Spontaneous Abortion and Chikungunya Infection: Pathological Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Procedures and Sample Collection
2.2. Clinical Case Descriptions
2.3. Histopathological Analysis
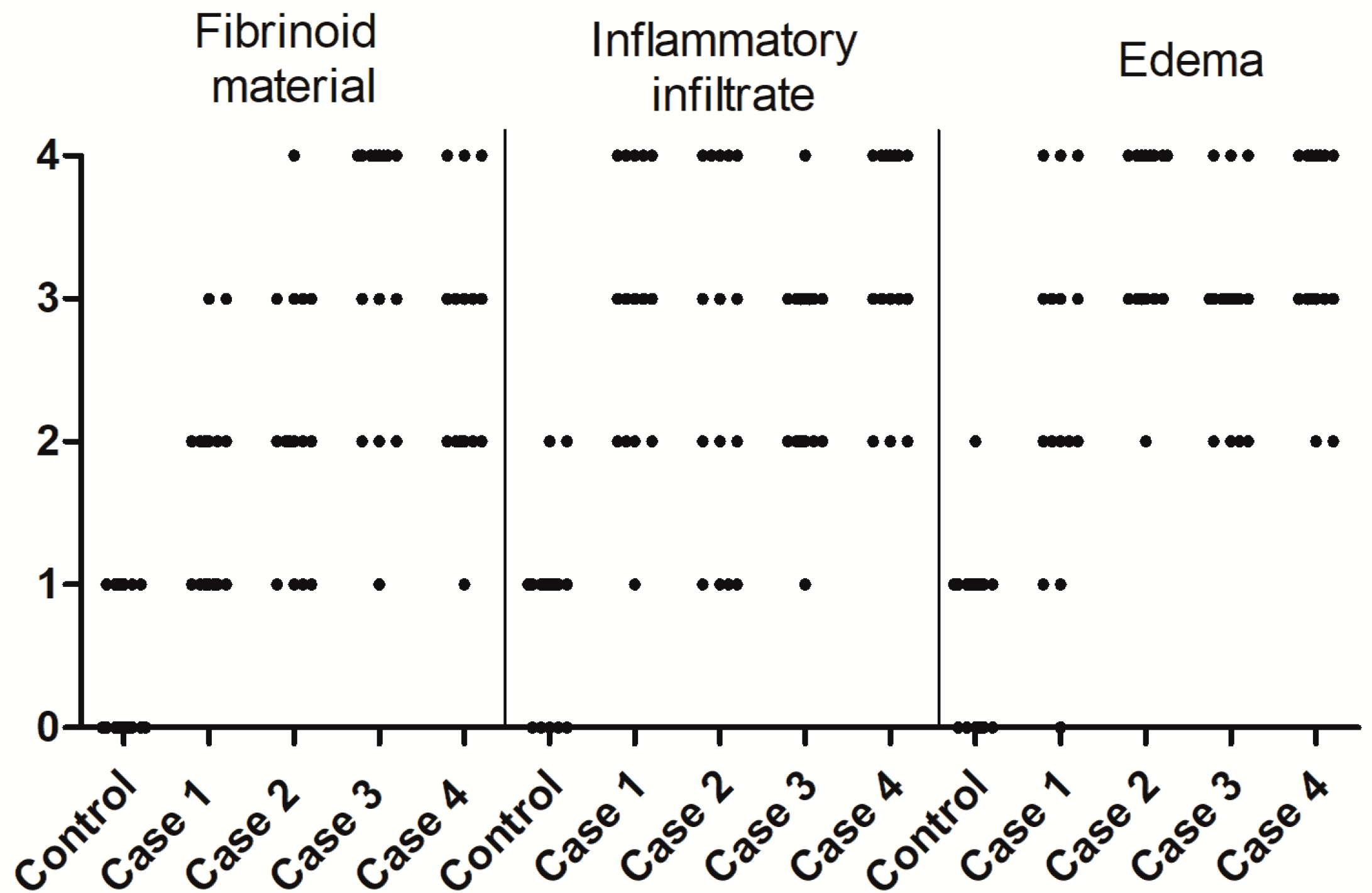
2.4. Quantitative Analysis
2.5. Immunohistochemistry Techniques
2.6. Electron Microscopy Assay
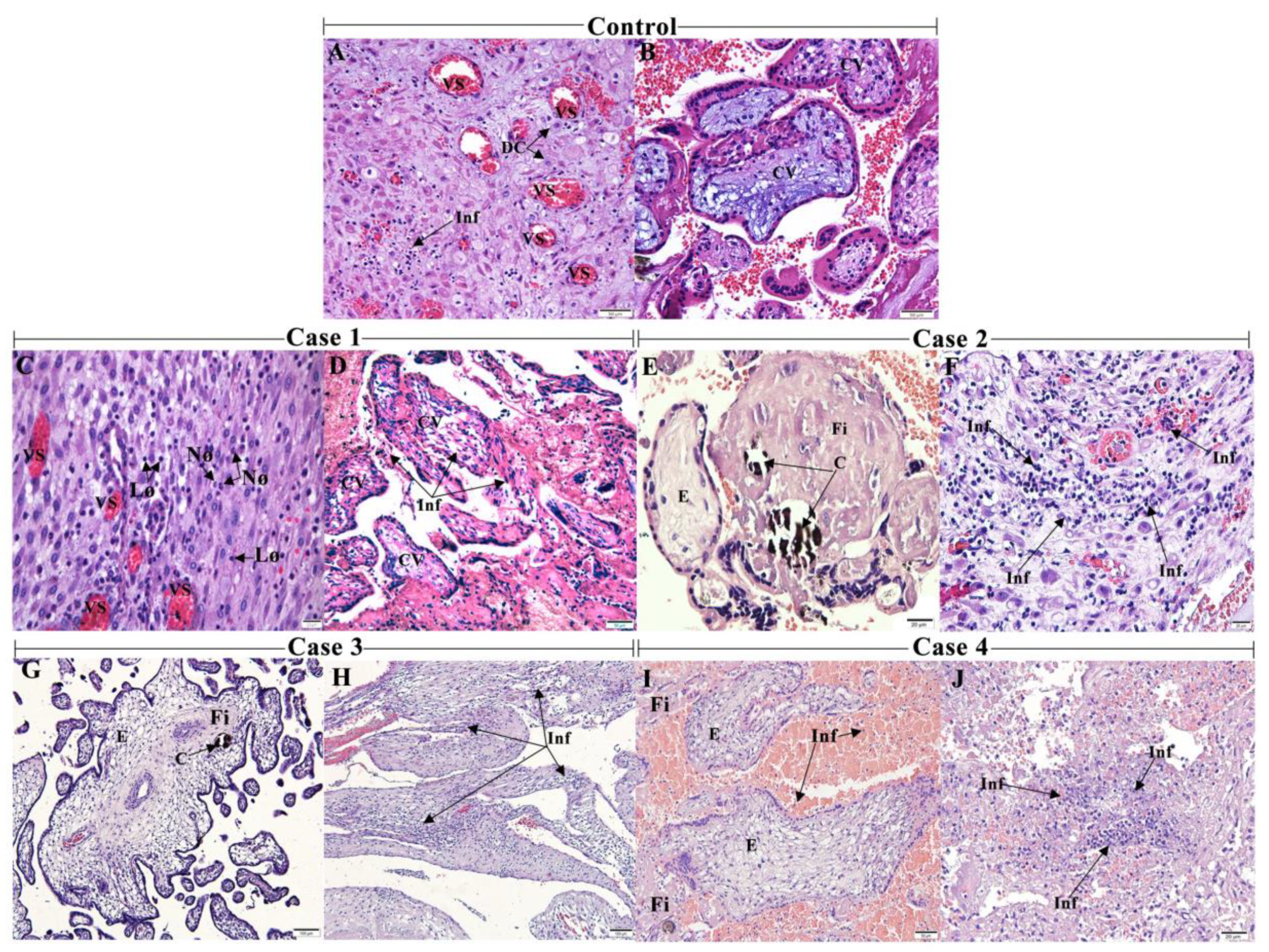
3. Results
3.1. CHIKV Infection Led to an Inflammatory Environment
3.2. CHIKV Antigen Detection in Abortion Material
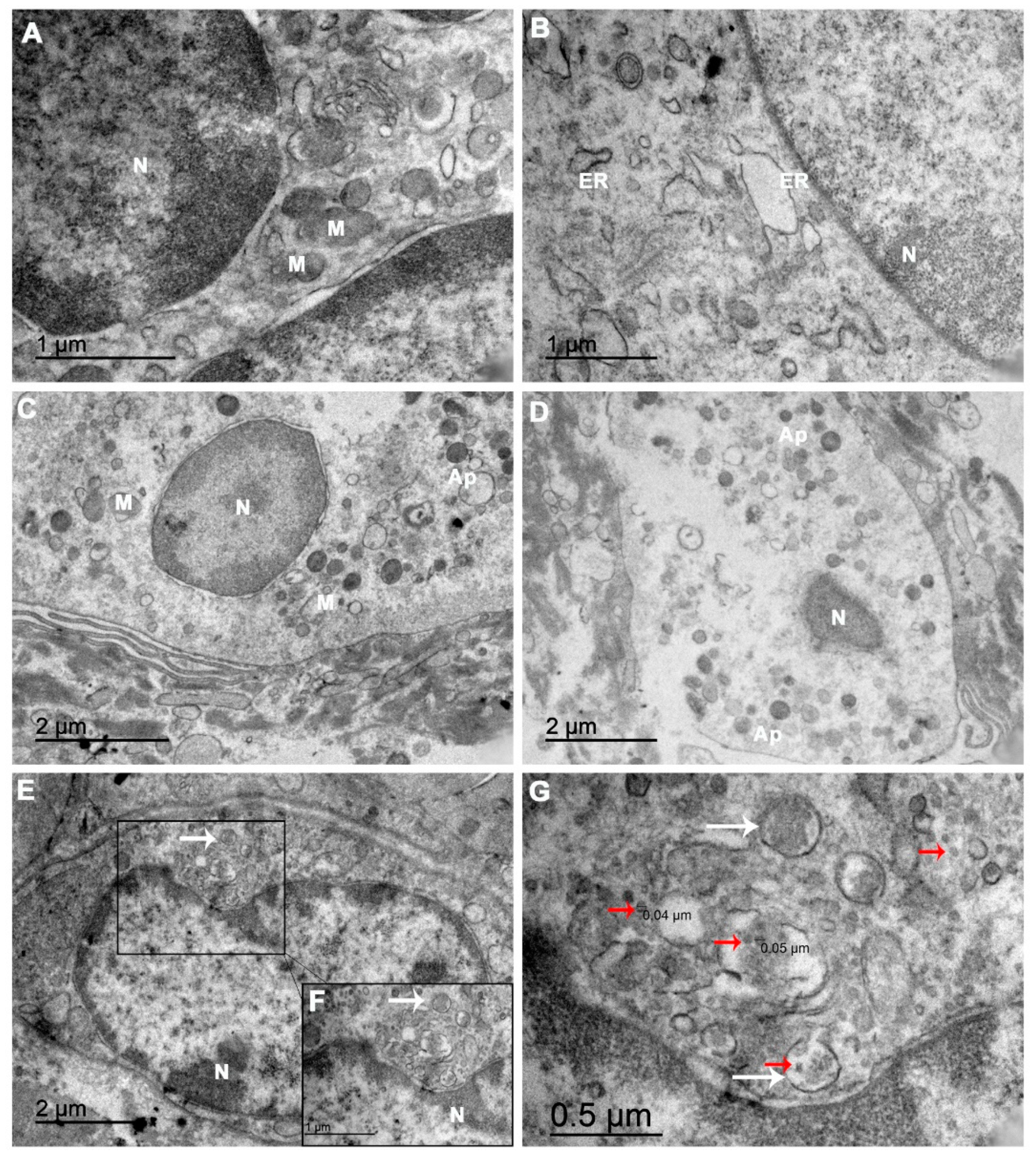
3.3. Ultrastructure of the Abortion Material
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S. Evolutionary influences in arboviral disease. Curr. Top. Microbiol. Immunol. 2006, 299, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.D.; Suarez, L.-A.C.; Labayo, H.K.M.; Liles, V.R.; Salvoza, N.C.; Klinzing, D.C.; Daroy, M.L.G.; Matias, R.R.; Natividad, F.F. Complete genome sequence of Chikungunya virus isolated in the Philippines. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, M.M.; Kannan, S.; Kawalekar, O.U.; Shedlock, D.J.; Khan, A.S.; Sarangan, G.; Srikanth, P.; Weiner, D.B.; Muthumani, K. Chikungunya: A potentially emerging epidemic? PLoS Negl. Trop. Dis. 2010, 4, e623. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Genet. 2010, 8, 491–500. [Google Scholar] [CrossRef]
- Chhabra, M.; Mittal, V.; Bhattacharya, D.; Rana, U.; Lal, S. Chikungunya fever: A re-emerging viral infection. Indian J. Med. Microbiol. 2008, 26, 5–12. [Google Scholar] [CrossRef]
- Borgherini, G.; Poubeau, P.; Staikowsky, F.; Lory, M.; Le Moullec, N.; Becquart, J.P.; Wengling, C.; Michault, A.; Paganin, F. Outbreak of Chikungunya on Reunion Island: Early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 2007, 44, 1401–1407. [Google Scholar] [CrossRef]
- Paganin, F.; Borgherini, G.; Staikowsky, F.; Arvin-Berod, C.; Poubeau, P. Chikungunya à l’île de la Réunion: Chronique d’une épidémie annoncée. Presse Médicale 2006, 35, 641–646. [Google Scholar] [CrossRef]
- Ramful, D.; Carbonnier, M.; Pasquet, M.; Bouhmani, B.; Ghazouani, J.; Noormahomed, T.; Beullier, G.; Attali, T.; Samperiz, S.; Fourmaintraux, A.; et al. Mother-to-child transmission of Chikungunya virus infection. Pediatr. Infect. Dis. J. 2007, 26, 811–815. [Google Scholar] [CrossRef]
- Robillard, P.-Y.; Boumahni, B.; Gérardin, P.; Michault, A.; Fourmaintraux, A.; Schuffenecker, I.; Carbonnier, M.; Djémili, S.; Choker, G.; Roge-Wolter, M.; et al. Vertical maternal fetal transmission of the chikungunya virus. Ten cases among 84 pregnant women. Presse Med. 2006, 35, 785–788. [Google Scholar] [CrossRef]
- Gérardin, P.; Sampériz, S.; Ramful, D.; Boumahni, B.; Bintner, M.; Alessandri, J.-L.; Carbonnier, M.; Tiran-Rajaoefera, I.; Beullier, G.; Boya, I.; et al. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: The CHIMERE Cohort Study on Reunion Island. PLoS Negl. Trop. Dis. 2014, 8, e2996. [Google Scholar] [CrossRef]
- Bandeira, A.C.; Campos, G.S.; Sardi, S.I.; Rocha, V.F.D.; Rocha, G.C.M. Neonatal encephalitis due to Chikungunya vertical transmission: First report in Brazil. IDCases 2016, 5, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Barau, G.; Michault, A.; Bintner, M.; Randrianaivo, H.; Choker, G.; Lenglet, Y.; Touret, Y.; Bouveret, A.; Grivard, P.; et al. Multidisciplinary prospective study of mother-to-child Chikungunya virus infections on the Island of La Réunion. PLoS Med. 2008, 5, e60. [Google Scholar] [CrossRef] [PubMed]
- Evans-Gilbert, T. Case report: Chikungunya and neonatal immunity: Fatal vertically transmitted chikungunya infection. Am. J. Trop. Med. Hyg. 2017, 96, 913–915. [Google Scholar] [PubMed]
- Touret, Y.; Randrianaivo, H.; Michault, A.; Schuffenecker, I.; Kauffmann, E.; Lenglet, Y.; Barau, G.; Fourmaintraux, A. Early maternal-fetal transmission of the Chikungunya virus. Presse Médicale 2006, 35, 1656. [Google Scholar] [CrossRef]
- Nunes, P.; Nogueira, R.; Coelho, J.; Rodrigues, F.; Salomão, N.; José, C.; De Carvalho, J.; Rabelo, K.; De Azeredo, E.; Basíl-io-De-oliveira, R.; et al. A stillborn multiple organs’ investigation from a maternal denv-4 infection: Histopathological and in-flammatory mediators characterization. Viruses 2019, 11, 319. [Google Scholar] [CrossRef]
- Nunes, P.C.G.; Paes, M.V.; de Oliveira, C.A.B.; Soares, A.C.G.; de Filippis, A.M.B.; Lima, M.d.R.Q.; de Barcelos Alves, A.M.; da Silva, J.F.A.; de Oliveira Coelho, J.M.C.; de Carvalho Rodrigues, F.d.C.; et al. Detection of dengue NS1 and NS3 proteins in placenta and umbilical cord in fetal and maternal death. J. Med. Virol. 2016, 88, 1448–1452. [Google Scholar] [CrossRef]
- Platt, D.J.; Smith, A.M.; Arora, N.; Diamond, M.S.; Coyne, C.; Miner, J.J. Zika virus–related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 2018, 10, eaao7090. [Google Scholar] [CrossRef]
- Rabelo, K.; de Souza Campos Fernandes, R.C.; De Souza, L.J.; De Souza, T.L.; Dos Santos, F.B.; Guerra Nunes, P.C.; De Azeredo, E.L.; Salomão, N.G.; Trindade, G.F.; Basílio-De-Oliveira, C.A.; et al. Placental histopathology and clinical presentation of severe congenital Zika Syndrome in a human immunodeficiency virus-exposed uninfected infant. Front. Immunol. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Kumanomido, T.; Wada, R.; Kanemaru, T.; Kamada, M.; Akiyama, Y.; Matumoto, M. Transplacental infection in mice inocu-lated with Getah virus. Vet. Microbiol. 1988, 16, 129–136. [Google Scholar] [CrossRef]
- Shibata, I.; Hatano, Y.; Nishimura, M.; Suzuki, G.; Inaba, Y. Isolation of Getah virus from dead fetuses extracted from a nat-urally infected sow in Japan. Vet. Microbiol. 1991, 27, 385–391. [Google Scholar] [CrossRef]
- Milner, A.R.; Marshall, I.D. Pathogenesis of in utero infections with abortogenic and non-abortogenic alphaviruses in mice. J. Virol. 1984, 50, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Lai Kuen, R.; Malassiné, A.; Tricottet, V.; Blain, P.; Vidaud, M.; Evain-Brion, D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab. Investig. 2001, 81, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Grivard, P.; Le Roux, K.; Laurent, P.; Fianu, A.; Perrau, J.; Gigan, J.; Hoarau, G.; Grondin, N.; Staikowsky, F.; Favier, F.; et al. Molecular and serological diagnosis of Chikungunya virus infection. Pathol. Biol. 2007, 55, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Prata-Barbosa, A.; Cleto-Yamane, T.L.; Robaina, J.R.; Guastavino, A.B.; De Magalhães-Barbosa, M.C.; Brindeiro, R.D.M.; Medronho, R.D.A.; Da Cunha, A.J.L.A. Co-infection with Zika and Chikungunya viruses associated with fetal death—A case report. Int. J. Infect. Dis. 2018, 72, 25–27. [Google Scholar] [CrossRef]
- Dulay, A.T. Spontaneous Abortion. 2020. MSD Manual. Available online: https://www.msdmanuals.com/professional/gynecology-and-obstetrics/abnormalities-of-pregnancy/spontaneous-abortion (accessed on 15 January 2021).
- Andersen, A.-M.N.; Wohlfahrt, J.; Christens, P.; Olsen, J.; Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 2000, 320, 1708–1712. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Updat. 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Kovalovszki, L.; Villányi, E.; Benkó, G. Placental villous edema: A possible cause of antenatal hypoxia. Acta Paediatr. Hung. 1990, 30, 209–215. [Google Scholar]
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS 2018, 126, 570–588. [Google Scholar] [CrossRef]
- Tindall, V.R.; Scott, J.S. Placental calcification a study of 3,025 singleton and multiple pregnancies. BJOG Int. J. Obstet. Gynaecol. 1965, 72, 356–373. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, L.R.; Lee, Y.H. Exploring the relationship between preterm placental calcification and adverse maternal and fetal outcome. Ultrasound Obstet. Gynecol. 2011, 37, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Mehrjardi, M.Z.; Shobeirian, F. The role of the placenta in prenatally acquired Zika virus infection. VirusDisease 2017, 28, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, R.; Lisman, B.; Boer, K.; Bleker, O.; Groningen, K.; Van Wely, M.; Exalto, N. Histological classification of chorionic villous vascularization in early pregnancy. Hum. Reprod. 2006, 21, 1291–1294. [Google Scholar] [CrossRef]
- Redline, R.W.; Pappin, A. Fetal thrombotic vasculopathy: The clinical significance of extensive avascular villi. Hum. Pathol. 1995, 26, 80–85. [Google Scholar] [CrossRef]
- Heider, A. Fetal vascular malperfusion. Arch. Pathol. Lab. Med. 2017, 141, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Scheimberg, I. Deaths: Placental and maternal conditions—Pathology. In Encyclopedia of Forensic and Legal Medicine, 2nd ed.; Elsevier: Amsterdarm, The Netherlands, 2015; ISBN 9780128000557. [Google Scholar]
- Redline, R.W. Classification of placental lesions. Am. J. Obstet. Gynecol. 2015, 213, S21–S28. [Google Scholar] [CrossRef]
- Pereira, L. Congenital viral infection: Traversing the uterine-placental interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. hCG: Biological functions and clinical applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araújo, I.M.T.C.; Cavalcante, M.C.A.; Lima, A.R.V.; Câmara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and Molecular Immune Response to Chikungunya Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jose, J.; Chipman, P.; Zhang, W.; Kuhn, R.J.; Baker, T.S. Molecular links between the E2 envelope glycoprotein and nucleocapsid core in Sindbis virus. J. Mol. Biol. 2011, 414, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Noranate, N.; Takeda, N.; Chetanachan, P.; Sittisaman, P.; A-Nuegoonpipat, A.; Anantapreecha, S. Characterization of Chikungunya virus-like particles. PLoS ONE 2014, 9, e108169. [Google Scholar] [CrossRef] [PubMed]
- Simizu, B.; Yamamoto, K.; Hashimoto, K.; Ogata, T. Structural proteins of Chikungunya virus. J. Virol. 1984, 51, 254–258. [Google Scholar] [CrossRef]
- Kantor, A.M.; Grant, D.G.; Balaraman, V.; White, T.A.; Franz, A.W.E. Ultrastructural analysis of chikungunya virus dissem-ination from the midgut of the yellow fever mosquito, aedes aegypti. Viruses 2018, 10, 571. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomão, N.; Brendolin, M.; Rabelo, K.; Wakimoto, M.; de Filippis, A.M.; dos Santos, F.; Moreira, M.E.; Basílio-de-Oliveira, C.A.; Avvad-Portari, E.; Paes, M.; et al. Spontaneous Abortion and Chikungunya Infection: Pathological Findings. Viruses 2021, 13, 554. https://doi.org/10.3390/v13040554
Salomão N, Brendolin M, Rabelo K, Wakimoto M, de Filippis AM, dos Santos F, Moreira ME, Basílio-de-Oliveira CA, Avvad-Portari E, Paes M, et al. Spontaneous Abortion and Chikungunya Infection: Pathological Findings. Viruses. 2021; 13(4):554. https://doi.org/10.3390/v13040554
Chicago/Turabian StyleSalomão, Natália, Michelle Brendolin, Kíssila Rabelo, Mayumi Wakimoto, Ana Maria de Filippis, Flavia dos Santos, Maria Elizabeth Moreira, Carlos Alberto Basílio-de-Oliveira, Elyzabeth Avvad-Portari, Marciano Paes, and et al. 2021. "Spontaneous Abortion and Chikungunya Infection: Pathological Findings" Viruses 13, no. 4: 554. https://doi.org/10.3390/v13040554
APA StyleSalomão, N., Brendolin, M., Rabelo, K., Wakimoto, M., de Filippis, A. M., dos Santos, F., Moreira, M. E., Basílio-de-Oliveira, C. A., Avvad-Portari, E., Paes, M., & Brasil, P. (2021). Spontaneous Abortion and Chikungunya Infection: Pathological Findings. Viruses, 13(4), 554. https://doi.org/10.3390/v13040554






