Abstract
Large-scale vegetation restoration campaigns have been implemented on the Chinese Loess Plateau, which have resulted in higher soil moisture deficits in this region. This, in turn, has hampered the healthy growth of planted trees, leading to a decline and, in severe cases, mortality of trees. Therefore, the rational regulation and utilization of rainwater, the primary water source in this region, may alleviate drought stress, ensuring the sustainability of the ecosystem. In this study, we investigated the impact of in situ rainwater collection and infiltration systems (IRCISs) on soil water, fine-root distribution, xylem vascular, and hydraulic conductivity characteristics in declining Robinia pseudoacacia forests. The results demonstrated that the application of an IRCIS can effectively increase plant available moisture content (0–5.0 m) of declining Robinia pseudoacacia forests. In particular, IRCIS treatment significantly increased xylem conduit diameter and plant hydraulic conductivity while substantially reducing the percentage loss of hydraulic conductivity in both roots and branches. Furthermore, IRCIS treatment significantly reduced the root biomass and distribution depth of Robinia pseudoacacia during both wet and dry years. This implies that IRCISs are beneficial for plant growth and survival. The findings of this study are significant for devising strategic methodologies for the planning and management of rainwater resources.
1. Introduction
Land degradation is one of the most pressing ecological issues in the world and a constraint to ecosystem services of terrestrial ecosystems [1]. In order to restore degraded land, planting trees is encouraged worldwide as forests are vital in averting desertification, and enhancing biodiversity and ecosystem services [2]. However, forest trees consume more water than other vegetation species, such as grasslands [3], and tend to deplete soil moisture in both shallow and deep layers post-tree planting [4]. This, in turn, will hamper normal plant growth, affecting fine-root traits and plant hydraulic conductivity characteristics, and may result in plant mortality and forest degradation [5]. Therefore, the balance between soil water supply and plant water uptake is fundamental for the healthy development and sustainability of forest ecosystems [6], especially in water-limiting regions.
Situated in the upper and middle reaches of the Yellow River basin, the semi-arid Loess Plateau of China spans an area of 64 × 104 km2, representing one of the most severely eroded regions throughout the world. The severe water loss and soil erosion in this region have increased the fragility of the ecosystem [7]. To mitigate these challenges and improve ecosystem services, larger-scale afforestation campaigns have been implemented by the Chinese government in the past few decades, including the Grain for Green Project, which has increased forest cover by 4.9% from 2000 to 2008 [8]. Robinia pseudoacacia L., recognized for its strong nitrogen-fixing ability and fast growth, is extensively cultivated, comprising 90% of afforestation trees within this area [9]. However, due to the imbalance between soil water supply and plant water consumption, the formation of dry soil layers has been documented across the majority of regions in the Loess Plateau [4,10]. This has led to the widespread early degradation of Robinia pseudoacacia trees, characterized by evenly dried shoots on the top of tree crowns—affected trees are commonly referred to as “dwarf-aged trees”—typically initiating around the age of 30 years [11]. Thus, the repaid development of larger scale Robinia pseudoacacia trees is sustainable only if there is sufficient soil moisture content. Inadequate management of soil water resources may lead to a severe soil water deficit, negatively impacting tree growth and survival rates, exacerbating land degradation [6,12].
Plants primarily depend on their fine roots and vascular structures to acquire and transport water and nutrient resources within forest ecosystems [13]. Therefore, understanding the characteristics of fine-root distribution and vascular features is essential for unraveling plant water-use patterns and plant survival strategies. Previous studies have indicated that fine-root distribution and water transport characteristics through xylem conduits are influenced by various factors, with drought stress being a significant one [14,15,16,17]. During drought periods, soil moisture is reduced severely, which may negatively affect fine-root distribution and xylem vascular structures. This potentially leads to plant hydraulic transfer failure and negatively impacts plant growth and photosynthesis, eventually causing plant mortality if drought persists [18]. Even though in the past few decades many studies have been conducted to explore the response of fine-root distribution or plant hydraulic conductivity to drought stress for different tree species, there remains a knowledge gap regarding how drought concurrently affects plant fine-root distribution, vascular structure, and the corresponding hydraulic conductivity. Knowledge of this will be helpful in deciphering plant water use patterns and survival strategies, and also pivotal in addressing critical issues in forests, especially in regions with limited water resources.
In recent years, a novel rainwater-saving method, referred to as the in situ rainwater collection and infiltration system (IRICIS), has been developed and implemented in apple and Chinese jujube orchards across the Loess Plateau region [19]. The primary objective of this rainwater-saving system is to improve the infiltration of rainfall into root zones, thereby increasing soil moisture content to meet the water demands of trees during dry periods. The theoretical framework and advantages of this system, compared to traditional management practices, have been extensively documented in previous studies [20]. However, research is limited regarding the influence of IRICISs on soil moisture content, fine-root distribution traits, xylem vascular properties, and associated plant hydraulic conductivity characteristics for common afforested trees, such as Robinia pseudoacacia trees. Additionally, the impact of IRICISs on the physiological and water transport characteristics of declining Robinia pseudoacacia trees remains unexplored. Knowledge of this holds significant importance in developing rational plantation management strategies and in fostering the healthy and sustainable development of these forests. Leveraging this background, we measured soil water content, fine-root distribution, xylem anatomical properties, and corresponding plant hydraulic conductivity of six declining Robinia pseudoacacia forest stands (35 years old) in the semi-arid Loess Plateau for two successive years, in 2015 (a wet year with above-average precipitation) and 2016 (a drought year with below-average precipitation). We hypothesized that the construction of the in situ rainwater collection and infiltration system (IRICIS) would positively influence plant available moisture content, fine-root distribution, xylem vascular characteristics, and plant hydraulic conductivity in declining Robinia pseudoacacia forests during drought conditions. We also expected that the IRICIS system, designed to enhance rainfall infiltration and soil moisture retention, would mitigate the adverse effects of drought on the physiological and water transport characteristics of the declining Robinia pseudoacacia trees, ultimately mitigating the processes of tree degradation.
2. Materials and Methods
2.1. Site Area
The study was conducted from 2015 to 2016 in the Yeheshan catchment, Fufeng County (34°33′ N, 107°54′ E, 1090 m a.s.l, Figure 1) in Shaanxi Province on the southern Chinese Loess Plateau. The average annual precipitation of the area is 580 mm with significant seasonal variations. Approximately 80% of the precipitation falls between May and October. The annual mean air temperature is 12.7 °C. The soil is silt loam according to the USDA (United States Department of Agriculture) classification, with average values of sand, 5.8%, silt, 73.4%, and clay, 20.9%.
Figure 1.
Map of the study area.
Vegetation restoration has been extensively implemented since the late of 1980s, when Robinia pseudoacacia was widely planted. The forest coverage of the Yeheshan catchment is approximately 90%, and the Robinia pseudoacacia spans an area of ~87 km2. The main understory vegetation species in this area include Stipa bungeana and A. codonocephala.
2.2. Experiment Design
In this study, six declining Robinia pseudoacacia (35 years) experimental plots, each 20 × 20 m2 in size and with a density of 1200 trees/ha, were chosen. The mean height and diameter at breast height of these trees were 9.0 m and 15.5 cm, respectively. The ratio of leaf area index to sapwood area for these trees was 0.05 m2 cm−2. The mean crown length (m), the mean crown height (m), leaf area index (m2 m−2), and cumulative sapwood area (m2/ha) values of these selected trees were 4.2 m, 5.8 m, 1.96 m2 m−2, and 3.3 m2/ha, respectively. These sites were strategically constructed on sun-facing middle slopes (5–10°), where rain-fed agriculture was formerly conducted.
Three experimental sites were implemented with IRCISs at treated sites, while another three experimental sites were designated as control sites. In the treated sites, an IRCIS system was strategically installed upslope of individual trees. The IRCISs consisted of a semi-circular ridge, 1.0 m in radius and 0.2 m in height (Figure 2). During ridge construction, soil was excavated and repositioned to establish the main stem at the summit of the ridge, ensuring a mostly flat soil surface in the semicircle area. Inside the semicircular ridge, a soil pit measuring 0.8 m × 0.8 m × 0.8 m deep was excavated, and the downslope wall was positioned 1.0 m from the bole. A rainwater storage pit, covered with synthetic fiber fabric and containing grasses, leaves, and brushwood debris, was established. The pit, lined with polythene sheet, had a central hole measuring 3 cm in diameter, chaneling the rainwater accumulated from the fish-scale ridge into the pit. Rainwater collected in the pit could then infiltrate into the surrounding soil. This design effectively reduces runoff, leading to an increase in soil moisture content. In this study, the IRCISs were installed at the end of 2014 and the measurements were carried out in 2015–2016, and when constructing the rainfall harvesting system, the understory herbaceous plants around the IRCISs were cleared to eliminate the potential influence of understory vegetation on the experimental results.
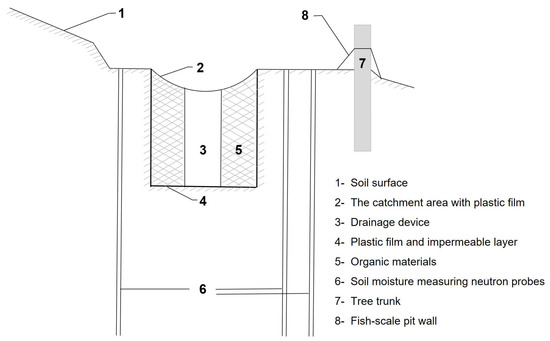
Figure 2.
Diagram of the rainwater harvesting system.
Rainfall was measured utilizing a Geonor T-200B weighing precipitation gauge (Geonor, Eiksmarka, Norway). Based on rainfall data from 1958–2016, a curve depicting the distribution of rainfall frequencies was formulated. Every year was categorized as wet, normal, or dry, based on the percentage of total precipitation (<25% for dry, >75% for wet, and the remaining for normal years). The experimental sites treated in 2015 were designated as RC2015, and those treated in 2016 were labeled as RC2016, while the comparison experimental sites in 2015 were labeled as WT2015, and those in 2016 were designated as DT2016.
2.3. Soil Water Measurement
At each experimental site, three neutron probes (5.0 m) were randomly positioned between the tree and rainwater harvesting system in the treated plots and randomly in the control treatment. The volumetric soil moisture content was measured using a neutron probe. To minimize the influence of rainfall on soil water movement, a rain-free period of five successive days was designated as the measurement period. Sampling was conducted 12 times/year throughout the sampling period. Slow neutron counts were counted at 0.15 m intervals down to a depth of 0.8 m and then at 0.2 m intervals down to a soil depth of 5.0 m. The volumetric soil moisture content (θv) at each depth was determined by applying the calibration curve to the slow neutron count rate (CR):
Plant available moisture storage (PAMS) denoted the maximum quantity of soil water accessible for plant use, and was determined by subtracting the soil moisture content (SWC) from the plant permanent wilting point (PWP):
where ΔZ denotes the increment in soil depth. Based on measurement, the average value of PWP in this study was 0.072 cm3 cm−3.
2.4. Fine-Root Measurement
Fine-root distribution was examined employing the soil auger technique. Within every experimental site, soil samples (n = 10) were gathered using a cylindrical metal auger (0.09 m diameter and 0.1 m long). Soil cores were randomly selected between the tree and IRCIS pit in the treated plots and randomly in the control treatment areas. Moreover, soil cores were gathered at 0.2 m increments, extending to a depth of 5.0 m beyond which fine roots were not detected.
A two-stage process was employed to separate root samples from the soil. Firstly, soil samples were meticulously washed over a 5 mm sieve. Vegetation roots and other plant remnants were visually identified and removed. Secondly, Robinia pseudoacacia roots were categorized based on their size classes (diameter > 2 mm and diameter ≤ 2 mm) using a microscope with 10–40× magnification. Root samples underwent digital scanning using an Epson Perfection v700 photo scanner (Seiko Epson Corporation, Suwa, Nagano-ken, Japan) with a resolution of 600 dpi. The length of roots was determined utilizing WinRhizo Software version Pro 3.5 (Régent Instrument Inc., Quebec, QC, Canada, www.regent.qc.ca). The length density of fine roots (<2 mm), denoted as FRLD (cm dm−3), was estimated as
where RL represents the length of fine root; RVs is the volume of soil samples. Additionally, the cumulative length density of fine roots (CFRLD) for each experimental site was calculated.
2.5. Xylem Anatomical Measurements
At each site, six trees were randomly selected, and sapwood samples were gathered from the diameter at breast height (DBH) of stems. Two cores for both current year sapwood (south facing and north facing; only current year sapwood is able to conduct water), each 5 mm in diameter and spaced 30 mm apart, were extracted using a Suunto increment borer (SUUNTO, Vantaa, Finland). Each core was sectioned transversely (20 μm thick), and 12 samples were obtained using a sliding microtome (Leica RM2235; Leica Microsystems Nussloch GmbH, Nussloch, Germany). The sections were then treated with a 1% safranin solution to improve the contrast between wood and conduit void space. After staining, section samples were placed in glycerol and prepared for microscopic analysis. Depending on the size and abundance of conduits, 4–6 images of each section were taken through a CCD digital camera (Guangzhou Mingmei Technology Co., Ltd., Guangzhou, China) linked to an Olympus CX 31 microscope (×40 magnification, Olympus Corp., Tokyo, Japan). Subsequently, conduit abundance and conduit diameter were obtained with the ImageJ software (version 1.8.0, US National Institutes of Health, Stapleton, NY, USA).
2.6. Hydraulic Conductivity Characteristics
The hydraulic conductivity KTH (Kg m MPa−1 s−1) of plant xylem conduit was calculated following Hagen–Poiseuille’s law as:
where η represents water viscosity (1.002 × 10−9 MPa s) and Dh represents the hydraulically weighted mean conduit diameter, estimated as follows:
where D represents the equivalent conduit diameter, and N is the number of conduits.
During this study period, branches aged one year old, positioned in the mid-upper canopy on the sun-exposed side of the plant, and roots from shallow soil layers (0–1.0 m), were utilized to evaluate the percentage loss of hydraulic conductivity (PLC). The values of PLC for both branches and roots were examined using the method suggested by Sperry et al. [21]. During the period of fine-root sampling, branch and root samples (~0.3–0.4 m in length, ~1.0 cm in diameter) were gathered. To mitigate embolism caused by excision, all branch and root samples were excised underwater. Subsequently, the collected samples were carefully enclosed in polyethylene film and transported to the experimental platform for further analysis. The values of PLC at each site were determined by averaging measurements from three segments (each 4-cm long) across three biological replicates. More detailed information about the measurement processes of PLC can be found in Ma et al. [13].
2.7. Statistical Analysis
One-way ANOVA was performed to test the difference between treatments for PAMS, FRLD, Dh, KTH, and PLC. All statistical analyses were assessed using SPSS software (version 25.0; SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered statistically significant.
3. Results
3.1. Plant Available Moisture Storage (PAMS)
The vertical distribution of PAMS in all experimental treatments is shown in Figure 3. Generally, PAMS followed similar trends with increasing soil depth in all treatments. With the increase of soil depth, PAMS exhibited a fluctuating increasing trend, with the fluctuation more prominent in the shallow soil layer (<2.0 m) and relatively less pronounced in the deep soil layer (>2.0 m). Specifically, PAMS was significantly affected by the IRCIS treatment. RC2015 and RC2016 had significantly higher PAMS than WT2015 and DT2016, respectively, being 12.2% in 2015 and 17.1% in 2016 (Figure 3B).
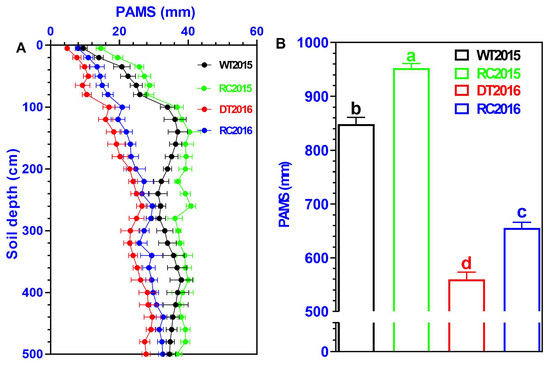
Figure 3.
Vertical distribution of plant available moisture storage (PAMS) (A) and the cumulative plant available moisture storage (B) within the 0–5 m soil layer in 2015 and 2016 of the six Robinia pseudoacacia forest stands. Varied letters represent statistically significant differences (p < 0.05) among treatments, with whiskers depicting ±1 SD.
3.2. Fine-Root Distribution Pattern
The distribution of FRLD within the 0–5 m soil layer of the six Robinia pseudoacacia forest stands is displayed in Figure 4. Generally, FRLD exhibited a declining trend with an increase in soil depth, particularly in the 0–1.0 m soil profile (Figure 4A). Fine roots were predominantly concentrated in the top 0–1.0 m of soil. WT2015 constituted 82.8% of the overall FRLD, whereas RC2015 comprised 92.97%. In 2016, DT2016 accounted for 61.9% of the total FRLD, and RC2016 made up 70.7%. The use of IRCISs markedly decreased the total FRLD. RC2015 showed 8.0% lower FRLD compared to WT2015, and RC2016 exhibited 12.1% lower FRLD compared to DT2016 (Figure 4B).
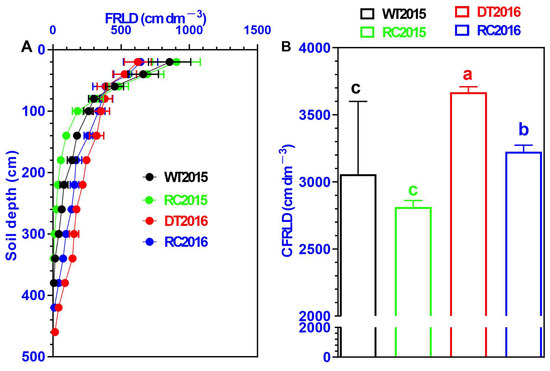
Figure 4.
Distribution of Robinia pseudoacacia fine-root length density (FRLD, cm/dm3) in 2015 and 2016 within the 0–5 m soil layer (A) and the cumulative fine-root length density (CFRLD) (B) in Robinia pseudoacacia forest stands. Varied letters denote statistically significant differences at p < 0.05 between treatments, with error bars depicting ±1 SD.
The cumulative fine-root length density (CFRLD) from surface down to a particular depth to the total CFRLD exhibited higher values in the shallow soil (<2.0 m) layer compared to the deeper soil layer (>2.0 m) in both study years (Figure 5). The implementation of the IRCIS treatment substantially influenced the distribution of CFRLD of Robinia pseudoacacia forest stands. With the increase of soil depth, CRFLD exhibited an increasing trend, with the increments being more prominent in the IRCIS treatments and being relatively less pronounced in control treatments. Forest stands treated with IRCIS showed shallower D95 (the depth above which 95% of the root mass is present) compared to the controlled sites. Overall, RC2015 had a value of 1.0 m, WT2015 had a value of 1.7 m, RC2016 had a value of 2.4 m, and DT2016 had a value of 2.9 m.
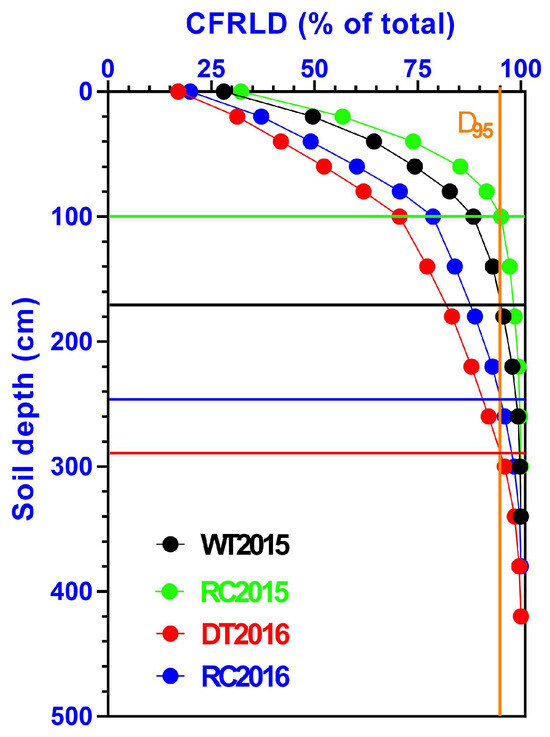
Figure 5.
Vertical distribution of cumulative fine-root length density (CFRLD) in Robinia pseudoacacia forest stands. Data points represent the mean values of fine-root length densities. The intersection of horizontal lines and vertical lines at D95 denotes soil depth above which 95% of fine-root length density occurred.
3.3. Xylem Conduit Diameter Distribution and Hydraulic Conductivity
The vessel diameter distribution and cumulative percentage of total theoretical hydraulic conductivity contributed by each conduit size class in stem sapwood for experimental forest trees is illustrated in Figure 6. Generally, IRCIS significantly affected the xylem conduit diameter and plant hydraulic conductivity of Robinia pseudoacacia trees in both study years. The conduit systems of Robinia pseudoacacia trees treated with IRCIS displayed a positive skewness, but exhibited a near normal distribution. In contrast, the control treatments for Robinia pseudoacacia developed a narrow conduit system with only a few wide conduit (μm) (largely positively skewed distribution) (Figure 6a). IRCIS treatments effectively reduced the skewness in conduit diameter distribution: with RC2015 and RC2016 displaying 78.8% and 57.0% lower skewness coefficients than WT2015 and DT2016. Furthermore, IRCIS treatments significantly influenced xylem conduit diameter. Specifically, RC2015 (164.4 μm) and RC2016 (146.4 μm) exhibited 7.5% and 9.4% greater xylem conduit diameters, respectively, compared to WT2015 (153.0 μm) and DT2016 (133.8 μm) (Figure 6a).
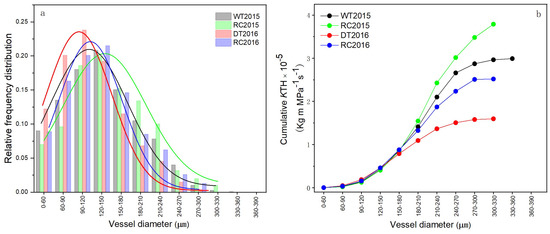
Figure 6.
Xylem conduit distribution (a) and cumulative percentage of total theoretical hydraulic conductivity by conduit size class (b) in stem sapwood for experimental Robinia pseudoacacia forest stands. For WT2015 and RC2015, the conduit diameter distributions were slightly skewed (γ = +0.18 and +0.10 for WT2015 and RC2016, respectively) and approached a normal distribution; those of DT2016 and RC2016 were positively skewed (γ = +0.37 and +0.23 for DT2016 and RC2016, respectively).
The differences in conduit diameter distribution among the four forest stands were statistically significant (p < 0.05), clearly illustrating the divergence in the cumulative theoretical hydraulic conductivity (KTH) for larger conduit classes (Figure 6b). The mean KTH for WT2015 and RC2015 in this study was 2.99 kg m MPa−1 s−1 and 3.79 kg m MPa−1 s−1, respectively, while values for DT2016 and RC2016 were 1.59 kg m MPa−1 s−1 and 2.52 kg m MPa−1 s−1, respectively.
3.4. The Percentage Loss of Hydraulic Conductivity
Generally, PLC was significantly influenced by IRCIS treatments. Throughout both study periods, the IRCISs consistently reduced the PLC at all experimental forest sites. In roots, RC2015 showed a 48.8% lower value of PLC compared to WT2015, and RC2016 exhibited a 35.7% lower value compared to DT2016 (Figure 7A). In branches, RC2015 displayed a 43.9% lower value of PLC compared to WT2015, and RC2016 demonstrated a 32.9% lower value compared to DT2016 (Figure 7B). Overall, the impact of IRCIS on PLC was more significant on roots than on branches. This observation implies that roots are more sensitive to external factors, such as drought stress and rainwater harvesting measures, compared to branches in terms of plant hydraulic conductivity.
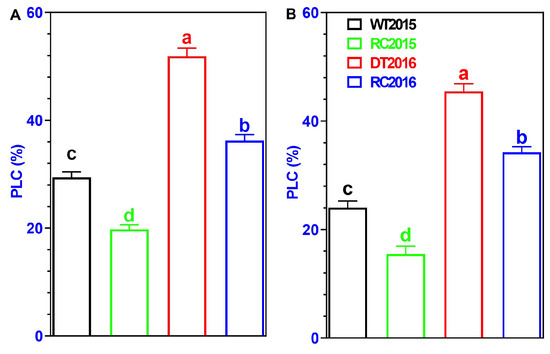
Figure 7.
The PLC values of roots (A) and branches (B) in Robinia pseudoacacia trees subjected to various treatments. Varied letters represent statistically significant differences (p < 0.05) among treatments, with whiskers depicting ±1 SD.
4. Discussion
4.1. Soil Moisture Content
Land conservation, measured as the increase in vegetation cover, biodiversity, and decrease in soil loss and water erosion, has been achieved after nearly 70 years of sustainable vegetation restoration efforts on China’s Loess Plateau. With the implementation of a large-scale afforestation campaign, forest coverage has increased from 8.0% in 1982 to 18.9% in 2015 [22]. This has adversely affect soil moisture content in this region, as illustrated by the significant decline of soil water content and the development of a dry soil layer in most area of this region [23]. These conditions pose a severe threat to the health of forest ecosystems on the Loess Plateau, leading to a reduction in forest growth (declining trees) and, in severe cases, forest mortality [15,24]. Therefore, there is an urgent need for rainwater-saving and harvesting management strategies to increase soil moisture content in this region, as the limited rainfall serves as the sole source of soil moisture given that groundwater tables are typically >50 m below the land surface.
This study highlights the importance of on-site rainwater collection and infiltration systems for rainwater retention in afforestation ecosystems, as they notably increased plant-available soil moisture content (Figure 3). This finding supports the results of previous studies [25,26,27], highlighting the effectiveness of rainfall harvesting techniques in increasing soil moisture content. For instance, Ma et al. [27] observed a substantial increase in plant-available water content in afforested forest stands in the Loess Plateau region when employing IRCIS treatment. Furthermore, our investigation revealed that shallow soil water content (0–2 m) is more influenced by the implementation of IRCIS than deep soil water content (2–5 m) (Figure 3), aligning well with the findings of Song et al. [12]. Their research showed that the shallow soil layer (0–2 m) exhibited more significant variations in soil water content compared to the deeper soil (>2 m) during both wet and drought years when an IRCIS was implemented in orchard plants. This is because shallow soil water content is more susceptible to the influences of rainfall, vegetation utilization, and forest management practices [28,29]. As a result, it leads to greater variability in shallow soil water compared to deep soil water. This simultaneously indicates that rainwater collected by an IRCIS is mainly stored in the shallow soil layer in this study region, making it readily available for forest use. It should be noted that deep soil moisture is also an important source of water for plants in water-limited regions, as it usually functions as a soil reservoir and plays a vital role in plant productivity and ecosystem sustainability [30]. Therefore, further research on the content and variability of deep soil water is urgently needed. This is particularly essential for developing effective forest management strategies to enhance forest growth and health.
4.2. Root Distribution Pattern
Fine roots (≤2 mm) are critical for the acquisition of water and mineral nutrients from soil [31], thus playing a significant role in forest ecosystems. Given their sensitivity to environmental changes and remarkable plasticity, the distribution pattern of fine roots determines a plant’s potential capacity for water and nutrient uptake [32]. During drought periods, when shallow soil water is insufficient to meet plant transpiration demands, plant roots tend to grow deeper, extracting additional water from the deep soil [33]. In agreement with these findings, declining Robinia pseudoacacia trees increased both their fine-root biomass and distribution depth during drought periods (Figure 4). This implies that declining Robinia pseudoacacia trees also possess the capability to absorb and utilize substantial amounts of soil moisture and nutrients from deep soil layers during dry periods, similar to actively growing trees (without declining). This may enable them to transition between shallow and deep soil moisture resources, depending on the availability of soil water. It strengthens their resilience to drought stress, consequently decelerating the tree mortality process. When subjected to IRCIS treatment, Robinia pseudoacacia exhibited a significant reduction in both fine-root biomass and depth. This was evident in the lower content of FRLD, reduced maximum depth, and D95 in both study years (Figure 4 and Figure 5). This reduction can be attributed to the effective collection and storage of rainfall by the IRCISs within the shallow soil layer (0–2 m). As a result, Robinia pseudoacacia trees accessed shallow soil moisture readily without the need for additional energy and resources to develop a deeper root system. Instead, these resources could be allocated to the growth of other organs, such as leaves, vessels, and phloem, facilitating better water and nutrient transport and mitigating the progresses of tree mortality.
4.3. Anatomical and Hydraulic Conductive Characteristics
Due to secondary growth, plants, especially trees, are able to adapt to low soil moisture conditions by modifying their xylem anatomical structures, including conduit size and distribution [34,35]. In previous studies, smaller vessel conduits were detected in stems of various tree species, such as Quercus robur and Robinia pseudoacacia, under drought conditions [13,36]. Therefore, smaller conduit vessels were less susceptible to drought-induced xylem embolism, contributing to water flow regulation during drought [37]. Supporting these findings, our results indicated that drought significantly reduced xylem conduit diameter at breast height in Robinia pseudoacacia, increased the number of smaller-diameter conduits, and significantly increased the skewness of conduit distribution, ultimately regulating hydraulic conductivity (Figure 6a and Figure 7).This adaptation is expected to better equip declining Robinia pseudoacacia to withstand drought stress, ensuring efficient water transport and slowing down the progress of tree mortality. When subjected to IRCIS treatments, the diameter of xylem conduits increased, and their distribution tended toward a more normal pattern (Figure 6b). This suggests that IRCIS treatments can effectively increase soil moisture content, modify xylem vascular structures (xylem conduit diameter and distribution) of declining Robinia pseudoacacia trees, facilitate water and nutrient transport to reproductive and productive organs, enhance plant vitality, and delay the mortality processes.
Previous studies indicated that there is a strong correlation between conduit diameter and resistance to cavitation at the individual conduit level [38]. Larger diameter xylem conduits possess greater hydraulic conductivity ability but are more vulnerable to cavitation, and vice versa [39]. This challenge arises from the structural characteristics of the xylem vascular network, as vegetation seeks to maximize water transport while minimizing drought-induced cavitation risk during water-limited time [40]. This held true in our study, where the decline in hydraulic conductivity was more pronounced in roots (with broader conduits) than in branches (with smaller conduits) during water-limited periods for all declining Robinia pseudoacacia trees. This adversely impacted plant growth processes such as plant cell enlargement, enzyme activity, plant photosynthesis, and plant transpiration, resulting in slowed plant growth and even mortality of plants. On the other hand, when subjected to IRCIS treatment, a decrease in roots and branches PLC was observed for both study years. This suggests that IRCIS treatment significantly improves plant resistance to drought and hydraulic transfer failure, particularly in water-limited situations.
4.4. Implications for Afforest Management
With the larger-scale implementation of afforestation campaigns, soil water deficits have become apparent in the Loess Plateau region, adversely affecting plant growth, leading to decreased plant growth rate and, in extreme cases, tree mortality [9,24]. This poses a threat to the health and services of the ecosystem. Therefore, maintaining a balance between soil water supply and plant water uptake is essential for the sustainable development of the ecosystem [6]. As rainfall serves as the only source of water essential for vegetation survival on the Loess Plateau, optimizing the utilization of rainwater resources is crucial for the health and sustainable growth of afforested trees. Recently, various afforestation management techniques, including engineering techniques such as the construction of fish-scale pits and agricultural management practices like covering soil with agricultural litter and the use of moisture-holding chemical and biological substances, have been extensively documented to effectively harness rainwater to enhance healthy and sustainable development of forest in the Loess Plateau region [12,20]. Our study confirms that implementing a rainwater harvesting system (IRCIS) can significantly increase plant-available soil moisture content. This, in turn, results in an enlargement of xylem conduit diameter and plant hydraulic conductivity, a reduction in water transmission losses, improved growth of declining Robinia pseudoacacia, and a slowing down of the decline and mortality processes. Moreover, over the two-year study period, IRCIS treatment resulted in an increase in tree height (m), DBH, mean crown length (m), mean crown height (m), leaf area index (m2 m−2), and cumulative sapwood area (m2/ha) by 4.4%, 5.8%, 14.3%, 12.1%, 29.6%, and 12.1%, respectively. IRCISs, therefore, can be promoted as a crucial forest management measure to increase soil moisture content, mitigate the decline of afforested trees, and achieve healthy and sustainable development of declining forests.
5. Conclusions
Due to the higher water demand of Robinia pseudoacacia trees, most Robinia pseudoacacia forest stands had significantly lower plant-available moisture content on the Loess Plateau, resulting in higher soil moisture deficits, especially in drought years. This, in turn, has hampered the healthy growth of Robinia pseudoacacia, leading to a decline and, in severe cases, mortality of trees. To minimize these negative effects, enhance the efficient use of soil water, and improve the resistance and resilience of forests to drought stress, diverse afforestation strategies, including engineering practices and rainfall harvesting measures, have been documented for sustainable artificial forest development. Our study demonstrates that the application of a rainfall harvesting system—the IRCIS—can effectively increase plant available moisture content of declining Robinia pseudoacacia forest stands. In particular, IRCIS treatment significantly increased xylem conduit diameter and plant hydraulic conductivity while substantially reducing the percentage loss of hydraulic conductivity in both roots and branches. Furthermore, IRCIS treatment significantly reduced the root biomass and distribution depth of Robinia pseudoacacia during different rainfall years. This implies that IRCISs are beneficial for plant growth and survival.
The response to rainwater harvesting may vary in different soil types due to variations in water retention, drainage, and other soil characteristics. The study’s findings may be specific to the soil conditions on the Loess Plateau. However, the results of this study can serve as a valuable reference for research on other soil types. The findings of this study are significant for devising strategic methodologies for the planning and management of rainwater resources. The adoption of these strategies, especially the incorporation of rainwater harvesting systems (IRCISs), offers a viable solution to counteract forest degradation induced by drought stress.
Author Contributions
Conception and design, C.M. and M.S.; acquisition of data, C.M. and W.Y.; analysis and interpretation of data, W.Y., B.Z. and Q.W.; drafting the article, C.M., W.Y., M.S. and Q.W.; conception and design, W.Y., Q.W. and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (42107326), the Scientific Research Foundation of Xi’an University of Technology (256082313), the National Natural Science Foundation–Outstanding Youth Foundation (52222903) and the China Three Gorges Corporation-funded “Research on Soil Improvement Plant Selection, and Slope Ecological Restoration Techniques in the Arid Hot Valley Area of the Baihetan Hydropower Station” (No: BHT/0869).
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weeraratna, S. Control of Land Degradation. In Understanding Land Degradation: An Overview; Weeraratna, S., Ed.; Springer: Cham, Switzerland, 2022; pp. 39–51. [Google Scholar]
- Smith, P.; Calvin, K.; Nkem, J.; Campbell, D.; Cherubini, F.; Grassi, G.; Korotkov, V.; Le Hoang, A.; Lwasa, S.; McElwee, P.; et al. Which practices co-deliver food security, climate change mitigation and adaptation, and combat land degradation and desertification? Glob. Change Biol. 2020, 26, 1532–1575. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.X.; Chen, L.; Yu, X.X. Impact of China’s Grain for Green Project on the landscape of vulnerable arid and semi-arid agricultural regions: A case study in northern Shaanxi Province. J. Appl. Ecol. 2009, 46, 536–543. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qiu, K.; López-Vicente, M.; Shen, W.; Wu, G.L. Soil-water deficit in deep soil layers results from the planted forest in a semi-arid sandy land: Implications for sustainable agroforestry water management. Agric. Water Manag. 2021, 254, 106985. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.C.; Dang, T.H.; Zhu, O.Y.; Li, Z.; Wang, J.; Wang, R.; Gao, C.Q. Soil water dynamics and deep soil recharge in a record wet year in the southern Loess Plateau of China. Agric. Water Manag. 2010, 97, 1133–1138. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Huang, L.; Shao, M. Advances and perspectives on soil water research in China’s Loess Plateau. Earth-Sci. Rev. 2019, 199, 102962. [Google Scholar] [CrossRef]
- Lü, Y.; Fu, B.; Feng, X.; Zeng, Y.; Liu, Y.; Chang, R.; Sun, G.; Wu, B. A policy-driven large scale ecological restoration: Quantifying ecosystem services changes in the Loess Plateau of China. PLoS ONE 2012, 7, e31782. [Google Scholar] [CrossRef]
- Kang, D.; Zou, S. Factors limiting the recruitment of artificial black locust forests in extremely arid environment. Ecol. Eng. 2020, 150, 105813. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M.; Shao, H. A preliminary investigation of the dynamic characteristics of dried soil layers on the Loess Plateau of China. J. Hydrol. 2010, 381, 9–17. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.P.; Horton, R.; Shao, M. Effects of vegetation and slope aspect on water budget in the hill and gully region of the Loess Plateau of China. Catena 2011, 87, 90–100. [Google Scholar] [CrossRef]
- Song, X.; Gao, X.; Wu, P.; Zhao, X.; Zhang, W.; Zou, Y.; Siddique, K.H.M. Drought responses of profile plant-available water and fine-root distributions in apple (Malus pumila Mill.) orchards in a loessial, semi-arid, hilly area of China. Sci. Total Environ. 2020, 723, 137739. [Google Scholar] [CrossRef] [PubMed]
- Bayala, J.; Prieto, I. Water acquisition, sharing and redistribution by roots: Applications to agroforestry systems. Plant Soil 2020, 453, 17–28. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Bauerle, T.L. Repetitive seasonal drought causes substantial species-specific shifts in fine-root longevity and spatio-temporal production patterns in mature temperate forest trees. New Phytol. 2021, 231, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, M.; Yang, Y.; Zhao, X. Evaluating drought-induced mortality risk for Robinia pseudoacacia plantations along the precipitation gradient on the Chinese Loess Plateau. Agric. For. Meteorol. 2020, 284, 107897. [Google Scholar] [CrossRef]
- Guérin, M.; von Arx, G.; Martin-Benito, D.; Andreu-Hayles, L.; Griffin, K.L.; McDowell, N.G.; Pockman, W.; Gentine, P. Distinct xylem responses to acute vs prolonged drought in pine trees. Tree Physiol. 2020, 40, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.L.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Ding, W.; Wang, F.; Dong, Y.; Jin, K.; Cong, C.; Han, J.; Ge, W. Effects of rainwater harvesting system on soil moisture in rain-fed orchards on the Chinese Loess Plateau. Agric. Water Manag. 2021, 243, 106496. [Google Scholar] [CrossRef]
- Song, X.; Gao, X.; Zhao, X.; Wu, P.; Dyck, M. Spatial distribution of soil moisture and fine roots in rain-fed apple orchards employing a Rainwater Collection and Infiltration (RWCI) system on the Loess Plateau of China. Agric. Water Manag. 2017, 184, 170–177. [Google Scholar] [CrossRef]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef]
- Kong, D.; Miao, C.; Wu, J.; Zheng, H.; Wu, S. Time lag of vegetation growth on the Loess Plateau in response to climate factors: Estimation, distribution, and influence. Sci. Total Environ. 2020, 744, 140726. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Duan, G.; Lu, J.; Zhou, R.; Ren, H.; Wen, Z. Response relationship between vegetation structure and runoff-sediment yield in the hilly and gully area of the Loess Plateau, China. Catena 2023, 227, 107107. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Z.; Huang, M.; Zhao, X.; Yang, F.; Wu, X. The impact of climate change on growth and drought-induced mortality risk of Robinia pseudoacacia plantations along a precipitation gradient on the Chinese Loess Plateau. Agric. For. Meteorol. 2022, 325, 109160. [Google Scholar] [CrossRef]
- Song, X.; Gao, X.; Dyck, M.; Zhang, W.; Wu, P.; Yao, J.; Zhao, X. Soil water and root distribution of apple tree (Malus pumila Mill) stands in relation to stand age and rainwater collection and infiltration system (RWCI) in a hilly region of the Loess Plateau, China. Catena 2018, 170, 324–334. [Google Scholar] [CrossRef]
- Chen, G.; Wu, P.; Wang, J.; Zhang, P.; Jia, Z. Ridge–furrow rainfall harvesting system helps to improve stability, benefits and precipitation utilization efficiency of maize production in Loess Plateau region of China. Agric. Water Manag. 2022, 261, 107360. [Google Scholar] [CrossRef]
- Ma, C.K.; Meng, H.B.; Xie, B.; Li, Q.; Li, X.D.; Zhou, B.B.; Wang, Q.J.; Luo, Y. In situ rainwater collection and infiltration system alleviates the negative effects of drought on plant-available water, fine root distribution and plant hydraulic conductivity. Forests 2022, 13, 2082. [Google Scholar] [CrossRef]
- Škerlep, M.; Steiner, E.; Axelsson, A.-L.; Kritzberg, E.S. Afforestation driving long-term surface water browning. Glob. Change Biol. 2020, 26, 1390–1399. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Shao, M.; Zhang, X. Using pedo-transfer functions to estimate dry soil layers along an 860-km long transect on China’s Loess Plateau. Geoderma 2020, 369, 114320. [Google Scholar] [CrossRef]
- Li, B.-B.; Li, P.-P.; Zhang, W.-T.; Ji, J.-Y.; Liu, G.-B.; Xu, M.-X. Deep soil moisture limits the sustainable vegetation restoration in arid and semi-arid Loess Plateau. Geoderma 2021, 399, 115122. [Google Scholar] [CrossRef]
- Addo-Danso, S.D.; Defrenne, C.E.; McCormack, M.L.; Ostonen, I.; Addo-Danso, A.; Foli, E.G.; Borden, K.A.; Isaac, M.E.; Prescott, C.E. Fine-root morphological trait variation in tropical forest ecosystems: An evidence synthesis. Plant Ecol. 2020, 221, 1–13. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Qian, S.; He, M.; Cai, X.; Ding, J. Root characteristics explain greater water use efficiency and drought tolerance in invasive Compositae plants. Plant Soil 2023, 483, 209–223. [Google Scholar] [CrossRef]
- Gori, A.; Moura, B.B.; Sillo, F.; Alderotti, F.; Pasquini, D.; Balestrini, R.; Ferrini, F.; Centritto, M.; Brunetti, C. Unveiling resilience mechanisms of Quercus ilex seedlings to severe water stress: Changes in non-structural carbohydrates, xylem hydraulic functionality and wood anatomy. Sci. Total Environ. 2023, 878, 163124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, C.; Liu, Y.; Li, S.; Li, X.; Xi, B.; Duan, J. Xylem anatomical and hydraulic traits vary within crown but not respond to water and nitrogen addition in Populus tomentosa. Agric. Water Manag. 2023, 278, 108169. [Google Scholar] [CrossRef]
- Fonti, P.; Heller, O.; Cherubini, P.; Rigling, A.; Arend, M. Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol. 2013, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Isasa, E.; Link, R.M.; Jansen, S.; Tezeh, F.R.; Kaack, L.; Sarmento Cabral, J.; Schuldt, B. Addressing controversies in the xylem embolism resistance–vessel diameter relationship. New Phytol. 2023, 238, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Harrison Day, B.L.; Johnson, K.M.; Tonet, V.; Bourbia, I.; Blackman, C.; Brodribb, T.J. The root of the problem: Diverse vulnerability to xylem cavitation found within the root system of wheat plants. New Phytol. 2023, 239, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Phillips, N.; Lunch, C.; Bond, B.J.; Ryan, M.G. An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol. 2002, 22, 763–774. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Berry, J.A.; Field, C.B. Loss of whole-tree hydraulic conductance during severe drought and multi-year forest die-off. Oecologia 2014, 175, 11–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).