosphorylated by TYK2. The phosphorylated STAT1 and STAT2 proteins form a ternary complex with methylated interferon regulatory factor 9 (IRF9), which is subsequently demethylated to signal for the migration of the STAT1–STAT2–IRF9 (SSI) complex to the nucleus. In the nucleus, the SSI complex binds to the ISG promoter region of the interferon-stimulated response element (ISRE), resulting in the transcriptional upregulation of hundreds of ISGs that augment the antiviral immune response by halting ribosomal protein synthesis [12,13], inducing the activation of regulatory cell death pathways [14,15,16,17,18], and carrying out the post-translational modification (PTM) of host and viral proteins that impact viral replication [19,20,21,22,23,24,25].
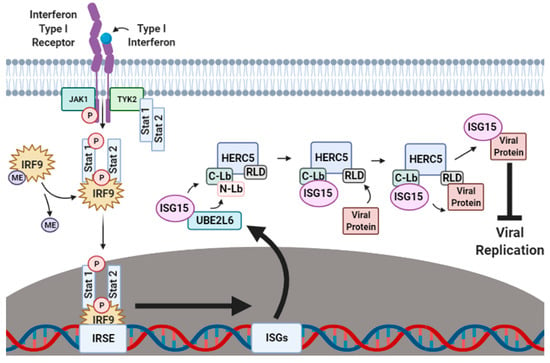
Figure 1.
Interferon-induced ISGylation of viral proteins. During the initial stages of infection, cellular IFN-α/β surface receptors are bound by IFN-α/β-specific ligands that expose receptor phosphorylation sites to the cytoplasm. Janus kinase 1 (JAK1) and tyrosine-protein kinase 2 (TYK2) phosphorylate exposed cytoplasmic IFN-α/β receptor sites, thus recruiting the nuclear transcriptional regulators signal transducer and activator of transcription proteins 1 and 2 (STAT1 and STAT2) to also be phosphorylated by TYK2. In the cytoplasm, phosphorylated STAT1 and STAT2 form a ternary complex with methylated IRF9 which is then demethylated to signal for the migration of the STAT1–STAT2–IRF9 (SSI) complex to the nucleus. Upon nuclear entry, the SSI complex binds to the ISG promoter region interferon-stimulated response element (ISRE) to upregulate the transcription of several hundred ISGs, including ISG15, UBE1L, UBE2L6 and HERC5. Translated UBE1L, UBE2L6 and HERC5 proteins are subsequently targeted to the perinuclear regions of the cytoplasm where they work in tandem to charge, transfer and attach ISG15 onto respective viral protein substrates to inhibit viral stability, transport and reassembly. ME, methylation. This figure was created with Biorender™.
HECT and RCC1-containing protein 5 (HERC5) and interferon-stimulated gene 15 (ISG15) are two antiviral immune proteins that are induced following IFN-α/β signal transduction. Multiple studies have established that HERC5 plays a central role in mammalian innate immunity by ISGylating viral proteins to disrupt viral replication [26,27]. The antiviral function of HERC5 was first discovered by researchers who found that its co-expression with the E1 ubiquitin-like modifier activating enzyme UBE1L (Uba7; E.C. 6.2.1.45) and UBE2L6 (UbcH8; E.C. 2.3.2.23) resulted in normal rates of cellular ISGylation activity, despite the absence of IFN-α/β stimulation [28].
The functional characterization of phosphokinase signal transduction pathways has now clarified that UBE1L, UBE2L6, HERC5 and ISG15 expression are predominantly induced by the JAK–STAT signaling pathway (Figure 1) [29,30]. However, in recent years, additional modes of ISG induction have been identified in cells. For example, the ISG15-specific cascade enzymes are thought to be induced by interferon regulatory factors 3, 5 and 7 (IRF3, IRF5 and IRF7) [31,32]. HERC5 expression is also regulated by the cytokine interleukin 1 β (IL-1β) and tumor necrosis factor α (TNFα) in distinct signal transduction pathways. Moreover, recent studies have shown that ISG15, UBE1L, UBE2L6 and HERC5 are tightly regulated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)–an immunological protein complex that is involved in controlling diverse cellular responses to viral infection including cytokine production, DNA transcriptional regulation and interferon activation [33].
2. The ISGylation Pathway
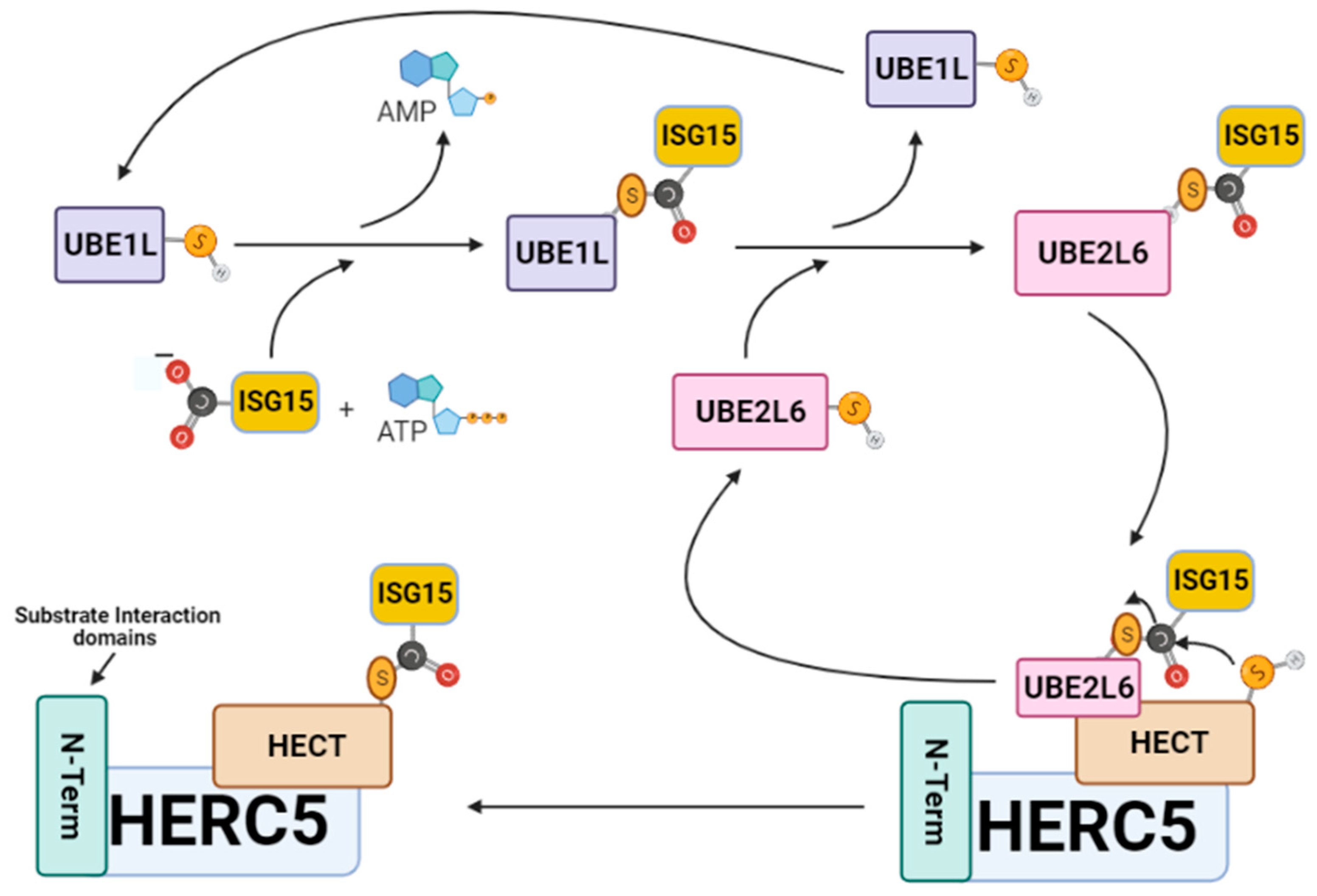
ISGylation involves the covalent posttranslational attachment of ISG15 to host and viral target substrates by a specialized group of IFN-α/β induced E1-E2-E3 ubiquitin cascade enzymes. For example, protein–protein interaction studies have shown that ISG15 only coordinates with five of the over 600 identified E1–E2–E3 enzymatic members [34]. The ISGylation pathway begins when UBE1L activates ISG15 through an ATP-dependent mechanism to form a thioester bond between the C-terminal carboxyl of ISG15 and the catalytic cysteine of UBE1L [35,36] (Figure 2). Following activation, ISG15 is transferred by UBE1L to UBE2L6 via a trans-thiolation reaction that forms a thioester bond between the C-terminus of ISG15 and the conserved catalytic cysteine residue of UBE2L6 [37,38]. The UBE2L6–ISG15 thioester complex then transfers its ISG15 cargo to the catalytic cysteine (C994) of the ISG15-specific homologous to E6AP C-terminus (HECT) E3 ligase HERC5 (EC 2.3.2.-). Thereafter, HERC5 catalyzes the covalent attachment of ISG15 onto host and viral substrate proteins by forming an isopeptide bond between the C-terminus of ISG15 and the ε-amino group on the lysine of the respective protein target [27,28].
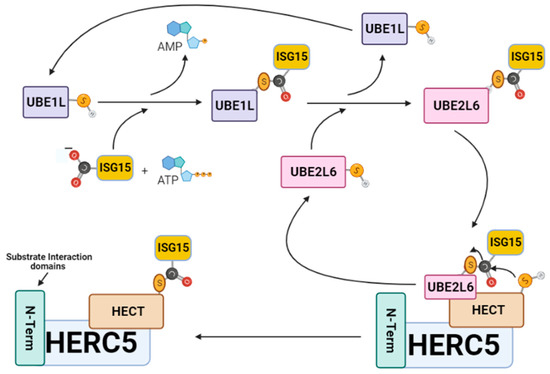
Figure 2.
The ISGylation signaling pathway. ISG15 is sequentially transferred from UBE1L to UBE2L6, then to HERC5 before its attachment to a viral protein. HERC5 is responsible for catalyzing the specific covalent attachment of ISG15 onto viral target proteins through the formation of a stable isopeptide bond between the C-terminus of ISG15 and the ε-amino group on the lysine viral protein target. This figure was created with Biorender™.
Studies have shown that the really interesting new gene (RING) E3 ligase tripartite motif-containing protein 25 (TRIM25; EC: 2.3.2.27) and the RING-between-RING (RBR) E3 ubiquitin ligase human homolog of Ariadne (HHARI) can also mediate the conjugation of ISG15 to cellular substrates [39,40]. TRIM25 achieves this catalytic activity by using its N-terminal RING domain as a scaffold to coordinate the transfer of ISG15 from UBE2L6 to the target protein via the ubiquitylation mechanism used by other members of the RING E3 ubiquitin ligase family [39]. Likewise, HHARI catalyzes the post-translational attachment of ISG15 to its target substrates using a canonical RBR ubiquitylation mechanism [40]. In this model, HHARI catalyzes substrate ISGylation by using a RING-like mechanism to coordinate ISG15-charged UBE2L6, followed by the formation of a HECT-like thioester intermediate between ISG15 and the HHARI required for the catalysis domain (Rcat; RING2) to complete the ISG15 transfer onto the target substrate [40].
Studies on the ubiquitylation pathway have found that E1–E2–E3 cascade enzymes are constitutively expressed and can form a variety of ubiquitin chain linkages with their target substrates [41,42,43,44,45]. Interestingly, the ISG15-specific E1–E2–E3 cascade enzymes are only induced following IFN-α/β signal transduction and exclusively form monomer isopeptide linkages between their ISG15 cargo and target substrate [46]. Accordingly, the ISG15 signaling pathway provides the cell with a highly specialized antiviral function at the expense of regulating vast cellular activities [47]. Intriguingly, a recent publication observed that hybrid ISG15–ubiquitin chains can also be formed by the cell to regulate protein homeostasis [48]. As novel ISG15 signaling mechanisms continue to be discovered, it will be important to refine the current understanding of how UBE1L, UBE2L6 and HERC5 target host and viral substrates for protein ISGylation.
4. HERC5: A Unique HECT E3 Ubiquitin Ligase That ISGylates Viral Proteins
HERC5 is a multidomain 114 kDa protein that belongs to both the HECT E3 ubiquitin ligase family and the RCC1 superfamily [72]. To date, 28 HECT E3 ubiquitin ligases have been identified in humans, with each having a conserved HECT domain found near their C-termini [73,74,75]. The HECT domain consists of two lobes—the N-terminal lobe that contains the binding site for E2 cognate enzymes, and the C-terminal lobe that contains the E3′s absolutely conserved catalytic cysteine [76]. Coordination of the HERC5 C-terminal lobe to ISG15, as opposed to ubiquitin, provides HERC5 with a potent anti-viral activity that is achieved by the covalent attachment of ISG15 to viral proteins. Structural studies on members of the HECT family have also revealed that there is a flexible hinge region located between the N- and C-terminal lobes of the HECT domain [77,78,79,80,81]. In the context of ISGylation, this inherent flexibility appears to be critical for allowing the juxtaposition of the catalytic cysteines in UBE2L6 and HERC5 for efficient ISG15 transfer [82], as well as for moving the ISG15-charged HECT C-terminal lobe of HERC5 into the proximity of viral substrates [83]. Previous domain-mapping experiments have determined that the HERC5 N-terminal region contains five regulator of chromatin condensation 1 (RCC1) motifs which collectively form an RCC1-like domain (RLD)—a substructure of HERC5 that plays a direct role in the recognition and coordination of viral substrates [84]. For example, studies have shown that HERC5 uses its RLD domain to bind the influenza A virus (IAV) non-structural protein 1 (NS1) [47], the hepatitis C virus (HCV) non-structural protein 5A (NS5A) [85] and multiple human immunodeficiency virus (HIV) gag particle precursor proteins [86]. Given the expansive amount of new research being conducted on HERC5 ISGylation, it is likely that additional HERC5 pathogenic substrates will be discovered, and the immunological consequences of these interactions will need to be resolved.
HERC5 is the only human HECT E3 ubiquitin ligase that is known to conjugate ISG15 to host and viral substrates. The immunological significance of HERC5 has become well established, with recent studies beginning to reveal what proteins are targeted by the enzyme during HCV, IAV, HIV-1 and other viral replication cycles, and whether viral protein activities are inhibited or augmented following ISG15 attachment (Table 1).

Table 1.
HERC5-dependent ISGylation of viral proteins and their downstream consequences.
Sequence alignment studies have found that the HERC5 domain architecture is paralogous to another HECT E3 ubiquitin ligase, HERC6, but this enzyme demonstrates a limited capability to coordinate ISG15 substrate transfer except in murine models [108]. These findings are surprising, particularly with the high level of sequence conservation between HERC5 and HERC6, and thus leaving many questions on the structural mechanisms used by HERC5 to mediate viral protein ISGylation. To uncover these mysteries, new research is required to clarify how HERC5 use its N-terminal RLD domain to recognize and bind a wide range of viral substrates. Furthermore, now that there is a consensus as to what structures and mechanisms are used by HERC5 to attach ISG15 onto viral substrates, it will be important to explore the molecular dynamics involved with HERC5-substrate targeting, as well as how these dynamics allow for certain viral protein antagonists to subvert ISG-related immune responses.
HERC5 has recently been shown to carry out viral protein ISGylation to (i) stall IAV replication by blocking NS1 protein homodimerization [47], (ii) promote HCV proliferation via improved cyclophilin A recruitment by NS5A proteins [85] and, alternatively, (iii) inhibit an early stage of HIV assembly by attenuating gag-particle production [86,109]. In the upcoming sections we discuss some of the recent discoveries involving the HERC5-dependent ISGylation of viral protein targets.
4.1. HERC5-Dependent ISGylation of HCV
The antiviral effects of HERC5 and ISG15 on HCV were first observed by Jung Kim et al., who were examining mechanisms of HCV replication cycle inhibition [110]. By conducting site-directed mutagenesis experiments on the HCV protein NS5A, the group was able to demonstrate that HERC5 inhibits HCV by ISGylating NS5A at lysine 379 (K379) to result in NS5A becoming targeted for K48 polyubiquitylation by an unknown E3 ligase [110]. The researchers confirmed this antiviral activity by demonstrating that HCV replication was unrestrained when ISG15 and its corresponding E1–E2–E3 enzyme cascade were expressed in the presence of HCV NS5A K379R variant proteins. Taken together, these findings suggest that NS5A is the primary HCV target substrate of HERC5-dependent ISGylation, and that K379 is the sole NS5A residue that HERC5 targets for ISGylation. More recently, Abe et al. demonstrated that the HCV NS5A protein is prone to ISGylation at five Lys residues (K44, K68, K166, K215 and K308) [85]. In fact, one of the ISG15 attachment points on NS5A, K308, is located within the NS5A-binding region for cyclophilin A (CypA), a virulence factor that is required for efficient HCV cellular propagation. Thus, HERC5 ISGylation of NS5A at K308 enhances HCV recruitment of CypA to provide a pro-virulent replication activity, a direct contradiction of previous findings from Kim et al. [110]. Moreover, a stand-alone study conducted by Domingues et al. found that ISG15-related forms of HCV inhibition occur independently of HERC5 activity [111]. To date, no follow up research has been conducted on the HERC5–HCV ISGylation system to confirm whether HERC5 ISGylation activity results in a pro- or anti-virulent response to HCV infection, and whether HERC5 is necessary for catalyzing the modes of ISG15 inhibition that have been observed for HCV NS5A proteins.
4.2. Influenza Viruses and ISGylation by HERC5
New research by Tang et al. has shown that HERC5 targets IAV NS1 for ISGylation to prevent IAV capsid formation [47]. Using pulldown assays and immunoprecipitation analysis, the researchers found that HERC5 interacts with the ribosomal-binding (RBD) and C-terminal effector domains (ED) of NS1, and that both interactions were required to form stable HERC5–NS1 complexes [47]. Lysine residue substitutions in IAV NS1 also revealed that HERC5 attaches ISG15 at multiple NS1 lysine residues (K20, K41, K217, K219, K108, K110 and K126), with the strongest inhibitory effect coming from the ISGylation of the K126 and K217 residues in the ED and RBD domains, respectively [56]. The ISGylation of NS1 subsequently abolished the ability of NS1 to interact with protein kinase R (PKR) and blocked NS1 RBD-dependent homodimerization, thereby significantly inhibiting IAV capsid assembly in vivo [47]. Interestingly, it was found that certain avian flu IAV strains, such as H5N1, demonstrated a higher rate of NS1 K217R mutation compared to most seasonal flu strains [47,112]. It was also observed that avian IAV variants were less susceptible to ISGylation at K126, suggesting that, unlike other IAV strains, avian IAV NS1 proteins may adopt a new structure that obstructs the K126 ISGylation site from HERC5. Given that K126 and K217 have been determined as the primary ISGylation sites involved with preventing IAV capsid formation, these discoveries provide a possible explanation for why avian strains of the flu are more infectious and lethal than other IAV strains. Despite HERC5 demonstrating minimal antiviral activity against certain avian IAV strains, these findings from Tang et al. indicate that HERC5 and its ISGylation activity could serve as prime drug targets for the development of treatments that are used to combat most seasonal IAV strains.
4.3. HERC5 Modes of Action Against HIV
A pivotal study by Woods et al. in 2011 confirmed that HERC5 prevents an early stage of HIV-1 viral assembly by ISGylating proteins involved with Gag polyprotein (Pr55Gag) particle production [86]. The researchers used confocal immunofluorescence microscopy to reveal that IFN-I-induced HERC5 localizes to the cytoplasm where it forms punctuate bodies in a variety of cell lineages, and that these bodies interact with polyribosomes [86]. These findings were consistent with a previous study conducted by Durfee et al., who observed that HERC5 associates with the 60S ribosomal subunit of the polyribosome using cell fractionation [113]. Additionally, Woods et al. found that IFN-I-induced HERC5 and HIV Gag proteins colocalize to the plasma membrane where HERC5 ISGylates Gag particles to prevent HIV-1 viral budding. Expanding on these findings, the research group showed HERC5 possesses a second distinct mode of HIV inhibition that is independent of its HECT ISGylation activity [60]. Typically, in eukaryotic cells, nascent RNA molecules remain in the nucleus until their introns are spliced to reach maturation. However, HIV-1 can circumvent this cellular activity by expressing the nuclear trans-activator protein Rev, which binds to the Rev-response element (RRE) located within the HIV-1 intron [114]. These Rev–RRE nuclear interactions permit the nuclear export of unspliced HIV-1 RNA constructs via a translocation mechanism that involves exportin-1 (CRM1) and Ras-related nuclear protein guanidine triphosphate (RanGTP) [114]. Focusing specifically on mechanisms involved with HIV transcription, Woods et al. determined that the HERC5 RLD domain localizes to the perinuclear area and reduces intracellular RanGTP levels [60]. This correlated with the subcellular mis-localization of Rev/RRE following their nuclear export. These findings led Woods et al. to conclude that HERC5 acts as a host restriction factor during HIV replication by preventing an early stage of HIV-1 Pr55Gag particle production through a HECT-dependent mechanism, and that HERC5 affects nuclear export of unspliced HIV RNA constructs via an RLD-dependent mechanism [60]. Importantly, the same research group has shown that other retroviruses such as murine leukemia virus (MLV) and simian immunodeficiency virus (SIV) are also inhibited by HERC5 [86]. Interestingly, although SIV was inhibited by human HERC5, an ancestral version of HERC5 found in coelacanth fish was unable to inhibit SIV replication, suggesting that the HERC5 gene has evolved to combat lentiviruses in primates [115].
4.4. HERC5 Combating Other Viruses
New reports have suggested that HERC5 and ISG15 play a significant role in preventing the replication of many viral pathogens; however, the modes of viral inhibition exhibited by HERC5 and ISG15 on these pathogens are still being characterized. For example, HERC5 has been shown to ISGylate the major capsid protein L1 of human papilloma virus 16 (HPV16) to reduce the rate of viral budding, but the specific protein interactions that occur between HERC5, ISG15 and the L1 capsid protein of HPV16 are unknown [113]. Another study conducted by Kim et al. found a similar function for HERC5 and ISG15 during cytomegalovirus (CMV) replication, whereby viral budding was halted at the cellular membrane via the HERC5-dependent ISGylation of multiple CMV factors [93]. It was also observed that HERC5 and ISG15 are upregulated in response to infection caused by the Zika virus [116,117,118,119], Ebola virus [120,121,122,123], vaccinia virus [107], Kaposi’s sarcoma-associated herpesvirus (KSHV) [101], influenza B virus [124] and others (please see Table 1); however, the specific structural and biochemical modes of inhibition enacted by HERC5 and ISG15 in response to these pathogens is not fully understood.
Interestingly, new studies on the Ebola virus have shown that HERC5 and ISG15 exert two independent mechanisms of inhibition. These studies show that HERC5 reduces intracellular Ebola virus RNA through an RLD-dependent mechanism or, alternatively, free ISG15 can inhibit viral budding at the plasma membrane [125]. ISG15 specifically inhibits VP40 budding at the plasma membrane by blocking neural precursor cell-expressed developmentally down-regulated protein 4 (NEDD4)-mediated ubiquitylation of the VP40 late domain—a PTM that usually signals for the host endosomal sorting complexes required for transport (ESCRT) machinery to initiate budding of viral particles [125].
Studies in mice have found that vaccinia virus replication is enhanced in ISG15 knockout cells and inhibited in cells expressing wildtype levels of ISG15 [107]. ISG15-deficient cells were more resistant to apoptosis and had impaired phagocytic activity when coming in contact with infected cells [105]. These studies demonstrated that control of vaccinia virus is dependent on the level of cellular ISGylation activity, where either ISG15 knockout or the deISGylation of proteins can lead to enhanced infection kinetics.
Although it is well established that ISG15 and the ISGylation cascade enzymes are induced by cytomegalovirus infection, their role and direct interaction with viral proteins were only recently described [93]. Kim et al. demonstrated that knocking down ISG15 or HERC5 lead to a significant increase in viral titers, whereas expression of ISG15 and HERC5 inhibited viral replication by reducing viral gene expression and viral budding at the plasma membrane [93]. To date, much of the research into cytomegalovirus infection and its inhibition by HERC5 and ISG15 has focused on human cytomegalovirus (HCMV) protein antagonism. However, little is known about the specific effect that ISGylation has on viral proteins other than it is has a dominant inhibitory effect. Further studies are needed to elucidate the precise mechanism of HCMV inhibition demonstrated by HERC5 and ISG15.
HERC5 has been shown to inhibit KSHV in an ISGylation-dependent manner, with the knockdown of either HERC5 or ISG15 prior to viral reactivation resulting in higher titers of infectious viral particles [101]. For example, ISG15 and HERC5 were identified as interactors of the KSHV viral homolog of interferon regulatory factor 1 (vIRF1), which is known to decrease total protein ISGylation. Later studies suggested that adequate ISG15 expression can result in viral latency, while the knockdown of ISG15 and ISG20 leads to lytic reactivation of the virus [100]. A more recent study implicated CRM1 in the inhibition of KSHV by promoting nuclear retention of the autophagy adaptor protein p62 (SQSTM1) to elevate the expression of antiviral genes [99]. Interestingly, it was shown that HERC5 inhibits nuclear export of HIV RNA in a CRM1-dependent manner [59]. Although it has not been directly studied, HERC5 may inhibit the lytic phase of KSHV replication through a mechanism involving the CRM1-dependent nuclear export pathway.
Most of the viral pathogens we have discussed in this review can also resist HERC5 and ISG15 antiviral activity by employing viral protein machinery to block the host immune response. As viral–host interactions continue to progress, it is likely that more pathogens will evolve and acquire new strategies to antagonize HERC5 antiviral function. These predictions make it critical that additional studies be done to determine the mechanisms and structures that dictate the pathogen-dependent processes of HERC5 antagonism. Such knowledge will be fundamental to the development of new antiviral treatments aimed at counteracting viral antagonistic strategies employed against HERC5 in the cell.
4.5. Viral Antagonism of HERC5 and ISGylation
HERC5 and ISGylation are essential regulators of the antiviral immune response. Accordingly, viruses have evolved diverse strategies to disrupt HERC5 and ISG15 immune activity. For example, viral ovarian tumor domains are common among the Nairovirus family, each retaining at least a small level of deubiquitylation activity. Moreover, a pathogenic clade containing the Crimean–Congo hemorrhagic fever virus (CCHFV), Nairobi sheep disease virus (NSDV), Ganjam virus (GANV) and Erve virus (ERVEV) also possess significant deISGylation activity [126,127,128]. Experimental evidence suggests that viral ovarian tumor family-like domains (vOTUs) have adapted to deISGylate in their most common host species and that deISGylation is likely a major hurdle that viruses must cross to change host specificity [127,129]. Interestingly, the most fatal virus in humans, CCHFV, is capable of both deubiquitylating and deISGylating target proteins, likely resulting in subversion of both the antiviral and inflammatory responses [126,130]. The inhibition of the CCHFV vOTU resulted in impaired viral replication and infectivity [131,132], suggesting that this deISGylase may be a prime target for further therapeutics to treat CCHFV.
In general, it has been difficult for researchers to delineate vOTU deISGylation activity from deubiquitylation activity and to specifically measure the contribution of each process to viral fitness. However, recent structural analysis and mutagenesis studies on these viral proteins have led to the identification of the binding pocket for ISG15 and ubiquitin. Interestingly, a single amino acid substitution can change the specificity of Hazara virus from ubiquitin to ISG15, resulting in a virus that was able to selectively deISGylate while not being able to cleave ubiquitin from certain proteins [133]. These discoveries provide a useful tool for examining the effect of deISGylation and deubiquitylation in the context of innate immune activation and sensing.
Unlike the vOTUs that promiscuously bind to both ubiquitylated and ISGylated substrates, the papain-like protease of foot and mouth disease virus (FMDV), leader protease (Lbpro), has been shown to have stronger affinity for ISGylated proteins than for ubiquitylated proteins [91,134]. FMDV acts to cleave ISG15 molecules from the target substrate adjacent to the C-terminal Gly–Gly motif, which renders it unable to be recycled and attached to other target proteins [91,134]. Likewise, the loss of Lbpro deISGylation activity significantly inhibited the ability of FMDV to grow in mice, although the researchers did not observe an increase in antiviral gene expression to suggest that the more likely mechanism of inhibition is through the direct ISGylation of viral proteins [91].
The inhibition of HERC5 ISGylation by influenza virus is generally species-specific. For instance, human and non-human primate ISGylation is antagonized by influenza B virus, but not in mice [124]. More recent studies have determined that influenza virus NS1B protein counteracts ISGylation by sequestering ISGylated proteins and inhibiting their incorporation in the viral ribonucleic complex [135]. Further studies are required to clarify the specific mechanisms employed by influenza viruses in different host organisms.
Intriguingly, the E3 ligase protein of vaccinia virus is a known antagonist of ISGylation [106]. When vaccinia E3 protein is expressed, there is a significant decrease in ISGylated proteins within the cell. This marked decrease corresponds with a reduction in ISGylated mitochondrial proteins and impaired mitophagy activity in the infection model. This suggests that the ISGylation of mitochondrial proteins is important for the regulation of oxidative phosphorylation and minimizing reactive oxygen species production [105].
Due to the COVID-19 pandemic, the coronavirus papain-like proteases (PLpro) have received considerable attention from the scientific community. Zhang et al. recently showed that expression of ubiquitin variants (UbVs) led to tight and specific binding of the PLpro of Middle Eastern respiratory syndrome coronavirus (MERS-CoV) [98]. Following PLpro–UbV complex formation, PLpro deubiquitylation, deISGylation and protease activities were significantly hindered and viral progeny were much less infectious [98]. Small-molecule inhibitor screens have subsequently identified the substrate-binding pocket and the ISG15 binding site of PLpro as important determinants of viral fitness and could serve as attractive targets for antiviral drug development [136,137,138,139,140,141,142]. For example, the small molecule GRL0617 is one of the most promising SARS-CoV-2 inhibitors currently being studied that sterically blocks the binding of ISG15 and ubiquitin to PLpro [137,142]. Furthermore, SARS-CoV-2 PLpro has a higher affinity for ISG15 than monomeric ubiquitin and its activity is important for viral pathogenesis [143]. In cell culture and mouse models it was observed that ISGylation of the caspase-recruiting domain (CARD) of the MDA5 domain by HERC5 was important for its oligomerization and activation [104]. Knockdown of either ISG15 or MDA5 resulted in loss of IRF3 phosphorylation, which is a downstream target of MDA5 [104]. Fascinatingly, researchers have noted that naturally emerging mutations causing residue substitutions in the ISG15-binding pocket of PLpro results in less pathogenic strains of SARS-CoV-2 [144]. Thus far, the primary role of PLpro deISGylation appears to be suppression of the immune response, with more studies needed to determine if SARS-CoV-2 proteins are directly ISGylated by host cell machinery and if this has an overall inhibitory effect on the virus.
5. HERC5 and ISGylation: Moving Forward
Although it is clear that HERC5 and ISGylation represent a formidable first line of defense against viral pathogens, our knowledge of how HERC5 uses its highly conserved domain architecture to recognize and bind a diverse range of evolving viral substrates remains unclear. Future structure–function studies involving HERC5 and its protein targets will be important for determining the structural requirements necessary for these interactions. Such knowledge will help to shed light on how viral proteins evolve to evade these interactions with the host’s ISGylation machinery. Moreover, the HECT-dependent and -independent antiviral mechanisms of HERC5 raise interesting questions about the evolutionary origins of HERC5 and its biological role in vertebrate evolution. Future research in these areas will help foster the development of new therapeutic approaches using HERC5 as a prominent modulator of cellular antiviral activity to combat a wide range of infectious diseases in vertebrates.
Author Contributions
Conceptualization, N.A.M. and D.E.S.; writing—original draft preparation, review and editing, N.A.M., E.P., S.D.B. and D.E.S.; supervision, S.D.B. and D.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institutes of Health, grant R15GM126432 (to D.E.S.), the Natural Sciences and Engineering Research Council of Canada, grant 386404-2012 (to S.D.B.) and the Canadian Institutes of Health Research, grants HBF134179 and HBF137693 (to S.D.B).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All discussed literature and figures are found in the main text of this mini-review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swanson, M.S.; Kokot, N.; Sinha, U.K. The Role of HPV in Head and Neck Cancer Stem Cell Formation and Tumorigenesis. Cancers (Basel) 2016, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Senba, M.; Mori, N. Mechanisms of virus immune evasion lead to development from chronic inflammation to cancer formation associated with human papillomavirus infection. Oncol. Rev. 2012, 6, e17. [Google Scholar] [CrossRef] [PubMed]
- Kheimar, A.; Trimpert, J.; Groenke, N.; Kaufer, B.B. Overexpression of cellular telomerase RNA enhances virus-induced cancer formation. Oncogene 2019, 38, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Ilarraza, R.; Wu, Y.; Davoine, F.; Ebeling, C.; Adamko, D.J. Human dendritic cells promote an antiviral immune response when stimulated by CVT-E002. J. Pharm. Pharmacol. 2011, 63, 670–678. [Google Scholar] [CrossRef]
- Renn, C.N.; Sanchez, D.J.; Ochoa, M.T.; Legaspi, A.J.; Oh, C.K.; Liu, P.T.; Krutzik, S.R.; Sieling, P.A.; Cheng, G.; Modlin, R.L. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J. Immunol. 2006, 177, 298–305. [Google Scholar] [CrossRef]
- Graef, K.M.; Vreede, F.T.; Lau, Y.F.; McCall, A.W.; Carr, S.M.; Subbarao, K.; Fodor, E. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. J. Virol. 2010, 84, 8433–8445. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.B.; Sorensen, L.N.; Malmgaard, L.; Ank, N.; Baines, J.D.; Chen, Z.J.; Paludan, S.R. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007, 81, 13315–13324. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Song, W.Q.; Li, Y.J.; Qian, J.; Zhai, A.X.; Wu, J.; Li, A.M.; He, J.M.; Zhao, J.Y.; Yu, X.; et al. Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production. Virol. J. 2012, 9, 312. [Google Scholar] [CrossRef]
- Smith, J.K.; Siddiqui, A.A.; Krishnaswamy, G.A.; Dykes, R.; Berk, S.L.; Magee, M.; Joyner, W.; Cummins, J. Oral use of interferon-alpha stimulates ISG-15 transcription and production by human buccal epithelial cells. J. Interferon Cytokine Res. 1999, 19, 923–928. [Google Scholar] [CrossRef]
- Zhao, Y.; Valbuena, G.; Walker, D.H.; Gazi, M.; Hidalgo, M.; DeSousa, R.; Oteo, J.A.; Goez, Y.; Brasier, A.R. Endothelial Cell Proteomic Response to Rickettsia conorii Infection Reveals Activation of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT)-Inferferon Stimulated Gene (ISG)15 Pathway and Reprogramming Plasma Membrane Integrin/Cadherin Signaling. Mol. Cell. Proteomics 2016, 15, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Baranek, T.; Manh, T.P.; Alexandre, Y.; Maqbool, M.A.; Cabeza, J.Z.; Tomasello, E.; Crozat, K.; Bessou, G.; Zucchini, N.; Robbins, S.H.; et al. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe 2012, 12, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Smith, N.; Yu, L.; Paton, I.R.; Gutowska, M.W.; Forrest, H.L.; Danner, A.F.; Seiler, J.P.; Digard, P.; Webster, R.G.; et al. A comparative analysis of host responses to avian influenza infection in ducks and chickens highlights a role for the interferon-induced transmembrane proteins in viral resistance. BMC Genom. 2015, 16, 574. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Su, Z.; Wu, Y.; Wu, C.; Jin, S.; Xie, W.; Jiang, W.; Zhou, R.; Cui, J. NLRP11 disrupts MAVS signalosome to inhibit type I interferon signaling and virus-induced apoptosis. EMBO Rep. 2017, 18, 2160–2171. [Google Scholar] [CrossRef]
- El Maadidi, S.; Faletti, L.; Berg, B.; Wenzl, C.; Wieland, K.; Chen, Z.J.; Maurer, U.; Borner, C. A novel mitochondrial MAVS/Caspase-8 platform links RNA virus-induced innate antiviral signaling to Bax/Bak-independent apoptosis. J. Immunol. 2014, 192, 1171–1183. [Google Scholar] [CrossRef]
- Choi, Y.B.; Shembade, N.; Parvatiyar, K.; Balachandran, S.; Harhaj, E.W. TAX1BP1 Restrains Virus-Induced Apoptosis by Facilitating Itch-Mediated Degradation of the Mitochondrial Adaptor MAVS. Mol Cell Biol 2017, 37. [Google Scholar] [CrossRef]
- Kaneda, Y. The RIG-I/MAVS signaling pathway in cancer cell-selective apoptosis. Oncoimmunology 2013, 2, e23566. [Google Scholar] [CrossRef]
- Renson, P.; Blanchard, Y.; Le Dimna, M.; Felix, H.; Cariolet, R.; Jestin, A.; Le Potier, M.F. Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains. Vet. Res. 2010, 41, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, C. E3 ubiquitin ligases, the powerful modulator of innate antiviral immunity. Cell Immunol. 2019, 340, 103915. [Google Scholar] [CrossRef]
- Li, S.Z.; Shu, Q.P.; Song, Y.; Zhang, H.H.; Liu, Y.; Jin, B.X.; Liuyu, T.Z.; Li, C.; Huang, X.C.; Du, R.L.; et al. Phosphorylation of MAVS/VISA by Nemo-like kinase (NLK) for degradation regulates the antiviral innate immune response. Nat. Commun. 2019, 10, 3233. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Imamura, H.; Taku, T.; Kuroki, T.; Kawaguchi, A.; Ishikawa, K.; Nakada, K.; Koshiba, T. RLR-mediated antiviral innate immunity requires oxidative phosphorylation activity. Sci. Rep. 2017, 7, 5379. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Pan, W.; Wang, P. ISG15 facilitates cellular antiviral response to dengue and west nile virus infection in vitro. Virol. J. 2011, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J. Antiviral Properties of ISG15. Viruses 2010, 2, 2154–2168. [Google Scholar] [CrossRef]
- Perng, Y.C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Harty, R.N.; Pitha, P.M.; Okumura, A. Antiviral activity of innate immune protein ISG15. J. Innate. Immun. 2009, 1, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Inoue, S.; Yokosawa, H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem. Biophys. Res. Commun. 2006, 348, 473–477. [Google Scholar] [CrossRef]
- Wong, J.J.; Pung, Y.F.; Sze, N.S.; Chin, K.C. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc. Natl. Acad. Sci. USA 2006, 103, 10735–10740. [Google Scholar] [CrossRef]
- Dastur, A.; Beaudenon, S.; Kelley, M.; Krug, R.M.; Huibregtse, J.M. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J. Biol. Chem. 2006, 281, 4334–4338. [Google Scholar] [CrossRef]
- Cheon, H.; Holvey-Bates, E.G.; Schoggins, J.W.; Forster, S.; Hertzog, P.; Imanaka, N.; Rice, C.M.; Jackson, M.W.; Junk, D.J.; Stark, G.R. IFNβ-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 2013, 32, 2751–2763. [Google Scholar] [CrossRef]
- Cheon, H.; Stark, G.R. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9373–9378. [Google Scholar] [CrossRef]
- Lu, G.; Reinert, J.T.; Pitha-Rowe, I.; Okumura, A.; Kellum, M.; Knobeloch, K.P.; Hassel, B.; Pitha, P.M. ISG15 enhances the innate antiviral response by inhibition of IRF-3 degradation. Cell. Mol. Biol. (Noisy-le-grand) 2006, 52, 29–41. [Google Scholar]
- Lazear, H.M.; Lancaster, A.; Wilkins, C.; Suthar, M.S.; Huang, A.; Vick, S.C.; Clepper, L.; Thackray, L.; Brassil, M.M.; Virgin, H.W.; et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Hollingsworth, J.; Lau, A.; Tone, A.; Kollara, A.; Allen, L.; Colgan, T.J.; Dube, V.; Rosen, B.; Murphy, K.J.; Greenblatt, E.M.; et al. BRCA1 Mutation Status and Follicular Fluid Exposure Alters NFκB Signaling and ISGylation in Human Fallopian Tube Epithelial Cells. Neoplasia 2018, 20, 697–709. [Google Scholar] [CrossRef]
- Kim, W.; Bennett, E.J.; Huttlin, E.L.; Guo, A.; Li, J.; Possemato, A.; Sowa, M.E.; Rad, R.; Rush, J.; Comb, M.J.; et al. Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome. Molecular Cell. 2011, 44, 325–340. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Sun, Q.; Chen, Z.J. E1-L2 activates both ubiquitin and FAT10. Mol. Cell. 2007, 27, 1014–1023. [Google Scholar] [CrossRef]
- Kim, K.I.; Yan, M.; Malakhova, O.; Luo, J.K.; Shen, M.F.; Zou, W.; de la Torre, J.C.; Zhang, D.E. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol. Cell. Biol. 2006, 26, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Beaudenon, S.L.; Kelley, M.L.; Waddell, M.B.; Yuan, W.; Schulman, B.A.; Huibregtse, J.M.; Krug, R.M. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc. Natl. Acad. Sci. USA 2004, 101, 7578–7582. [Google Scholar] [CrossRef]
- Kim, K.I.; Giannakopoulos, N.V.; Virgin, H.W.; Zhang, D.E. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol. Cell. Biol. 2004, 24, 9592–9600. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhang, D.E. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J. Biol. Chem. 2006, 281, 3989–3994. [Google Scholar] [CrossRef]
- Okumura, F.; Zou, W.; Zhang, D.E. ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP. Genes Dev 2007, 21, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.D.; Spyracopoulos, L.; Glover, J.N. Ubc13: The Lys63 ubiquitin chain building machine. Oncotarget 2016, 7, 64471–64504. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Harrison, J.S.; Lewis, S.M.; Kuhlman, B.; Kleiger, G. Mechanism of Lysine 48 Selectivity during Polyubiquitin Chain Formation by the Ube2R1/2 Ubiquitin-Conjugating Enzyme. Mol. Cell. Biol. 2016, 36, 1720–1732. [Google Scholar] [CrossRef]
- Xu, P.; Peng, J. Characterization of polyubiquitin chain structure by middle-down mass spectrometry. Anal. Chem. 2008, 80, 3438–3444. [Google Scholar] [CrossRef] [PubMed]
- Fujimuro, M.; Nishiya, T.; Nomura, Y.; Yokosawa, H. Involvement of polyubiquitin chains via specific chain linkages in stress response in mammalian cells. Biol. Pharm. Bull. 2005, 28, 2315–2318. [Google Scholar] [CrossRef][Green Version]
- Sowa, M.E.; Harper, J.W. From loops to chains: Unraveling the mysteries of polyubiquitin chain specificity and processivity. ACS Chem. Biol. 2006, 1, 20–24. [Google Scholar] [CrossRef]
- Minakawa, M.; Sone, T.; Takeuchi, T.; Yokosawa, H. Regulation of the nuclear factor (NF)-kappaB pathway by ISGylation. Biol. Pharm. Bull. 2008, 31, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhong, G.; Zhu, L.; Liu, X.; Shan, Y.; Feng, H.; Bu, Z.; Chen, H.; Wang, C. Herc5 attenuates influenza A virus by catalyzing ISGylation of viral NS1 protein. J. Immunol. 2010, 184, 5777–5790. [Google Scholar] [CrossRef]
- Fan, J.B.; Arimoto, K.; Motamedchaboki, K.; Yan, M.; Wolf, D.A.; Zhang, D.E. Identification and characterization of a novel ISG15-ubiquitin mixed chain and its role in regulating protein homeostasis. Sci. Rep. 2015, 5, 12704. [Google Scholar] [CrossRef]
- Yim, H.Y.; Yang, Y.; Lim, J.-S.; Lee, M.S.; Zhang, D.-E.; Kim, K.I. The mitochondrial pathway and reactive oxygen species are critical contributors to interferon-α/β-mediated apoptosis in Ubp43-deficient hematopoietic cells. Biochem. Biophys. Res. Commun. 2012, 423, 436–440. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Bravo García-Morato, M.; Gruber, C.; Murias Loza, S.; Malik, M.N.H.; Alsohime, F.; Alakeel, A.; Valdez, R.; Buta, S.; Buda, G.; et al. Systemic Type I IFN Inflammation in Human ISG15 Deficiency Leads to Necrotizing Skin Lesions. Cell Rep. 2020, 31, 107633. [Google Scholar] [CrossRef]
- Speer, S.D.; Li, Z.; Buta, S.; Payelle-Brogard, B.; Qian, L.; Vigant, F.; Rubino, E.; Gardner, T.J.; Wedeking, T.; Hermann, M.; et al. ISG15 deficiency and increased viral resistance in humans but not mice. Nat. Commun. 2016, 7, 11496. [Google Scholar] [CrossRef]
- Zhang, X.; Bogunovic, D.; Payelle-Brogard, B.; Francois-Newton, V.; Speer, S.D.; Yuan, C.; Volpi, S.; Li, Z.; Sanal, O.; Mansouri, D.; et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 2015, 517, 89–93. [Google Scholar] [CrossRef]
- Mallette, F.A.; Richard, S. K48-linked ubiquitination and protein degradation regulate 53BP1 recruitment at DNA damage sites. Cell Res. 2012, 22, 1221–1223. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wee, S.; Gunaratne, J.; Lane, D.P.; Bulavin, D.V. Isg15 controls p53 stability and functions. Cell Cycle 2014, 13, 2200–2210. [Google Scholar] [CrossRef]
- Malakhova, O.A.; Zhang, D.E. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J. Biol. Chem. 2008, 283, 8783–8787. [Google Scholar] [CrossRef]
- Okumura, A.; Pitha, P.M.; Harty, R.N. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc. Natl. Acad. Sci. USA 2008, 105, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Yokosawa, H. ISG15 modification of Ubc13 suppresses its ubiquitin-conjugating activity. Biochem. Biophys. Res. Commun. 2005, 336, 9–13. [Google Scholar] [CrossRef]
- Komander, D.; Reyes-Turcu, F.; Licchesi, J.D.; Odenwaelder, P.; Wilkinson, K.D.; Barford, D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep 2009, 10, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, J.; Wang, M.; Fu, Z.; Klein, J.M.; Haas, A.L.; Kim, J.J. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J. Biol. Chem. 2005, 280, 27356–27365. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.W.; Tong, J.G.; Tom, S.K.; Szabo, P.A.; Cavanagh, P.C.; Dikeakos, J.D.; Haeryfar, S.M.; Barr, S.D. Interferon-induced HERC5 is evolving under positive selection and inhibits HIV-1 particle production by a novel mechanism targeting Rev/RRE-dependent RNA nuclear export. Retrovirology 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Malakhov, M.P.; Kim, K.I.; Malakhova, O.A.; Jacobs, B.S.; Borden, E.C.; Zhang, D.E. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J. Biol. Chem. 2003, 278, 16608–16613. [Google Scholar] [CrossRef]
- Przanowski, P.; Loska, S.; Cysewski, D.; Dabrowski, M.; Kaminska, B. ISG’ylation increases stability of numerous proteins including Stat1, which prevents premature termination of immune response in LPS-stimulated microglia. Neurochem. Int. 2018, 112, 227–233. [Google Scholar] [CrossRef]
- Shi, H.X.; Yang, K.; Liu, X.; Liu, X.Y.; Wei, B.; Shan, Y.F.; Zhu, L.H.; Wang, C. Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification. Mol. Cell. Biol. 2010, 30, 2424–2436. [Google Scholar] [CrossRef]
- Koike, A.; Tsujinaka, K.; Fujimori, K. Statins attenuate antiviral IFN-beta and ISG expression via inhibition of IRF3 and JAK/STAT signaling in poly(I:C)-treated hyperlipidemic mice and macrophages. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thery, F.; Wu, N.C.; Luhmann, E.K.; Dussurget, O.; Foecke, M.; Bredow, C.; Jiménez-Fernández, D.; Leandro, K.; Beling, A.; et al. The in vivo ISGylome links ISG15 to metabolic pathways and autophagy upon Listeria monocytogenes infection. Nat. Commun. 2019, 10, 5383. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.; Konishi, H.; Shimotohno, K. UbcH8 regulates ubiquitin and ISG15 conjugation to RIG-I. Mol. Immunol. 2008, 45, 1078–1084. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hwang, S.-Y.; Imaizumi, T.; Yoo, J.-Y. Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J. Virol. 2008, 82, 1474–1483. [Google Scholar] [CrossRef]
- Chiang, C.; Liu, G.; Gack, M.U. Viral Evasion of RIG-I-Like Receptor-Mediated Immunity through Dysregulation of Ubiquitination and ISGylation. Viruses 2021, 13, 182. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, T.; Xiao, J.; Zhu, K.; Wei, R.; Wu, Z.; Meng, H.; Li, Y.; Yuan, J. Modification of BECN1 by ISG15 plays a crucial role in autophagy regulation by type I IFN/interferon. Autophagy 2015, 11, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, J.; Radke, J.B.; Perng, Y.C.; McAllaster, M.; Lenschow, D.J.; Virgin, H.W.; Sibley, L.D. ISG15 Connects Autophagy and IFN-γ-Dependent Control of Toxoplasma gondii Infection in Human Cells. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Hadjebi, O.; Casas-Terradellas, E.; Garcia-Gonzalo, F.R.; Rosa, J.L. The RCC1 superfamily: From genes, to function, to disease. Biochim. Biophys. Acta 2008, 1783, 1467–1479. [Google Scholar] [CrossRef]
- Wang, Y.; Argiles-Castillo, D.; Kane, E.I.; Zhou, A.; Spratt, D.E. HECT E3 ubiquitin ligases-emerging insights into their biological roles and disease relevance. J. Cell. Sci. 2020, 133. [Google Scholar] [CrossRef]
- Lorenz, S. Structural mechanisms of HECT-type ubiquitin ligases. Biol Chem 2018, 399, 127–145. [Google Scholar] [CrossRef]
- Weber, J.; Polo, S.; Maspero, E. HECT E3 Ligases: A Tale With Multiple Facets. Front. Physiol. 2019, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kinnucan, E.; Wang, G.; Beaudenon, S.; Howley, P.M.; Huibregtse, J.M.; Pavletich, N.P. Structure of an E6AP-UbcH7 complex: Insights into ubiquitination by the E2-E3 enzyme cascade. Science 1999, 286, 1321–1326. [Google Scholar] [CrossRef]
- Verdecia, M.A.; Joazeiro, C.A.; Wells, N.J.; Ferrer, J.L.; Bowman, M.E.; Hunter, T.; Noel, J.P. Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol. Cell. 2003, 11, 249–259. [Google Scholar] [CrossRef]
- Ogunjimi, A.A.; Briant, D.J.; Pece-Barbara, N.; Le Roy, C.; Di Guglielmo, G.M.; Kavsak, P.; Rasmussen, R.K.; Seet, B.T.; Sicheri, F.; Wrana, J.L. Regulation of Smurf2 Ubiquitin Ligase Activity by Anchoring the E2 to the HECT Domain. Mol. Cell. 2005, 19, 297–308. [Google Scholar] [CrossRef]
- Maspero, E.; Mari, S.; Valentini, E.; Musacchio, A.; Fish, A.; Pasqualato, S.; Polo, S. Structure of the HECT:ubiquitin complex and its role in ubiquitin chain elongation. EMBO Rep. 2011, 12, 342–349. [Google Scholar] [CrossRef]
- Maspero, E.; Valentini, E.; Mari, S.; Cecatiello, V.; Soffientini, P.; Pasqualato, S.; Polo, S. Structure of a ubiquitin-loaded HECT ligase reveals the molecular basis for catalytic priming. Nat. Struct. Mol. Biol. 2013, 20, 696–701. [Google Scholar] [CrossRef]
- Pandya, R.K.; Partridge, J.R.; Love, K.R.; Schwartz, T.U.; Ploegh, H.L. A Structural Element within the HUWE1 HECT Domain Modulates Self-ubiquitination and Substrate Ubiquitination Activities*. J. Biol. Chem. 2010, 285, 5664–5673. [Google Scholar] [CrossRef] [PubMed]
- Kamadurai, H.B.; Souphron, J.; Scott, D.C.; Duda, D.M.; Miller, D.J.; Stringer, D.; Piper, R.C.; Schulman, B.A. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol. Cell. 2009, 36, 1095–1102. [Google Scholar] [CrossRef]
- Kamadurai, H.B.; Qiu, Y.; Deng, A.; Harrison, J.S.; Macdonald, C.; Actis, M.; Rodrigues, P.; Miller, D.J.; Souphron, J.; Lewis, S.M.; et al. Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. Elife 2013, 2, e00828. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Abe, T.; Minami, N.; Bawono, R.G.; Matsui, C.; Deng, L.; Fukuhara, T.; Matsuura, Y.; Shoji, I. ISGylation of Hepatitis C Virus NS5A Protein Promotes Viral RNA Replication via Recruitment of Cyclophilin A. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Woods, M.W.; Kelly, J.N.; Hattlmann, C.J.; Tong, J.G.K.; Xu, L.S.; Coleman, M.D.; Quest, G.R.; Smiley, J.R.; Barr, S.D. Human HERC5 restricts an early stage of HIV-1 assembly by a mechanism correlating with the ISGylation of Gag. Retrovirology 2011, 8, 95. [Google Scholar] [CrossRef]
- Scholte, F.E.M.; Zivcec, M.; Dzimianski, J.V.; Deaton, M.K.; Spengler, J.R.; Welch, S.R.; Nichol, S.T.; Pegan, S.D.; Spiropoulou, C.F.; Bergeron, É. Crimean-Congo Hemorrhagic Fever Virus Suppresses Innate Immune Responses via a Ubiquitin and ISG15 Specific Protease. Cell Rep. 2017, 20, 2396–2407. [Google Scholar] [CrossRef]
- Akutsu, M.; Ye, Y.; Virdee, S.; Chin, J.W.; Komander, D. Molecular basis for ubiquitin and ISG15 cross-reactivity in viral ovarian tumor domains. Proc. Natl. Acad. Sci. USA 2011, 108, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Elkin, B.A.; van Kasteren, P.B.; Snijder, E.J.; Kikkert, M.; Mark, B.L. Viral OTU deubiquitinases: A structural and functional comparison. PLoS Pathog. 2014, 10, e1003894. [Google Scholar] [CrossRef] [PubMed]
- Deaton, M.K.; Dzimianski, J.V.; Daczkowski, C.M.; Whitney, G.K.; Mank, N.J.; Parham, M.M.; Bergeron, E.; Pegan, S.D. Biochemical and Structural Insights into the Preference of Nairoviral DeISGylases for Interferon-Stimulated Gene Product 15 Originating from Certain Species. J. Virol. 2016, 90, 8314–8327. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.N.; Azzinaro, P.; Ramirez-Medina, E.; Gutkoska, J.; Fang, Y.; Diaz-San Segundo, F.; de Los Santos, T. Impairment of the DeISGylation Activity of Foot-and-Mouth Disease Virus Lpro Causes Attenuation In Vitro and In Vivo. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Swaim, C.D.; Canadeo, L.A.; Monte, K.J.; Khanna, S.; Lenschow, D.J.; Huibregtse, J.M. Modulation of Extracellular ISG15 Signaling by Pathogens and Viral Effector Proteins. Cell. Rep. 2020, 31, 107772. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, E.T.; Kim, Y.E.; Lee, M.K.; Kwon, K.M.; Kim, K.I.; Stamminger, T.; Ahn, J.H. Consecutive Inhibition of ISG15 Expression and ISGylation by Cytomegalovirus Regulators. PLoS Pathog. 2016, 12, e1005850. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Mohr, I. Restriction of Human Cytomegalovirus Replication by ISG15, a Host Effector Regulated by cGAS-STING Double-Stranded-DNA Sensing. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, Y.J.; Kim, Y.E.; Han, T.H.; Milbradt, J.; Marschall, M.; Ahn, J.H. Transmembrane Protein pUL50 of Human Cytomegalovirus Inhibits ISGylation by Downregulating UBE1L. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hsiang, T.-Y.; Kuo, R.-L.; Krug, R.M. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2253–2258. [Google Scholar] [CrossRef]
- Clasman, J.R.; Everett, R.K.; Srinivasan, K.; Mesecar, A.D. Decoupling deISGylating and deubiquitinating activities of the MERS virus papain-like protease. Antiviral Res. 2020, 174, 104661. [Google Scholar] [CrossRef]
- Zhang, W.; Bailey-Elkin, B.A.; Knaap, R.C.M.; Khare, B.; Dalebout, T.J.; Johnson, G.G.; van Kasteren, P.B.; McLeish, N.J.; Gu, J.; He, W.; et al. Potent and selective inhibition of pathogenic viruses by engineered ubiquitin variants. PLoS Pathog. 2017, 13, e1006372. [Google Scholar] [CrossRef]
- Meng, W.; Gao, S.J. Targeting XPO1 enhances innate immune response and inhibits KSHV lytic replication during primary infection by nuclear stabilization of the p62 autophagy adaptor protein. Cell Death Dis. 2021, 12, 29. [Google Scholar] [CrossRef]
- Dai, L.; Bai, L.; Lin, Z.; Qiao, J.; Yang, L.; Flemington, E.K.; Zabaleta, J.; Qin, Z. Transcriptomic analysis of KSHV-infected primary oral fibroblasts: The role of interferon-induced genes in the latency of oncogenic virus. Oncotarget 2016, 7, 47052–47060. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.R.; Stopford, C.M.; West, J.A.; Bennett, C.L.; Giffin, L.; Damania, B. Kaposi’s Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway. J. Virol. 2015, 89, 11572–11583. [Google Scholar] [CrossRef]
- Ratia, K.; Kilianski, A.; Baez-Santos, Y.M.; Baker, S.C.; Mesecar, A. Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating activity of SARS-CoV papain-like protease. PLoS Pathog. 2014, 10, e1004113. [Google Scholar] [CrossRef]
- Báez-Santos, Y.M.; St John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Liu, G.; Lee, J.-H.; Parker, Z.M.; Acharya, D.; Chiang, J.J.; van Gent, M.; Riedl, W.; Davis-Gardner, M.E.; Wies, E.; Chiang, C.; et al. ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nat. Microbiol. 2021, 6, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Baldanta, S.; Fernández-Escobar, M.; Acín-Perez, R.; Albert, M.; Camafeita, E.; Jorge, I.; Vázquez, J.; Enríquez, J.A.; Guerra, S. ISG15 governs mitochondrial function in macrophages following vaccinia virus infection. PLoS Pathog. 2017, 13, e1006651. [Google Scholar] [CrossRef] [PubMed]
- Eduardo-Correia, B.; Martínez-Romero, C.; García-Sastre, A.; Guerra, S. ISG15 is counteracted by vaccinia virus E3 protein and controls the proinflammatory response against viral infection. J. Virol. 2014, 88, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Cáceres, A.; Knobeloch, K.-P.; Horak, I.; Esteban, M. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog. 2008, 4, e1000096. [Google Scholar] [CrossRef] [PubMed]
- Oudshoorn, D.; van Boheemen, S.; Sanchez-Aparicio, M.T.; Rajsbaum, R.; Garcia-Sastre, A.; Versteeg, G.A. HERC6 is the main E3 ligase for global ISG15 conjugation in mouse cells. PLoS ONE 2012, 7, e29870. [Google Scholar] [CrossRef]
- Okumura, A.; Lu, G.; Pitha-Rowe, I.; Pitha, P.M. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 2006, 103, 1440–1445. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, J.Y. Inhibition of hepatitis C virus replication by IFN-mediated ISGylation of HCV-NS5A. J. Immunol. 2010, 185, 4311–4318. [Google Scholar] [CrossRef]
- Domingues, P.; Bamford, C.G.G.; Boutell, C.; McLauchlan, J. Inhibition of hepatitis C virus RNA replication by ISG15 does not require its conjugation to protein substrates by the HERC5 E3 ligase. J. Gen. Virol. 2015, 96, 3236–3242. [Google Scholar] [CrossRef]
- Zhao, C.; Collins, M.N.; Hsiang, T.Y.; Krug, R.M. Interferon-induced ISG15 pathway: An ongoing virus-host battle. Trends Microbiol. 2013, 21, 181–186. [Google Scholar] [CrossRef]
- Durfee, L.A.; Lyon, N.; Seo, K.; Huibregtse, J.M. The ISG15 conjugation system broadly targets newly synthesized proteins: Implications for the antiviral function of ISG15. Mol. Cell. 2010, 38, 722–732. [Google Scholar] [CrossRef]
- Sherpa, C.; Rausch, J.W.; Le Grice, S.F.J.; Hammarskjold, M.-L.; Rekosh, D. The HIV-1 Rev response element (RRE) adopts alternative conformations that promote different rates of virus replication. Nucleic Acids Res. 2015, 43, 4676–4686. [Google Scholar] [CrossRef]
- Paparisto, E.; Woods, M.W.; Coleman, M.D.; Moghadasi, S.A.; Kochar, D.S.; Tom, S.K.; Kohio, H.P.; Gibson, R.M.; Rohringer, T.J.; Hunt, N.R.; et al. Evolution-Guided Structural and Functional Analyses of the HERC Family Reveal an Ancient Marine Origin and Determinants of Antiviral Activity. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, K.; Li, S.; Yang, C.; Chen, L. Interferon stimulated gene 15 promotes Zika virus replication through regulating Jak/STAT and ISGylation pathways. Virus Res. 2020, 287, 198087. [Google Scholar] [CrossRef]
- Singh, P.K.; Guest, J.-M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.-S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Singh, S.; Farr, D.; Kumar, A. Interferon-stimulated gene 15 (ISG15) restricts Zika virus replication in primary human corneal epithelial cells. Ocul. Surf. 2019, 17, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Ling, L.; Nichol, S.T.; Hibberd, M.L. Whole-genome expression profiling reveals that inhibition of host innate immune response pathways by Ebola virus can be reversed by a single amino acid change in the VP35 protein. J. Virol. 2008, 82, 5348–5358. [Google Scholar] [CrossRef]
- Caballero, I.S.; Honko, A.N.; Gire, S.K.; Winnicki, S.M.; Melé, M.; Gerhardinger, C.; Lin, A.E.; Rinn, J.L.; Sabeti, P.C.; Hensley, L.E.; et al. In vivo Ebola virus infection leads to a strong innate response in circulating immune cells. BMC Genom. 2016, 17, 707. [Google Scholar] [CrossRef]
- Kotliar, D.; Lin, A.E.; Logue, J.; Hughes, T.K.; Khoury, N.M.; Raju, S.S.; Wadsworth, M.H., 2nd; Chen, H.; Kurtz, J.R.; Dighero-Kemp, B.; et al. Single-Cell Profiling of Ebola Virus Disease In Vivo Reveals Viral and Host Dynamics. Cell 2020, 183, 1383–1401.e1319. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.; Huber, B.R.; Liu, D.X.; Logue, J.P.; Hischak, A.M.W.; Hart, R.J.; Abbott, M.; Isic, N.; Hisada, Y.M.; Mackman, N.; et al. Quantification of Viral and Host Biomarkers in the Liver of Rhesus Macaques: A Longitudinal Study of Zaire Ebolavirus Strain Kikwit (EBOV/Kik). Am. J. Pathol. 2020, 190, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Hale, B.G.; van Boheemen, S.; Wolff, T.; Lenschow, D.J.; García-Sastre, A. Species-specific antagonism of host ISGylation by the influenza B virus NS1 protein. J. Virol. 2010, 84, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Sagum, C.A.; Bedford, M.T.; Sidhu, S.S.; Sudol, M.; Han, Z.; Harty, R.N. Chaperone-Mediated Autophagy Protein BAG3 Negatively Regulates Ebola and Marburg VP40-Mediated Egress. PLoS Pathog. 2017, 13, e1006132. [Google Scholar] [CrossRef]
- Dzimianski, J.V.; Beldon, B.S.; Daczkowski, C.M.; Goodwin, O.Y.; Scholte, F.E.M.; Bergeron, É.; Pegan, S.D. Probing the impact of nairovirus genomic diversity on viral ovarian tumor domain protease (vOTU) structure and deubiquitinase activity. PLoS Pathog. 2019, 15, e1007515. [Google Scholar] [CrossRef]
- Dzimianski, J.V.; Scholte, F.E.M.; Williams, I.L.; Langley, C.; Freitas, B.T.; Spengler, J.R.; Bergeron, É.; Pegan, S.D. Determining the molecular drivers of species-specific interferon-stimulated gene product 15 interactions with nairovirus ovarian tumor domain proteases. PLoS ONE 2019, 14, e0226415. [Google Scholar] [CrossRef]
- Frias-Staheli, N.; Giannakopoulos, N.V.; Kikkert, M.; Taylor, S.L.; Bridgen, A.; Paragas, J.; Richt, J.A.; Rowland, R.R.; Schmaljohn, C.S.; Lenschow, D.J.; et al. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2007, 2, 404–416. [Google Scholar] [CrossRef]
- Davies, F.G. A survey of Nairobi sheep disease antibody in sheep and goats, wild ruminants and rodents within Kenya. J. Hyg. (Lond) 1978, 81, 251–258. [Google Scholar] [CrossRef]
- James, T.W.; Frias-Staheli, N.; Bacik, J.-P.; Levingston Macleod, J.M.; Khajehpour, M.; García-Sastre, A.; Mark, B.L. Structural basis for the removal of ubiquitin and interferon-stimulated gene 15 by a viral ovarian tumor domain-containing protease. Proc. Natl. Acad. Sci. USA 2011, 108, 2222–2227. [Google Scholar] [CrossRef]
- Scholte, F.E.M.; Hua, B.L.; Spengler, J.R.; Dzimianski, J.V.; Coleman-McCray, J.D.; Welch, S.R.; McMullan, L.K.; Nichol, S.T.; Pegan, S.D.; Spiropoulou, C.F.; et al. Stable Occupancy of the Crimean-Congo Hemorrhagic Fever Virus-Encoded Deubiquitinase Blocks Viral Infection. mBio 2019, 10. [Google Scholar] [CrossRef]
- Devignot, S.; Kromer, T.; Mirazimi, A.; Weber, F. ISG15 overexpression compensates the defect of Crimean-Congo hemorrhagic fever virus polymerase bearing a protease-inactive ovarian tumor domain. PLoS Negl. Trop. Dis. 2020, 14, e0008610. [Google Scholar] [CrossRef] [PubMed]
- Dzimianski, J.V.; Mace, S.L.; Williams, I.L.; Freitas, B.T.; Pegan, S.D. Flipping the substrate preference of Hazara virus ovarian tumour domain protease through structure-based mutagenesis. Acta Cryst. D Struct. Biol. 2020, 76, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Aumayr, M.; Pruneda, J.N.; Visser, L.J.; Berryman, S.; Kueck, A.F.; Geurink, P.P.; Ovaa, H.; van Kuppeveld, F.J.M.; Tuthill, T.J.; et al. Irreversible inactivation of ISG15 by a viral leader protease enables alternative infection detection strategies. Proc. Natl. Acad. Sci. USA 2018, 115, 2371–2376. [Google Scholar] [CrossRef]
- Zhao, C.; Sridharan, H.; Chen, R.; Baker, D.P.; Wang, S.; Krug, R.M. Influenza B virus non-structural protein 1 counteracts ISG15 antiviral activity by sequestering ISGylated viral proteins. Nat. Commun. 2016, 7, 12754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, X.; Duan, Y.; Pan, X.; Sun, Y.; You, T.; Han, L.; Jin, Z.; Shang, W.; Yu, J.; et al. High-throughput screening identifies established drugs as SARS-CoV-2 PLpro inhibitors. Protein Cell 2021. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Huang, B.; Tang, J.; Liu, S.; Liu, M.; Ye, Y.; Liu, Z.; Xiong, Y.; Zhu, W.; Cao, D.; et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, R.; Park, Y.-I.; Cha, Y.-E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef]
- Li, D.; Luan, J.; Zhang, L. Molecular docking of potential SARS-CoV-2 papain-like protease inhibitors. Biochem. Biophys. Res. Commun. 2021, 538, 72–79. [Google Scholar] [CrossRef]
- Clemente, V.; D’Arcy, P.; Bazzaro, M. Deubiquitinating Enzymes in Coronaviruses and Possible Therapeutic Opportunities for COVID-19. Int. J. Mol. Sci. 2020, 21, 3492. [Google Scholar] [CrossRef]
- Lei, J.; Hilgenfeld, R. Structural and mutational analysis of the interaction between the Middle-East respiratory syndrome coronavirus (MERS-CoV) papain-like protease and human ubiquitin. Virol. Sin. 2016, 31, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Pitsillou, E.; Liang, J.; Hung, A.; Karagiannis, T.C. Inhibition of interferon-stimulated gene 15 and lysine 48-linked ubiquitin binding to the SARS-CoV-2 papain-like protease by small molecules: In silico studies. Chem. Phys. Lett. 2021, 771, 138468. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Hossain, M.U.; Bhattacharjee, A.; Emon, M.T.H.; Chowdhury, Z.M.; Ahammad, I.; Mosaib, M.G.; Moniruzzaman, M.; Rahman, M.H.; Islam, M.N.; Ahmed, I.; et al. Novel mutations in NSP-1 and PLPro of SARS-CoV-2 NIB-1 genome mount for effective therapeutics. J. Genet. Eng. Biotechnol. 2021, 19, 52. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).