The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It—When to Use It—How Does It Compare to PCR Based Assays?
Abstract
1. Introduction
2. Main Body
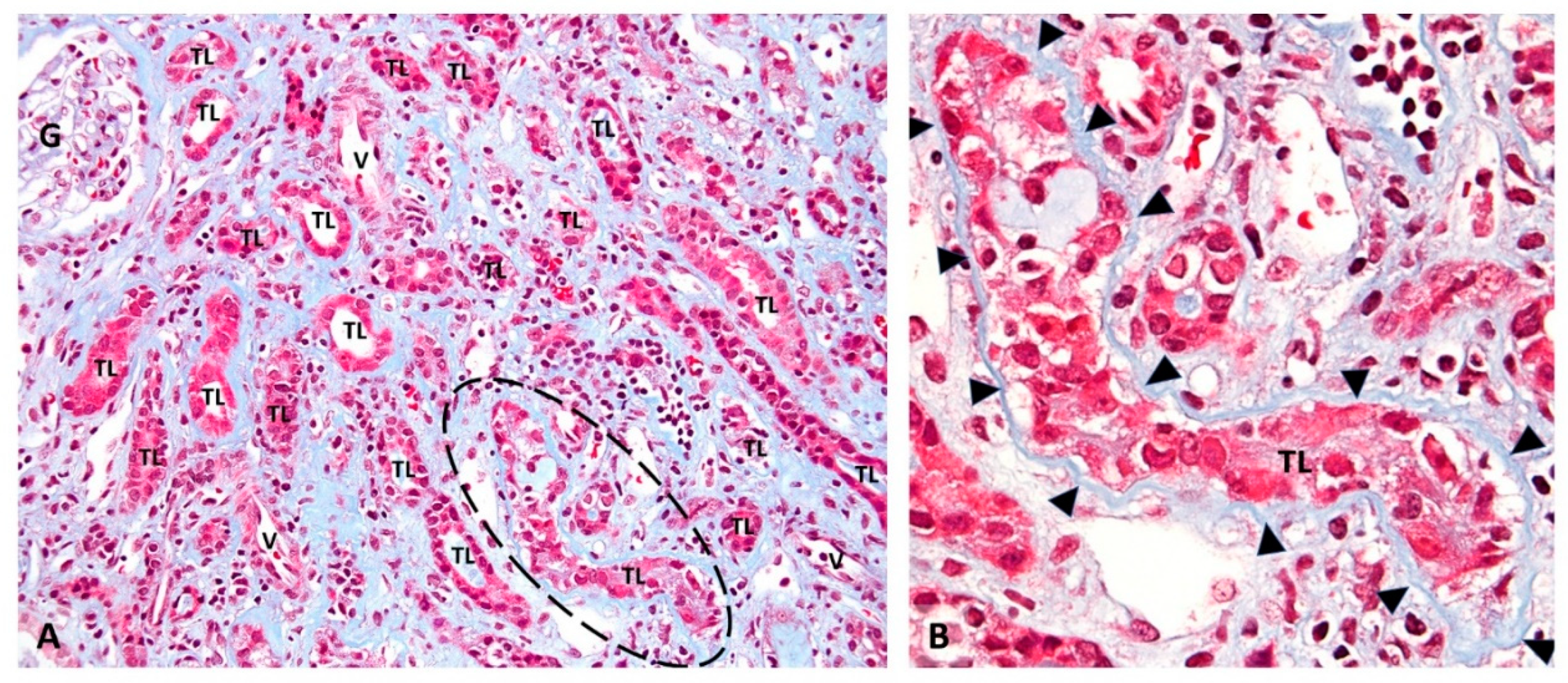
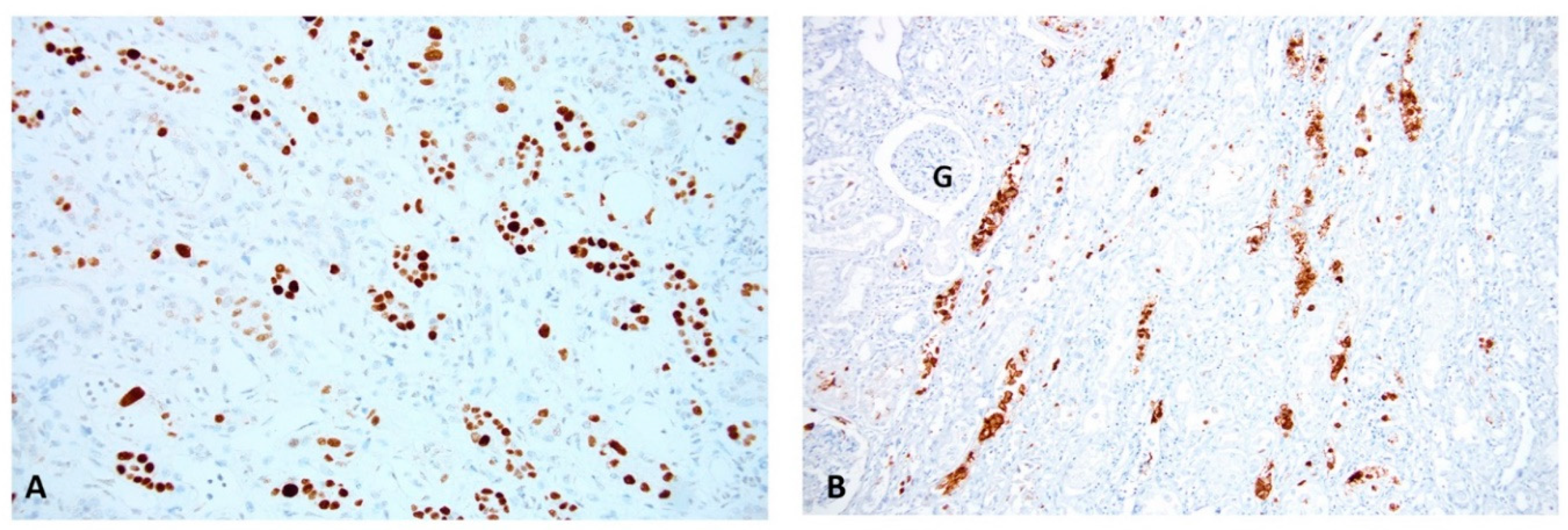
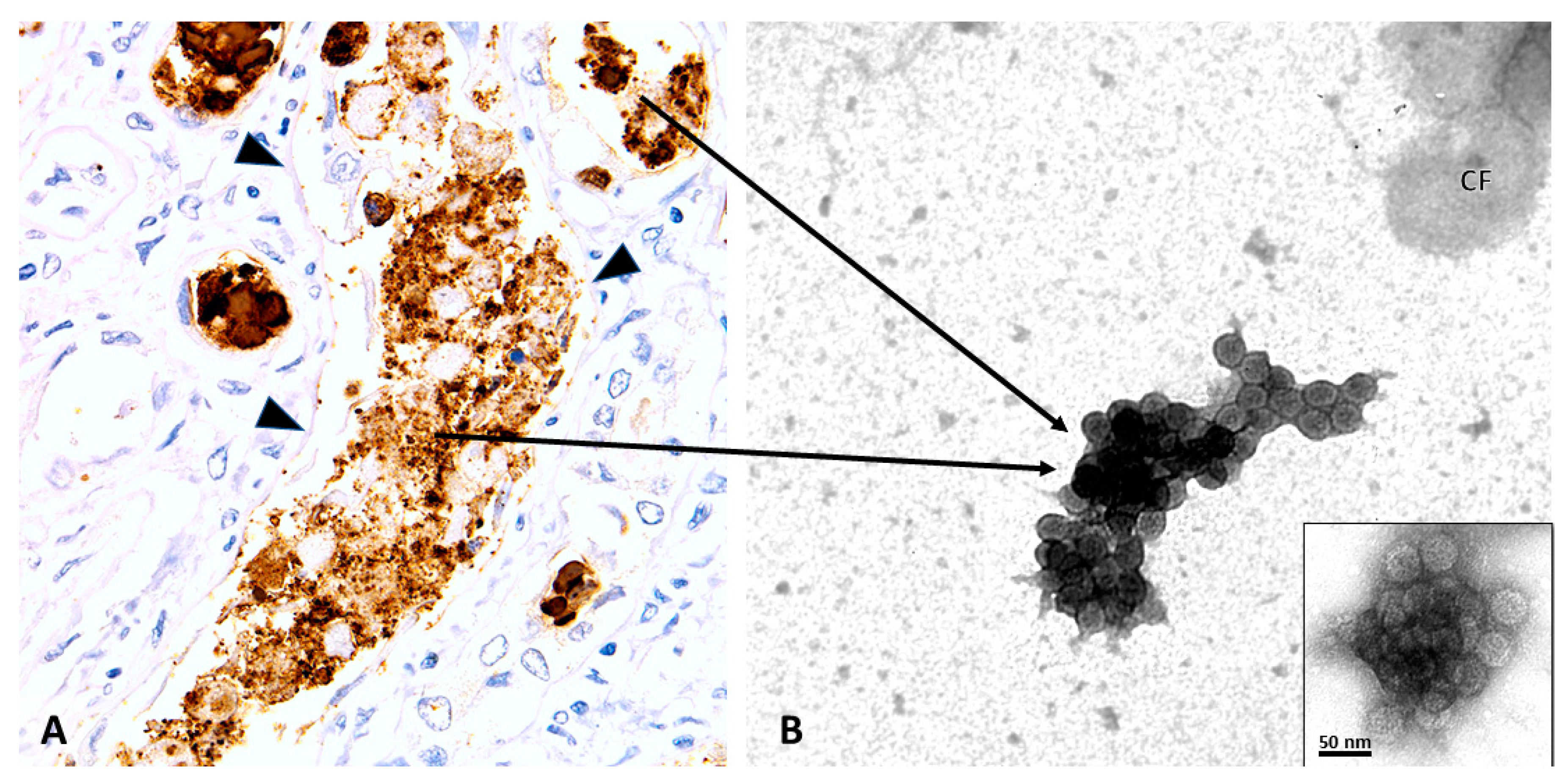
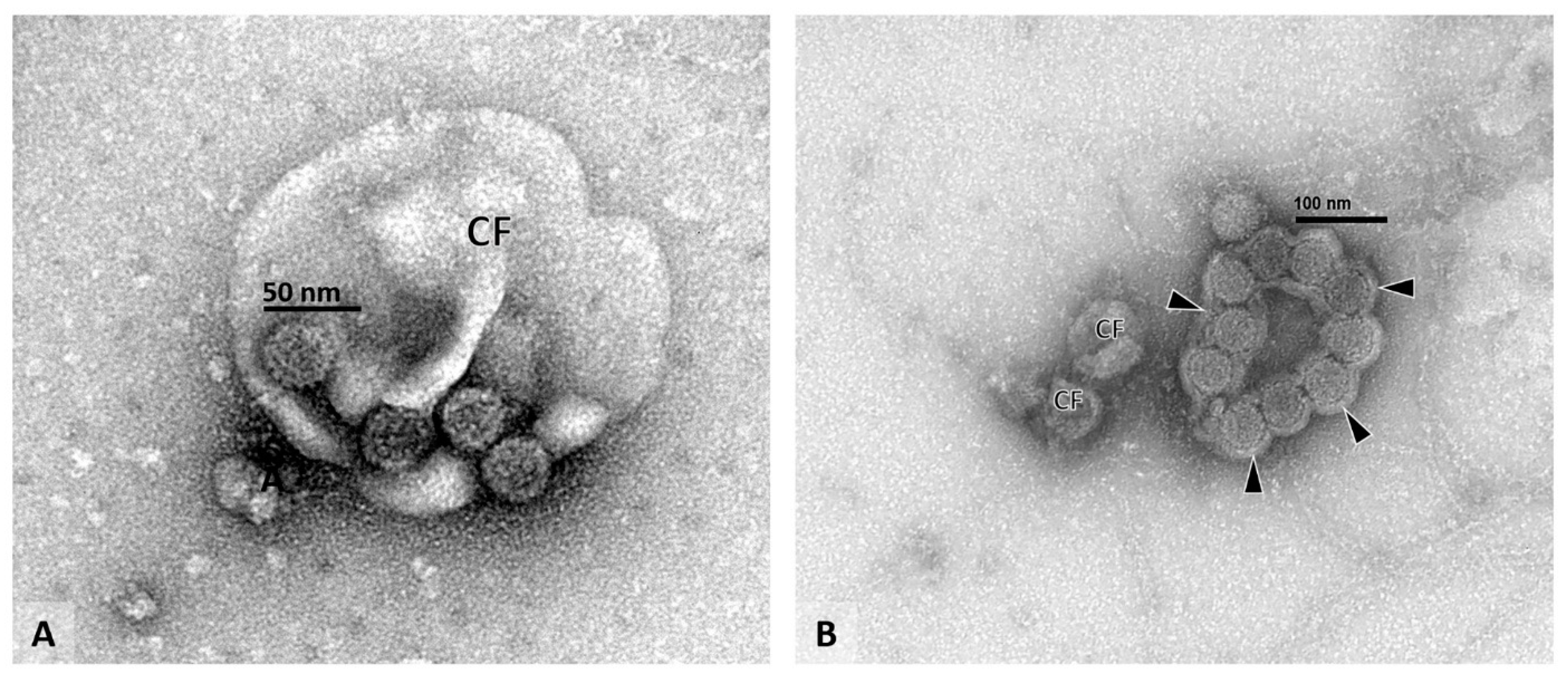
2.1. PyV-Haufen—Definition and Characteristics
2.2. Urinary PyV-Haufen Assays: A Qualitative, Quantitative and Comparative Test Analysis
2.2.1. PyV-Haufen Testing: Qualitative Assay
- Group 1, no PyV activation: 581/809 patients (72%):
- Decoy cell analysis negative or with no more than one positive decoy-test(cut-off for positive test ≥10 decoy cells per ThinPrep cytology preparation);
- BKPy-viremia undetectable or always less than 250 viral copies/mL plasma;
- No “definitive” PyVN in all available biopsies.
- Group 2, PyV activation with positive decoy-cell tests: 37/809 patients (5%):
- Decoy cell analysis positive with two or more positive decoy-tests(cut-off for positive test ≥10 decoy cells per ThinPrep cytology preparation);
- BKPy-viremia undetectable or always less than 250 viral copies/mL plasma;
- No “definitive” PyVN in all available biopsies.
- Group 3, PyV activation with low level BKPy-viremia: 78/809 patients (10%):
- Decoy cell test positive or negative;
- BKPy-viremia between 250 and 9999 viral copies/mL plasmain one or more tests, and no test ≥104 viral copies/mL plasma;
- No “definitive” PyVN in all available biopsies.
- Group 4, PyV activation with high level BKPy-viremia: 32/809 patients (4%):
- Decoy cell tests positive or negative;
- BKPy-viremia variable with at least one test ≥104 viral copies/mL plasma;
- No “definitive” PyVN in all available biopsies.
- Group 5, biopsy proven “definitive” PyVN: 81/809 patients (10%):
- Decoy cell tests positive or negative;
- BKPy-viremia variable;
- Biopsy diagnosis of PyVN in all patients.
2.2.2. PyV-Haufen Testing: Quantitative Assay
2.3. Urinary PyV-Haufen Testing: Clinical Indications
2.4. Urinary PyV-Haufen Testing: Technical Guidelines and Recommendations
2.4.1. Urine Collection and Storage
2.4.2. Grid Preparation for EM and Negative Staining Protocols
2.4.3. PyV-Haufen Detection by EM: Best Practice Recommendations
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BKPyV- | BK-polyomavirus strain |
| EM- | (transmission) electron microscopy |
| LM- | (standard) light microscopy |
| PCR- | (quantitative) polymerase chain reaction |
| PyV- | polyomavirus |
| Pvl- | histologic Banff score of intra renal polyomavirus load levels ranging from “0” (no PyV detected) to “3” (marked PyV load with >10% infected renal tubules) |
| PyVN- | polyomavirus nephropathy (biopsy proven definitive renal disease) |
| UNC | The University of North Carolina at Chapel Hill. |
References
- Boldorini, R.; Veggiani, C.; Barco, D.; Monga, G. Kidney and urinary tract polyomavirus infection and distribution: Molecular biology investigation of 10 consecutive autopsies. Arch. Pathol. Lab. Med. 2005, 129, 69–73. [Google Scholar] [PubMed]
- Dalianis, T.; Hirsch, H.H. Human polyomaviruses in disease and cancer. Virology 2013, 437, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Babel, N.; Comoli, P.; Friman, V.; Ginevri, F.; Jardine, A.; Lautenschlager, I.; Legendre, C.; Midtvedt, K.; Muñoz, P.; et al. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin. Microbiol. Infect. 2014, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.H.; Tylden, G.D.; Sharma, B.N. The human polyomavirus BK (BKPyV): Virological background and clinical implications. APMIS 2013, 121, 728–745. [Google Scholar] [CrossRef]
- Nickeleit, V.; Hirsch, H.H.; Zeiler, M.; Gudat, F.; Prince, O.; Thiel, G.; Mihatsch, M.J. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol. Dial. Transplant. 2000, 15, 324–332. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Kenan, D.J.; Mieczkowski, P.A. The two-faced nature of BK polyomavirus: Lytic infection or non-lytic large-T-positive carcinoma. J. Pathol. 2018, 246, 7–11. [Google Scholar] [CrossRef]
- Kenan, D.J.; Mieczkowski, P.A.; Calderon, R.B.; Singh, H.K.; Nickeleit, V. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J. Pathol. 2015, 237, 379–389. [Google Scholar] [CrossRef]
- Kenan, D.J.; Mieczkowski, P.A.; Latulippe, E.; Cote, I.; Singh, H.K.; Nickeleit, V. BK Polyomavirus Genomic Integration and Large T Antigen Expression: Evolving Paradigms in Human Oncogenesis. Am. J. Transplant. 2017, 17, 1674–1680. [Google Scholar] [CrossRef]
- Papadimitriou, J.C.; Randhawa, P.; Rinaldo, C.H.; Drachenberg, C.B.; Alexiev, B.; Hirsch, H.H. BK Polyomavirus Infection and Renourinary Tumorigenesis. Am. J. Transplant. 2016, 16, 398–406. [Google Scholar] [CrossRef]
- Gilis, L.; Morisset, S.; Billaud, G.; Leprêtre, S.D.; Wallet, H.L.; Nicolini, E.F.; Barraco, F.; Detrait, M.; Thomas, X.; Tedone, N. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2014, 49, 664–670. [Google Scholar] [CrossRef]
- Drachenberg, R.C.; Drachenberg, C.B.; Papadimitriou, J.C.; Ramos, E.; Fink, J.C.; Wali, R.; Weir, M.R.; Cangro, C.B.; Klassen, D.K.; Khaled, A.; et al. Morphological spectrum of polyoma virus disease in renal allografts: Diagnostic accuracy of urine cytology. Am. J. Transplant. 2001, 1, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Nickeleit, V.; Hirsch, H.H.; Binet, I.F.; Gudat, F.; Prince, O.; Dalquen, P.; Thiel, G.; Mihatsch, M.J. Polyomavirus infection of renal allograft recipients: From latent infection to manifest disease. J. Am. Soc. Nephrol. 1999, 10, 1080–1089. [Google Scholar] [PubMed]
- Mackenzie, E.F.; Poulding, J.M.; Harrison, P.R.; Amer, B. Human polyoma virus (HPV) a significant pathogen in renal transplantation. Proc. Eur. Dial. Transpl. Assoc. 1978, 15, 352–360. [Google Scholar]
- Hirsch, H.H.; Brennan, D.C.; Drachenberg, C.B.; Ginevri, F.; Gordon, J.; Limaye, A.P.; Mihatsch, M.J.; Nickeleit, V.; Ramos, E.; Randhawa, P.; et al. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 2005, 79, 1277–1286. [Google Scholar] [CrossRef]
- Nickeleit, V.; Mihatsch, M.J. Polyomavirus nephropathy in native kidneys and renal allografts: An update on an escalating threat. Transpl. Int. 2006, 19, 960–973. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Dadhania, D.; Cornea, V.; El-Husseini, A.; Castellanos, A.; Davis, V.G.; Waid, T.; Seshan, S.V. The 2018 Banff Working Group Classification of Definitive Polyomavirus Nephropathy: A Multi Center Validation Study in the Modern Era. Am. J. Transplant. 2020, 11. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Randhawa, P.; Drachenberg, C.B.; Bhatnagar, R.; Bracamonte, E.; Chang, A.; Chon, W.J.; Dadhania, D.; Davis, V.G.; et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J. Am. Soc. Nephrol. 2018, 29, 680–693. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020, 28, 15898. [Google Scholar]
- Sharma, S.G.; Nickeleit, V.; Herlitz, L.C.; de Gonzalez, A.K.; Stokes, M.B.; Singh, H.K.; Markowitz, G.S.; D’Agati, V.D. BK polyoma virus nephropathy in the native kidney. Nephrol. Dial. Transplant. 2013, 28, 620–631. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Knowles, W.; Dickenmann, M.; Passweg, J.; Klimkait, T.; Mihatsch, M.J.; Steiger, J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 2002, 347, 488–496. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Hirsch, H.H.; Papadimitriou, J.C.; Gosert, R.; Wali, R.K.; Munivenkatappa, R.; Nogueira, J.; Cangro, C.B.; Haririan, A.; Mendley, S.; et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: A prospective evaluation. Transplantation 2007, 84, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Milstone, A.; Vilchez, R.A.; Geiger, X.; Fogo, A.B.; Butel, J.S.; Dummer, S. Polyomavirus simian virus 40 infection associated with nephropathy in a lung-transplant recipient. Transplantation 2004, 77, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Wiegley, N.; Walavalkar, V.; Aujla, H.; Chen, L.X.; Huang, Y.; Lee, B.K.; Jen, K.Y. Clinicopathologic Characteristics of JC Virus Nephropathy in Kidney Transplant Recipients. Transplantation 2020, 17. [Google Scholar] [CrossRef]
- Binet, I.; Nickeleit, V.; Hirsch, H.H.; Prince, O.; Dalquen, P.; Gudat, F.; Mihatsch, M.J.; Thiel, G. Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation 1999, 67, 918–922. [Google Scholar] [CrossRef]
- Mengel, M.; Marwedel, M.; Radermacher, J.; Eden, G.; Schwarz, A.; Haller, H.; Kreipe, H. Incidence of polyomavirus-nephropathy in renal allografts: Influence of modern immunosuppressive drugs. Nephrol. Dial. Transplant. 2003, 18, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, L.; Liang, X.; Qiu, J.; Deng, R.; Li, J.; Chen, G.; Dong, Y.; Chen, L. Risk factors for BK virus infection and BK virus-associated nephropathy under the impact of intensive monitoring and pre-emptive immunosuppression reduction. Transplant. Proc. 2014, 46, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Weng, C.H.; Lai, W.C.; Wu, H.H.; Chen, Y.C.; Hung, C.C.; Yang, C.W.; Tian, Y.C. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation 2010, 89, 299–306. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Randhawa, P.S. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K. Polyomaviruses and disease: Is there more to know than viremia and viruria? Curr. Opin. Organ. Transplant. 2015, 20, 348–358. [Google Scholar] [CrossRef]
- Nickeleit, V.; Klimkait, T.; Binet, I.F.; Dalquen, P.; del Zenero, V.; Thiel, G.; Mihatsch, M.J.; Hirsch, H.H. Testing for polyomavirus type BK DNA in plasma to identify renal allograft recipients with viral nephropathy. N. Engl. J. Med. 2000, 342, 1309–1315. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Steiger, J. Polyomavirus BK. Lancet Infect. Dis. 2003, 3, 611–623. [Google Scholar] [CrossRef]
- Nickeleit, V.; True, K.; Detwiler, R.; Kozlowski, T.; Singh, H. Risk assessment for polyomavirus nephropathy using urine cytology and the detection of decoy cells: Cheap and efficient. Transplantation 2012, 94, e42–e44, author reply e45. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.K.; Bubendorf, L.; Mihatsch, M.J.; Drachenberg, C.B.; Nickeleit, V. Urine cytology findings of polyomavirus infections. Adv. Exp. Med. Biol. 2006, 577, 201–212. [Google Scholar] [PubMed]
- Hirsch, H.H.; Drachenberg, C.B.; Steiger, J.; Ramos, E. Polyomavirus Associated Nephropathy in Renal Transplantation: Critical Issues of Screening and Management. In Polyomaviruses and Human Diseases, 1st ed.; Ahsan, N., Ed.; Springer: New York, NY, USA, 2006; pp. 160–173. [Google Scholar]
- Ginevri, F.; Azzi, A.; Hirsch, H.H.; Basso, S.; Fontana, I.; Cioni, M.; Bodaghi, S.; Salotti, V.; Rinieri, A.; Botti, G.; et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am. J. Transplant. 2007, 7, 2727–2735. [Google Scholar] [CrossRef]
- Hardinger, K.L.; Koch, M.J.; Bohl, D.J.; Storch, G.A.; Brennan, D.C. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am. J. Transplant. 2010, 10, 407–415. [Google Scholar] [CrossRef]
- Schaub, S.; Hirsch, H.H.; Dickenmann, M.; Steiger, J.; Mihatsch, M.J.; Hopfer, H.; Mayr, M. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am. J. Transplant. 2010, 10, 2615–2623. [Google Scholar] [CrossRef]
- Huang, G.; Chen, L.Z.; Qiu, J.; Wang, C.X.; Fei, J.G.; Deng, S.X.; Li, J.; Chen, G.D.; Zhang, L.; Fu, Q.; et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: A single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin. Transplant. 2010, 24, 599–609. [Google Scholar] [CrossRef]
- Sawinski, D.; Forde, K.A.; Clark, J.T.; Patel, P.; Olivera, B.; Goral, S.; Bloom, R.D. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J. Am. Soc. Nephrol. 2015, 26, 966–975. [Google Scholar] [CrossRef]
- Devresse, A.; Tinel, C.; Vermorel, A.; Snanoudj, R.; Morin, L.; Avettand-Fenoel, V.; Amrouche, L.; Scemla, A.; Zuber, J.; Devresse, C.L. No clinical benefit of rapid versus gradual tapering of immunosuppression to treat sustained BK virus viremia after kidney transplantation: A single-center experience. Transpl. Int. 2019, 32, 481–492. [Google Scholar] [CrossRef]
- Dieplinger, G.; Everly, M.J.; Briley, K.P.; Haisch, C.E.; Bolin, P.; Maldonado, A.Q.; Kendrick, W.T.; Kendrick, S.A.; Morgan, C.; Terasaki, P.I.; et al. Onset and progression of de novo donor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl. Infect. Dis. 2015, 17, 848–858. [Google Scholar] [CrossRef]
- Singh, H.K.; Andreoni, K.A.; Madden, V.; True, K.; Detwiler, R.; Weck, K.; Nickeleit, V. Presence of urinary Haufen accurately predicts polyomavirus nephropathy. J. Am. Soc. Nephrol. 2009, 20, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.K.; Reisner, H.; Derebail, V.K.; Kozlowski, T.; Nickeleit, V. Polyomavirus nephropathy: Quantitative urinary polyomavirus-Haufen testing accurately predicts the degree of intrarenal viral disease. Transplantation 2015, 99, 609–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, H.K.; Donna Thompson, B.; Nickeleit, V. Viral Haufen are urinary biomarkers of polyomavirus nephropathy: New diagnostic strategies utilizing negative staining electron microscopy. Ultrastruct. Pathol. 2009, 33, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P. BK virus in solid organ transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 4), S136–S146. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef]
- Solez, K.; Axelsen, R.A.; Benediktsson, H.; Burdick, J.F.; Cohen, A.H.; Colvin, R.B.; Croker, B.P.; Droz, D.; Dunnill, M.S.; Halloran, P.F. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 1993, 44, 411–422. [Google Scholar] [CrossRef]
- Laskin, B.L.; Singh, H.K.; Beier, U.H.; Moatz, T.; Furth, S.L.; Bunin, N.; Witte, D.; Goebel, J.; Davies, S.M.; Dandoy, C.; et al. The Noninvasive Urinary Polyomavirus Haufen Test Predicts BK Virus Nephropathy in Children After Hematopoietic Cell Transplantation: A Pilot Study. Transplantation 2016, 100, e81–e87. [Google Scholar] [CrossRef]
- Nickeleit, V.; Brylawski, B.; Singh, H.; Rivier, L. Urinary Polyomavirus-Haufen Shedding in Mouse and Man: A Proof-of-Concept Study for a Non-Invasive Urine Biomarker for Polyomavirus Nephropathy. Lab. Investig. 2013, 93, 390A–391A. [Google Scholar]
- Nickeleit, V.; Singh, H.K.; Goldsmith, C.S.; Miller, S.E.; Kenan, D.J. BK virus-associated urinary bladder carcinoma in transplant recipients: Productive or nonproductive polyomavirus infections in tumor cells? Hum. Pathol. 2013, 44, 2870–2871. [Google Scholar] [CrossRef][Green Version]
- Singh, H.K.; Madden, V.; Shen, Y.J.; Thompson, D.; Nickeleit, V. Negative Staining Electron Microscopy of Urine for the Detection of Polyomavirus Infections. Ultrastruct. Pathol. 2006, 30, 329–338. [Google Scholar]
- Hayat, M.; Miller, S. Negative Staining; McGraw-Hill Publishing Company: New York, NY, USA, 1990; Volume 1, pp. 114–120. [Google Scholar]

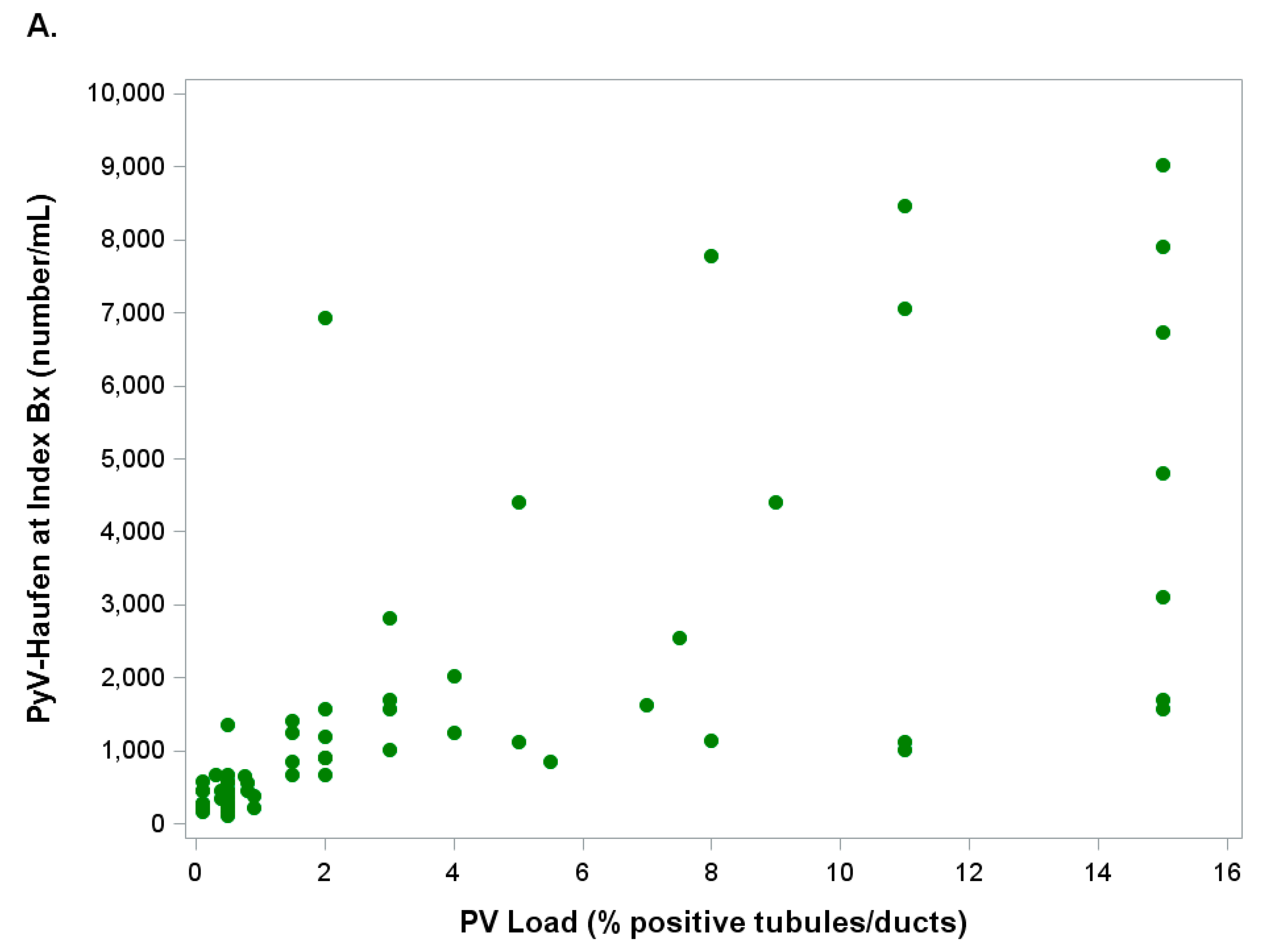
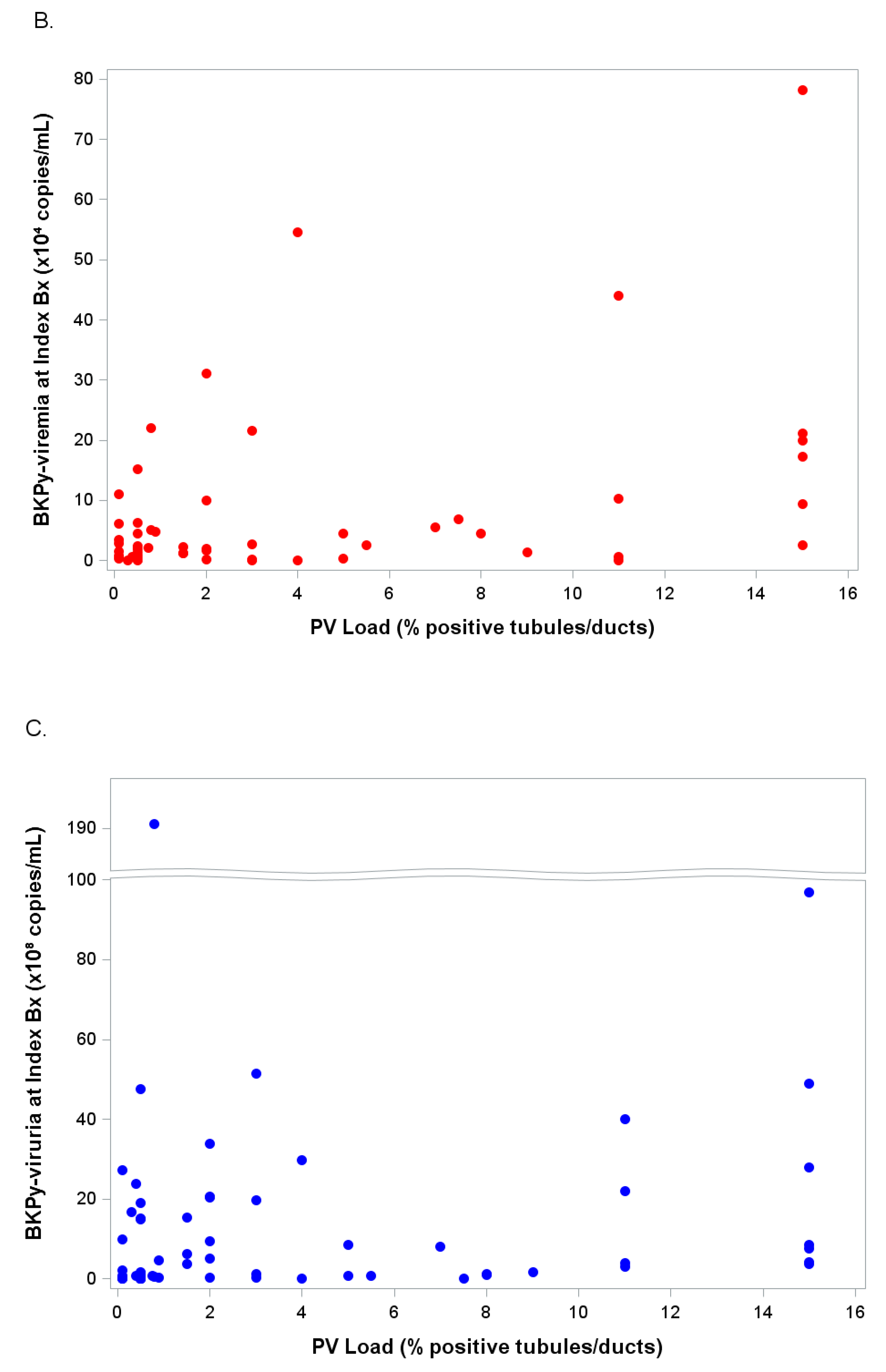
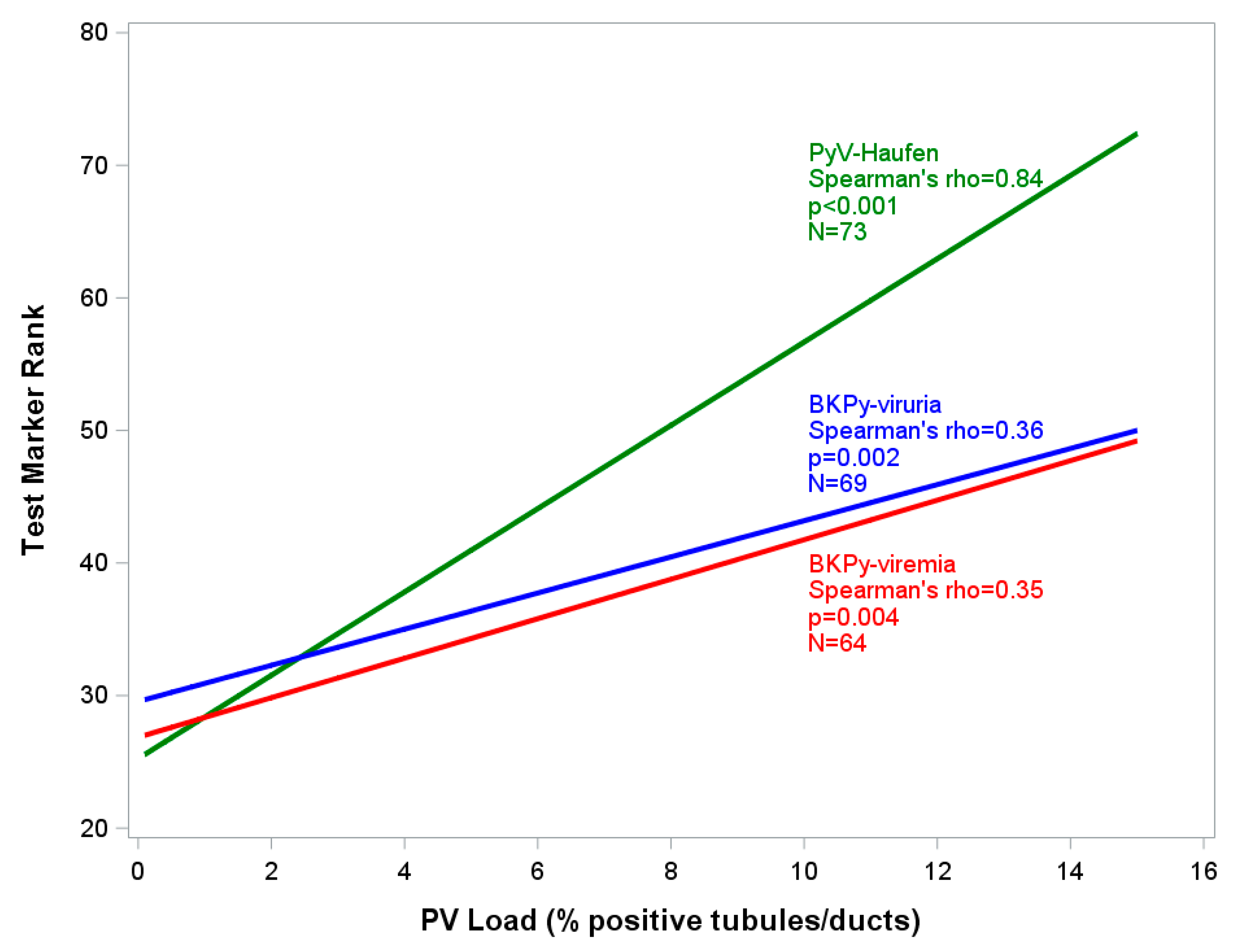
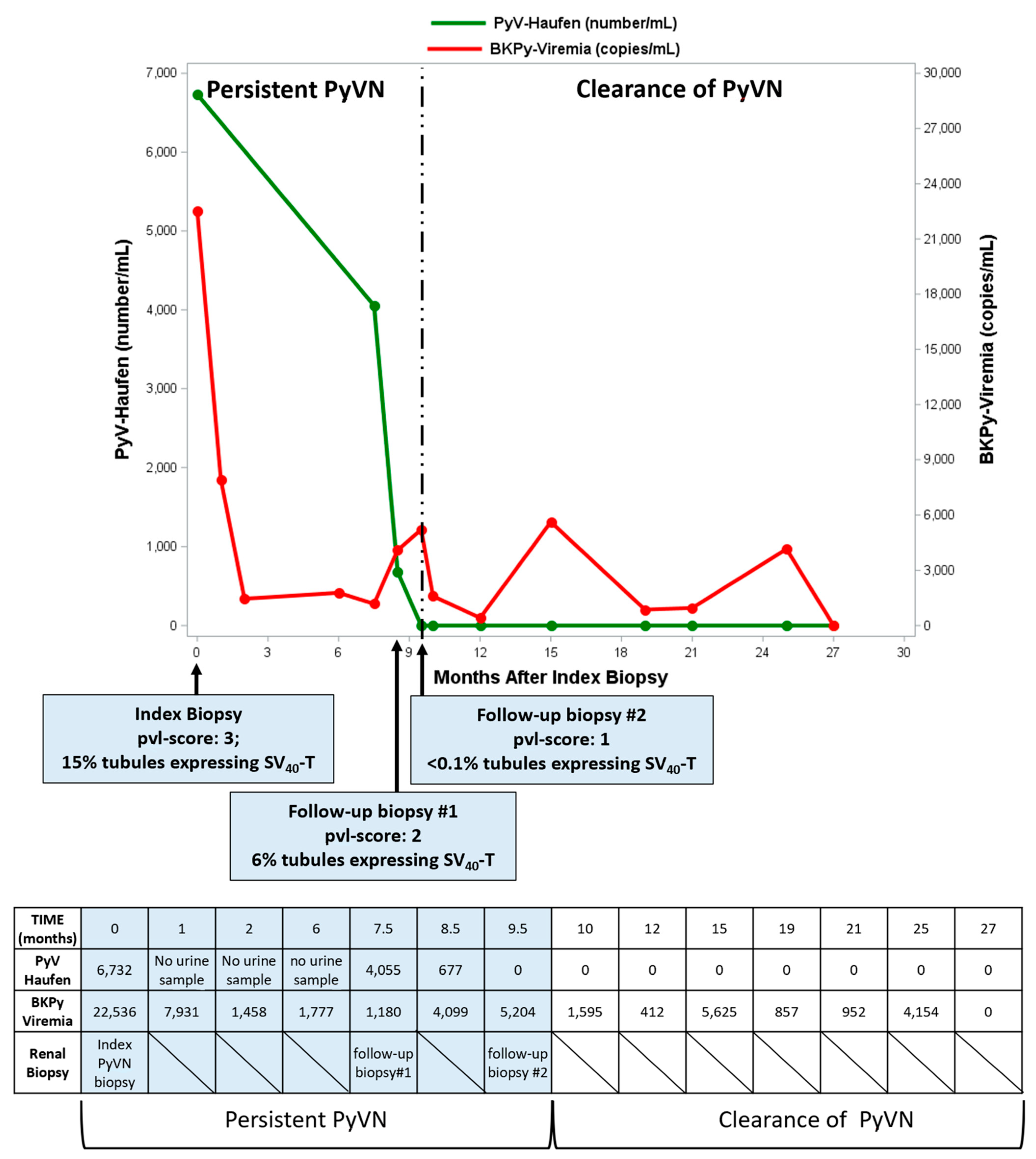
| Groups N (Patients in Group) | PyV-Haufen Positive N (%) | PyV Haufen Negative N (%) | N Patients Tested for PyV-Haufen |
|---|---|---|---|
| Group 1 N = 581 No PyV activation | 1 (6) | 16 (94) | 17 |
| Group 2 N = 37 PyV activation with positive decoy-cell tests | 0 (0) | 24 (100) | 24 |
| Group 3 N = 78 PyV activation with low level BKPy-viremia | 1 (2) | 56 (98) | 57 |
| Group 4 N = 32 PyV activation with high level BKPy-viremia | 0 (0) | 31 (100) | 31 |
| Group 5 N = 81 Biopsy proven “definitive” PyVN | 80 (99) | 1 (1) | 81 |
| N Patients tested | 82 | 128 | 210 |
| Test | Patients with “Definitive” PyVN N 2 | Patients with “Definitive” PyVN -and- Positive Test 3 N | Sensitivity (%) | Patients without “Definitive” PyVN N | Patients without “Definitive” PyVN -and- Negative Test 4 N | Specificity (%) |
|---|---|---|---|---|---|---|
| Urine cytology/Decoys | ||||||
| ≥2 tests with ≥10 cells | 60 | 30 | 50 | 114 | 62 | 54 |
| BKPy-viremia by PCR | ||||||
| ≥104 viral copies/mL | 61 | 40 | 66 | 102 | 82 | 80 |
| ≥250 viral copies/mL | 61 | 59 | 97 | 102 | 33 | 32 |
| Urinary PyV-Haufen | 64 | 64 | 100 | 118 | 116 | 98 |
| Test Marker (At Time of Index Biopsy) | Statistic | PyVN Disease Class (In Index Biopsy) | |||
|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | p-Value * | ||
| PyV Haufen | Median | 416.5 | 1411 | 7881 | <0.001 |
| (number/mL urine) | IQR | 282–451 | 903–3104 | 6732–9030 | |
| N | 32 | 39 | 2 | ||
| BKPy-viremia | Median | 1.39 | 2.74 | 11.83 | 0.098 |
| (×104 viral copies/mL) | IQR | 0.31–3.48 | 0.59–10.02 | 2.52–21.13 | |
| N | 27 | 35 | 2 | ||
| BKPy-viruria | Median | 0.82 | 5.70 | 3.84 | 0.086 |
| (×108 viral copies/mL) | IQR | 0.28–14.90 | 0.80–20.70 | 3.60–4.08 | |
| N | 29 | 38 | 2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nickeleit, V.; Davis, V.G.; Thompson, B.; Singh, H.K. The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It—When to Use It—How Does It Compare to PCR Based Assays? Viruses 2021, 13, 135. https://doi.org/10.3390/v13010135
Nickeleit V, Davis VG, Thompson B, Singh HK. The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It—When to Use It—How Does It Compare to PCR Based Assays? Viruses. 2021; 13(1):135. https://doi.org/10.3390/v13010135
Chicago/Turabian StyleNickeleit, Volker, Vicki G. Davis, Bawana Thompson, and Harsharan K. Singh. 2021. "The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It—When to Use It—How Does It Compare to PCR Based Assays?" Viruses 13, no. 1: 135. https://doi.org/10.3390/v13010135
APA StyleNickeleit, V., Davis, V. G., Thompson, B., & Singh, H. K. (2021). The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It—When to Use It—How Does It Compare to PCR Based Assays? Viruses, 13(1), 135. https://doi.org/10.3390/v13010135







