Abstract
Mangrove ecosystems play a critical role in global carbon cycling, serving as significant carbon sinks by storing carbon in both aboveground biomass (ACG) and soil carbon stock (SOC). However, the temporal dynamics of ACG and SOC, as well as their spatial variations across different mangrove age stages, remain poorly understood, particularly under the influence of introduced species such as Sonneratia apetala Buch.-Ham. To address these gaps, our study used a long-term series of NDVI from Landsat (from 1990 to 2024) and the mangrove product of China (1990, 2000, 2010, and 2018) to estimate the mangrove age stage ( 10–24 years, 24–34 years, and > 34 years). UAV-LiDAR and in-situ surveys were applied to measure mangrove canopy height to calculate ACG and measure the belowground soil carbon stock, respectively. Combined with the mangrove age stage, ACG, and SOC, our results reveal that ACG accumulates rapidly in younger mangroves dominated by Sonneratia apetala, peaking early (<20 years) and then stabilizing as mangroves, indicating that the introduction of Sonneratia apetala changed the increase in ACG with age. In contrast, SOC increases more gradually over time, with only older mangroves (over 30 years) storing significantly higher SOC. Root structure, TN, and TP were sensitive to the SOC. The different root structures (pneumatophore, plank, pop, and knee root) had different SOC results, and the pneumatophore had the lowest SOC. Remote sensing data revealed that the introduction of Sonneratia apetala altered the species composition of younger mangroves, leading to its predominance within these ecosystems. This shift in species composition not only altered the temporal dynamics of aboveground carbon (ACG) but also favored pneumatophore-dominated root structures, which were associated with the lowest soil organic carbon (SOC). Consequently, younger stands may require more time to accumulate SOC to levels comparable to older mangrove forests. These results suggest that restoration targets for vegetation carbon and soil carbon should be set on different timelines, explicitly accounting for stand age, species composition, and root functional types.
1. Introduction
Mangrove ecosystems are critical carbon sinks that store substantial amounts of carbon in both aboveground biomass (ACG) and belowground soil carbon stocks (SOC), playing a vital role in mitigating climate change [1,2,3]. However, the temporal dynamics of ACG and SOC—i.e., how these carbon pools change over time and interact with forest age, species traits, and environmental factors—remain insufficiently understood. Understanding these dynamics is crucial for optimizing mangrove restoration strategies, particularly in light of increasing restoration efforts globally [4,5]. For instance, fast-growing introduced species such as Sonneratia apetala Buch.-Ham are widely used in restoration projects due to their rapid aboveground biomass accumulation [6,7,8]; however, their long-term effects on ACG and SOC trajectories remain unclear. Without a clear understanding of the mechanisms driving ACG and SOC changes, restoration policies may fail to maximize carbon sequestration or, worse, disrupt natural carbon cycles.
Recent technological advancements, including Landsat satellite imagery and UAV-LiDAR, have provided powerful tools for large-scale and long-term monitoring of mangrove carbon stocks [9,10]. UAV-LiDAR captures high-resolution structural data essential for accurately quantifying ACG [11,12]. Previous studies have demonstrated that structural metrics derived from UAV-LiDAR can retrieve vegetation biomass with higher accuracy than satellite-based remote sensing [12,13]. Landsat, with its multi-decadal archive since the 1980s, enables the estimation of forest age and land cover changes, and numerous studies have used these datasets to assess forest age and evaluate the potential of forest carbon stocks for mitigating climate change [13,14]. Integrating these technologies with in situ measurements offers a robust approach to calculating ACG and dynamics with mangrove age increasing and identifying their driving mechanisms [15,16]. By combining remote sensing data with field surveys, this study investigates how mangrove carbon pools evolve over three forest age stages, focusing on the temporal divergence between ACG and SOC, thereby offering insights to support government efforts in monitoring the spatial and temporal dynamics of mangrove carbon storage.
Aboveground carbon stock (ACG) generally increases with forest age due to biomass accumulation, but this growth is not uniform [17,18]. Young mangroves often exhibit rapid biomass accumulation due to high growth rates, while older forests tend to stabilize as they approach their biomass saturation point [14,19]. Species-specific traits—such as the exceptionally rapid growth of Sonneratia apetala—can substantially modify ACG accumulation patterns [6,7]. As this fast-growing exotic species has been widely replanted, it has gradually become dominant in many restoration areas [6,20], thereby accelerating aboveground carbon (ACG) accumulation and altering the temporal trajectory of ACG compared to older, native mangrove stands. However, it remains unclear whether these species-level differences influence the temporal distribution and dynamics of ACG across different forest stages.
In contrast, soil carbon stock (SOC), which is primarily stored in sediments, is more strongly influenced by soil environmental factors and root traits than by species level differences [21]. Mangrove root systems—by trapping sediments and organic matter—serve as key drivers of SOC accumulation [22,23]. Root architecture varies significantly among species, with shallow, spreading roots and deep, vertical roots influencing both the rate and depth of SOC accumulation [21]. Furthermore, environmental factors such as soil salinity, water-table depth, and microbial activity regulate root growth and organic matter decomposition, thereby shaping SOC dynamics [24,25]. Nevertheless, further research is needed to clarify whether root structural traits and environmental conditions play a more decisive role in SOC regulation than species-level differences.
Recent studies have shown that the sequestration and turnover of ACG and SOC are fundamentally different, resulting in distinct temporal trajectories for these two carbon pools [1,26]. For instance, while ACG may peak and plateau within decades, SOC can accumulate over much longer timescales [24,27,28]. Moreover, the introduction of fast-growing species such as Sonneratia apetala may further amplify this divergence by rapidly altering ACG dynamics while exerting minimal or delayed effects on SOC [6]. These discrepancies underscore the importance of examining the asymmetrical and asynchronous temporal changes in ACG and SOC and their underlying drivers. The asynchronous behavior between aboveground and belowground carbon also suggests that SOC in restored or disturbed mangroves requires substantially longer recovery periods to reach levels comparable to those in natural, mature forests.
In this study, we aimed to reveal the differences in the temporal dynamics of SOC and ACG, as well as the key factors influencing them. We integrated time-series Landsat NDVI data (1990–2024) with national mangrove maps (1990, 2000, 2010, and 2018) to classify mangroves into three age stages (Stage I: 10–24 years; Stage II: 24–34 years; Stage III: >34 years). Aboveground carbon (ACG) was estimated from UAV-LiDAR-derived canopy height, while SOC was quantified through field sampling. We then analyzed the temporal patterns of both ACG and SOC and identified their dominant driving factors, providing insights to guide restoration planning and determine appropriate timescales for achieving ACG and SOC recovery.
We hypothesized:
- (1)
- The temporal changes in ACG and SOC are asymmetric and asynchronous.
- (2)
- Species-level differences play a dominant role in determining ACG and root structure, whereas soil environmental factors are the primary drivers of SOC dynamics
2. Methods
2.1. Study Area
Zhanjiang, located in the southernmost region of mainland China, spans from 109°40′ E to 110°58′ E and 20°13′ N to 21°57′ N. The region experiences a tropical monsoon climate with considerable rainfall, receiving between 1500 and 2000 mm of precipitation annually and with an average temperature of about 21 °C [6,26]. Zhanjiang, renowned for its abundant marine resources, features a coastline stretching over 2023.6 km and encompasses a mangrove forest area surpassing 6000 hectares. Within Zhanjiang’s mangrove ecosystems, there exist 28 distinct mangrove species, among which the dominant ones include Avicennia marina (Forssk.) Vierh., Aegiceras corniculatum (L.) Blanco, Sonneratia apetala Buch.-Ham., Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong, and Rhizophora stylosa Griff. [10].
2.2. Field Sampling
Two field surveys were conducted, in May 2022 and September 2022, covering a total of 60 plots (Figure 1), each measuring 10 × 10 m (Table 1), distributed across eight areas in Zhanjiang. During these surveys, detailed data on mangrove species and tree height were collected. Although the soil survey was conducted at the same time and in the same locations as the vegetation survey, the number of vegetation quadrats exceeded that of soil quadrats (55 samples) due to the labor-intensive process of collecting soil samples, which followed procedures outlined in previous studies [5,14]. Within each area, 2 to 4 plots, each measuring 5 m × 5 m, were selected to represent the primary mangrove communities in that specific area. A total of 55 plots were chosen across the 8 areas, with their respective geographical coordinates (longitude and latitude) and species names meticulously recorded. The primary mangrove communities identified included Avicennia marina, Aegiceras corniculatum, Sonneratia apetala, Kandelia obovata, Rhizophora stylosa, and Bruguiera gymnorhiza. All the geographical coordinates of soil and vegetation plots were monitored using the handheld GPS (UniStrong).
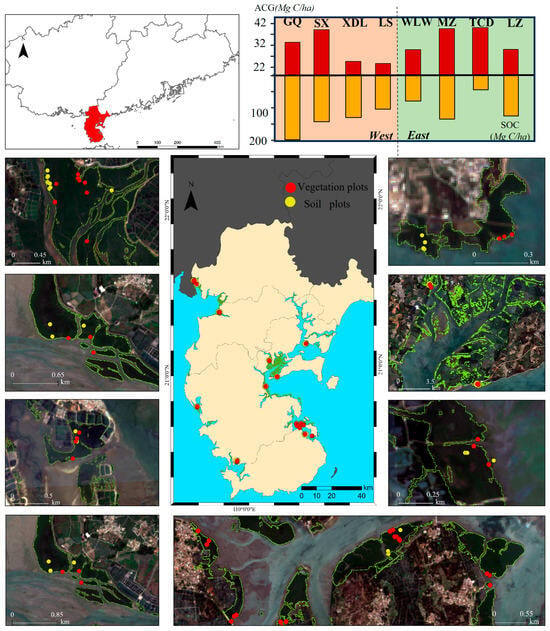
Figure 1.
Overview of the study area and survey plots in Zhanjiang.

Table 1.
In situ survey calculation of mangrove aboveground (ACG) and soil carbon stocks (SOC) for each sampling site in Zhanjiang, China.
During low tide, we collected three 1 m soil cores per plot using a 5 cm (diameter) stainless-steel corer. We avoided respiratory roots, crab burrows, and trampling traces and removed the surface apoplastic layer and living tissue prior to coring. To correct for compaction, we recorded both the core length and penetration depth, calculated a compression coefficient (core length ÷ penetration depth), and applied this correction factor to each depth interval [29]. In the field, soil cores were sectioned into eight depth intervals: 0–5, 5–10, 10–20, 20–30, 30–45, 45–60, 60–80, and 80–100 cm [30]. All soil cores were used as an undisturbed profile sample. Samples were placed in zip-top polyethylene bags, kept at −4 °C, and transferred to a −20 °C laboratory freezer within four hours of collection. Samples were sealed in zip-lock bags, chilled at −4 °C, and transferred to a −20 °C freezer within four hours.
2.3. Data Preparation
2.3.1. Collecting the UAV-LiDAR and Extracting the Canopy Height
The LiDAR data were collected during the two-field surveys encompassing 60 sampling plots. To ensure data quality, all flights were conducted during low tide under clear and windless weather conditions. A Zenmuse L1 LiDAR sensor (DJI, Shenzhen, China) mounted on a DJI UAV was used, with a flight altitude of approximately 50 m. The raw LiDAR data were processed in DJI Terra (DJI, Shenzhen, China) to generate point clouds, with the point cloud density set to High and the elevation metric defined as the 95th percentile. All outputs were projected to the WGS-84 coordinate system. Canopy height was subsequently derived from the LiDAR point clouds following standard procedures described in previous studies [10,16,31,32]. Previous studies have demonstrated that canopy height derived from UAV-LiDAR data is highly consistent with field-measured canopy height, with strong correlations and low estimation errors [10,31]. To improve data quality, a hierarchical denoising workflow was applied before subsequent analysis using LiDAR 360 (version 5.4). This process combined statistical outlier removal (1σ threshold within a 1 m neighborhood) with morphological filtering. After preprocessing, a 10 m Digital Surface Model (DSM) was generated by extracting the highest LiDAR returns [33]. A Digital Elevation Model (DEM) was then derived by separating ground and non-ground points with the improved progressive TIN densification algorithm [34], supplemented with manual adjustments, also at 10 m resolution. Finally, the Canopy Height Model (CHM) was obtained by subtracting the DEM from the DSM, where each pixel represents mangrove canopy height [16]. To assess accuracy, we compared UAV-LiDAR-derived canopy height (CH) with in situ measurements, confirming the robustness of the UAV-LiDAR estimates (Figure A1).
2.3.2. Auxiliary Data
For analysis of the relationship between climate factors and mangrove carbon stock, ECMWF/Copernicus Climate Change Service (2 km) was used to obtain climate factors. According to Meng et al. [35] and Lu et al. [36], air temperature (Tem), precipitation (Preci), and vapor pressure deficit (VPD) were important factors in the carbon fluxes of China’s coastal wetlands. Those climate factors were chosen and calculated for the mean value of 2000–2023 (Tem, Preci, and VPD) on the Google Earth Engine platform.
HSL_MangroveChina_LASAC_share provides the spatial distribution of China’s mangrove resources in Shapefile format across five epochs (1978, 1990, 2000, 2013, and 2018). The 2018 mangrove map was produced from domestic 2 m satellite imagery (including ZY-3 01/02, GF-1, and GF-1 B/C/D) using a combined workflow of automated computer interpretation, expert refinement, and field verification, achieving an overall accuracy of 98% [37,38].
The Landsat NDVI time series (1990–2024) and the China mangrove dataset (HSL_MangroveChina_LASAC_share) were used to estimate mangrove age stage (Table 2). We utilized the Google Earth Engine (GEE) platform’s full archive to compile stacks of Surface Reflectance Tier 1 Landsat TM/ETM+/OLI images spanning from 1990 to 2024. To reduce phenological and solar-geometry effects, images were restricted to summer (1 June–1 September) across Zhanjiang. We then generated annual median composites with minimal cloud contamination and computed NDVI from each composite to track vegetation dynamics from 1990 to 2024.

Table 2.
Datasets used in this study.
To understand the composition of mangrove species across different age stages, a spatial map of mangrove species is essential (Table 2). Spatial distribution data of mangrove species in China (10 m) were utilized to support the analysis, which was extracted from synthesized Sentinel-2 images [20,39,40]. The spatial distribution of mangroves in China was originally classified into ten types, five of which—Kandelia obovata, Aegiceras corniculatum, Sonneratia apetala, Rhizophora stylosa, and Avicennia marina—were consistent with our study. For subsequent analysis, we extracted those five mangrove species: Kandelia obovata, Aegiceras corniculatum, Sonneratia apetala, Rhizophora stylosa, Avicennia marina.
2.4. Calculations of the Aboveground Vegetation and Soil Carbon Stocks
2.4.1. Calculation of the Aboveground Vegetation Carbon Stocks
To estimate aboveground carbon stock (ACG), we derived canopy height (CH) from UAV-LiDAR data, following the methodology described by Maimaitijiang et al. [9] and Lu et al. [31], and we applied a commonly used allometric growth equation [41,42]. This involved calculations of aboveground biomass for each plant within the sampled quadrats. Subsequently, the total aboveground biomass for each quadrat was determined by aggregating the individual plant biomass values. This approach enabled us to account for the varied characteristics of mangrove species and conduct a comprehensive assessment of biomass across the study area.
is the vegetation carbon stock, is the canopy height from UAV-LiDAR, and 0.47 is the transform parameter from aboveground vegetation biomass to the vegetation carbon stock, according to Meng et al. (2022) [35]. Within each area, a total of 60 plots were chosen across the 8 areas.
2.4.2. Laboratory Analysis
The soil samples were subjected to oven-drying at 65 °C until reaching a constant weight [43,44]. Following the removal of plant debris and shells, the soil samples were ground and sieved through a 0.2 mm sieve for the determination of Total Nitrogen (TN), Total Phosphorus (TP), salinity, and organic carbon content. Soil salinity was assessed using a YSI-proplus multi-probe sensor (YSI Incorporated, Yellow Springs, OH, USA) with a soil-to-deionized water ratio of 1:5 (w/v). TP was determined through H2SO4-HClO4 digestion and analyzed using a UV-1900i spectrophotometer (Shimadzu (Hong Kong) Ltd., Hong Kong, China). TN was determined via Kjeldahl digestion using an A AA3 continuous flow analyzer (SEAL Analytical, Norderstedt, Germany) [7]. Soil bulk density (BD) was calculated based on the mass of the completely dried sample and the soil volume using the formula:
BD (g cm−3) = Dry weight of soil (g)/Soil volume (cm3)
Soil volume = 3.14 × (soil auger radius2 (cm)× soil thickness (cm))
Soil organic carbon content was analyzed using a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany; C% accuracy: ±0.5%). The soil carbon stock was computed by multiplying BD by the soil carbon content and then by the corresponding corrected soil depth, as per the formula proposed by Kauffman and Donato [45]:
The temporal dynamics of soil carbon stocks were found to be significant within the 0–60 cm soil layer [8]. In this study, we first calculated SOC for eight individual depth intervals and then aggregated them into five standardized layers (0–20, 20–40, 40–60, 60–80, and 80–100 cm) to analyze temporal variations. The raw data are presented in Table A1 and Table A2.
SOC = BD (g cm−3) × Carbon content (%) ×corrected soil column (cm)
2.5. Extracting the Age Stage of Mangroves
Combined with the long-term NDVI time series and the HSL_MangroveChina_LASAC_share, we extracted mangrove age information. Understanding the influence of the introduction of exotic species—Sonneratia apetala—on the temporal dynamic of ACG and SOC is the main purpose of our study. Previous studies reported that Sonneratia apetala was first introduced into China in the 1990s and was later incorporated into mangrove replantation programs in the 2000s [46,47]. By the 2010s, several studies had reported that Sonneratia apetala possesses a more tolerant and invasive niche than native mangrove species [48,49,50]. To define an appropriate temporal window for assessing changes in ACG and SOC, we drew on published estimates of carbon stocks for dominant mangrove species across age stages (Table 3). For Sonneratia apetala, aboveground biomass can exceed ~180 Mg C·ha−1 within approximately 15 years and remains at a comparable level by ~40 years, indicating an early growth plateau.
Briefly, we chose the spatial distribution of mangroves in 1990, 2000, 2013, and 2018 to filter three stages of mangrove vegetation. To identify three distinct mangrove age stages, we used historical proxy mangrove classification datasets (1990, 2000, 2013, and 2018) with high overall accuracy and determined their intersections across time periods. Long-term NDVI imagery was then applied to further validate and refine the classification, ensuring that the three mangrove stages corresponded accurately to their respective temporal periods. Previous studies have demonstrated that an NDVI threshold of 0.63 can effectively differentiate dwarf shrub mangroves from mature mangrove stands, making it a sensitive indicator for species or structural classification [51]. This conservative cutoff minimizes the risk of overestimating stand age, particularly in areas dominated by Sonneratia apetala, a fast-growing species [6,50]. Specifically, Stage III, Stage II, and Stage I were signals of the pixels with mangrove ages over 34, 24, and 11 years, respectively. Those different stages of mangrove vegetation were filtered based on three equations:
is the mangrove area in 1990, 2000, 2013, and 2018, respectively, and , and are signals of the pixel’s NDVI at different stages.

Table 3.
The aboveground vegetation biomass and belowground vegetation biomass and soil carbon stock at different ages.
Table 3.
The aboveground vegetation biomass and belowground vegetation biomass and soil carbon stock at different ages.
| Location | Latitude | Types | Age | Aboveground Vegetation Biomass (Mg C/ha) | Belowground Vegetation Biomass (Mg C/ha) | Soil Carbon Stock (Mg C/ha) | Reference |
|---|---|---|---|---|---|---|---|
| Qiao Island | 22° N | S. apetela (SA) | 1 | 1.04 | 0.13 | 77.09 | |
| 4 | 97.66 | 22.46 | 81.62 | ||||
| 9 | 146.87 | 39.42 | 85.2 | [6] | |||
| 15 | 184.88 | 47.94 | 101.22 | ||||
| 40 | 194.66 | 58.39 | |||||
| Leizhou Bay | 20–21° N | SA | 5 | 12.81 (AGB + BGB) | 68.81 | ||
| 13 | 65.04 (AGB + BGB) | 75.12 | [52] | ||||
| 20 | 65.48 (AGB + BGB) | 89.86 | |||||
| Futian Bay | 22.5° | SA | 6 | 144 | 86 | 44 | |
| 20 | 258 | 58 | 48 | [53] | |||
| Malaysia | 2°06′ N | natural | 3 | 37.8 | 42.05 | ||
| 12 | 66.01 | 32.81 | |||||
| 25 | 75.61 | 32.64 | [54] | ||||
| A. marina (AM) | 6 | 69.74 | 38.25 | ||||
| 12 | 66.01 | 32.81 | |||||
| Qiao Island | 22° N | SA | 4 | 99.42 | 24 | 92.06 | |
| SA | 9 | 147.42 | 37.71 | 92.66 | [55] | ||
| SA | 15 | 212.57 | 53.71 | 101.08 | |||
| SA | 40 | 188.57 | 87.99 | 170.88 | |||
| Yingluo Bay | 21° N | AM | 15 | 29.64 | 21.34 | 150.41 | [56] |
| AM | 45 | 84.17 | 34.3 | 234.45 | |||
| Leizhou, Guangdong | 109°03′ E, 20°30′ N | SA | 4 | 16.3 | 3.6 | 27.7 (0–20 cm) | [57] |
| 10 | 37.2 | 11. 8 | 67.5 (0–20 cm) | ||||
| Gaoqiao, Guangdong | 21°30′ N | AM | 5 | 16.4 (AGB + BGB) | |||
| Bruguiera gymnorhiza (BG) | 10 | 41.4 (AGB + BGB) | [58] | ||||
| BG | 30 | 96.1 (AGB + BGB) | |||||
2.6. Data Analysis
ACG and SOC were quantified using UAV-LiDAR data and in situ measurements. By integrating long-term NDVI imagery with the HSL_MangroveChina_LA-SAC_share dataset, we further estimated mangrove age stages and revealed the temporal dynamics of both ACG and SOC. Finally, structural equation modeling (SEM) was applied to identify the key drivers of SOC (Figure 2).
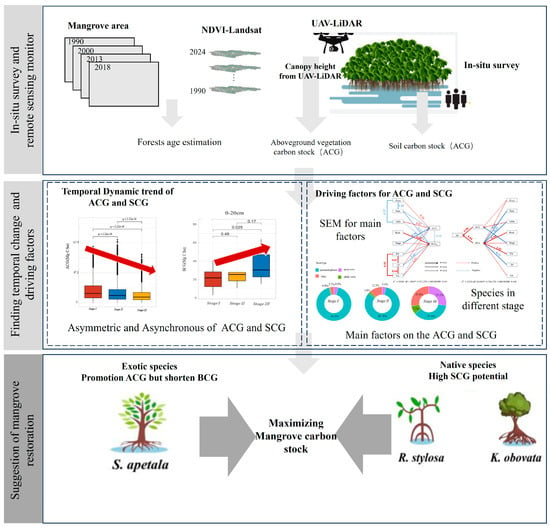
Figure 2.
Workflow for analyzing the temporal changes of ACG and SOC.
The Standard Deviational Ellipse was used to detect the temporal–spatial change trend of the mangrove area on ArcMap 10.3. The mean center was applied to demonstrate the temporal change of different mangrove stages [59]. To compare mangrove canopy height and soil carbon stocks, we performed a one-way ANOVA, and statistically significant results (p < 0.05) were further examined using LSD post-hoc tests. Results are presented as mean values with error bars indicating standard deviation (SD).
Structural equation modeling (SEM) was used to quantify the direct and indirect effects of eight primary drivers (TN, TP, SA, Stage, Root, VPD, Temp, and Pre) on SOC and ACG. In the SEM framework, Root and Stage represented species functional type and stand age, respectively. Models were implemented using the psem function in the ‘piecewiseSEM’ package (R version 4.3.3 (R Core Team, Vienna, Austria), with generalized least squares (GLS) employed to account for spatial autocorrelation among age stages and to improve model robustness [60,61]. The best-fitting model was identified as the one with the lowest AIC and a statistically significant chi-square test (p < 0.05) [62,63].
3. Results
3.1. ACG and SOC Based on Field Survey
Our findings revealed distinct variations in the spatial distribution of mangrove canopy height (CH) in Zhanjiang (Figure 2). Specifically, mangroves along the western coast of Zhanjiang City exhibited a mean CH of 3.67 m, whereas those in the eastern coastal zone had a lower mean CH of 2.48 m. The spatial distribution of aboveground carbon stock (ACG) showed a similar pattern. Mazhang had the highest ACG (41.38 Mg C/ha), while Luishawan recorded the lowest value (21.83 Mg C/ha). In contrast, the spatial pattern of soil carbon stock (SOC) differed from that of ACG. Gaoqiao exhibited the highest SOC (193.42 Mg C/ha), whereas Leizhou showed the lowest (45.25 Mg C/ha). Overall, SOC was several times greater than ACG across all regions. At the species level, Sonneratia apetala had the highest ACG (52.45 Mg C/ha) but the lowest SOC (79.87 Mg C/ha). Other species displayed no significant differences in ACG, while Bruguiera gymnorhiza and Kandelia obovata exhibited relatively high SOC levels (Table 1 and Figure 3).
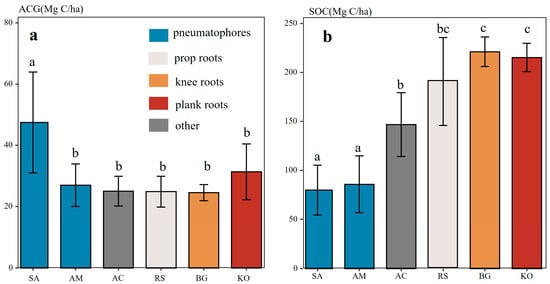
Figure 3.
(a) Aboveground carbon stocks (ACG) of mangrove species with different root structures, and (b) soil carbon stocks (SOC, 0–100 cm). Species abbreviations: AM—Avicennia marina (Forssk.) Vierh.; RS—Rhizophora stylosa Griff. BG—Bruguiera gymnorrhiza (L.) Lam.; KO—Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong; AC—Aegiceras corniculatum (L.) Blanco; SA—Sonneratia apetala Buch.-Ham. Different superscript letters indicate significant differences among groups (p < 0.05).
3.2. Remote-Sensing-Based Extraction of Forest Age Stages and Species Composition
Based on the classification of three mangrove age stages, we analyzed the spatiotemporal changes in mangrove distribution across Zhanjiang (Figure 4). The areas of Stage III, Stage II, and Stage I mangroves were 1259.45 ha, 3763.05 ha, and 630.27 ha, respectively. Stage III mangroves were primarily distributed in the northwest of Zhanjiang (around GQ) and the central region (around TCD). Stage II mangroves were mainly located in the western (around SX) and southeastern parts (around LZ), whereas Stage I mangroves were concentrated in the central and southeastern regions (around WLW). The centroid of mangrove distribution gradually shifted from northern Zhanjiang toward the middle-eastern region (around LZ). Overall, older mangroves were concentrated in the northwest (GQ) and central (TCD) areas, while younger mangroves were mainly found in the southeast (WLW and LZ). These results are consistent with previous studies: Wang et al. (2024) [16] also reported that mangroves in GQ were in an older developmental stage; Huang et al. (2024) [7] found that the mangroves in TCD were stable and mature; and Zhao et al. [39] identified Sonneratia apetala—an exotic species introduced around 2000—in the LZ and WLW regions, confirming that these areas represent younger mangrove stands (<34 years).
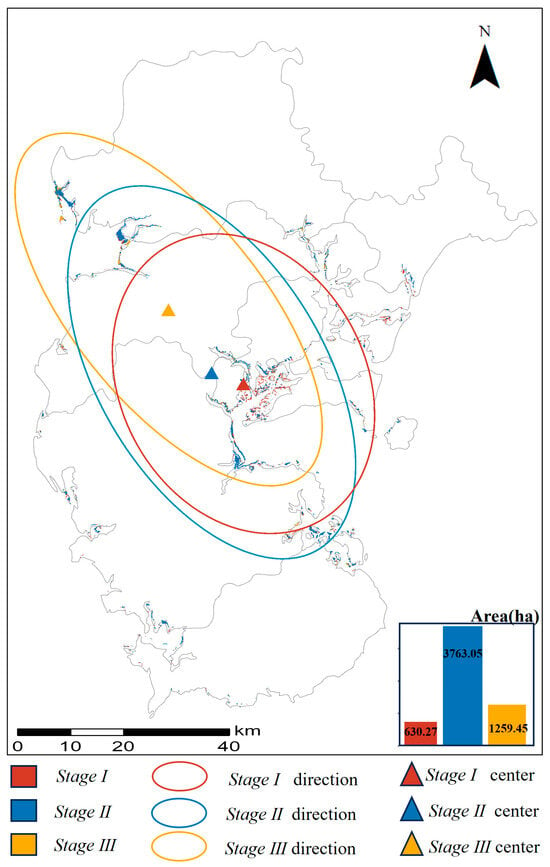
Figure 4.
The spatial–temporal variation in mangrove area in Zhanjiang (ellipses and rectangles in different colors indicate the different stages of mangrove direction and center change).
By combining the spatial distribution of mangrove species with forest age stages, we analyzed the area occupied by five species—Kandelia obovata, Aegiceras corniculatum, Sonneratia apetala, Rhizophora stylosa, and Avicennia marina—across different successional stages. According to Figure 5, species composition varied significantly across stages. In the old-growth stage (Stage III), Sonneratia apetala accounted for only 9% of the total area, while native species such as Rhizophora stylosa, Aegiceras corniculatum, and Avicennia marina dominated. In contrast, Sonneratia apetala increased sharply in the younger stages (Stage I and Stage II), emerging as the second most dominant species. The area occupied by Avicennia marina in the younger stages was also greater than that in the old-growth stage. Overall, Sonneratia apetala and Avicennia marina were the dominant species in younger mangroves, whereas the native species Avicennia marina, Aegiceras corniculatum, and Rhizophora stylosa were more dominant in older stands. As shown in Figure 6, notable differences in root-type composition were observed between younger (Stages I and II) and older mangroves (Stage III). Younger stands exhibited a higher proportion of pneumatophores compared with older mangroves.
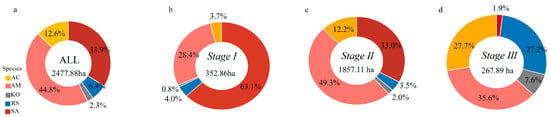
Figure 5.
Species composition of mangroves at different age stages. (a) All mangroves; (b) Stage I; (c) Stage II; (d) Stage III (AM—Avicennia marina, RS—Rhizophora stylosa, KO—Kandelia obovata; AC—Aegiceras corniculatum; and SA—Sonneratia apetala).
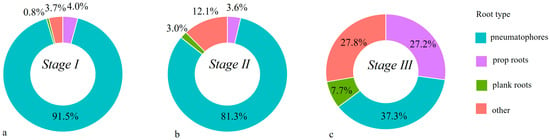
Figure 6.
Root types observed in mangroves at different age stages. (a) Stage I; (b) Stage II; (c) Stage III.
3.3. Temporal Dynamics in the Aboveground and Soil Carbon Stocks of Mangroves
Based on the classification of mangrove age stages, significant differences were observed among the three stages (Figure 7). Canopy height (CH) showed a clear negative relationship with forest age, with Stage I exhibiting the highest CH, followed by Stage II and Stage III. Because aboveground carbon stock (ACG) was derived from CH, its temporal pattern across age stages was consistent with that of CH.
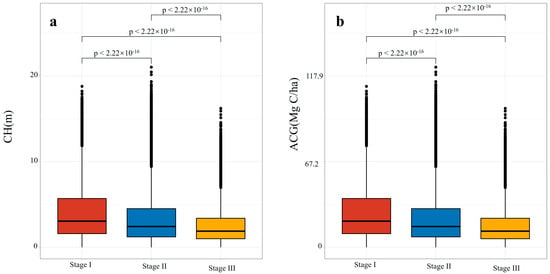
Figure 7.
(a) Canopy height (CH, m) and (b) aboveground carbon stock (ACG, Mg C/ha) of mangrove stages extracted from UAV-LiDAR.
Mangrove age exerted a significant effect on SOC in the upper soil profile (0–60 cm), whereas deeper layers (60–100 cm) showed no age-dependent differences (Figure 8). Stage III mangroves stored consistently higher SOC than Stage I and Stage II, whereas SOC in younger stands (<30 years) showed no significant differences between stages.
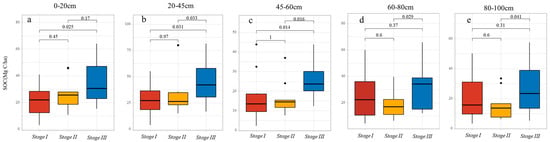
Figure 8.
Soil carbon stock (SOC, Mg C/ha) values at different depths for the three mangrove age stages. (a) 0–20 cm; (b) 20–45 cm; (c) 45–60 cm; (d) 60–80 cm; (e) 80–100 cm.
3.4. Driving Factors of Temporal Variations in the Aboveground and Soil Carbon Stocks
Based on the results of the in situ survey results, Sonneratia apetala had the highest ACG density (Table 1 and Figure 3). Figure 5 and Figure 6 indicate that Sonneratia apetala was the predominant species in younger mangroves (Stage I and Stage II), while it accounted for only a minor proportion in older stands (Stage III). Zan et al. [47] found that S. apetala adapts well to coastal environments in southern China, including the Shenzhen coastline near Zhanjiang, where it can reach heights of up to 10 m. As a non-native species, S. apetala was widely introduced in the 1990s for mangrove restoration due to its rapid growth and high environmental tolerance [44,47], and it has since become increasingly prevalent along China’s coastline [46]. Taken together, these findings suggest that the widespread introduction of S. apetala has enabled younger mangroves to accumulate greater ACG than older stands, resulting in the observed negative relationship between ACG and mangrove age stage. We further analyzed the influence of mangrove species and age on the ACG using SEM (Figure 9) and found that the forest age had a significant impact on ACG, whereas species identity did not play a major direct role. This finding suggests that most native mangrove species exhibit similar ACG values, consistent with the patterns observed in Figure 3. Overall, the gradual expansion of Sonneratia apetala over the past two decades has reshaped the temporal trajectory of ACG due to its high tolerance and rapid growth.
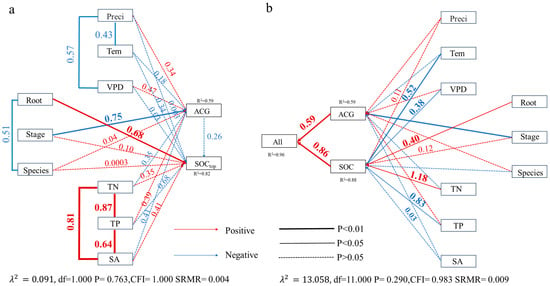
Figure 9.
(a) Structural equation model showing direct and indirect effects of environmental factors, stage, species, and root structure on mangrove aboveground carbon stock (ACG), the top of soil carbon stock (SOCtop, 0–5 cm), and (b) the total soil carbon stock (SOC, 0–100 cm) carbon stock (SA—Soil salinity, TN—Total Nitrogen, TP—Total Nitrogen, Tem—Air temperature, Preci—precipitation, and VPD—vapor pressure deficit).
We applied structural equation modeling (SEM) to identify the primary drivers of SOC dynamics. Root structure was the most influential factor affecting surface SOC (0–5 cm), with a strong positive path coefficient (0.68, p < 0.01). Forest age (Stage), TN, TP, SA, and species identity also exhibited significant positive correlations with surface SOC. At the 0–100 cm profile, root structure and TN remained significantly positively correlated with SOC, whereas TP showed a significant negative correlation (Figure 9). Across all soil depths, mangrove species dominated by pneumatophores had the lowest SOC. At the 20–60 cm depth interval, SOC differed significantly among the three other root types (Figure 10). These results indicate that differences in root structure restrict carbon accumulation in species with pneumatophores, thereby amplifying temporal variation in SOC among mangrove communities.
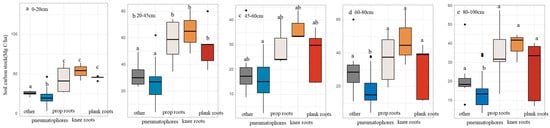
Figure 10.
Soil carbon stock (SOC, Mg C·ha−1) at different soil depths across root-structure types. (a) 0–20 cm; (b) 20–45 cm; (c) 45–60 cm; (d) 60–80 cm; (e) 80–100 cm. (Different lowercase letters above the boxes indicate significant differences among age stages (p < 0.05)).
4. Discussion
By integrating in situ surveys with UAV-LiDAR and long-term NDVI series, we identified significant asymmetry and asynchrony in the temporal dynamics of aboveground carbon (ACG) and soil organic carbon (SOC), reflecting distinct sequestration mechanisms in the above- and belowground components. The introduction of Sonneratia apetala, a fast-growing exotic species, accelerated and reshaped the temporal trajectory of ACG, whereas SOC accumulation was mainly regulated by root structure and soil nutrient availability. Notably, SOC in younger mangroves (10–24 and 24–34 years) did not increase substantially, suggesting that soil carbon restoration requires longer time scales to reach levels comparable to those in older stands (>34 years). RS further revealed that the introduction of S. apetala altered species composition in younger mangroves and reshaped the temporal dynamics of ACG. This shift was accompanied by a transition in root structure toward pneumatophores, which contributed to slower SOC accumulation. As a result, younger stands will require more time to develop soil carbon stocks equivalent to those of mature forests. These findings highlight the fundamental differences between aboveground and belowground carbon sequestration processes and underscore the critical roles of species composition, root architecture, and environmental factors in regulating mangrove carbon dynamics.
Our study shows clear asymmetry and asynchrony in the temporal trajectories of ACG and SOC, revealing distinct restoration time lags between these pools. The rapid growth of Sonneratia apetala accelerates ACG turnover and can produce ACG levels comparable to older native stands within <20 years. In contrast, SOC recovery proceeds much more slowly: the soil carbon pool requires substantially longer to approach that of older native mangroves and may remain lower within the several frames of typical restoration projects. Yu et al. [6] also found that the ACG of younger Sonneratia apetala was higher than older native mangroves, while the SOC of Sonneratia apetala was lower than older native mangroves. The younger mangrove (<30 years) had a similar SOC, while the older mangrove (>40 years) had a higher SOC than the younger mangrove. These findings highlight the need for restoration strategies to explicitly prioritize SOC recovery and to establish long-term baselines and monitoring frameworks that can effectively track belowground carbon trajectories.
The differing sensitivities of ACG and SOC indicate distinct mechanisms of above- and belowground carbon sequestration in mangroves. ACG can accumulate rapidly when highly competitive species are introduced, as they capture resources more efficiently to support fast growth. The sequestration of soil carbon was a complex mechanism, since the soil carbon pool is supplied by autochthonous inputs (aboveground litter and root production) and by allochthonous carbon delivered via tidal exchange and lateral water movement from adjacent ecosystems [54,55]. Using structural equation modeling (SEM), we further identified root structure as the most significant factor influencing SOC, with environmental variables such as total nitrogen (TN) and total phosphorus (TP) also playing key roles, which was consistent with previous studies [7,21,64,65]. These results suggest that effective SOC restoration should explicitly consider belowground processes (e.g., root production) and site-specific soil conditions.
Sonneratia apetala could rapidly accumulate ACG due to its special growth strategy, compared to other native species. Sonneratia apetala exhibits rapid early structural development, high specific leaf area (SLA), and elevated leaf nitrogen content, coupled with relatively low to moderate wood density [66,67]. Such traits promote fast canopy closure, high photosynthetic rates, and rapid ACG accumulation in the first two decades after planting, as shown in Table 3. In contrast, native species generally possess higher wood density and greater leaf construction costs [68,69], resulting in a more conservative growth strategy and slower biomass accumulation.
The RS data revealed clear alterations in mangrove species composition, indicating a shift toward the dominance of Sonneratia apetala. Zhao et al. [40] similarly reported that S. apetala is now the predominant mangrove species in Zhanjiang. However, the introduction and widespread colonization of this species may reduce the soil carbon sequestration capacity of mangrove ecosystems. Its gradual dominance should therefore be viewed as a potential early warning signal of ecological degradation. Moreover, the transition toward pneumatophore-dominated root systems appears to further weaken belowground carbon storage potential. Although species-specific differences in SOC were not directly detected in Zhanjiang, previous studies have shown that stands dominated by S. apetala can exhibit lower SOC than native communities [8]. Nevertheless, it is premature to conclude that S. apetala inherently suppresses soil carbon sequestration without direct evidence on SOC turnover (e.g., repeated depth-resolved coring, root production and decomposition rates, or δ13C/14C and biomarker constraints), particularly given that SOC dynamics are strongly age-dependent. In contrast, mixed native communities with greater functional trait diversity generally exhibit higher SOC levels [45]. Because Sonneratia apetala introductions can simplify community composition and homogenize root architectures, they may reduce belowground functional heterogeneity, thereby dampening SOC sequestration and increasing ecological vulnerability. Taken together, these considerations argue for diversity-oriented restoration strategies and long-term, process-based monitoring of soil carbon.
5. Conclusions
Our study highlights the asymmetry and asynchrony in the temporal dynamics of ACG and SOC in mangrove ecosystems. ACG accumulates rapidly in younger mangroves dominated by Sonneratia apetala but plateaus with forest maturity, while SOC increases more gradually over time due to root structure and environmental factors. Our findings emphasize the need for stage-specific restoration strategies that enhance SOC through native species while managing the rapid ACG growth in younger mangroves. Future research should refine temporal analyses to identify critical transition points and optimize strategies for sustaining long-term carbon storage. In particular, quantitative measurements of SOC and ACG turnover rates, as well as stand age, are essential for pinpointing key time windows and informing rational planning for mangrove restoration.
Author Contributions
Conceptualization Z.W.; methodology, Z.W. and F.G.; normal analysis, Z.W.; investigation, Z.W., X.Z., Z.H. and H.X.; data curation, X.Z. and Y.Z.; writing—original draft preparation, Z.W.; writing—review and editing, F.G. and X.O.; supervision, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Key Research and Development Program of China (2021YFC3201004), the National Natural Science Foundation of China (42477489, 52471276, 52388101, and 52271280), the Program for Guangdong Introducing Innovative and Entrepreneurial Team (2021ZT090543), Marine Economy Development Project of Guangdong Province (GDNRC[2023]43).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Summary of soil carbon storage in different layers of mangrove forests at different forest ages.
Table A1.
Summary of soil carbon storage in different layers of mangrove forests at different forest ages.
| Age Stage | 0–5 cm (Mg C/ha) | 5–10 cm (Mg C/ha) | 10–20 cm (Mg C/ha) | 20–30 cm (Mg C/ha) | 30–45 cm (Mg C/ha) | 45–60 cm (Mg C/ha) | 60–80 cm (Mg C/ha) | 80–100 cm (Mg C/ha) |
|---|---|---|---|---|---|---|---|---|
| 4.88 ± 2.63 | 5.53 ± 3.23 | 10.78 ± 5.29 | 11.19 ± 5.22 | 17.35 ± 11.18 | 16.93 ± 11.88 | 25.38 ± 17.21 | 20.72 ± 14.75 | |
| 6.48 ± 3.14 | 6.78 ± 4.02 | 13.25 ± 4.98 | 12.86 ± 5.33 | 18.86 ± 13.63 | 16.37 ± 8.54 | 17.81 ± 9.44 | 15.18 ± 9.64 | |
| 8.92 ± 4.61 | 8.72 ± 3.41 | 17.6 ± 8.36 | 18.81 ± 8.02 | 25.89 ± 10.99 | 25.1 ± 8.33 | 30.08 ± 15.08 | 24.85 ± 14.52 |

Table A2.
Summary of soil carbon storage in different mangrove species at different layers.
Table A2.
Summary of soil carbon storage in different mangrove species at different layers.
| Species | 0–5 cm (Mg C/ha) | 5–10 cm (Mg C/ha) | 10–20 cm (Mg C/ha) | 20–30 cm (Mg C/ha) | 30–45 cm (Mg C/ha) | 45–60 cm (Mg C/ha) | 60–80 cm (Mg C/ha) | 80–100 cm (Mg C/ha) |
|---|---|---|---|---|---|---|---|---|
| Sonneratia apetala | 4.02 ± 0.56 | 4.2 ± 1.65 | 10.92 ± 1.46 | 7.67 ± 1.32 | 13.76 ± 3.54 | 11.08 ± 3.23 | 11.02 ± 4.67 | 11.06 ± 3.42 |
| Avicennia marina | 4.77 ± 2.82 | 5.41 ± 3.06 | 10.26 ± 4.92 | 10.73 ± 4.11 | 12.7 ± 4.96 | 13.64 ± 5.15 | 16.31 ± 8.83 | 11.76 ± 7.43 |
| Aegiceras corniculatum | 6.95 ± 2.4 | 7.56 ± 2.64 | 14.91 ± 4.24 | 15.22 ± 4.24 | 24.13 ± 7.59 | 23.87 ± 8.07 | 29.72 ± 12.73 | 24.62 ± 11.58 |
| Rhizophora stylosa | 13.35 ± 2.46 | 9.63 ± 2.38 | 24.21 ± 10.53 | 29.42 ± 3.05 | 33.68 ± 8.32 | 30.05 ± 7.06 | 39.55 ± 12.91 | 36.21 ± 16.05 |
| Bruguiera gymnorrhiza | 13.84 ± 3.28 | 13.31 ± 1 | 26.12 ± 4.84 | 25.03 ± 5.51 | 39.84 ± 8.1 | 36.76 ± 5.04 | 47.79 ± 13.51 | 38.83 ± 6.26 |
| Kandelia obovata | 11.57 ± 1.79 | 12.84 ± 0.25 | 20.19 ± 1.79 | 22.56 ± 1.96 | 40.9 ± 10.48 | 33.07 ± 3.01 | 41.09 ± 2.53 | 37.41 ± 2.83 |
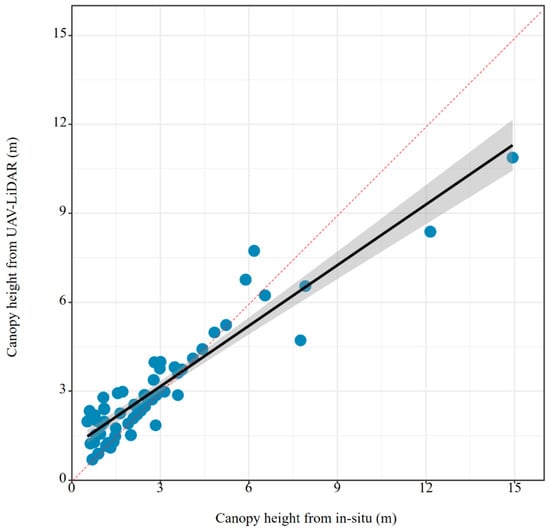
Figure A1.
Comparison of the measure Canopy Height (m) between in situ survey and UAV-LiDAR (the red dotted line represents the best performance; the black line represents the real performance).
References
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Alongi, D.M. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Ai, J.; Cai, S.; Dong, J. Predicting Mangrove Distributions in the Beibu Gulf, Guangxi, China, Using the MaxEnt Model: Determining Tree Species Selection. Forests 2023, 14, 149. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, S.; Li, H.; Liang, J.; Liu, W.; Piao, S.; Tian, H.; Zhou, G.; Lu, C.; You, W.; et al. Maximizing Carbon Sequestration Potential in Chinese Forests through Optimal Management. Nat. Commun. 2024, 15, 3154. [Google Scholar] [CrossRef] [PubMed]
- Delfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of Structural and Functional Diversity of Macrofaunal Communities in a Subtropical Mangrove Ecosystem. Estuar. Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Yu, C.; Feng, J.; Liu, K.; Wang, G.; Zhu, Y.; Chen, H.; Guan, D. Changes of Ecosystem Carbon Stock Following the Plantation of Exotic Mangrove Sonneratia apetala in Qi’ao Island, China. Sci. Total Environ. 2020, 717, 137142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Mangrove Soil Carbon Stocks Varied Significantly across Community Compositions and Environmental Gradients in the Largest Mangrove Wetland Reserve, China. Reg. Environ. Change 2024, 24, 140. [Google Scholar] [CrossRef]
- Pham, T.D.; Yoshino, K.; Bui, D.T. Biomass Estimation of Sonneratia caseolaris (L.) Engler at a Coastal Area of Hai Phong City (Vietnam) Using ALOS-2 PALSAR Imagery and GIS-Based Multi-Layer Perceptron Neural Networks. GIScience Remote Sens. 2017, 54, 329–353. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Hartling, S.; Esposito, F.; Fritschi, F.B. Soybean Yield Prediction from UAV Using Multimodal Data Fusion and Deep Learning. Remote Sens. Environ. 2020, 237, 111599. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, H.; Zhou, G.; Zhang, Q.; Tao, J.; Zhang, Y.; Lin, J. Aboveground Mangrove Biomass Estimation in Beibu Gulf Using Machine Learning and UAV Remote Sensing. Sci. Total Environ. 2021, 781, 146816. [Google Scholar] [CrossRef]
- Li, M.S.; Mao, L.J.; Shen, W.J.; Liu, S.Q.; Wei, A.S. Change and Fragmentation Trends of Zhanjiang Mangrove Forests in Southern China Using Multi-Temporal Landsat Imagery (1977–2010). Estuar. Coast. Shelf Sci. 2013, 130, 111–120. [Google Scholar] [CrossRef]
- Pasquarella, V.J.; Arévalo, P.; Bratley, K.H.; Bullock, E.L.; Gorelick, N.; Yang, Z.; Kennedy, R.E. Demystifying LandTrendr and CCDC Temporal Segmentation. Int. J. Appl. Earth Obs. Geoinf. 2022, 110, 102806. [Google Scholar] [CrossRef]
- Shang, R.; Chen, J.M.; Xu, M.; Lin, X.; Li, P.; Yu, G.; He, N.; Xu, L.; Gong, P.; Liu, L.; et al. China’s Current Forest Age Structure Will Lead to Weakened Carbon Sinks in the near Future. Innovation 2023, 4, 100515. [Google Scholar] [CrossRef]
- Leng, Y.; Li, W.; Ciais, P.; Sun, M.; Zhu, L.; Yue, C.; Chang, J.; Yao, Y.; Zhang, Y.; Zhou, J.; et al. Forest Aging Limits Future Carbon Sink in China. One Earth 2024, 7, 822–834. [Google Scholar] [CrossRef]
- Valencia, E.; De Bello, F.; Galland, T.; Adler, P.B.; Lepš, J.; E-Vojtkó, A.; Van Klink, R.; Carmona, C.P.; Danihelka, J.; Dengler, J.; et al. Synchrony Matters More than Species Richness in Plant Community Stability at a Global Scale. Proc. Natl. Acad. Sci. USA 2020, 117, 24345–24351. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, F.; Gao, W.; Guo, F.; Li, Z.; Yang, Z. Regional Mangrove Vegetation Carbon Stocks Predicted Integrating UAV-LiDAR and Satellite Data. J. Environ. Manag. 2024, 368, 122101. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, N.; Moskwa, L.-M.; Schmidt, K.; Oeser, R.A.; Aburto, F.; Bader, M.Y.; Baumann, K.; Von Blanckenburg, F.; Boy, J.; van den Brink, L. Pedogenic and Microbial Interrelations to Regional Climate and Local Topography: New Insights from a Climate Gradient (Arid to Humid) along the Coastal Cordillera of Chile. Catena 2018, 170, 335–355. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, L.; Zhou, G.; SiQing, B.; Du, W.; Meng, S.; Yu, J.; Sun, Z.; Liu, Q. Exploring Carbon Sequestration in Broad-Leaved Korean Pine Forests: Insights into Photosynthetic and Respiratory Processes. Sci. Total Environ. 2024, 906, 167421. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass Resilience of Neotropical Secondary Forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, C.-Z.; Wang, Z.; Mao, D.; Wang, Y.; Jia, M. Decision Surface Optimization in Mapping Exotic Mangrove Species (Sonneratia apetala) across Latitudinal Coastal Areas of China. ISPRS J. Photogramm. Remote Sens. 2022, 193, 269–283. [Google Scholar] [CrossRef]
- Arnaud, M.; Krause, S.; Norby, R.J.; Dang, T.H.; Acil, N.; Kettridge, N.; Gauci, V.; Ullah, S. Global Mangrove Root Production, Its Controls and Roles in the Blue Carbon Budget of Mangroves. Glob. Change Biol. 2023, 29, 3256–3270. [Google Scholar] [CrossRef] [PubMed]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal Landforms and Accumulation of Mangrove Peat Increase Carbon Sequestration and Storage. Proc. Natl. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Midway, S.R.; Friess, D.A.; Trettin, C.C.; Bukoski, J.J.; Stovall, A.E.L.; Pagliosa, P.R.; Fonseca, A.L.; et al. Macroecological Patterns of Forest Structure and Allometric Scaling in Mangrove Forests. Global Ecol. Biogeogr. 2021, 30, 1000–1013. [Google Scholar] [CrossRef]
- Sun, S.; Song, Z.; Guo, L.; Duarte, C.M.; Macreadie, P.I.; Zhang, Z.; Fang, Y.; Chen, B.; Wang, Z.; Wang, W.; et al. A Spatial Baseline of China’s Blue Carbon Stocks for Improved Monitoring, Management, and Protection. Earth’s Future 2025, 13, e2024EF005380. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Sillanpää, M.; Hayes, M.A.; Bachri, S.; Saragi-Sasmito, M.F.; Sidik, F.; Hanggara, B.B.; Mofu, W.Y.; Rumbiak, V.I.; Hendri; et al. Mangrove Blue Carbon Stocks and Dynamics Are Controlled by Hydrogeomorphic Settings and Land-use Change. Glob. Change Biol. 2020, 26, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.L.; Megonigal, J.P. Tidal Wetland Stability in the Face of Human Impacts and Sea-Level Rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I. Global Patterns in Mangrove Soil Carbon Stocks and Losses. Nat. Clim. Change 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W.; et al. Mangrove Reforestation Provides Greater Blue Carbon Benefit than Afforestation for Mitigating Global Climate Change. Nat. Commun. 2023, 14, 756. [Google Scholar] [CrossRef]
- Morton, R.; White, W. Characteristics of and Corrections for Core Shortening in Unconsolidated Sediments. J. Coast. Res. 1997, 13, 761–769. [Google Scholar]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the Most Carbon-Rich Forests in the Tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Lu, L.; Luo, J.; Xin, Y.; Duan, H.; Sun, Z.; Qiu, Y.; Xiao, Q. How Can UAV Contribute in Satellite-Based Phragmites Australis Aboveground Biomass Estimating? Int. J. Appl. Earth Obs. Geoinf. 2022, 114, 103024. [Google Scholar] [CrossRef]
- Cao, J.; Liu, K.; Zhuo, L.; Liu, L.; Zhu, Y.; Peng, L. Combining UAV-Based Hyperspectral and LiDAR Data for Mangrove Species Classification Using the Rotation Forest Algorithm. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102414. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, R.; Zhao, C.; Zhou, Y.; Ren, C.; Mao, D.; Li, H.; Sun, G.; Zhang, H.; Yu, W.; et al. Synergistic Estimation of Mangrove Canopy Height across Coastal China: Integrating SDGSAT-1 Multispectral Data with Sentinel-1/2 Time-Series Imagery. Remote Sens. Environ. 2025, 323, 114719. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Q.; Su, Y.; Xue, B. Improved Progressive TIN Densification Filtering Algorithm for Airborne LiDAR Data in Forested Areas. ISPRS J. Photogramm. Remote Sens. 2016, 117, 79–91. [Google Scholar] [CrossRef]
- Meng, Y.; Gou, R.; Bai, J.; Moreno-Mateos, D.; Davis, C.C.; Wan, L.; Song, S.; Zhang, H.; Zhu, X.; Lin, G. Spatial Patterns and Driving Factors of Carbon Stocks in Mangrove Forests on Hainan Island, China. Global Ecol. Biogeogr. 2022, 31, 1692–1706. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Gao, H.; Jia, Q.; Li, Z.; Liang, J.; Xing, Q.; Mao, D.; Li, H.; Chu, X.; et al. Carbon Fluxes of China’s Coastal Wetlands and Impacts of Reclamation and Restoration. Glob. Change Biol. 2024, 30, e17280. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, S.; He, Y.; You, S.; Yang, X.; Gan, Y.; Liu, A. A Fine-Scale Mangrove Map of China Derived from 2-Meter Resolution Satellite Observations and Field Data. Int. J. Geo-Inf. 2021, 10, 92. [Google Scholar] [CrossRef]
- Zhang, B.; Ye, H.; Lu, W.; Huang, W.; Wu, B.; Hao, Z.; Sun, H. A Spatiotemporal Change Detection Method for Monitoring Pine Wilt Disease in a Complex Landscape Using High-Resolution Remote Sensing Imagery. Remote Sens. 2021, 13, 2083. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, M.; Zhang, R.; Wang, Z.; Mao, D.; Zhong, C.; Guo, X. Distribution of Mangrove Species Kandelia obovata in China Using Time-Series Sentinel-2 Imagery for Sustainable Mangrove Management. J. Remote Sens. 2024, 4, 0143. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, M.; Zhang, R.; Wang, Z.; Ren, C.; Mao, D.; Wang, Y. Mangrove Species Mapping in Coastal China Using Synthesized Sentinel-2 High-Separability Images. Remote Sens. Environ. 2024, 307, 114151. [Google Scholar] [CrossRef]
- Aslan, A.; Aljahdali, M.O. Characterizing Global Patterns of Mangrove Canopy Height and Aboveground Biomass Derived from SRTM Data. Forests 2022, 13, 1545. [Google Scholar] [CrossRef]
- Tang, W.; Zheng, M.; Zhao, X.; Shi, J.; Yang, J.; Trettin, C. Big Geospatial Data Analytics for Global Mangrove Biomass and Carbon Estimation. Sustainability 2018, 10, 472. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, F.; Ouyang, X.; Xiong, L.; Zhu, Z.; Zhang, Y. Differential Carbon Stocks and Burial Rates in Natural versus Planted Mangrove Forests under Varied Hydrogeomorphic Conditions. Catena 2025, 254, 108981. [Google Scholar] [CrossRef]
- Huang, M.; Guo, F.; Ouyang, X.; Gao, X.; Zhu, Z.; Zeng, X.; Zhang, Y. Exploring Soil Carbon Drivers across Natural Mangroves, Restored Mangroves, and Tidal Flats: Implications for Subtropical Coastal Carbon Management. Catena 2025, 252, 108875. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; Cifor Bogor: Jawa Barat, Indonesia, 2012; Volume 86. [Google Scholar]
- Huang, X.; Yang, Q.; Feng, J.; Yang, Z.; Yu, C.; Zhang, J.; Ling, J.; Dong, J. Introduction of Exotic Species Sonneratia apetala Alters Diazotrophic Community and Stimulates Nitrogen Fixation in Mangrove Sediments. Ecol. Indic. 2022, 142, 109179. [Google Scholar] [CrossRef]
- Zan, Q.J.; Wang, B.S.; Wang, Y.J.; Li, M.G. Ecological Assessment on the Introduced Sonneratia caseolaris and S-Apetala at the Mangrove Forest of Shenzhen Bay, China. Acta Bot. Sin. 2003, 45, 544–551. [Google Scholar]
- Hongwiset, S.; Rodtassana, C.; Poungparn, S.; Umnouysin, S.; Suchewaboripont, V. Synergetic Roles of Mangrove Vegetation on Sediment Accretion in Coastal Mangrove Plantations in Central Thailand. Forests 2022, 13, 1739. [Google Scholar] [CrossRef]
- Chen, J.; Du, H.; Mao, F.; Huang, Z.; Chen, C.; Hu, M.; Li, X. Improving Forest Age Prediction Performance Using Ensemble Learning Algorithms Base on Satellite Remote Sensing Data. Ecol. Indic. 2024, 166, 112327. [Google Scholar] [CrossRef]
- Feng, X.; Deng, Y.; Zhong, W.; Xie, Z.; Liu, H.; Li, Z.; Jia, Y.; Li, X.; Chen, R.; Peng, X. Tracking the Expansion of Sonneratia apetala and Its Impact on Local Mangroves Using Time-Series Remote Sensing Data. Sustainability 2025, 17, 1069. [Google Scholar] [CrossRef]
- Pirasteh, S.; Mafi-Gholami, D.; Li, H.; Fang, Z.; Nouri-Kamari, A.; Khorrami, B. Precision in Mapping and Assessing Mangrove Biomass: Insights from the Persian Gulf Coasts. Int. J. Appl. Earth Obs. Geoinf. 2024, 128, 103769. [Google Scholar] [CrossRef]
- Gao, T.; Guan, W.; Mao, J.; Jiang, Z.; Liao, B. Carbon Storage and Influence Factors of Major Mangrove Communities in Fucheng, Leizhou Peninsula, Guangdong Province. Ecol. Environ. Sci. 2017, 26, 985–990. [Google Scholar]
- Lunstrum, A.; Chen, L. Soil Carbon Stocks and Accumulation in Young Mangrove Forests. Soil Biol. Biochem. 2014, 75, 223–232. [Google Scholar] [CrossRef]
- Azman, M.S.; Sharma, S.; Shaharudin, M.A.M.; Hamzah, M.L.; Adibah, S.N.; Zakaria, R.M.; MacKenzie, R.A. Stand Structure, Biomass and Dynamics of Naturally Regenerated and Restored Mangroves in Malaysia. For. Ecol. Manag. 2021, 482, 118852. [Google Scholar] [CrossRef]
- Wang, G.; Yu, C.; Singh, M.; Guan, D.; Xiong, Y.; Zheng, R.; Xiao, R. Community Structure and Ecosystem Carbon Stock Dynamics along a Chronosequence of Mangrove Plantations in China. Plant Soil 2021, 464, 605–620. [Google Scholar] [CrossRef]
- Yu, C.; Guan, D.; Gang, W.; Lou, D.; Wei, L.; Zhou, Y.; Feng, J. Development of Ecosystem Carbon Stock with the Progression of a Natural Mangrove Forest in Yingluo Bay, China. Plant Soil 2021, 460, 391–401. [Google Scholar] [CrossRef]
- Ren, H.; Chen, H.; Li, Z.; Han, W. Biomass Accumulation and Carbon Storage of Four Different Aged Sonneratia apetala Plantations in Southern China. Plant Soil 2010, 327, 279–291. [Google Scholar] [CrossRef]
- Mu, S.; Chen, G; Chen, Z.; Wu, H. Biomasses and Distributive Patterns of Mangrove Populations in Zhanjiang Nature Reserves, Guangdong, China. Guangxi Zhiwu 1998, 18, 19–23. [Google Scholar]
- Wang, Z.; Liu, M.; Liu, X.; Meng, Y.; Zhu, L.; Rong, Y. Spatio-Temporal Evolution of Surface Urban Heat Islands in the Chang-Zhu-Tan Urban Agglomeration. Phys. Chem. Earth Parts A/B/C 2020, 117, 102865. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise Structural Equation Modelling in r for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best Practices in Metabarcoding of Fungi: From Experimental Design to Results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of Structural Equation Modeling (SEM) in Ecological Studies: An Updated Review. Ecol. Process. 2016, 5, 19. [Google Scholar] [CrossRef]
- Zhang, M.; Delgado-Baquerizo, M.; Li, G.; Isbell, F.; Wang, Y.; Hautier, Y.; Wang, Y.; Xiao, Y.; Cai, J.; Pan, X. Experimental Impacts of Grazing on Grassland Biodiversity and Function Are Explained by Aridity. Nat. Commun. 2023, 14, 5040. [Google Scholar] [CrossRef]
- Hongwiset, S.; Rodtassana, C.; Poungparn, S.; Umnouysin, S.; Komiyama, A. Spatiotemporal Heterogeneity of Mangrove Root Sphere under a Tropical Monsoon Climate in Eastern Thailand. Forests 2021, 12, 966. [Google Scholar] [CrossRef]
- Feng, J.; Cui, X.; Zhou, J.; Wang, L.; Zhu, X.; Lin, G. Effects of Exotic and Native Mangrove Forests Plantation on Soil Organic Carbon, Nitrogen, and Phosphorus Contents and Pools in Leizhou, China. Catena 2019, 180, 1–7. [Google Scholar] [CrossRef]
- Zhu, D.; Hui, D.; Wang, M.; Yang, Q.; Yu, S. Light and Competition Alter Leaf Stoichiometry of Introduced Species and Native Mangrove Species. Sci. Total Environ. 2020, 738, 140301. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Zan, Q.-J.; Hu, Z.-Y.; Shin, P.-K.S.; Cheung, S.-G.; Wong, Y.-S.; Tam, N.F.-Y.; Lei, A.-P. Are Photosynthetic Characteristics and Energetic Cost Important Invasive Traits for Alien Sonneratia Species in South China? PLoS ONE 2016, 11, e0157169. [Google Scholar] [CrossRef]
- Li, F.; Yang, Q.; Zan, Q.; Tam, N.F.Y.; Shin, P.K.S.; Vrijmoed, L.L.P.; Cheung, S.G. Differences in Leaf Construction Cost between Alien and Native Mangrove Species in Futian, Shenzhen, China: Implications for Invasiveness of Alien Species. Mar. Pollut. Bull. 2011, 62, 1957–1962. [Google Scholar] [CrossRef]
- Zhu, D.; Hui, D.; Wang, M.; Yang, Q.; Li, Z.; Huang, Z.; Yuan, H.; Yu, S. Allometric Growth and Carbon Storage in the Mangrove Sonneratia apetala. Wetl. Ecol. Manag. 2021, 29, 129–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).