Decoupled Leaf Physiology and Branch-Level BVOC Emissions in Two Tree Species Under Water and Nitrogen Treatments
Abstract
1. Introduction
2. Materials and Methods
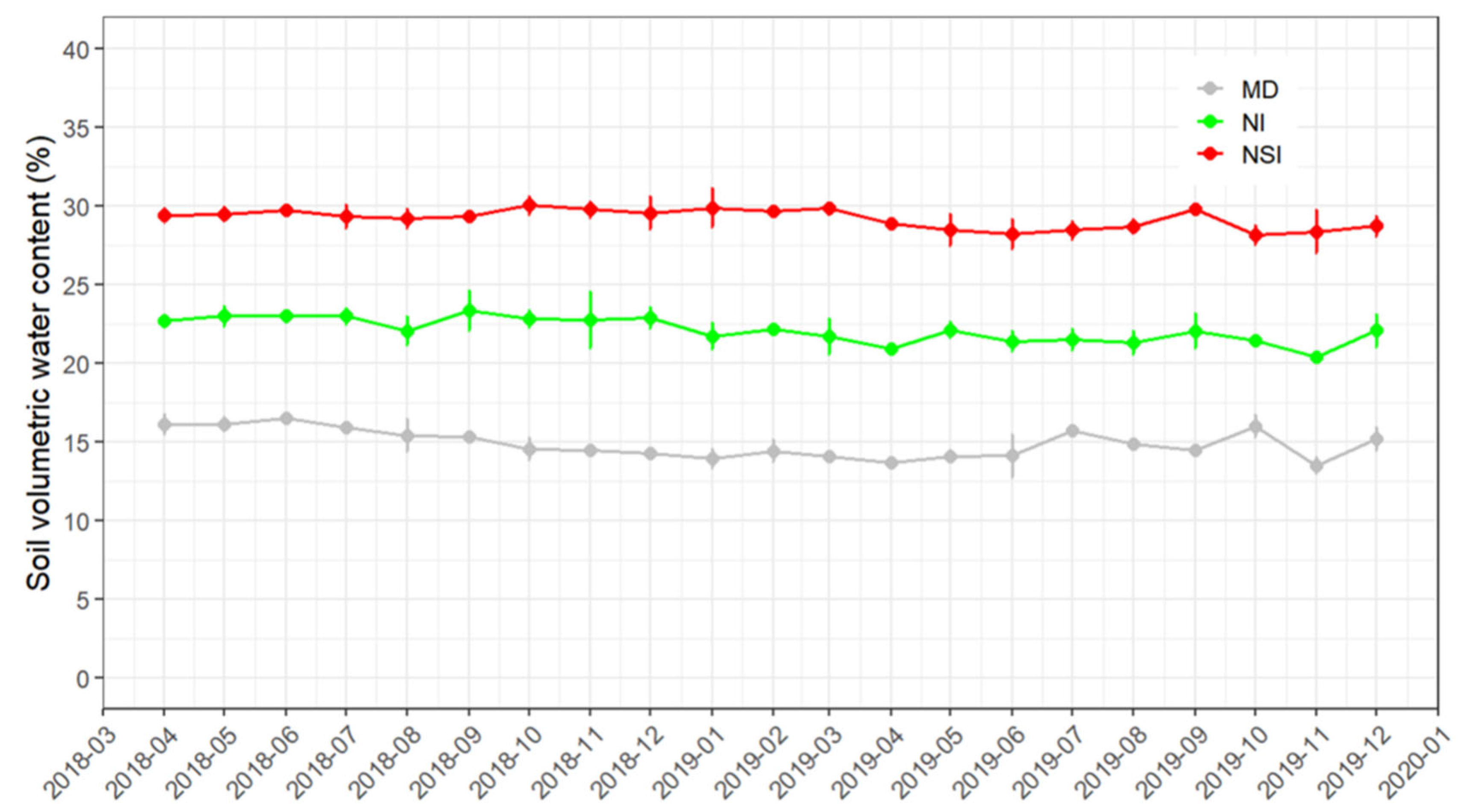
2.1. Experimental Treatments
2.2. BVOC Emissions and Gas Exchange Measurements
2.3. BVOCs Quantification and Emission Determination
2.4. Enzyme Activity Assays of BVOC Biosynthetic and Photosynthetic Enzymes
2.5. Statistical Analysis
3. Results
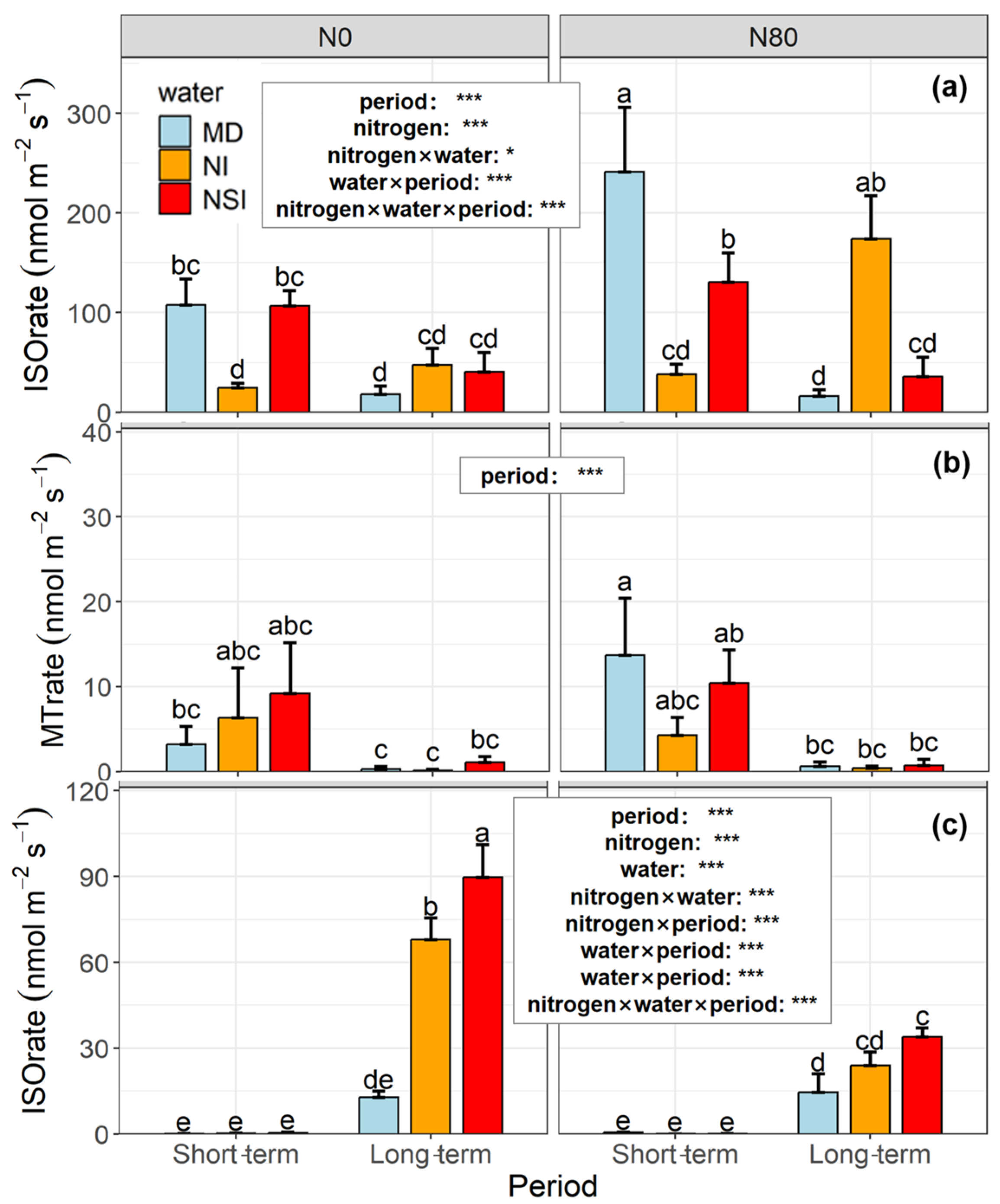
3.1. Effects of Water and Nitrogen Treatments on Isoprene and Total Monoterpene Emission Across Two Sampling Periods
3.2. Differences in BVOC Composition Under Short- and Long-Term Water and Nitrogen Treatments
3.3. Effects of Water and Nitrogen Treatments on Leaf Physiological Parameters
3.4. Correlations Between BVOC Emissions and Leaf Physiological and Biochemical Parameters
4. Discussion
4.1. Responses of BVOC Emissions to Short-Term Water and Nitrogen Treatments
4.2. Responses of BVOC Emissions to Long-Term Water and Nitrogen Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef]
- Peñuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global dataset of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN 2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Gómez, M.C.; Durana, N.; García, J.A.; de Blas, M.; de Cámara, E.S.; García-Ruiz, E.; Gangoiti, G.; Torre-Pascual, E.; Iza, J. Long-term measurement of biogenic volatile organic compounds in a rural background area: Contribution to ozone formation. Atmos. Environ. 2020, 224, 117315. [Google Scholar] [CrossRef]
- Qin, M.M.; Wang, X.S.; Hu, Y.T.; Ding, X.; Song, Y.; Li, M.M.; Vasilakos, P.; Nenes, A.; Russell, A.G. Simulating biogenic secondary organic aerosol during summertime in China. J. Geophys. Res. Atmos. 2018, 123, 11100–11119. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Lee, M.; Shim, H.; Wolfe, G.M.; Guenther, A.B.; He, A.; Hong, Y.; Han, J. Impact of isoprene and HONO chemistry on ozone and OVOC formation in a semirural South Korean forest. Atmos. Chem. Phys. 2015, 15, 4357–4371. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mojzes, A.; Kalapos, T.; Kovács-Láng, E. Plant ecophysiological responses to drought, nocturnal warming and variable climate in the Pannonian sand forest-steppe: Results of a six-year climate manipulation experiment. Biologia 2017, 72, 1431–1445. [Google Scholar] [CrossRef]
- Haberstroh, S.; Kreuzwieser, J.; Lobo-do-Vale, R.; Caldeira, M.C.; Dubbert, M.; Werner, C. Terpenoid emissions of two Mediterranean woody species in response to drought stress. Front. Plant Sci. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Leite, A.T.; Costa, M.H.; Fu, R. The southern Amazon rainy season: The role of deforestation and its interactions with large-scale mechanisms. Int. J. Climatol. 2020, 40, 2328–2341. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2007, 131, 211–225. [Google Scholar] [CrossRef]
- Svendsen, S.H.; Lindwall, F.; Michelsen, A.; Rinnan, R. Biogenic volatile organic compound emissions along a high arctic soil moisture gradient. Sci. Total Environ. 2016, 573, 131–138. [Google Scholar] [CrossRef]
- Otu-Larbi, F.; Bolas, C.G.; Ferracci, V.; Staniaszek, Z.; Jones, R.L.; Malhi, Y.; Harris, N.R.P.; Wild, O.; Ashworth, K. Modelling the effect of the 2018 summer heatwave and drought on isoprene emissions in a UK woodland. Glob. Change Biol. 2020, 26, 2320–2335. [Google Scholar] [CrossRef]
- Parveen, S.; Rashid, M.H.; Inafuku, M.; Iwasaki, H.; Oku, H. Molecular regulatory mechanism of isoprene emission under short-term drought stress in the tropical tree Ficus septica. Tree Physiol. 2019, 39, 440–453. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Calatayud, V.; Gao, F.; Fares, S.; Paoletti, E.; Tian, Y.; Feng, Z. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 2016, 39, 2276–2287. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Yuan, X.Y.; Fares, S.; Loreto, F.; Li, P.; Hoshika, Y.; Paoletti, E. Isoprene is more affected by climate drivers than monoterpenes: A meta-analytic review on plant isoprenoid emissions. Plant Cell Environ. 2019, 42, 1939–1949. [Google Scholar] [CrossRef]
- Lavoir, A.V.; Staudt, M.; Schnitzler, J.P.; Landais, D.; Massol, F.; Rocheteau, A.; Rodriguez, R.; Zimmer, I.; Rambal, S. Drought reduced monoterpene emissions from the evergreen Mediterranean oak Quercus ilex: Results from a throughfall displacement experiment. Biogeosciences 2009, 6, 1167–1180. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Feng, Z.Z.; Shang, B.; Calatayud, V.; Paoletti, E. Ozone exposure, nitrogen addition and moderate drought dynamically interact to affect isoprene emission in poplar. Sci. Total Environ. 2020, 734, 139368. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Wu, Y.; Liu, S.; Kong, F.L.; Li, L.Y. Effects of soil drought and nitrogen deposition on BVOC emissions and their O3 and SOA formation for Pinus thunbergii. Environ. Pollut. 2023, 316, 120693. [Google Scholar] [CrossRef]
- Silver, G.M.; Fall, R. Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J. Biol. Chem. 1995, 270, 13010–13016. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Ghirardo, A.; Juurola, E.; Schnitzler, J.P.; Zimmer, I.; Hellén, H.; Bäck, J. Long-term dynamics of monoterpene synthase activities, monoterpene storage pools and emissions in boreal Scots pine. Biogeosciences 2018, 15, 5047–5060. [Google Scholar] [CrossRef]
- Li, S.J.; Feng, Z.; Yuan, X.; Wang, M.; Agathokleous, E. Elevated ozone inhibits isoprene emission of a diploid and a triploid genotype of Populus tomentosa by different mechanisms. J. Exp. Bot. 2022, 73, 6449–6462. [Google Scholar] [CrossRef]
- Li, S.J.; Yuan, X.; Li, S.L.; Zhou, Y.Q.; Wang, S.L.; Zhang, K.; Agathokleous, E.; Blande, J.D.; Feng, Z. Extreme heat event alters BVOC responses to elevated ozone: From physiology to emission patterns. Environ. Sci. Technol. 2025, 59, 12132–12144. [Google Scholar] [CrossRef]
- Street, R.A.; Hewitt, C.N.; Mennicken, S. Isoprene and monoterpene emissions from a Eucalyptus plantation in Portugal. J. Geophys. Res. Atmos. 1997, 102, 15875–15887. [Google Scholar] [CrossRef]
- He, C.R.; Murray, F.; Lyons, T. Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos. Environ. 2000, 34, 645–655. [Google Scholar] [CrossRef]
- Winters, A.J.; Adams, M.A.; Bleby, T.M.; Rennenberg, H.; Steigner, D.; Steinbrecher, R.; Kreuzwieser, J. Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmos. Environ. 2009, 43, 3035–3043. [Google Scholar] [CrossRef]
- Liu, J.X.; Li, Y.Y.; Xu, Y.; Liu, S.E.; Huang, W.J.; Fang, X.; Yin, G. Phosphorus uptake in four tree species under nitrogen addition in subtropical China. Environ. Sci. Pollut. Res. 2017, 24, 20005–20014. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, T.; Ge, X.; Cao, Y.; Li, Z.C.; Zhou, B.Z. Emission trade-off between isoprene and other BVOC components in Pinus massoniana saplings may be regulated by content of chlorophylls, starch and NSCs under drought stress. Int. J. Mol. Sci. 2023, 24, 8946. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Wang, X.M.; Wu, T. Release of isoprene and monoterpenes during the aerobic decomposition of orange wastes from laboratory incubation experiments. Environ. Sci. Technol. 2008, 42, 3265–3270. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Klinger, L.F.; Li, Q.J.; Guenther, A.B.; Greenberg, J.P.; Baker, B.; Bai, J.H. Assessment of volatile organic compound emissions from ecosystems of China. J. Geophys. Res.-Atmos. 2002, 107, 4603. [Google Scholar] [CrossRef]
- Aydin, Y.M.; Yaman, B.; Koca, H.; Dasdemir, O.; Kara, M.; Altiok, H.; Dumanoglu, Y.; Bayram, A.; Tolunay, D.; Odabasi, M.; et al. Biogenic volatile organic compound (BVOC) emissions from forested areas in Turkey: Determination of specific emission rates for thirty-one tree species. Sci. Total Environ. 2014, 490, 239–253. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Grote, R.; Monson, R.K.; Niinemets, Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–355. [Google Scholar]
- Ryan, A.C.; Hewitt, C.N.; Possell, M.; Vickers, C.E.; Purnell, A.; Mullineaux, P.M.; Davies, W.J.; Dodd, I.C. Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol. 2014, 201, 205–216. [Google Scholar] [CrossRef]
- Monson, R.K.; Neice, A.A.; Trahan, N.A.; Shiach, I.; McCorkel, J.T.; Moore, D.J.P. Interactions between temperature and intercellular CO2 concentration in controlling leaf isoprene emission rates. Plant Cell Environ. 2016, 39, 2404–2413. [Google Scholar] [CrossRef]
- Nogues, I.; Medori, M.; Fortunati, A.; Lellei-Kovacs, E.; Kroel-Dulay, G.; Calfapietra, C. Leaf gas exchange and isoprene emission in poplar in response to long-term experimental night-time warming and summer drought in a forest-steppe ecosystem. Environ. Exp. Bot. 2018, 152, 60–67. [Google Scholar] [CrossRef]
- Haworth, M.; Catola, S.; Marino, G.; Brunetti, C.; Michelozzi, M.; Riggi, E.; Avola, G.; Cosentino, S.L.; Loreto, F.; Centritto, M. Moderate drought stress induces increased foliar dimethylsulphoniopropionate (DMSP) concentration and isoprene emission in two contrasting ecotypes of Arundo donax. Front. Plant Sci. 2017, 8, 1016. [Google Scholar] [CrossRef]
- Ahrar, M.; Doneva, D.; Tattini, M.; Brunetti, C.; Gori, A.; Rodeghiero, M.; Wohlfahrt, G.; Biasioli, F.; Varotto, C.; Loreto, F.; et al. Phenotypic differences determine drought stress responses in ecotypes of Arundo donax adapted to different environments. J. Exp. Bot. 2017, 68, 2439–2451. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Rocca, G.; Centritto, M.; Parenti, A.; Monti, A. Giant reed genotypes from temperate and arid environments show different response mechanisms to drought. Physiol. Plant. 2018, 163, 490–501. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Arneth, A.; Kuhn, U.; Monson, R.K.; Penuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef]
- Bruggemann, N.; Schnitzler, J.P. Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens Willd. and Quercus robur L.) saplings. Plant Biol. 2002, 4, 456–463. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Shang, B.; Xu, Y.S.; Xin, Y.; Tian, Y.; Feng, Z.Z.; Paoletti, E. No significant interactions between nitrogen stimulation and ozone inhibition of isoprene emission in cathay poplar. Sci. Total Environ. 2017, 601–602, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Litvak, M.E.; Loreto, F.; Harley, P.C.; Sharkey, T.D.; Monson, R.K. The response of isoprene emission rate and photosynthetic rate to photon flux and nitrogen supply in aspen and white oak trees. Plant Cell Environ. 1996, 19, 549–559. [Google Scholar] [CrossRef]
- Carriero, G.; Brunetti, C.; Fares, S.; Hayes, F.; Hoshika, Y.; Mills, G.; Tattini, M.; Paoletti, E. BVOC responses to realistic nitrogen fertilization and ozone exposure in silver birch. Environ. Pollut. 2016, 213, 988–995. [Google Scholar] [CrossRef]
- Llusià, J.; Bermejo-Bermejo, V.; Calvete-Sogo, H.; Peñuelas, J. Decreased rates of terpene emissions in Ornithopus compressus L. and Trifolium striatum L. by ozone exposure and nitrogen fertilization. Environ. Pollut. 2014, 194, 69–77. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.P.; Sharkey, T.D. Leaf isoprene emission as a trait that mediates the growth-defense tradeoff in the face of climate stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- Pollastri, S.; Baccelli, I.; Loreto, F. Isoprene: An antioxidant itself or a molecule with multiple regulatory functions in plants? Antioxidants 2021, 10, 684. [Google Scholar] [CrossRef]
- Malik, T.G.; Gajbhiye, T.; Pandey, S.K. Some insights into composition and monoterpene emission rates from selected dominant tropical tree species of Central India: Plant-specific seasonal variations. Ecol. Res. 2019, 34, 821–834. [Google Scholar] [CrossRef]
- Sørensen, M.; Rinnan, R.; Woodrow, I.; Moller, B.L.; Neilson, E.H.J. The entangled dynamics of eucalypt leaf and flower volatile emissions. Environ. Exp. Bot. 2020, 176, 104032. [Google Scholar] [CrossRef]
- Purser, G.; Heal, M.R.; White, S.; Morison, J.I.L.; Drewer, J. Differences in isoprene and monoterpene emissions from cold-tolerant eucalypt species grown in the UK. Atmos. Pollut. Res. 2020, 11, 2011–2021. [Google Scholar] [CrossRef]
- Nagalingam, S.; Seco, R.; Kim, S.; Guenther, A. Heat stress strongly induces monoterpene emissions in some plants with specialized terpenoid storage structures. Agric. For. Meteorol. 2023, 333, 109400. [Google Scholar] [CrossRef]
- Pollastri, S.; Jorba, I.; Hawkins, T.J.; Llusià, J.; Michelozzi, M.; Navajas, D.; Peñuelas, J.; Hussey, P.J.; Knight, M.R.; Loreto, F. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 2019, 223, 1307–1318. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Monson, R.K.; Arneth, A.; Ciccioli, P.; Kesselmeier, J.; Kuhn, U.; Noe, S.M.; Peñuelas, J.; Staudt, M. The leaf-level emission factor of volatile isoprenoids: Caveats, model algorithms, response shapes and scaling. Biogeosciences 2010, 7, 1809–1832. [Google Scholar] [CrossRef]
- Wiberley, A.E.; Linskey, A.R.; Falbel, T.G.; Sharkey, T.D. Development of the capacity for isoprene emission in kudzu. Plant Cell Environ. 2005, 28, 898–905. [Google Scholar] [CrossRef]
- Centritto, M.; Brilli, F.; Fodale, R.; Loreto, F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef]


| Species | Pn | gs | Ci | Tr | IspS | MtpS | RuBisCO | CA | PEPCK | |
|---|---|---|---|---|---|---|---|---|---|---|
| O. pinnata | P | <0.01 | <0.001 | <0.01 | <0.001 | 0.890 | / | 0.411 | 0.981 | <0.01 |
| W | 0.260 | <0.05 | <0.05 | 0.075 | 0.918 | / | 0.075 | 0.139 | 0.379 | |
| N | 0.423 | 0.899 | <0.05 | 0.837 | 0.205 | / | 0.006 | 0.582 | <0.05 | |
| W × N | 0.09 | 0.455 | 0.789 | 0.437 | <0.01 | / | 0.571 | 0.910 | 0.569 | |
| P × N | 0.518 | 0.826 | 0.597 | 0.745 | 0.397 | / | 0.998 | 0.890 | 0.653 | |
| W × P | 0.394 | 0.210 | 0.122 | 0.292 | 0.774 | / | 0.987 | 0.687 | 0.373 | |
| P × W × N | 0.297 | 0.252 | 0.957 | 0.165 | 0.392 | / | 0.388 | 0.257 | 0.781 | |
| P. massoniana | P | 0.182 | 0.123 | 0.233 | 0.608 | <0.01 | <0.001 | <0.001 | <0.01 | 0.296 |
| W | 0.285 | <0.001 | <0.001 | 0.065 | 0.555 | 0.707 | 0.152 | 0.697 | 0.028 | |
| N | 0.560 | 0.734 | 0.713 | 0.705 | 0.923 | 0.340 | 0.901 | 0.146 | 0.564 | |
| W × N | 0.975 | 0.780 | 0.698 | 0.662 | 0.163 | 0.256 | 0.053 | 0.514 | <0.01 | |
| P × N | 0.910 | 0.803 | <0.05 | 0.972 | 0.679 | 0.117 | 0.622 | 0.655 | 0.928 | |
| W × P | 0.824 | 0.308 | 0.068 | 0.877 | 0.441 | 0.911 | 0.673 | 0.251 | <0.01 | |
| P × W × N | 0.752 | 0.972 | 0.670 | 0.510 | 0.406 | 0.558 | 0.385 | 0.538 | 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yan, D.; Liu, X.; Lin, M.; Yi, Z. Decoupled Leaf Physiology and Branch-Level BVOC Emissions in Two Tree Species Under Water and Nitrogen Treatments. Forests 2025, 16, 1708. https://doi.org/10.3390/f16111708
Li S, Yan D, Liu X, Lin M, Yi Z. Decoupled Leaf Physiology and Branch-Level BVOC Emissions in Two Tree Species Under Water and Nitrogen Treatments. Forests. 2025; 16(11):1708. https://doi.org/10.3390/f16111708
Chicago/Turabian StyleLi, Shuangjiang, Diao Yan, Xuemei Liu, Maozi Lin, and Zhigang Yi. 2025. "Decoupled Leaf Physiology and Branch-Level BVOC Emissions in Two Tree Species Under Water and Nitrogen Treatments" Forests 16, no. 11: 1708. https://doi.org/10.3390/f16111708
APA StyleLi, S., Yan, D., Liu, X., Lin, M., & Yi, Z. (2025). Decoupled Leaf Physiology and Branch-Level BVOC Emissions in Two Tree Species Under Water and Nitrogen Treatments. Forests, 16(11), 1708. https://doi.org/10.3390/f16111708









