Abstract
Bovine herpesvirus 1 (BoHV-1) infected cell protein 0 (bICP0) is an important transcriptional regulatory protein that stimulates productive infection. In transient transfection assays, bICP0 also inhibits interferon dependent transcription. bICP0 can induce degradation of interferon stimulatory factor 3 (IRF3), a cellular transcription factor that is crucial for activating beta interferon (IFN-β) promoter activity. Recent studies also concluded that interactions between bICP0 and IRF7 inhibit trans-activation of IFN-β promoter activity. The C3HC4 zinc RING (really important new gene) finger located near the amino terminus of bICP0 is important for all known functions of bICP0. A recombinant virus that contains a single amino acid change in a well conserved cysteine residue of the C3HC4 zinc RING finger of bICP0 grows poorly in cultured cells, and does not reactivate from latency in cattle confirming that the C3HC4 zinc RING finger is crucial for viral growth and pathogenesis. A bICP0 deletion mutant does not induce plaques in permissive cells, but induces autophagy in a cell type dependent manner. In summary, the ability of bICP0 to stimulate productive infection, and repress IFN dependent transcription plays a crucial role in the BoHV-1 infection cycle.
1. Introduction
BoHV-1 infection can cause conjunctivitis, pneumonia, genital disorders, abortions, and an upper respiratory infection known as bovine respiratory disease (BRD) or “Shipping Fever” [136]. BoHV-1 initiates BRD by immunosuppressing cattle [24,48-50,152], which can lead to pneumonia as a result of secondary bacterial infections. BoHV-1 induced immune-suppression can result in secondary bacterial infections (Pasteurella haemolytica, Pasteurella multocida, and Haemophilus somnus for example) that cause life-threatening pneumonia [152]. BRDC and BoHV-1 infections costs the cattle industry at least $3 billion/year in the United States [1,15,24,48-50,71,79,122,136]. Modified live vaccines are available, and in general, they prevent clinical disease in adults. However, the same vaccine strains are immunosuppressive and can cause serious disease in young calves or abortions in pregnant cows.
Like other α-herpesvirinae subfamily members, BoHV-1 establishes lifelong latency in ganglionic neurons of the peripheral nervous system following acute replication in mucosal epithelium [146]. Virus reactivation and spread to other susceptible cattle occur after stress or corticosteroid treatment, which resembles stress [124,134]. There have been increases in BoHV-1 outbreaks in vaccinated feedlot cattle, which are the result of vaccine strains reactivating from latency [33,66,137,139]. The viral protein, bICP0, is crucial for productive infection and reactivation from latency. The bICP0 protein has two known functions that are important for stimulating productive infection and promoting viral pathogenesis: stimulating the activity of all viral promoters and inhibiting interferon dependent transcription. These bICP0 functions are the focus of this review.
2. Results and Discussion
2.1. bICP0 is encoded within immediate early transcript unit 1 (IEtu1)
Infection of permissive cells [28] or calves [145] leads to rapid cell death, in part due to apoptosis. Viral gene expression is temporally regulated in 3 distinct phases: immediate early (IE), early (E), or late (L). IE gene expression is stimulated by a virion component, bTIF, which interacts with a cellular transcription factor (Oct-1). The bTIF/Oct-1 complex binds to IE promoters and stimulates transcription [49].
Two IE transcription units exist: IEtu1 and IEtu2. IEtu1 encodes homologues of two human herpesvirus-1 (HHV-1) proteins, ICP0 and ICP4. IEtu2 encodes a protein similar to the HSV ICP22 protein [69]. IE proteins activate E gene expression, and viral DNA replication occurs. L gene expression then occurs, culminating in virion assembly and release. bICP0 is crucial for productive infection because it activates all viral promoters [43,147,149]. Relative to other alpha-herpesvirus members, the organization of the BoHV-1 ICP4 and ICP0 genes is unique because a common IE promoter (IEtu1) drives expression of bICP0 and bICP4 [147] (Figure 1B). bICP0 also contains an E promoter located near the 5’ end of the coding exon of bICP0, which is positively regulated by bICP0. Expression of bICP4 leads to repression of IEtu1 promoter activity. Thus bICP0 is considered to be the major viral regulatory protein that stimulates productive infection [43,147-149]. The bICP0 E promoter is activated by dexamethasone [150], a stimulus that induces reactivation from latency suggesting the E promoter plays a role in the early phases of reactivation from latency [75,76].
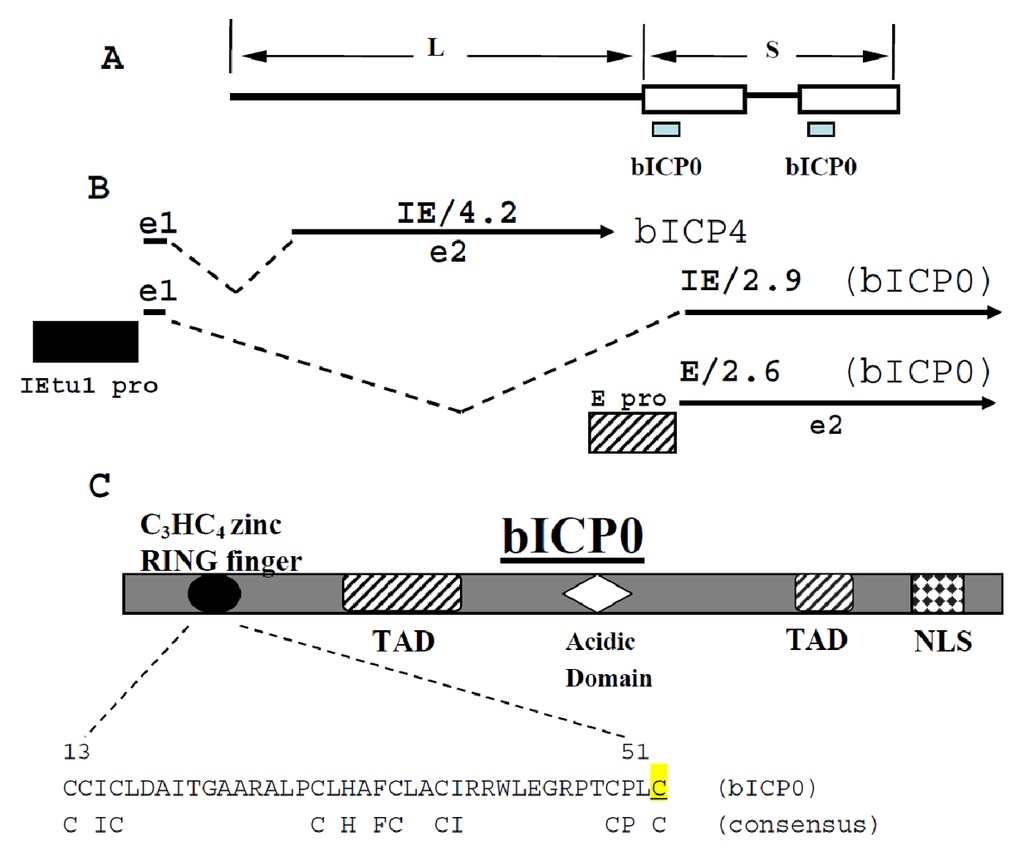
Figure 1.
Schematic of bICP0 gene within the BoHV-1 genome. Panel A: Schematic of BoHV-1 genome and location of bICP0 gene. The Unique Long (L) and Unique Short (S) regions of BoHV-1 are denoted. Panel B: Position of bICP4 and bICP0 transcripts is shown. The immediate early transcription unit 1 (IEtu1) encodes bICP4 (IE/4.2) and bICP0 (IE/2.9) [148,149]. The IEtu1 promoter activates IE expression of IE/4.2 and IE/2.9 (IEtu1 pro). E/2.6 is the early transcript that encodes bICP0 and an early promoter (E pro) activates expression of this transcript [147]. Exon 2 (e2) of bICP0 contains all of the protein coding sequences of bICP0. The dashed lines are intron sequences. Panel C: Schematic of bICP0 protein and known functional domains. The functional domains include a NLS (nuclear localization sequence, TAD (transcriptional activation domain), Acidic Domain, and the C3HC4 zinc RING finger. Amino acid sequence (residue 13-51) of the C3HC4 zinc RING finger in bICP0 is shown. The consensus residues in a C3HC4 zinc RING finger are also shown. The mutated cysteine at position 51 is highlighted in yellow and is underlined.
2.2. bICP0 contains multiple functional domains
bICP0 [70], HHV-1 encoded ICP0 [34-37,93], and equine herpesvirus 1 encoded ICP0 [16,17] contain a C3HC4 zinc RING finger near their N terminus that activates productive infection (Figure 1C). ICP0 [38-40,102,103] and bICP0 [70,114] localize with and disrupt promyelocytic leukemia (PML) protein-containing nuclear domains. The C3HC4 zinc RING finger domains located in bICP0 [29] and ICP0 [13,14,140] possess intrinsic E3 ubiquitin ligase activity. Thus, bICP0 and ICP0 can induce ubiquitin dependent proteolysis of specific proteins [38,40,85,115]. The specific proteins that are targets for proteolysis by bICP0 have not been identified.
bICP0 contains two transcriptional activation domains (TAD) that were identified by transposon insertion studies [157] (Figure 1C). A nuclear localization signal (NLS) is also located near the C-terminus of bICP0 and deletion of sequences encompassing the NLS impairs bICP0 function [128,129,157]. An acidic domain, which can be found in many transcriptional activators, is adjacent to the C3HC4 zinc RING finger. To date, the acidic domain does not appear to play a role in stimulating transcription or inhibiting IFN-dependent transcription [129,157].
2.3. bICP0 interacts with chromatin remodeling enzymes
The ability of bICP0 to interact with chromatin-remodeling enzymes correlates with activating viral transcription. For example, bICP0 interacts with histone deacetylase 1 (HDAC1), and can relieve HDAC1 induced repression of a model promoter [158]. Secondly, bICP0 interacts with p300, a histone acetyl transferase (HAT), and this interaction correlates with activating a late viral promoter [159]. Interestingly, a histone deacetylase inhibitor, trichostatin A, and HHV-1 encoded ICP0 has similar effects on cellular and viral gene expression [62]. Finally, HHV-1 encoded ICP0 interacts with class II HDACs [94]. Furthermore, ICP0 can interfere with histone deacetylation, which correlates with stimulating viral gene expression [52,121].
In general, the eight HDAC family members repress transcription by deacetylating histones [41,51,82,107,141]. HDACs are recruited to specific promoters by interacting with sequence specific DNA binding proteins [10,27,31,57,97,98]. Conversely, HAT family members activate transcription by acetylating histones [83], and non-histone transcription factors [18,25,53,67,101,111,135,156]. These studies suggested that interacts with bICP0 and complexes that contain chromatin-remodeling enzymes play a crucial role with respect to the ability of bICP0 to activate viral transcription.
2.4. Activation of the interferon pathway following infection
Infection of bovine cells or calves with BoHV-1 leads to a robust interferon (IFN) response [116]. Relative to HHV-1 or HHV-2, BoHV-1 DNA contains high levels of unmethylated CpG DNA [96], which can trigger the innate immune response via TLR9 [4,55]. Thus, BoHV-1 DNA alone can induce innate immune responses. Although it seems likely that additional viral encoded products can induce innate immune responses, they have not been identified. To give insights into the regulation of BoHV-1, a brief description of how HHV-1 regulates an innate immune response is summarized below.
Infection of cultured human cells with HHV-1 leads to production and secretion of interferon (IFN). The HHV-1 glycoprotein gD induces IFN-α production in mononuclear cells [80], in part, because HHV-1 activates IRF-3 (interferon response factor 3) in certain cell types [123]. Entry of enveloped viruses into cells, including HHV-1 and BoHV-1, also induces innate immune responses that are IFN independent [113]. ICP0, ICP34.5, and Us11 are the known HHV-1 genes that inhibit IFN activation after infection [89,108-110,120]. It seems clear that multiple mechanisms lead to innate immune responses following infection with BoHV-1 and HHV-1.
2.5. bICP0 inhibits beta interferon promoter (IFN-β) promoter activity
2.5.a. Activation of the IFN-β promoter is an early event after virus infection
Activation of the IFN-β promoter is an early response to viral infection [2,80,86] (summarized in Figure 2A). The IFN-β promoter contains three distinct transcription factor binding sites, AP-1 (activating protein 1), the IFN response element (ISRE), and NF-κB. These transcription factor-binding sites are necessary for induction of the IFN response following virus infection. Induction of toll like receptors can activate IRF-3 and the transcription factor NF-κB, and consequently the IFN-β promoter is activated [7,32]. Following virus infection, two cellular protein kinases, IKK-ε (I Kappa kinase epsilon) or TBK-1 (tank binding kinase 1), phosphorylate serine residues at the C-terminus of IRF3 (interferon response factors 3), which induces IRF3 homodimerization and nuclear translocation [42,133]. Nuclear IRF3 associates with other transcriptional activators resulting in direct binding and stimulation of IFN-β promoter activity [142]. IRF3 also directly binds several consensus DNA binding sites, including ISRE (IFN response elements), which stimulates transcription of IFN-stimulated genes in the absence of IFN [54,109].
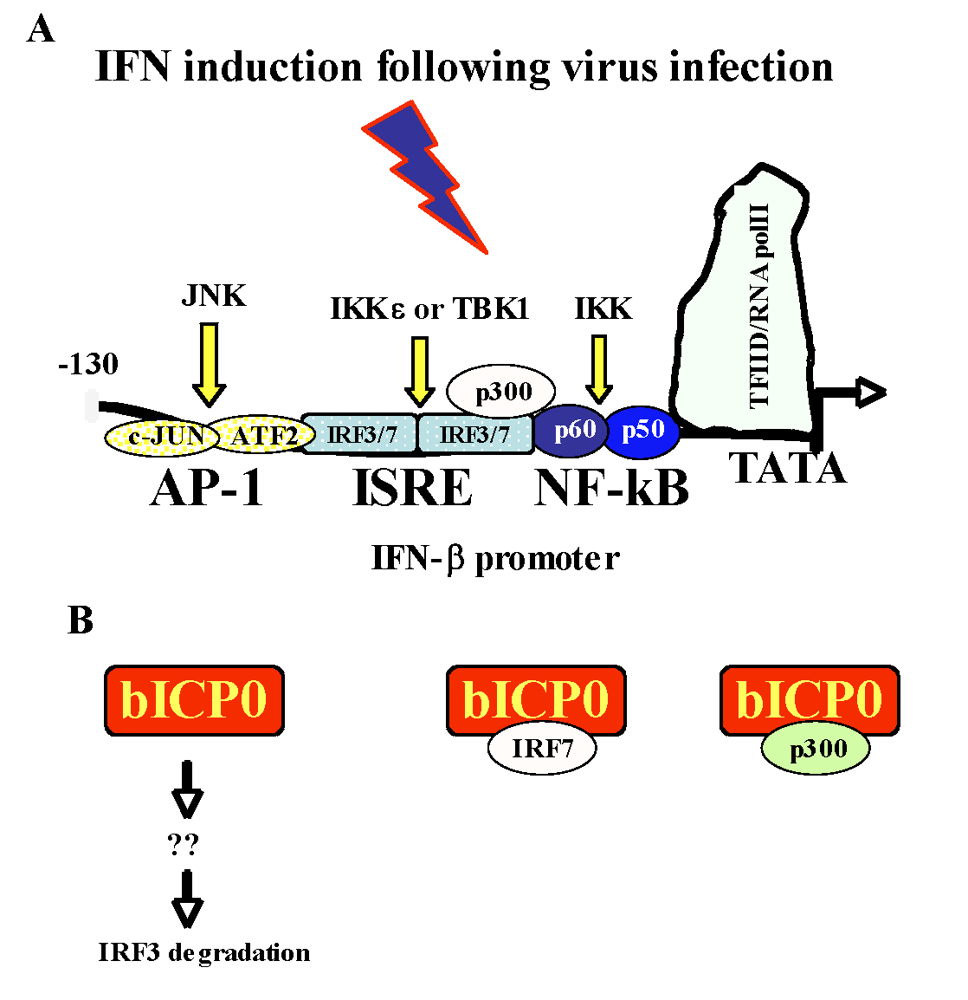
Figure 2.
Activation of IFN-β promoter activity and how bICP0 inhibits IFN-β promoter activity. Panel A: Schematic of the human IFN-β promoter necessary for inducing an IFN response to virus infection [2,80,86]. The NF-B binding site is bound by the two proteins, p60 and p50, that comprise the NF-B transcription factor. A summary of signaling pathways that induces IFN-β promoter activity is presented [7,32,42,54,72,109,119,133,142,143,153]. Panel B: A schematic of the known steps by which bICP0 inhibits IFN-β promoter activity. For details, see text.
The serine/threonine protein kinase family (IKK) regulates the activity of NF-κB and to a certain extent JNK (c-jun N-terminus kinases) [119]. JNK1 and JNK2, both serine/threonine protein kinases that are activated by stress can positively activate c-jun, a key component for stimulating AP-1 binding sites [72], including the AP-1 site in the IFN-β promoter. The histone acetylase, p300, is a co-activator for the IFN-β promoter and numerous IFN induced genes [143,153]. Collectively, the activation and association of these transcription factors with the IFN-β promoter is required for the IFN response following virus infection.
2.5.b. bICP0 inhibits IRF3 functions by inducing its degradation
Mice lacking type I and type II interferon receptors in combination with RAG-2 gene deletions die within a few days following BoHV-1 infection [3]. In contrast, BoHV-1 infection of wt mice does not lead to clinical symptoms or extensive viral replication highlighting the importance that IFN plays in controlling BoHV-1 replication and pathogenesis.
In the absence of other viral genes, bICP0 inhibits interferon signaling [59]. bICP0 (directly or indirectly) reduces IRF3 protein levels in human or bovine cells (Figure 3B), which consequently leads to reduced IFN-β promoter activity [127] (Figure 2B). The C3HC4 zinc RING finger of bICP0 [29,91] is important for inducing IRF3 degradation suggesting bICP0 can ubiquitinate IRF3. The finding that a functional proteasome is necessary for bICP0 induced IRF3 degradation [127] further supports the notion that the bICP0 RING finger mediates IRF3 degradation. However, direct evidence demonstrating that bICP0 ubiquitinates IRF3 is lacking. The C3HC4 zinc RING finger is not the sole component necessary for bICP0 induced IRF3 degradation because specific mutations near the C-terminus of bICP0 are also important [127].
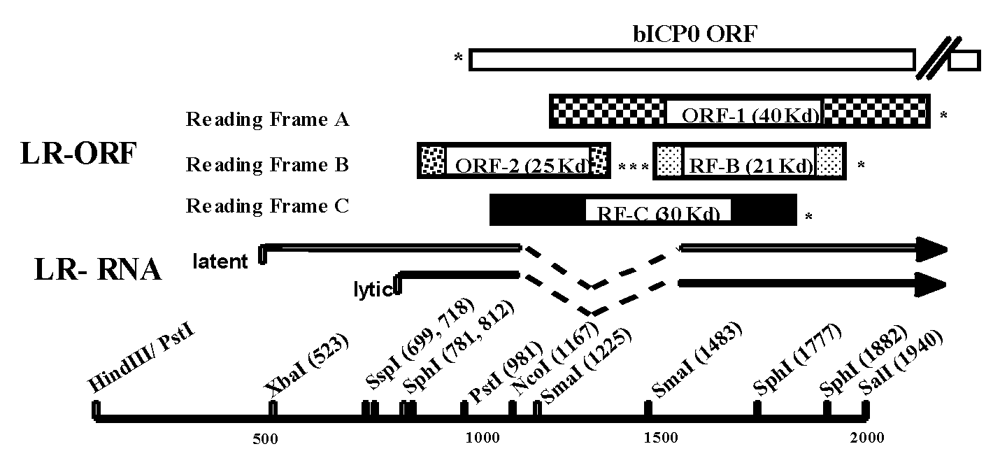
Figure 3.
Schematic of the BoHV-1 LR gene and primers used in this study. Partial restriction map, location of LR-RNA, organization of LR ORFs, and the bICP0 ORF. Start sites for LR transcription during latency and productive infection were previously described [44]. Reading Frames B and C contain an open reading frame, but lack an initiating Met. The (*) denotes the positions of stop codons that are in frame with the respective ORF.
2.5.c. bICP0 interacts with IRF7
IRF7 was originally identified as a protein that binds and represses the Epstein-Barr virus Qp promoter [155]. IRF7 stimulates IFN-α/β expression [9,100], and thus is an important regulator of the innate immune response [63]. IRF7, like IRF3, undergoes virus-induced activation and phosphorylation at its C-terminus [9,99,130]. Phosphorylation of IRF7 promotes retention in the nucleus, binding to ISRE sequences in promoters of IFN responsive genes, and interactions with IRF3. IRF3 activation is an immediate early regulator of the IFN response, whereas IRF7 is believed to be a component of the early response [131,153,154]. Surprisingly, IRF7 is more important with respect to inhibiting viral infection when IRF3 versus IRF7 knockout mice are compared [63].
Although bICP0 inhibited the ability of IRF7 to stimulate IFN-β promoter activity [127], it was not clear whether bICP0 had a direct effect on IRF7 or if reduced IRF3 levels inhibited the trans-activation potential of IRF7. To test whether bICP0 could inhibit IFN-β promoter activity in the absence of IRF3, undifferentiated embryonic murine teratocarcinoma (P19) cells were utilized. P19 cells, like most undifferentiated teratocarcinoma cell lines, do not express detectable levels of IRF3 or IRF7, and consequently IFN-β promoter activity is not induced following virus infection [151]. However, an IRF7 expression plasmid trans-activated the human IFN-β promoter in P19 cells [128]. The ability of bICP0 to inhibit the ability of IRF7 to trans-activate the IFN-β promoter indicated bICP0 had a direct effect on the functions of IRF7. In contrast to bICP0 inducing reduced levels of IRF3 [127], bICP0 had no effect on IRF7 protein levels in cells transfected with bICP0 or infected cells [127,128]. However, bICP0 interacted with IRF7 or a complex containing IRF7 (Figure 2B), and this interaction correlated with the ability of bICP0 to inhibit the ability of IRF7 to trans-activate IFN-β promoter activity. Finally, the ability of bICP0 to interact with p300 or p300 containing complexes (Figure 2B) may also interfere with IFN- promoter activity because p300 is crucial for stimulating IFN- promoter activity.
2.6. Growth properties of bICP0 mutant viruses
A bICP0 null mutant virus was generated by homologous recombination [47]. The bICP0 null mutant does not produce plaques; it grows poorly in bovine cells, and does not grow at all in calves. Interestingly, the bICP0 null mutant persists in bovine cells following infection. Additional studies suggested that the bICP0 null mutant induced cytoplasmic vesicles following infection of bovine kidney cells, but not rabbit skin cells [46]. These cytoplasmic vesicles appear to be autophagosomes suggesting that the bICP0 null mutant induced autophagy, a form of programmed cell death [6,11,22]. The bICP0 null mutant, unlike wild type BoHV-1, does not protect infected cells from UV induced apoptosis. The ability of bICP0 to induce viral gene expression appears to be the reason why the bICP0 null mutant does not protect cells from apoptosis because bICP0 is toxic to cells in transient transfection assays [60,70].
A bacterial artificial chromosome (BAC) that contains the Cooper strain of BoHV-1 (pBoHV-1BAC) was used to introduce a single cysteine to glycine mutation into the 51st amino acid of bICP0 (51g mutant), which is within the C3HC4 zinc RING finger of bICP0 [126]. The 51g mutant grows poorly compared to the rescued virus or the wt BAC derived Cooper strain of BoHV-1. However, the 51g mutant, unlike the bICP0 deletion mutant, induces plaques following infection of cultured bovine cells.
During transient transfection [70,157] or productive infection, higher levels of bICP0 are detected in cells expressing bICP0 proteins containing mutations within the C3HC4 zinc RING finger. Since it is well established that HHV-1 ICP0 zinc RING finger mutants have increased ½ lives because they cannot induce their own ubiquitination [14,23,34], we tested whether bICP0 regulates its own ½ life by self-ubiquitination. Not surprising, C3HC4 zinc RING finger of bICP0 [126] regulates the ½ life of bICP0. Furthermore, transient transfection assays and cell free assays demonstrated that the bICP0 protein possess E3 ubiquitin ligase activity, and that the C3HC4 zinc RING finger is crucial for this activity [29].
HHV-1 encoded ICP0 plays a crucial role in allowing virus replication in the face of an IFN response [108-110]. The ICP0 homologue (EP0) encoded by pseudorabies virus is also important for counteracting the antiviral state in swine cells, but not cells from non-natural-host cells [20]. The ability of the 51g mutant virus to grow in bovine cells pretreated with imiquimod is greatly reduced compared to the rescued virus (51gR). Imiquimod stimulates IFN as well as cytokine production [104], and IFN-β promoter activity in bovine cells [116]. Since bICP0 can inhibit IRF3 and IRF7 dependent activation of the IFN-β promoter [127,128], bICP0, directly or indirectly, overcomes the effects of imiquimod. It is tempting to conclude that the ability of bICP0 to inhibit IFN dependent transcription is the reason why the 51g mutant grows poorly in bovine cells after imiquimod treatment. However, the poor growth of the 51g mutant may reflect the fact that disabled viruses do not grow efficiently in suboptimal growth conditions.
In calves, the 51g mutant grows poorly and does not reactivate from latency following treatment with dexamethasone. As expected, the wt BAC derived Cooper strain and the 51g rescued virus reactivated from latency following dexamethasone treatment [92,129]. The current evidence supports a crucial role for wt expression of bICP0 for the growth of BoHV-1 in cultured cells and calves.
2.7. Regulation of immune responses by other BoHV-1 genes
2.7.a. Inhibition of CD8+ T cell recognition in infected cells
The BoHV-1 UL49.5 ORF impairs CD8+ T cell recognition of infected cells by repressing expression of the major histocompatibility complex class I and the transporter associated with antigen presentation (TAP) [56,61,112]. UL49.5, also referred to as glycoprotein N (gN), encodes a 96 amino acid protein with an apparent molecular mass of 9 kDa [88] that inhibits TAP (transporter associated antigen processing) functions. The gN proteins encoded by pseudorabies virus and BoHV-1 inhibit TAP-mediated transport of cytosolic peptides into ER, which consequently blocks the assembly of peptide containing ternary MHC-I complexes in vitro in virus-infected cells [81,90]. Furthermore, the BoHV-1 UL49.5 protein targets the TAP complex for proteosomal degradation [81]. The UL49.5 protein contains a 22 amino acid signal peptide at its N-terminus, an extracellular domain of 32 amino acids, a transmembrane region of 25 amino acids, and a 17 amino acid cytoplasmic tail [88]. The UL49.5 protein is a non-glycosylated type I membrane protein [87,88].
Peptide transport by TAP is a critical step in MHC class I antigen presentation [5,8,65,81]. In the absence of a functional TAP transporter, most MHC class I molecules are not loaded with peptides [65]. However, they are retained within the ER and ultimately directed for degradation by the proteasome [65]. A gN mutant that lacks the cytoplasmic tail can still bind to the TAP complex and block peptide transport, but this mutant gN protein does not degrade TAP [81]. If the transmembrane domain and cytoplasmic tail are truncated, this gN mutant protein does not block TAP functions [81]. Therefore, sequences within the transmembrane domain of gN appear to be necessary for interacting with TAP. The phenotype of a viral strain expressing the various gN mutant proteins has not been tested in cattle.
2.7.b. Inhibition of CD4+ T cell functions
CD4+ T cell functions are impaired during acute infection of calves, in part, because BoHV-1 infects CD4+ T cells and induces apoptosis [145]. It appears that CD4+ T cells are semi-permissive for BoHV-1 infection suggesting that binding of virus to a receptor or the early events of virus infection induces apoptosis. Unlike CD8 T cell functions that are specifically blocked by gN, it is not clear why CD4+ T cells are very susceptible to apoptosis after infection.
2.7.c. BoHV-1 encodes a protein that interacts with chemokines
Chemokines are small proteins (8-10 kd) that function as cytokines, and thus regulate trafficking and effector functions of leukocytes [12]. As such, chemokines are important regulators of inflammation, immune surveillance, and they have potent anti-viral functions. Functionally, chemokines can be divided into two groups: pro-inflammatory chemokines that are inducible and housekeeping chemokines that are constitutively expressed. Activation of chemokine functions are dependent on selective recognition and activation of chemokine receptors belonging to the seven-membrane domain, G protein-coupled receptor super family. Chemokines can also bind to glycosaminoglycans (GAGS). Chemokine binding to GAGS on cells, in particular endothelial cells, results in chemotactive chemokine gradients that allow the correct presentation of chemokines to leukocytes and therefore enable target cells to cross the endothelial barrier and migrate to tissues.
BoHV-1, BoHV-5, and equine herpesvirus 1 encode a glycoprotein (gG) that is secreted from infected cells, and can bind to a broad range of chemokines [21]. Interactions between gG and chemokines block chemokine activity by preventing their interactions with specific receptors and GAGS. By preventing chemokine-GAG interactions, gG disrupts chemokine gradients, which controls the local environment surrounding an infected cell. A BoHV-1 gG deletion mutant was reported to have reduced virulence [78] suggesting gG is a viral immune evasion gene. However, the exact role of gG in virulence requires additional studies because the gG mutant that was examined was not rescued, and expression of surrounding genes was not examined.
2.7.d. LR RNA can influence immune responses
The latency related (LR) gene is abundantly transcribed in trigeminal ganglia of latently infected calves [84,124,125], and is anti-sense with respect to the bICP0 gene [75-77] (Figure 3A). The LR gene has two open reading frames (ORF-1 and ORF-2), and two reading frames that lack an initiating ATG (RF-B and RF-C) (Figure 3A). A mutant BoHV-1 virus (LR mutant virus) that contains 3 stop codons at the beginning of ORF-2, and lacks 25 bp of wt sequences at the beginning of ORF2 was constructed [69] (Figure 3B). The LR mutant virus grows to similar titers as wt BoHV-1 or the LR rescued virus in cultured bovine cells indicating expression of LR proteins is not necessary for productive infection. When bovine cells are infected with the LR mutant virus, proteins containing ORF-2 or RF-C are not expressed [64,73,74]. Calves infected with the LR mutant virus exhibit diminished clinical symptoms, and reduced shedding of infectious virus in the eye, tonsil, or TG [68,69,118]. The LR mutant virus does not reactivate from latency following dexamethasone treatment [68] indicating LR protein expression is crucial for the latency-reactivation cycle. LR gene products inhibit mammalian cell growth [44,132], bICP0 expression [19,45,132], and apoptosis [26,58]. LR protein expression is necessary for inhibiting apoptosis, in part, because a LR protein binds to two proteins that induce apoptosis, Bid and Cdc42 [105]. LR protein expression is not necessary for inhibiting cell growth or bICP0 expression. It appears that a small regulatory RNA, perhaps a micro-RNA that is encoded by the LR gene inhibits cell growth.
The LR mutant virus induces higher levels of IFN-β3 RNA and to a lesser extent IFN-β2 RNA when compared to wild type BoHV-1 or the LR rescued virus that grows like wild type BoHV-1 [116]. In contrast to humans or mice, cattle contain three different IFN-β genes that are differentially regulated because they have distinct promoters [138,144]. The LR mutant virus prematurely expresses LR-RNA in productively infected cells [116]. Since LR-RNA has the potential to base pair with bICP0 RNA, the formation of double stranded RNA may induce higher levels of IFN-β during the early stages of virus infection. The ability of LR-RNA to induce IFN may promote establishment and maintenance of latency because of the anti-viral effects of IFN.
LR gene products inhibit infiltration of inflammatory cells into trigeminal ganglia of infected calves [117]. It is not clear whether this effect is a direct effect, but a protein encoded by the LR gene can interact with a B-cell and monocyte-activating chemokine precursor [106]. Enhanced infiltration of inflammatory cells into trigeminal ganglia during acute infection correlates with enhanced neuronal death and the inability of the LR mutant virus to reactivate from latency [68,95].
3. Conclusions
The ability of viruses to inhibit innate immune responses dictates host range, the pathogenic potential of a virus, and the ability of a given virus to survive in nature. The bICP0 protein has at least two important functions that play an important role during infection of cattle: 1) stimulates viral transcription, and 2) inhibits innate immune responses. bICP0 is a unique viral transcription factor because there is currently no evidence to indicate that it binds specifically (or directly) to DNA. The ability of bICP0 to regulate gene expression and innate immune responses is linked to its ability to interact with cellular regulatory factors. For example, bICP0 can interact with HDAC1, p300, and IRF7 [128,158,159]. The ability of bICP0 to function as an E3 ligase [29,30] correlates with its ability to activate transcription [70,157-159] and inhibit IFN dependent transcription [59,127]. To date, the specific cellular proteins that are targets for proteasomal degradation induced by bICP0 have not been identified. Identification of the cellular proteins that are degraded by bICP0 would greatly enhance our understanding of how bICP0 inhibits IFN dependent transcription and stimulates viral gene expression. In addition to bICP0, BoHV-1 has additional means by which is can regulate cellular immune responses. For example, the ability of BoHV-1 to infect CD4+ T cells and kill these cells would transiently repress immune responses. Furthermore, the ability of UL49.5 to inhibit CD8 T cell recognition of infected cells is an important mechanism by which BoHV-1 evades immune responses. Finally, the viral encoded chemokine, gG, would also appear to play an important role in immune evasion. In conclusion, BoHV-1 has multiple genes that inhibit specific arms of immune responses, which are necessary for growth in cattle.
Acknowledgments
The laboratory of C. Jones is supported by two USDA grants (08-00891 and 09-01653), a PHS grant (R21AI069176), and a PHS grant (1P20RR15635) to the Nebraska Center for Virology.
References and Notes
- 1995-96 Agricultural Statistics; National Agricultural Statistics Service (NASS): Agricultural Statistics Board, U.S. Department of Agriculture, 1996.
- Aaronson, D.S.; Horvath, C.M. A road map for those who don't know JAK-STAT. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Abril, C.; Engels, M.; Limman, A.; Hilbe, M.; Albini, S.; Franchini,M.; Suter, M.; Ackerman, M. Both viral and host factors contribute to neurovirulence of bovine herpesvirus 1 and 5 in interferon receptor-deficient mice. J. Virol. 2004, 78, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Hume, D.A. How do you see CG? Cell 2000, 103, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Meyer, T.H.; Uebel, S.; Sempe, P.; Djaballah, H.; Yang, Y.; Peterson, P.A.; Fruh, K.; Tampe, R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996, 15, 3247–3255. [Google Scholar] [PubMed]
- Akdemir, F.; Farkas, R.; Chen, P.; Juhasz, G.; Medved'ova, L; Saas, M.; Wang, L.; Wang, X.; Chittaranjan, S.; Gorski, S.M.; Rodriguez, A.; Abrams, J. M. Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development 2006, 133, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ambagala, A.P.; Gopinath, R.S.; Srikumaran, S. Peptide transport activity of the transporter associated with antigen processing (TAP) is inhibited by an early protein of equine herpesvirus-1. J. Gen. Virol. 2004, 66, 2383–2394. [Google Scholar]
- Au, W.C.; Moore, P.A.; LaFleur, D.W.; Tombal, B.; Pitha, P.M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 1998, 273, 29210–29217. [Google Scholar] [CrossRef] [PubMed]
- Ayer, D.E.; Kretzner, L.; Eisenman, R.N. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell 1993, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baehrecke, E.H. Autophagy: dual roles in life and death? Nat. Rev. Molec. Cell Biol. 2006, 6, 505–510. [Google Scholar] [CrossRef]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Boutell, C.; Everett, R.D. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with an ubiquitinates p53. J. Biol. Chem. 2003, 278, 36596–36602. [Google Scholar] [CrossRef] [PubMed]
- Boutell, C.; Sadis, S.; Everett, R.D. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 2002, 76, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bowland, S.L.; Shewen, P.E. Bovine respiratory disease: commercial vaccines currently available in Canada. Can. Vet. J. 2000, 41, 33–48. [Google Scholar]
- Bowles, D.E.; Holden, V.R.; Zhao, Y.; O'Callaghan, D.J. The ICP0 protein of equine herpesvirus 1 is an early protein that independently transactivates expression of all classes of viral promoters. J. Virol. 1997, 71, 4904–4914. [Google Scholar] [PubMed]
- Bowles, D.E.; Kim, S.K.; O'Callaghan, D.J. Characterization of the trans-activation properties of equine herpesvirus 1 EICP0 protein. J. Virol. 2000, 74, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Boyes, J.; Byfield, P.; Nakatani, Y.; Ogryzko, V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 1998, 396, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Bratanich, A.C.; Hanson, N.D.; Jones, C. The latency-related gene of bovine herpesvirus 1 inhibits the activity of immediate-early transcription unit 1. Virology 1992, 191, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Brukman, A; Enquist, L.W. Pseudorabies virus EP0 protein counteracts an interferon-induced antiviral state in a species-specific manner. J. Virol. 2006, 80, 10871–10873. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Davis-Poynter, N.; Vanderplasschen, A.; Alcami, A. Glycoprotein G isoforms from some alphaherpesvirus function as broad-spectrum chemokine binding proteins. EMBO J. 2003, 22, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Burch, W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death and Differentiation 2001, 8, 569–581. [Google Scholar] [CrossRef]
- Canning, M.; Boutell, C.; Parkinson, J.; Everett, R.D. A RING finger ubiquitin ligase activity is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP27. J. Biol. Chem. 2004, 279, 38160–38168. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Weinberg, A.D.; Pollard, A.; Reeves, R.; Magnuson, J.A.; Magnuson, N.S. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J. Virol. 1989, 63, 1525–1530. [Google Scholar] [PubMed]
- Chen, H.; Lin, R.J.; Xie, W. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 1999, 98, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Ciacci-Zanella, J.; Stone, M.; Henderson, G.; Jones, C. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J. Virol. 1999, 73, 9734–9740. [Google Scholar] [PubMed]
- Coull, J.J.; Romerio, F.; Sun, J.M.; Volker, J.L.; Galvin, K.M.; Davie, J.R.; Shi, Y.; Hansen, U.; Margolis, D.M. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 2000, 74, 6790–6799. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Jones, C. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J. Virol. 1999, 73, 3778–3788. [Google Scholar] [PubMed]
- Dia, L.; Zhang, B.; Fan, J.; Gao, X.; Sun, S.; Yang, K.; Xin, D.; Jin, N.; Geng, Y.; Wang, C. Herpes virus proteins ICP0 and BICP0 can activate NF-kB by catalyzing IkBa ubiquitination. Cellular Signalling 2005, 17, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Qiao, W.; Chen, Q.; Wang, C.; Geng, Y. bICP0 and its RING domain act as ubiquitin E3 ligases in vitro. Chinese Science Bulletin 2005, 50, 636–640. [Google Scholar] [CrossRef]
- Doetzlhofer, A.; Rotheneder, H.; Koranda, M.; Kurtev, V.; Brosch, G.; Wintersberger, E.; Seiser, C. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol. Cell. Biol. 1999, 19, 5504–5511. [Google Scholar] [PubMed]
- Doyle, S.; Vaidya, S.; O'Connell, R.; Dadgostar, H.; Dempsey, P.; Wu, T.; Rao, G.; Sun, R.; Haberland, M.; Modlin, R.; Cheng, G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 2002, 17, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Waldner, C.; Rhodes, C.; Ricketts, V. Longevity of protective immunity to experimental bovine herpesvirus-1 infection following inoculation with a combination modified-live virus vaccine in beef calves. J. Am. Vet. Med. Assoc. 2005, 227, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.; O'Hare, P.; O'Rourke, D.; Barlow, P.; Orr, A. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol. 1995, 69, 7339–7344. [Google Scholar] [PubMed]
- Everett, R.D. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 1988, 202, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 2000, 22, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D.; Barlow, P.; Milner, A.; Luisi, B.; Orr, A.; Hope, G.; Lyon, D. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J. Mol. Biol. 1993, 234, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D.; Earnshaw, W.C.; Findlay, J.; Lomonte, P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999, 18, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D.; Lomonte, P.; Orr, A. Cell cycle regulation of PML modification and ND10 composition. J. Cell Sci. 1999, 112, 4581–4588. [Google Scholar] [PubMed]
- Everett, R.D.; Meredith, M.; Orr, A.; Cross, A.; Kathoria, M.; Parkinson, J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein [corrected and republished article originally printed in EMBO J.1997, 16, 566-577]. EMBO J. 1997, 16, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Fischle, W.; Emiliani, S.; Hendzel, M.J.; Nagase, T.; Nomura, N.; Voelter, W.; Verdin, E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J. Biol. Chem. 1999, 274, 11713–11720. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faja, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKe and TBKI are essential components of the IRF3 signaling pathway. Nature 2003, 4, 491–496. [Google Scholar]
- Fraefel, C.; Zeng, J.; Choffat, Y.; Engels, M.; Schwyzer, M.; Ackermann, M. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICP0. J. Virol. 1994, 68, 3154–3162. [Google Scholar] [PubMed]
- Geiser, V.; Jones, C. The latency related gene encoded by bovine herpesvirus 1 encodes a small regulatory RNA that inhibits cell growth. J. Neurovirol. 2005, 11, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Geiser, V.; Inman, M.; Zhang, Y.; Jones, C. The latency related (LR) gene of bovine herpes virus 1 (BHV-1) can inhibit the ability of bICP0 to activate productive infection. J. Gen. Virol. 2002, 83, 2965–2971. [Google Scholar] [PubMed]
- Geiser, V.; Rose, S.; Jones, C. The bovine herpes virus 1 bICP0 protein regulates toxicity in a cell type dependent fashion. Molec. Path. 2008, 44, 459–466. [Google Scholar]
- Geiser, V.; Zhang, Y. Characterization of a BHV-1 strain that does not express the major regulatory protein, bICP0. J. Gen. Virol. 2005, 86, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.; Ohmann, H.B.; Lawman, M.J.; Babiuk, L.A. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J. Gen. Virol. 1990, 71, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.; Qualtiere, L.; Davis, W.C.; Gee, A.; Bielefeldt Ohmann, H.; Lawman, M.J.; Babiuk, L.A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral. Immunol. 1987, 1, 287–304. [Google Scholar] [CrossRef]
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Lawman, M.J.; Babiuk, L.A. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1987, 1, 267–286. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Hassig, C.A.; Schreiber, S.L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 4868–4873. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liang, Y.; Mandel, G.; Roizman, B. Components of the REST/CoREST/histone deacytlase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Nat. Acad. Sci. U.S.A. 2005, 102, 7571–7576. [Google Scholar] [CrossRef]
- Gu, W.; Roeder, R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 1997, 90, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peters, K.L.; Sen, G.C. Induction of the human protein P56 by interferon, double stranded RNA, or virus infection. Virology 2000, 267, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hacker, H.; Vabulas, R.M.; Takeuchi, O.; Hoshino, K.; Akira, S.; Wagner, H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF) 6. J. Exp. Med. 2000, 192, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, M.J.; Nataraj, C.; Srikumaran, S. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 1993, 6, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, T.; Lavinsky, R.M.; Mullen, T.M.; Soderstrom, M.; Laherty, C.D.; Torchia, J.; Yang, W.M.; Brard, G.; Ngo, S.D.; Davie, J.R.; Seto, E.; Eisenman, R.N.; Rose, D.W.; Glass, C.K.; Rosenfeld, M.G. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression [see comments]. Nature 1997, 387, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Perng, G.-C.; Nesburn, A.; Wechsler, S.; Jones, C. The latency related gene of bovine herpesvirus 1 can suppress caspase 3 and caspase 9 during productive infection. J. Neurovirol. 2004, 10, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Zhang, Y.; Jones, C. The bovine herpesvirus 1 gene encoding infected cell protein 0 (bICP0) can inhibit interferon-dependent transcription in the absence of other viral genes. J. Gen. Virol. 2005, 86, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Zhang, Y.; Inman, M.; D. Jones, C. Jones. The infected cell protein 0 encoded by bovine herpes virus 1 (bICP0) can activate caspase 3 when over-expressed in transfected cells. J. Gen. Virol. 2004, 85, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, S.; Hill, A.B.; Srikumaran, S. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 1998, 53, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, W.E.; DeLuca, N.A. 2nd; Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. . J. Virol. 1999, 73, 8245–8255. [Google Scholar] [PubMed]
- Honda, K.; Yanai, H.; Negishi, H.; Asagiri, M.; Saton, M.; Mizutani, T.; Shimada, N.; Ohba, Y.; Takaoka, A.; Yoshida, N.; Taniguchi, T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Schang, L.M.; Jones, C. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 1995, 69, 5345–5352. [Google Scholar] [PubMed]
- Hughes, E.A.; Hammond, C.; Cresswell, P. Misfolded major histocompatibilitycomplex class I heavy chains are translocated into the cytoplasm and degraded by the proteosome. Proc. Nat. Acad. Sci. 1997, 94, 1896–1901. [Google Scholar] [CrossRef]
- Irving, U.P. Reference of Dairy Health and Management in the United States; USDA:AHIS:VS, CEAH,National Animal Health Monitoring System, 2002. [Google Scholar]
- Imhof, A.; Yang, X.J.; Ogryzko, V.V.; Nakatani, Y.; Wolffe, A.P.; Ge, H. Acetylation of general transcription factors by histone acetyltransferases. Curr. Biol. 1997, 7, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Inman, M.; Lovato, L.; Doster, A.; Jones, C. A mutation in the latency related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J. Virol. 2002, 76, 6771–6779. [Google Scholar] [CrossRef] [PubMed]
- Inman, M.; Lovato, L.; Doster, A.; Jones, C. A mutation in the latency-related gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves. J. Virol. 2001, 75, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- Inman, M.; Zhang, Y.; Geiser, V.; Jones, C. The zinc ring finger in the bICP0 protein encoded by bovine herpes virus-1 mediates toxicity and activates productive infection. J. Gen. Virol. 2001, 82, 483–492. [Google Scholar] [PubMed]
- Ishmael, W. Gasping for dollars. Angus Beef Bulletin 2001, Available online: www.mycattle.com/health/updates/ gaspingfordollars/. [Google Scholar]
- Jaeschke, A.; Karasarides, M.; Ventura, J.; Ehrhardt, A.; Zhang, C.; Flavell, R.A.; Shokat, K.M.; Davis, R.J. JNK2 Is a Positive Regulator of the cJun Transcription Factor. Mol. Cell. 2006, 23, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hossain, A.; Winkler, M.T.; Holt, T.; Doster, A.; Jones, C. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 1998, 72, 8133–8142. [Google Scholar] [PubMed]
- Jiang, Y.; Inman, M.; Zhang, Y.; Posadas, N.A.; Jones, C. A mutation in the latency related gene of bovine herpesvirus 1 (BHV-1) inhibits protein expression of a protein from open reading frame 2 (ORF-2) and an adjacent reading frame during productive infection. J. Virol. 2004, 78, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 1998, 51, 81–133. [Google Scholar] [PubMed]
- Jones, C. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Micro. Rev. 2003, 16, 79–95. [Google Scholar] [CrossRef]
- Jones, C.; Geiser, V.; Henderson, G.; Jiang, Y.; Meyer, F.; Perez, S.; Zhang, Y. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet. Micro. 2006, 113, 199–210. [Google Scholar] [CrossRef]
- Kaashoek, M. J.; Fijsewijk, F.A.M.; Ruuls, R.C.; Keil, G.M.; Thiry, E.; Pastoret, P.P.; Van Oirschot, J.T. Virulence, immunogenicity and reactivation of bovine herpesvirus 1 mutants with a deletion in the gC, gG, gI, gE, or in both the gI and gE gene. Vaccine 1998, 16, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Basaraba, R.J. Infectious bovine rhinotracheitis, parainfluenza-3, and respiratory coronavirus. Bovine respiratory disease update. In Food Animal Practice; Veterinary Clinics of North America:, 1997; pp. 455–461. [Google Scholar]
- Katze, M.G.; Heng, Y.; Gale, M. Viruses and interferon: fight for supremacy. Nat. Rev. Immuno. 2002, 2, 675–686. [Google Scholar] [CrossRef]
- Koppers-Lalic, D.; Reits, E.A.J.; Ressing, M.E.; Lipinska, A.D.; Abele, R.; Koch, J.; Rezende, M.M.; Admiraal, P.; van Leeuwen, D.; Bienkowska-Szewczyk, K.; Mettenleiter, T.C.;Rijsewijk; Tampé, R.; Neefjes, J.; Wiertz, E.J.H J. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Nat. Acad. Sci. 2005, 102, 5144–5149. [Google Scholar] [CrossRef]
- Kouzarides, T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000, 19, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 1999, 9, 40–48. [Google Scholar] [CrossRef]
- Kutish, G.; Mainprize, T.; Rock, D. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 1990, 64, 5730–5737. [Google Scholar] [PubMed]
- Lees-Miller, S.P.; Long, M.C.; Kilvert, M.A.; Lam, V.; Rice, S.A.; Spencer, C.A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 1996, 70, 7471–7477. [Google Scholar] [PubMed]
- Levy, D.E.; Darnell, Jr., J.E. Stats: transcriptional control and biological impact. Nature Reviews Molecular Cell Biology 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chow, B.; Raggo, C.; Babiuk, L.A. Bovine herpesvirus 1 UL49.5 homolog gene encodes a novel viral envelope protein that forms a disulfide-linked complex with a second virion structural. J. Virol. 1996, 70, 1448–1454. [Google Scholar] [PubMed]
- Liang, X.; Tang, M.; Manns, B.; Babiuk, L.A.; Zamb, T.J. Identification and deletion mutagenesis of the bovine herpesvirus 1 dUTPase gene and a gene homologous to herpes simplex virus UL49.5. Virology 1993, 195, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Noyce, R.S.; Collins, S.E.; Everett, R.D.; Mossman, K.L. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 2004, 78, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, A.D.; Koppers-Lalic, D.; Rychlowski, M.; Admiraal, P.; Rijsewijk, F.A.M.; Bieńkowska-Szewczyk, K.; Wiertz, E.J. Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M. J. Virol. 2006, 81, 5822–5832. [Google Scholar] [CrossRef]
- Lirong, D.; Wenato, Q.; Qimin, C.; Chen, W.; Yunqi, G. bICP0 and its RING domain act as ubiquitin E3 ligases in vitro. Chinese Science Bulletin 2005, 50, 636–640. [Google Scholar] [CrossRef]
- Liu, S.F.; Brum, M.C.S.; Doster, A.; Jones, C.; Chowdhury, S.I. A bovine herpesvirus type 1 mutant virus specifying a carboxyl-terminal truncation of glycoprotein E is defective in anterograde neuronal transport in rabbits and calves. J. Virol. 2008, 82, 7432–7442. [Google Scholar] [CrossRef] [PubMed]
- Lium, E.K. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 1997, 71, 8602–8614. [Google Scholar] [PubMed]
- Lomonte, P.; Thomas, J..; Pascale, T.; Caron, C.; Khochbin, S.; Epstein, A.L. Interaction ebtween class II histone deacetylases and ICP0 of herpes simplex virus type 1. J. Virol. 2004, 78, 6744–6757. [Google Scholar] [CrossRef] [PubMed]
- Lovato, L.; Inman, M.; Henderson, G.; Doster, A.; Jones, C. Infection of cattle with a bovine herpesvirus 1 (BHV-1) strain that contains a mutation in the latency related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J. Virol. 2003, 77, 4848–4857. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Welander, P.; Han, X.; Cantin, E. Herpes simplex virus type 1 DNA is immunostimulatory in vitro and in vivo. J. Virol. 2003, 77, 11158–11169. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.X.; Postigo, A.A.; Dean, D.C. Rb interacts with histone deacetylase to repress transcription. Cell 1998, 92, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi-Jaulin, L.; Groisman, R.; Naguibneva, I.; Robin, P.; Lorain, S.; Le Villain, J.P.; Troalen, F.; Trouche, D.; Harel-Bellan, A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase [see comments]. Nature 1998, 391, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Marie, I.; Smith, E.; Prakash, A.; Levy, D.E. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Molec. Cell. Biol. 2000, 20, 8803–8814. [Google Scholar] [CrossRef]
- Marie, I.; Durbin, J.E.; Levy, D.E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998, 17, 6660–6669. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Balbas, M.A.; Bauer, U.M.; Nielsen, S.J.; Brehm, A.; Kouzarides, T. Regulation of E2F1 activity by acetylation. EMBO J. 2000, 19, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Maul, G.G.; Everett, R.D. The nuclear location of PML, a cellular member of the C3HC4 zinc- binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 1994, 75, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Maul, G.G.; Guldner, H.H.; Spivack, J.G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 1993, 74, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Megyer, K.; Au, W.-C.; Rosztoczy, I.; Babu, N.; Raj, K.; Miller, R.L.; Tomai, M.A.; Pitha, P.M. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Molec. Cell. Biol. 1995, 15, 2207–2218. [Google Scholar] [PubMed]
- Meyer, F.; Perez, S.; Jiang, Y.; Zhou, Y.; Henderson, G.; Jones, C. Identification of a novel protein encoded by the latency-related gene of bovine herpesvirus 1. J. Neurovirol. 2007, 13, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Perez, S.; Geiser, V.; Sintek, M.; Inman, M.; Jones, C. A protein encoded by the bovine herpes virus 1 (BHV-1) latency related gene interacts with specific cellular regulatory proteins, including the CCAAT enhancer binding protein alpha (C/EBP-a). J. Virol. 2007, 81, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Karlsson, C.; Langley, E.; Nielsen, S.J.; Pines, J.; Kouzarides, T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999, 18, 5099–5107. [Google Scholar] [CrossRef] [PubMed]
- Mossman, K.L.; Saffran, H.A.; Smiley, J.R. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 2000, 74, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Mossman, K.L.; Macgregor, P.F.; Rozmus, J.J.; Edwards, A.M.; Smiley, J.R. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 2001, 75, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Mossman, K.L.; Smiley, J.R. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon- induced barriers to virus replication. J. Virol. 2002, 76, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.; Merika, M.; Yie, J.; Senger, K.; Chen, G.; Thanos, D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol. Cell 1998, 2, 457–467. [Google Scholar] [CrossRef]
- Nataraj, C.; Eidmann, S.; Hariharan, M.J.; Sur, J.H.; Perry, G.A.; Srikumaran, S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Collins, S.E.; Mossman, K.L. Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral repsonse to enveloped virions. J. Virol. 2006, 80, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.; Everett, R.D. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 2000, 74, 10006–10017. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.; Lees-Miller, S.P.; Everett, R.D. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA- dependent protein kinase. J. Virol. 1999, 73, 650–657. [Google Scholar] [PubMed]
- Perez, S.; Meyer, F.; Saira, K.; Doster, A.; Jones, C. Premature expression of the latency-related RNA encoded by bovine herpesvirus 1 correlates with higher levels of beta interferon RNA expression in productively infected cells. J. Gen. Virol. 2008, 89, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Lovato, L.; Zhou, J.; Doster, A.; Jones, C. Comparison of inflammatory infiltrates in trigeminal ganglia of cattle infected with wild type BHV-1 versus a virus strain containing a mutaition in the LR (latency-related) gene. J. Neurovirol. 2006, 12, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Inman, M.; Doster, A.; Jones, C. Latency-related gene encoded by bovine herpesvirus 1 promotes virus growth and reactivation from latency in tonsils of infected calves. J. Clin. Micro. 2005, 43, 393–401. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kB and IKK functions. Nature Reviews Molecular Cell Biology 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Peters, G.A.; Khoo, D.; Mohr, I.; Sen, G.S. Inhibition of PACT-mediated activation of PKR by the herpes simplex simplex virus type 1 Us11 protein. J. Virol. 2002, 75, 11054–11064. [Google Scholar] [CrossRef]
- Poon, A.P.; Gu, H.; Roizman, B. ICP0 and the Us3 protein kinase of herpes simplex virus 1 independently block histone deactylation to enable gene expression. Proc. Nat. Acad. Sci. 2006, 103, 9993–9998. [Google Scholar] [CrossRef]
- Powell,J. Bovine Respiratory Disease, University of Arkansas Division of Agriculture Cooperative Extension Service, 2005, FSA:3082.
- Preston, C.M.; Harman, A.N.; Nicholl, M.J. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 2001, 75, 8909–8916. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.; Lokensgard, J.; Lewis, T.; Kutish, G. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 1992, 66, 2484–2490. [Google Scholar] [PubMed]
- Rock, D.L.; Beam, S.L.; Mayfield, J.E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 1987, 61, 3827–3831. [Google Scholar] [PubMed]
- Saira, K. Functional analysis of the bovine herpesvirus-1 gene encoding bICP0, a promiscuous trans-activator, that stimulates productive infection and interferon signaling pathways. In PhD Thesis; University of Nebraska,: Lincoln, 2008. [Google Scholar] [CrossRef] [PubMed]
- Saira, K.; Zhou, Y.; Jones, C. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 (IRF3), and consequently inhibits beta interferon promoter activity. J. Virol. 2007, 81, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Saira, K.; Jones, C. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) associates with interferon regulatory factor 7 (IRF7), and consequently inhibits beta interferon promoter activity. J. Virol. 2009, 83, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Saira, S.; Chowdhury, S.; Gaudreault, N.; Henderson, G.; Doster, A.; Jones, C. The zinc RING finger of the bovine herpesvirus 1 encoded bICP0 protein is crucial for viral replication and virulence. J. Virol. 2008, 82, 12060–12068. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, N.; Hata, N.; Oda, E.; Taniguchi, T. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 1998, 425, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Taniguchi, T.; Tanaka, N. The interferon system and interferon regulatory factor transcription factors-studies from gene knockout mice. Cytokine Growth Factor Rev. 2001, 12, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Schang, L.M. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J. Virol. 1996, 70, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; tenOever, B.R.; Grandvaux, N.; Zhou, G.-P.; Lin, R.; Hiscott, J. Triggering the interferon antiviral response through and IKK-related pathway. Science, 2003, 300, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Sheffy, B.E.; Davies, D.H. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc. Soc. Exp. Biol. Med. 1972, 140, 974–976. [Google Scholar] [PubMed]
- Sterner, R.; Vidali, G.; Allfrey, V.G. Studies of acetylation and deacetylation in high mobility group proteins. Identification of the sites of acetylation in HMG-1. J. Biol. Chem. 1979, 254, 11577–83. [Google Scholar] [PubMed]
- Tikoo, S.K.; Campos, M.; Babiuk, L.A. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv. Virus Res. 1995, 45, 191–223. [Google Scholar] [PubMed]
- USDA Health Management and Biosecurity in U.S. Feedlots, 1999.
- Valarcher, J.-F.; Furze, J.; Wyld, S.; Cook, R.; Conzelman, K.-K.; Taylor, G. Role of alpha/beta interferons in the attenuation and immunogenecity of recombinant bovine respiratory syncitial viruses lacking NS proteins. J. Virol. 2003, 77, 8426–8439. [Google Scholar] [CrossRef] [PubMed]
- van Drunen Littel-van den Hurk, S.; Myers, D.; Doig, P.A.; Karvonen, B.; Habermehl, M.; Babiuk, L.A.; Jelinski, M.; Van Donkersgoed, J.; Schlesinger, K.; Rinehart, C. Identification of a mutant bovine herpesvirus-1 (BHV-1) in post-arrival outbreaks of IBR in feedlot calves and protection with conventional vaccination. Can. J. Vet. Res. 2001, 65, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Van Sant, C.; Hagglund, R.; Lopez, P.; Roizman, B. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin- conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 8815–8820. [Google Scholar] [CrossRef] [PubMed]
- Verdel, A.; Khochbin, S. Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. J. Biol. Chem. 1999, 274, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Wathelet, M.G.; Lin, C.H.; Parekh, B.S.; Ronco, L.V.; Howley, P.M.; Maniatis, T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-b enhancer in vivo. Mol. Cell 1998, 1, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.K.; Kumar, K.P.; Reich, N.C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Molec. Cell. Biol. 1998, 18, 1359–1368. [Google Scholar] [PubMed]
- Wilson, V.; Jeffreys, A.J.; Barrie, P.A. A comparison of vertebrate interferon gene families deteced by hybridization with human interferon DNA. J. Mol. Biol. 1983, 166, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.T.; Doster, A.; Jones, C. Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 1999, 73, 8657–8668. [Google Scholar] [PubMed]
- Winkler, M.T.; Doster, A.; Jones C. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J .Virol. 2000, 74, 5337–5346. [Google Scholar] [CrossRef] [PubMed]
- Wirth, U.V.; Fraefel, C.; Vogt, B.; Vlcek, V.; Paces, C.; Schwyzer, M. Immediate-early RNA 2. 9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein . J. Virol. 1992, 66, 2763–2772. [Google Scholar] [PubMed]
- Wirth, U.V.; Gunkel, K.; Engels, M.; Schwyzer, M. Spatial and temporal distribution of bovine herpesvirus 1 transcripts. J. Virol. 1989, 63, 4882–4889. [Google Scholar] [PubMed]
- Wirth, U.V.; Vogt, B.; Schwyzer, M. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J. Virol. 1991, 65, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.; Perez, S.; Meyer, F.; Doster, A.; Jones, C. Dexamethasone treatment of calves latently infected with bovine herpesvirus 1 (BHV-1) leads to activation of the bICP0 early promoter. J Virol. 2009, In Press. [Google Scholar] [PubMed]
- Yang, H.; Ma, G.; Lin, C.H.; Orr, M.; Wathelet, M.G. Mechanism for transcriptional synergy between interferon regulatory factor (IRF)-3 and IRF-7 in activation of the interferon-beta gene promoter. Eur. J. Biochem. 2004, 271, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Yates, W.D.G. A review of infectious bovine rhinotracheitis, shipping fever pneumonia, and viral-bacterial synergism in respiratory diesease of cattle. Can. J. Comp. Med. 1982, 46, 225–263. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Suhara, W.; Fukuhara, M.; Fukuda, M.; Nishida, E.; Fujita, T. Direct triggering of the type 1 interferon system by virus infection: activation of a transcription factors containing IRF-3 and CBP/p300. EMBO J. 1998, 17, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yuang, Y.; Lowther, W.; Kellum, M.; Au, W.C.; Lin, R.; Hiscott, J.; Pitha, P.M. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 1998, 95, 9837–9842. [Google Scholar] [CrossRef]
- Zhang, L.; Pagano, J.S. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bieker, J.J. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 1998, 95, 9855–9860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jones, C. Identification of functional domains within the bICP0 protein encoded by BHV-1. J. Gen. Virol. 2005, 86, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jones, C. The bovine herpesvirus 1 immediate-early protein (bICP0) associates with histone deacetylase 1 to activate transcription. J. Virol. 2001, 75, 9571–9578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Zhou, J.; Jones, C. The bovine herpes virus 1 (BHV-1) immediate early protein (bICP0) interacts with the histone acetyltransferase p300, and these interactions correlate with stimulation of gC promoter activity. J. Gen. Virol. 2006, 87, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.