Abstract
The aim of this study was to determine the tolerance to metals (Zn, Cu) and drought of male and female Salix × fragilis L. under isolated and combined treatments, and to assess the metal uptake and profiling of metabolic plant responses. The 14-day experiment was performed in a hydroponic system, and metals were applied at 1.5 mM in a Knop’s solution. Drought simulation was achieved by adding sorbitol at a moderate level (200 mM). Isolated Zn treatment enhanced plant growth, more pronouncedly in females. Equimolar Cu treatment caused diverse reactions, and females exhibited significantly higher tolerance. Male specimens were less tolerant to isolated drought and to combined drought and metal presence. The highest contents of Cu and Zn were found in roots, compared to the aboveground tissues (wooden rods and leaves), of both female and male metal-treated plants. Simultaneously applied drought limited Zn accumulation in roots and elevated its translocation to leaves while increasing Cu accumulation, predominantly in females showing higher tolerance. Both isolated and combined drought and metals reduced leaf water content, caused the allocation of mineral nutrients (Ca, Mg, K, and Na), and affected metabolism in a stressor-specific and sex-dependent manner. For males, Cu accumulation in the leaves was significantly correlated with the majority of metabolites, while for both sexes, kaempferol and salicylic acid were strongly correlated, indicating their role in tolerance against the metal. The obtained results are an excellent starting point for the practical use of male and female Salix × fragilis L. in areas heavily polluted with Cu or Zn and exposed to drought, for the purpose of their recultivation.
1. Introduction
Soil pollution with heavy metals has become a serious problem worldwide. The mobilization of heavy metals through extraction from ores with subsequent processing and broad applications has led to their release into the environment. Being essentially nonbiodegradable, heavy metals tend to accumulate in the environment and pose an increasing risk to ecosystems and human health [1]. A technology with the potential to deal with soil pollution is phytoremediation: the use of green plants and associated soil microbes to reduce the concentrations or toxic effects of contaminants in the environment [2]. It is a cost-effective, environment-friendly, in situ-applicable, and solar-driven method for the removal of heavy metals and radionuclides, as well as for the degradation of a wide array of organic pollutants [3,4].
Although phytoremediation is a promising approach to tackle the heavy metal pollution of soils, and despite being supported by a vast research background, it suffers some limitations for large-scale applications [2,5]. Major limitations include the long time required for the soil clean-up process, and above all, difficulties in the proper selection of the efficient plant species. In particular, the low biomass of metal hyperaccumulators and the low efficiency of metal uptake by most plants, accompanied by limited adaptation to adverse environmental conditions, are major issues [1,6]. Ideal plant species for phytoremediation should be highly biomass-producing, easily adaptive to changing environments, tolerant to toxic metals and other contaminants, easy to cultivate, resistant to pests and diseases, have high metal accumulation capacity, and finally, be non-attractive to herbivores [7].
The increasing concern limiting the phytoremediation process is unpredictable changes in water availability, including prolonged no-rain periods or heavy precipitation events [8]. In particular, drought, as one of the most complex hydrological and climate disasters, affects all aspects of ecosystem structure and functioning [9,10]. Drought occurrence accompanied by high temperatures is critical for plant survival, and resulting water stress may interfere with metal toxicity, aggravating oxidative stress and damage to plants [11,12]. In heavily degraded lands, such as mine tailings, plants employed for phytoremediation should exhibit both metal and drought tolerance, and the interactions between these two stressors require evaluation to predict the success of the clean-up process [13].
In the present study, we investigated the phytoremediation potential of Salix × fragilis L., a hybrid of S. alba × S. euxina, commonly named “crack willow”. The main features of the plant meet the requirements for phytoremediation purposes; however, relatively high water demands may limit its application in view of increasing drought occurrence. The plant is a strong medium-sized to large deciduous tree, fast growing up to 10–20 m, and forms male and female flowers on separate specimens. It tolerates almost any soil, including sandy and infertile soil, and preferers wet to marshy habitats [14]. The native range of S. × fragilis is central Europe, southern Scandinavia, northern areas of Asia Minor, and the Caucasus; however, it is widely distributed across nearly all climatic zones due to its fast adaptation, low soil requirements, easy vegetative propagation, and previous introduction (mostly to prevent soil erosion) [15].
Natural riparian communities of S. × fragilis are important for restoring natural river-bank environments, regulating water flow, soil erosion control, improving water quality, and providing habitats for wildlife. They were evaluated as close to ecological indicators for soil moisture, light, and temperature [16] and used as accumulating bioindicators for river water pollution with heavy metals [17]. In the Himalayan cold desert (India), S. × fragilis is considered a multipurpose tree of high significance and is cultivated under a national agroforestry system as a main source of construction timber, fuel wood, agricultural implements, basket making, etc. [18,19].
However, in some regions, S. × fragilis, similar to many other willows, is considered an invasive species or weed, including in Australia and New Zealand, the northern regions of the United States, Canada, and South Africa. It is primarily of concern along rivers and streams, in wetlands, and around waterbodies, where it competes for space, water, and nutrients. It displaces native plants and changes stream hydrology, sedimentation rates, and flooding patterns [20,21]. Thus, in these areas, the hybrid is excluded from water remediation purposes, and for soil clean-up, monosexual plantings should be exclusively introduced.
The aim of this study was (i) to determine the tolerance to metals (Zn and Cu) and drought of male and female specimens of S. × fragilis under isolated and combined treatments; (ii) to evaluate the effect of drought on Zn and Cu uptake and the allocation of major mineral nutrients depending on the plant sex; and (iii) to survey the metabolic adjustment to applied treatments and to elucidate the role of particular compounds in plant responses to isolated and combined stressors. The experiment was performed in a sand-stabilized hydroponic system to exclude the effect of soil characteristics on examined features. The level of Zn and Cu additions was selected based on our previous studies on Salix sp. [22,23]. Drought simulation was achieved by the addition of sorbitol at the moderate level, as previously applied in short-term observations [24].
2. Materials and Methods
2.1. Plant Material and Experiment Set-Up
Two-year-old healthy stem cuttings of S. × fragilis collected from ten-year-old rootstock were used in the experiment. Male (M) and female (F) specimens without foliage were obtained for the study in February 2021, and they originated from the Willow Collection in Zielonka village (52°33′4″ N, 17°6′19.7″ E), governed by the Poznań University of Life Sciences and located within the Zielonka Forest Landscape Park in the central part of the Wielkopolska region, Poland.
Standardized rods (25 cm long and similar diameter, i.e., ~1.2 and 0.8 mm for M and F, respectively) were cut off and stored in a cold store at 4 °C until the experiment was set up. Before the experiment, rods were incubated in half-strength Knop’s medium to induce root formation. After 7 days, plants were selected to obtain a uniform group and placed in hydroponic pots equipped with a dashed insert and a water level indicator (two plants per pot). Rods were stabilized with steamed ultrapure quartz sand (1–3 mm particle size; 3.5 kg per pot), and 1 L of a full-strength Knop’s solution was added to each pot. For metal treatments, Knop’s solution was modified with zinc or copper nitrates (Zn(NO3)2 × 6H2O or Cu(NO3)2 × 3H2O, respectively) at a concentration of 1.5 mM. In order to simulate drought (“d”) conditions, D-sorbitol (Sigma-Aldrich, St. Louis, MO, USA; cat.-no. 85529) was added at a concentration of 200 mM. Plants cultivated in unmodified Knop’s solution were designated as control (“c”). The experiment set-up consisted of six variants per sex, including control, three single stressor treatments (“Zn”, “Cu” and “d”), and two combined metal + “d” treatments (“Zn + d”, “Cu + d”). The 14-day experiment was performed under greenhouse conditions during spring 2021. Plants were controlled daily and watered with distilled water to maintain a constant solution level. The experiment was performed in triplicates.
2.2. Biomass Measurements and Handling
After the experiment, plants were gently removed from pots and divided into roots, rods, and shoots (twigs). Roots were immersed in 0.01 M HCl to eliminate trace elements adsorbed at the root surface [25], washed with deionized water, and gently dried on a filter paper to remove excess water. The biomass of roots and shoots was determined by weighing (n = 6). The tolerance index (TI) of S. × fragilis plants was calculated for particular treatments (t) as a ratio of combined shoots and roots fresh weight vs. control (c) [26]:
For biochemical investigations, leaves and roots were in situ frozen in liquid nitrogen and stored at −80 °C until analyses. Before extraction, each sample was homogenized in liquid nitrogen to obtain a fine powder, and accurately weighed aliquots were taken to the extraction procedure. For the elemental investigations, plant samples were dried at 105 ± 5 °C in an electric oven (SLW 53 STD, Pol-Eko, Wodzisław Śląski, Poland) and then ground in a Cutting Boll Mill PM 200 (Retsch GmbH, Haan, Germany). To cover weight requirements for performed investigations, fresh tissue of plants from each pot was combined (n = 3).
2.3. Relative Water Content
To evaluate the relative water content (RWC), the middle leaf of each plant was weighed immediately after the harvest to determine fresh weight (FW) using an analytical balance. Afterward, leaves were placed in string bags with their petiole soaked in distilled water and left for 4 h in the dark to reach full turgidity. After measuring the turgid weight (TW), leaves were dried in an electric oven at 80 °C for 48 h to determine dry weight (DW). The relative water content of leaves was calculated using the formula [27]:
2.4. Metal Content and Uptake Ratios
Dry samples were digested using the microwave system Mars 6 (CEM, Matthews, NC, USA). An accurate weight of 0.050–0.500 (±0.001) g of a dry sample was mineralized with 5 mL of concentrated nitric acid (180 °C, 20 min ramp time, 20 min heating time, 20 min cooling). After digestion, samples were diluted with ultra-pure water (Mili-Q, Millipore, Molsheim, France) to the final volume of 10.0 mL and filtered using a paper filter.
The ICP-OES spectrometer Agilent 5110 (Agilent, Santa Clara, CA, USA) was used in the elemental analysis of samples. The following instrumental conditions were used: nebulizer gas flow 0.7 L min−1, auxiliary gas flow 1.0 L min−1, plasma gas flow 12.0 L min−1, Radio Frequency (RF) power 1.2 kW, Charge Coupled Device (CCD) temperature −40 °C, viewing height for radial plasma observation 8 mm, signal acquisition time 5 s, 3 replicates. The analytical wavelengths were: 327.395 nm for Cu (axial view), 213.857 nm for Zn (axial view), 422.673 nm for Ca (radial view), 285.213 nm for Mg (axial view), 766.491 nm for K (radial view), 589.592 nm for Na (radial view). The detection limits were set using 3-sigma criteria: 0.01 mg kg−1 for Cu, Zn and Mg, and 0.03 mg kg−1 for Ca, K and Na. The accuracy was checked using both CRM (INCT-OBTL-5 and NIST-SRM-1573a—tobacco leaves) and the standard addition method, and the recoveries at the 80%–125% level were found to be satisfactory.
For investigated metals, the translocation factor (TF) was calculated as a ratio of metal content in shoots to roots. The bioconcentration factor (BCF) was calculated as a ratio of metal content in roots (or rods) to its concentration in a cultivation solution at the experiment start point. According to the evaluated TF and BCF values, plants were further categorized as accumulators (BCF > 1 and TF > 1), stabilizers (BCF > 1 and TF < 1) or excluders (BCF < 1 and TF < 1) of the applied metal under given cultivation conditions [28,29]. In order to determine the effect of treatments on mineral nutrient allocation, the BCF and TF values were calculated for Ca, Mg, K (ingredients of the Knop’s solution), and TF for Na (not included in the cultivation medium).
2.5. Metabolic Investigations
2.5.1. Total Phenolic Content
The total phenolic content (TPC) was determined according to the Folin–Ciocalteu assay with some modifications [30]. The extracts (100 µL) were mixed with 1 mL of diluted Folin–Ciocalteu reagent (1:1 with water, v:v), and after 3 min, 3 mL of 10% Na2CO3 was added. After 30 min of incubation at room temperature in dark, the absorbance at λ = 765 nm was measured with a Cary 300 Bio UV–vis scanning spectrophotometer (Varian, Mulgrave, VIC, Australia). The results were expressed in mg of gallic acid equivalents per g of fresh tissue weight (mg GAeq g−1 FW).
2.5.2. Profiling of Phenolic Metabolites and Low-Molecular-Weight Organic Acids (LMWOAs)
Samples of S. × fragilis roots and leaves were ground to a homogenous powder in a chilled mortar using liquid nitrogen. Metabolites were extracted from roots with ultra-pure water (Mili-Q, Millipore, Molsheim, France) and from leaves with 80% methanol acidified with 2% HCl. The mixtures were sonicated for 60 min in a water bath at 40 °C and shaken for 5 h with an orbital shaker. Aqueous and methanolic extracts were centrifuged (10,000× g, 20 min), and the obtained supernatants were evaporated to dryness and stored frozen until analysis. Before Ultra Performance Liquid Chromatography (UPLC) analysis, extracts were dissolved in 1 mL of methanol and filtered with a syringe filter (0.22 μm cellulose membrane) (MilliporeSigma Filtration, Burlington, MA, USA).
Organic acids and phenolics were profiled using a Waters Acquity H-class Ultra Performance Liquid Chromatography (UPLC) system. Separations were achieved on an Acquity UPLC BEH C18 column (150 × 2.1 mm, 1.7 μm; Waters, Milford, MA, USA) at 35 °C. The elution was performed with ultra-pure water and acetonitrile (both containing 0.1% formic acid) at a flow rate of 0.4 mL min−1. Detection was carried out using a Waters Photodiode Array Detector (Waters Corporation, Milford, MA, USA) at λ = 280 and 320 nm, as previously described in detail [31]. The results were expressed in micrograms per gram of tissue fresh weight (FW). Exclusively for salicylic acid determination, chromatographic analyses were performed with a Waters Alliance 2695 Chromatograph coupled with a Waters 2475 Multi-λ Fluorescence Detector (Waters Corporation, Milford, MA, USA), using a Waters Spherisorb ODS2 column (100 × 4.6 mm, 3 μm). The isocratic elution was performed with a 1.5 mL min−1 flow rate of potassium-acetate buffer (0.2 M, pH 5.0) [32]. The fluorometric detection was carried out at λ = 295 and 405 nm for excitation and emission, respectively. In both cases, metabolites were identified according to the retention time and quantified by comparing peak areas using an appropriate calibration curve prepared for an array of external standard solutions.
2.6. Statistical Analysis
Statistical analyses were performed using the R software (ver. 4.2; R Development Core Team). Test assumptions were checked visually (Q-Q plots) and tested with formal methods; homogeneity of variance across groups was assessed by Levene’s Test. The relationship between the factors was tested by an interaction plot.
Principal component analysis (PCA) was performed to verify the relationship between the variables and the samples. Data normalization was performed to prevent one variable from dominating the others (clusterSim library), and variables were shifted so they were zero-centered. The cluster number was estimated using parallel analysis and a scree plot in the paran package. The PCA graph was plotted based on the top ten contributors to the first and second dimensions.
The differences concerning the specimen’s morphological features; phenolics concentration; translocation and bioconcentration factors; and relative water content were assessed by multi-way analysis of variance with treatment, sex, and in some cases, elements as fixed factors (stats library). Tukey’s HSD test was employed as a post-hoc analysis for significant effects. Data were visualized using ggplot2 package ver. 3.5.0 [33].
3. Results
3.1. Plant Growth and Toxicity Symptoms
3.1.1. Shoot Biomass
The biomass of shoots developed during the experiment differed significantly (p < 0.001) between male and female control plants, reaching 6.17 and 3.65 g, respectively (Figure 1). The addition of Zn led to an increase in shoot biomass, which was comparable for both sexes, i.e., by ~17% (M) and 28% (F). In contrast to Zn, equimolar Cu treatment caused a reduction in shoot biomass, and the inhibitory effect of Cu depended significantly on the S. × fragilis sex (~76 and 5% for M and F, respectively). Drought simulation led to the highest retardation of shoot growth and was comparable for M and F (by ~92% and 82%, respectively). The simultaneous action of metal and limited water availability caused a drop in shoot biomass compared to isolated metal treatments. In the case of male plants, the reduction effect was similar for “Zn + d” vs. “Zn” (p < 0.001) and “Cu + d” vs. “Cu” (p > 0.05) treatments (by ~76 and 67%, respectively). For female plants, the joint effect on shoot biomass depended significantly on the applied metal and reached a ~84% and 53% decrease in the case of “Zn + d” vs. “Zn” (p < 0.001) and “Cu + d” vs. “Cu” (p < 0.05) treatments, respectively.
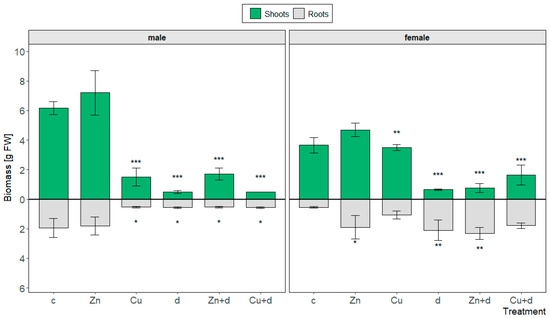
Figure 1.
Biomass of shoots and roots of male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control; data presented as mean ± SD; asterisks show significant differences between treatment and control: [*] p < 0.05, [**] p < 0.01, [***] p < 0.001).
3.1.2. Root Biomass
Similar to the shoots, the root biomass of control plants was higher for male specimens and reached 1.95, vs. the 0.55 g FW observed for female plants (p < 0.05) (Figure 1). Particular treatments caused diverse changes in root growth depending on the plant sex. In the case of male specimens, Cu addition, drought simulation, and simultaneous metal and drought treatments (“Zn + d” and “Cu + d”) led to a marked and comparable reduction of root biomass (~27%–29% of control). For isolated Zn treatment, root biomass remained similar to the control. In contrast to male specimens, the root growth of female plants was significantly induced by Zn, drought simulation, and their interaction (by ~344%–422% of control).
3.1.3. Tolerance Index and Toxicity Symptoms
The joined effect of “sex × treatment” was found to significantly (p < 0.001) differentiate tolerance index values considering the overall fresh biomass (shoots and roots). In the case of male plants, all treatments excluding “Zn” similarly reduced the TI values (p < 0.001 vs. control), with the lowest observed for drought (“d”) and combined drought and copper (“Cu + d”) treatments (Figure 2a). Plants treated with Zn showed slightly increased tolerance vs. control (p > 0.05). A similar positive effect of Zn addition was found for female plants, as well as significant vs. control or Zn-treated males (p < 0.001). Further, drought simulation significantly lowered the TI value (p < 0.05), while the remaining treatments caused a non-significant (p > 0.05) increase (“Cu”) or decrease (“Zn + d”, “Cu + d”) in the tolerance index of female specimens.
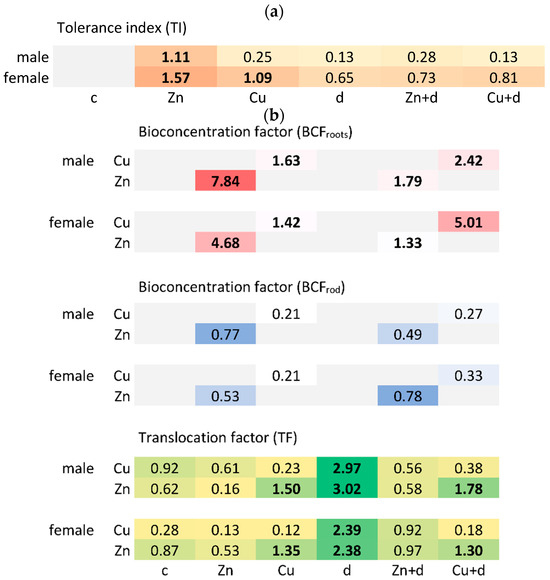
Figure 2.
Tolerance index (a), bioconcentration and translocation factors (b) of Zn and Cu for male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control).
Besides significant changes in S. × fragilis growth, visible symptoms of toxicity were also noted (Figure S1). The lower yield of shoot biomass caused by “d” and combined metal + “d” treatments, was accompanied by lower blade size, chlorosis, leaf edge necrosis and senescence of the oldest leaves, and tip burn necrosis of the youngest leaves, which proceeded to the edges in the case of female plants. For male specimens, the reduction of leaf blade area was also noted; however, negative symptoms dominantly concerned the root system architecture, for which the reduction of hairy root growth was noted.
3.2. Leaf RWC
Relative water content determined for leaves of control plants showed similar values (p > 0.05) for M (male) and F (female) specimens (96.7 and 98.4%, respectively) (Figure 3). Among isolated metal treatments, only Cu led to a significant reduction of leaf RWC observed for females (by 9.3% vs. control). For both sexes, applied drought simulation caused a comparable (p > 0.05) drop in leaf RWC by 12.8 and 11.1% for M and F, respectively. The simultaneous Cu and drought treatment of male plants caused the strongest reduction of the RWC value to 75.1%—by 21.7, 20.2, and 8.4% in relation to control, isolated metal, and drought, respectively (p < 0.001). On the contrary, the “Zn + d” co-treatment reduced leaf turgidity by 7.3% compared to the control, while a significant change was not observed compared to the isolated Zn treatment. Further, “Zn + d” led to a weaker reduction in the RWC value than drought simulation alone (p < 0.05). In the case of female plants, the simultaneous “Zn + d” treatment caused the lowest RWC value among treatments, which was strongly reduced compared to the control (by 13.5%) and isolated metal addition (by 11.5%, p < 0.001); however, it was similar to the RWC for drought simulation. Opposite to zinc, “Cu + d” did not alter leaf turgidity vs. control, and the RWC value was higher than for isolated drought (p < 0.05) and similar to Cu (p > 0.05) treatments.
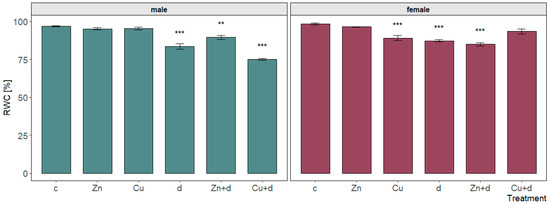
Figure 3.
Relative water content in leaves of male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control; data presented as mean ± SD; asterisks show significant differences between treatment and control: [**] p < 0.01, [***] p < 0.001).
3.3. Cu and Zn Uptake and Translocation
3.3.1. Roots
The mean content of Zn in roots of control male and female S. × fragilis (44.4 and 109 mg kg−1, respectively) was similar to that for the “d” treatment (44.4 and 45.7 mg kg−1, respectively) (Table S1). No significant differences between these variants were indicated. The addition of Zn elevated metal content in roots, which differed significantly depending on the plant sex (769 mg kg−1 and 459 mg kg−1 for M and F, respectively). Simultaneous “Zn + d” treatment resulted in a lower uptake of Zn in roots, without significant differences between sexes (176 and 131 mg kg−1 for M and F, respectively). The efficient uptake of Zn by roots was confirmed by high values of the BCF calculated in the case of isolated metal addition (7.8 and 4.7 for M and F, respectively). The simultaneous action of Zn and drought resulted in a less effective accumulation of this metal in roots, reflected by BCF = 1.8 and 1.3 (for M and F, respectively) (Figure 2b, Table S2).
The mean content of Cu in roots of control male and female S. × fragilis (10.9 and 29.8 mg kg−1, respectively) was similar to the “d” treatment (14.9 and 14.7 mg kg−1, respectively). Significantly higher contents were determined for plants treated with Cu (155 and 135 mg kg−1 for M and F, respectively) (Table S1). The combined “Cu + d” treatment contributed to more efficient uptake of Cu and, at the same time, the differentiation between male and female specimens (231 and 478 mg kg−1, respectively). This was reflected by the BCF values, and a significant increase was demonstrated in the case of the “Cu + d” co-treatment of female specimens vs. males (BCF = 5.0 vs. 2.4) (Figure 2b, Table S2).
3.3.2. Rod
The addition of Zn caused a significantly higher uptake of the metal in the wooden stem of male plants (75.1 mg kg−1) compared to the control (44.0 mg kg−1), while for simultaneous “Zn + d” treatment, the content of Zn was similar as for control (48.2 mg kg−1) (Table S1). The opposite was observed for females, where a similar content of Zn was noted for control and “Zn” (56.6 and 51.6 mg kg−1, respectively), while the “Zn + d” co-treatment caused significantly higher Zn uptake (76.5 mg kg−1). For both sexes, the uptake of Zn to the rods was limited, characterized by BCF values < 1 (Figure 2b, Table S2).
The addition of Cu resulted in a higher content of the metal in rods of S. × fragilis (~19.9 mg kg−1) compared to control plants (10.2 and 12.2 mg kg−1 for M and F, respectively) (Table S1). The simultaneous “Cu + d” treatment increased the metal uptake to wooden stems (25.6 and 31.7 mg kg−1, respectively). Still, the efficiency of Cu uptake to the rods was low, confirmed by BCF values < 1 for both sexes (Figure 2b, Table S2).
3.3.3. Leaves
The content of Zn in the leaves of control plants was higher for female than male specimens (92.7 and 28.1 mg kg−1, respectively). Elevated Zn content and similar relations were noted for plants cultivated with the addition of Zn (231 and 113 mg kg−1 for F and M, respectively) (Table S1). Under drought simulation, Zn content in leaves was similar for both sexes (133 and 106 mg kg−1 for M and F, respectively) and elevated vs. male control. Simultaneously, in the case of male specimens, it was similar to in leaves of Zn-treated plants. For the combined “Zn + d” treatment, similar Zn content was noted as in the case of “d”, and drought lowered Zn uptake to leaves when applied simultaneously. The TF values < 1 indicated limited Zn transfer from the roots to leaves in the case of the “Zn” and “Zn + d” treatments, while drought simulation elevated Zn content in leaves in relation to roots (TF > 2.38) (Figure 2b, Table S2).
The addition of Cu led to the increased content of the metal in the leaves of male plants compared to the control (33.7 vs. 9.82 mg kg−1, respectively), while the elevation was not observed for females (Table S1). Drought simulation increased Cu uptake to leaves compared to the control plants of both sexes and also compared to Cu-treated females. The “Cu + d” co-treatment caused the highest content in leaves and was comparable for M and F specimens (85.2 and 85.3 mg kg−1, respectively). The TF values for Cu were found to be <1 in the case of isolated metal additions and metal+”d” co-treatments, while drought simulation elevated Cu content in leaves in relation to roots (TF > 2.39) (Figure 4). Moreover, Cu addition led to an elevated translocation of Zn in both “Cu”- and “Cu + d”-treated plants, which was observed for both sexes (Figure 2b, Table S2).
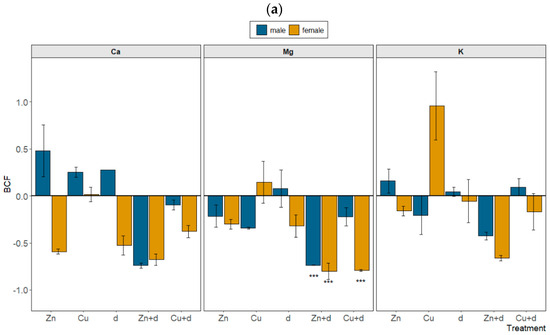
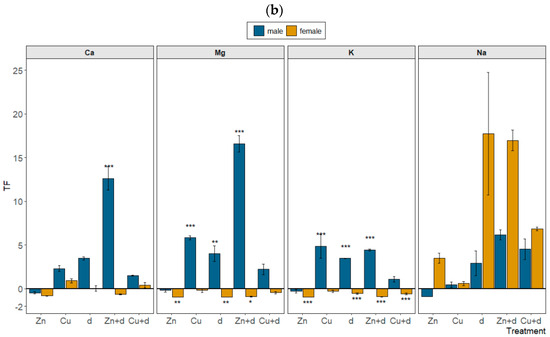
Figure 4.
Relative changes in bioconcentration factor (BCF) (a) and translocation factor (TF) (b) of mineral nutrients in male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“0” represents no change vs. control plants, “1” represents doubled value of the parameter, “−0.5” represents a decrease by a half vs. control; data presented as mean ± SD; asterisks show significant differences between treatment and control: [*] p < 0.05, [**] p < 0.01, [***] p < 0.001).
3.4. Mineral Nutrients Allocation
Particular treatments altered the uptake and transport of mineral nutrients, including bivalent (Ca, Mg) and monovalent (K, Na) metals, in a sex-dependent manner (Tables S1 and S2). In the case of the BCF, significant depletion was observed only for Mg under “Cu + d” (F) and “Zn + d” (M and F) treatments relative to the control (Figure 4a). The values of TF were significantly raised in male plants subjected to “Zn + d” (Ca, Mg, K), “d” and “Cu” (Ca, Mg and K) (Figure 4b). On the contrary, in female specimens, the translocation of mineral nutrients was significantly reduced compared to control plants for “d”, “Zn” and “Zn + d” treatments (Mg, K), and “Cu + d” (K). Simultaneously, the TF calculated for Na was increased vs. control (p > 0.05) for the majority of variants, and a more conspicuous increase was noted in the case of female plants under “d” and metal + “d” treatments (Figure 4b).
3.5. Metabolite Investigations
3.5.1. Total Phenolics Content
The TPC in roots ranged from 0.25 to 7.24 mg GAeq g−1 FW, with the lowest values found for control plants of both sexes and male specimens treated with Zn, and the highest values were found for the “Cu + d” co-treatment of males (Figure 5). Among isolated metal treatments, only in the case of male plants treated with Cu, a conspicuous increase in the TPC was noted. Drought simulation caused a similar increase in phenolic content in roots of both sexes, while Zn significantly lowered the effect, as noted for the “Zn + d” treatment. In the case of “Cu + d” simultaneous treatment, an additive elevation of the TPC was observed for male plants, while for females, the TPC value was lower vs. “d” and higher vs. “Cu” isolated treatments.
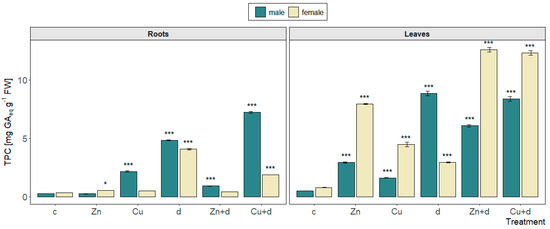
Figure 5.
Total phenolic content in male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control; data presented as mean ± SD; asterisks show significant differences between treatment and control: [*] p < 0.05, [***] p < 0.001).
In leaves, the TPC showed the lowest values for control plants of both sexes (<0.8 mg GAeq g−1 FW), while treatments led to their elevated accumulation up to 12.6 mg GAeq g−1 FW (“Zn + d” treatment of female plants) (Figure 5). Male specimens were characterized by lower TPC values compared to the females excluding drought simulation, for which the highest value among treatments was noted. On the contrary, female plants showed the lowest content of phenolics in leaves under drought stress compared to other treatments. For both sexes, isolated Zn addition caused a significantly higher accumulation of phenolics than Cu. In leaves of male specimens, combined metal and drought action caused an increase in the TPC vs. isolated metal additions; however, it did not exceed the level found in drought-treated plants. Contrastingly to male plants, female plants showed significantly higher values of the TPC in leaves for combined stressors (“Zn + d”, “Cu + d”) than for isolated metals or drought.
3.5.2. Profiling of Phenolic Metabolites
The profile of hydroxybenzoic acids (C6-C1), phenylpropanoids (C6-C3) and flavonoid (C6-C3-C6) structures in the leaves and roots of S. × fragilis varied between female and male specimens depending on the treatment. A detailed description of the absolute contents of phenolic metabolites is presented in Supplementary Materials (Table S3).
Considering relative changes in phenolic profile, roots of male plants showed relatively lower increases in particular phenolics than those of females (Figure 6a). The strongest induction of phenolic metabolism was noted under “d” and combined metal+”d” treatments. Among the structures, the highest increase compared to control was observed for flavonoids in the case of “Cu” and “Cu + d” treatments. Isolated metal additions and drought simulation induced catechin accumulation, while combined treatments depleted the effect.
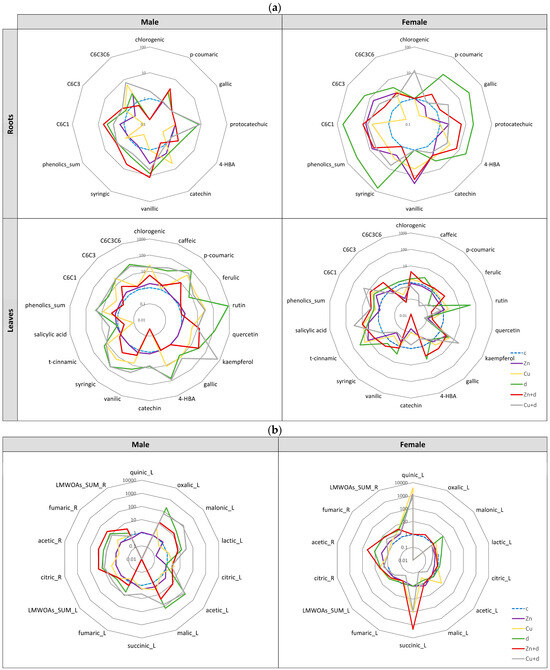
Figure 6.
Relative changes in profile of phenolic metabolites (a) and low-organic-weight organic acids (LMWOAs) (b) in male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control, L—leaves, R—roots, 4-HBA—4-hydroxybenzoic acid, C6-C1—hydroxybenzoic acids, C6-C3—phenylpropanoids, C6-C3-C6—flavonoids).
Roots of female plants showed the highest increase in the majority of phenolic metabolites when challenged with drought (excluding chlorogenic and vanillic acids), most pronounced for p-coumaric, gallic, protocatechuic, 4-HBA (4-hydroxybenzoic acid) and syringic acids (Figure 6a). This resulted in a highly elevated content of C6-C1 structures in relation to control plants. Vanillic acid was accumulated to a similar extent in the roots of females treated with “Zn” and “Zn + d”. The lowest induction of phenolics and a depletion in C6-C3 biosynthesis were observed in the case of “Cu” treatment. Catechin accumulation was induced under all applied treatments, most profoundly under drought simulation. In the roots of both male and female specimens, the “Cu + d” co-treatment increased the content of chlorogenic acid.
In leaves of male plants, most profiled compounds were induced by applied treatments, with the highest increase vs. control noted for drought simulation, “Cu + d” co-treatment, and isolated Cu addition (Figure 6a). The highest relative contents were noted for rutin, kaempferol, and phenolic acids (p-coumaric, 4-HBA, and syringic acids). Isolated Zn addition did not cause significant changes in phenolic profile, and in the case of “Zn + d”, an induction was noted for particular metabolites; however, this was lower than for “d” and combined “Cu + d” treatment. In contrast to roots, catechin content was reduced by “Zn + d” and “Cu” compared to the control.
In leaves of female plants, catechin content was depleted for nearly all treatments with the following decrease in C6-C3-C6 content, while a slight increase in flavonoids was noted only for drought simulation (Figure 6a). This was caused by the intense accumulation of rutin and kaempferol, also induced under “Cu” and “Cu + d”. Among phenolic acids, “d” and “Zn + d” treatments increased the content of vanillic acid (similarly to in the leaves), while the remaining additions restricted its accumulation. The content of trans-cinnamic acid was induced by drought simulation and isolated metal additions, and co-treatments lowered the induction effect. In contrast to male plants, salicylic acid was strongly accumulated under all treatments, and its relative content decreased in the following order: “Cu + d” > “Zn + d” ≥ “Cu” > “d” ≥ “Zn”.
3.5.3. Profiling of Low-Molecular-Weight Organic Acids
The profile of organic acids in the roots and leaves of S. × fragilis showed significant differences depending on the sex and applied treatment. A detailed description of absolute contents is presented in Supplementary Materials (Table S3).
Relative changes in organic acid profiles in response to treatments were strongly diverse between male and female plants (Figure 6b). In roots of male plants, a similar increase relative to control was noted for citric, acetic, and fumaric acids under “Zn + d” > “d” > “Cu + d” treatments. For females, an increased content was observed, particularly for acetic acid under “Zn + d” > “d” > “Zn”, while citric and fumaric acids were induced to a lesser extent in “d”- and metal+“d”-treated plants.
In leaves of males, treatments (excluding “Zn”) led to the induction of the majority of acids (oxalic, malonic, lactic, acetic, malic, and fumaric), predominantly in the case of “d” and metal + “d” treatments (Figure 6b). At the same time, quinic acid was suppressed, while succinic acid was induced to a lesser extent when compared to control plants. In contrast to males, in leaves of female plants, the accumulation of quinic acid was strongly induced by all treatments, excluding “Zn + d”, for which succinic acid was increased as the second affected acid. Additionally, the content of succinic acid was elevated for “Cu” and “Cu + d” treatments to a similar extent. Drought caused an increase in malonic acid content in the leaves of females, while the “Cu” treatment induced acetic acid accumulation.
3.6. Principal Components Analysis (PCA) and Simple Linear Correlations
The PCA analysis for all investigated parameters was reduced to ten for each axis, and the first two principal components explained 60.5% of the variability (Figure 7). The applied treatments were shown to differentiate the studied parameters compared to the control. Also, for isolated metal additions and drought simulation, the response was diverse and depended on the plant sex, predominantly in the case of drought. Combined metal and drought treatments caused similar changes to drought, proving its dominant effect on investigated parameters and increasing differentiation between male and female specimens. Considering correlations between variables, shoot biomass was strongly correlated with leaf RWC values and negatively related with Cu accumulation in leaves (Figure 7). Cu uptake to leaves was strongly correlated with the accumulation of LMWOAs and phenolic compounds (sum of profiled metabolites) and with the TPC in leaves and roots. On the contrary, the Zn level in roots was negatively correlated with the TPC. Disruption in mineral nutrient (Ca, Mg, K) uptake and translocation was negatively correlated with root biomass, and the reduction of root growth followed their increased content in leaves. A negative correlation was found between Cu content in leaves and mineral content (particularly Na) in roots. On the contrary, Na uptake to leaves is accompanied by an increased Cu level and the subsequent induction of metabolite accumulation in S. × fragilis leaves.
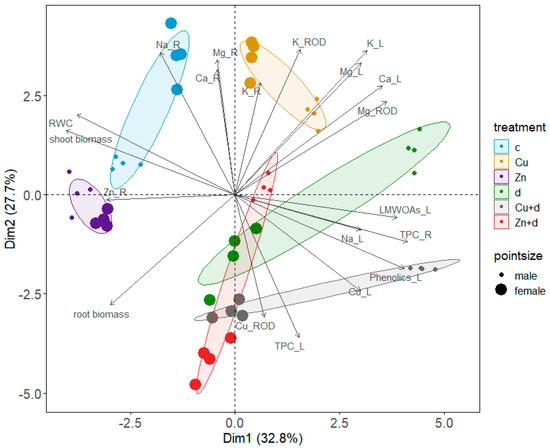
Figure 7.
Principal component analysis (PCA) of investigated parameters of male and female Salix × fragilis L. plants treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control; L—leaves, R—roots; RWC—relative water content, TPC—total phenolic content, Phenolics—sum of profiled phenolic metabolites, LMWOAs—sum of profiled low-molecular-weight organic acids). Only variables with high confidence were shown.
Linear correlation analysis performed for Cu and Zn accumulation and content of particular metabolites revealed positive and negative relations, reflecting the induction and suppression of their biosynthesis in treated plants. In the case of roots, Cu accumulation was negatively correlated with the content of 4-HBA (r = −0.55) in male specimens. For Zn accumulation, significant correlations were found only for female plants and concerned vanillic acids (r > 0.83) (Figure 8a).
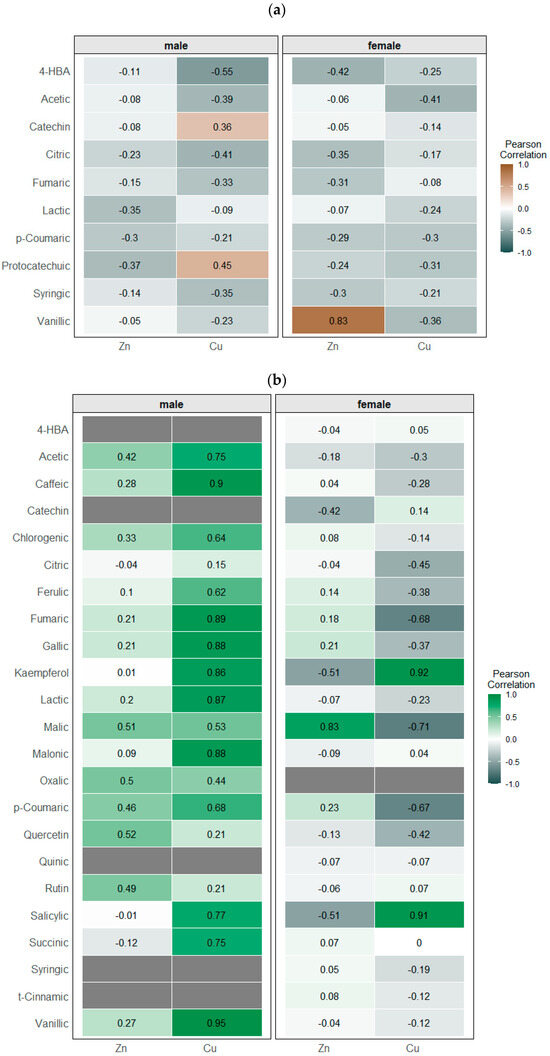
Figure 8.
Linear correlations between the content of Zn or Cu and profiled metabolites (phenolics and low-molecular-weight organic acids) in roots (a) and leaves (b) of Salix × fragilis L.
In leaves of male plants, Cu accumulation was significantly correlated with LMWOA content (r = 0.922 for sum, and r > 0.87 for malonic, lactic, and fumaric acids), while only malic and oxalic acids were correlated with Zn level in leaves (r = 0.50) (Figure 8b). Considering phenolic metabolites, the content of particular compounds was positively correlated with Cu accumulation in leaves, i.e., caffeic and vanillic acids (r > 0.90), and salicylic, chlorogenic, and ferulic acids (r > 0.62). Similar to LMWOAs, a limited number of phenolic compounds correlated with Zn accumulation, including rutin and quercetin (r > 0.49).
A significantly different pattern was observed for female plants (Figure 8b). Cu accumulation in leaves was accompanied by the increased biosynthesis of kaempferol and salicylic acid (r > 0.91), while a negative correlation was found for p-coumaric acid (r = −0.67) and among LWWOAs for malic and fumaric acids (r < −0.68). The opposite was noted for Zn content, for which negative correlations were found in the case of kaempferol and salicylic acid (r < −0.51), and positive correlations were found for malic acid content (r = 0.83).
4. Discussion
Plant growth is regulated by many factors, with particular emphasis on photosynthesis and transpiration. However, the critical nutrient is water, which makes up to 95% of plant tissue. Taken up by the roots, it determines plant photosynthesis by providing hydrogen, protects the plant from overheating, transports minerals and nutrients from the soil and within the plant, and generates turgor in cells [34]. Limited availability of water poses a serious threat, and a permanent deficit usually leads to ultimate plant death [35]. Drought is one of the main factors causing stress in plants, and accompanied by toxic elements in soil, it may contribute to the aggravation of unfavorable growth conditions [36]. However, the effect is related to the applied metal dose as documented for Quercus suber L. seedlings subjected to drought and additionally receiving Zn [37].
The present study revealed significant differences between male and female specimens of S. × fragilis in tolerance to Zn or Cu, drought, and combined metal and drought treatments. Considering isolated treatments, the presence of Zn enhanced plant growth, more pronouncedly in the case of females. Equimolar Cu treatment caused the diverse reaction of S. × fragilis, and females exhibited significantly higher tolerance to the metal than males, while for males, a strong reduction of biomass, both for shoots and roots, was observed. Male specimens were also less tolerant to drought, and consequently, to combined drought and metal presence. This observation confirmed previous studies on the impact of sex on mortality and biomass production of 127 willow taxa, including S. × fragilis [38]. Similar to the present study, the authors revealed differences in leaf traits (male specimens had longer and wider leaves) and adaptation to soil conditions (males showed higher biomass than females under favorable growth conditions, while at the poorer location, the mortality of males was higher than females). Considering metal and drought co-occurrence, the effect on plant growth depended on the applied metal, i.e., males were less tolerant to Cu, while females were less tolerant to Zn and drought combined treatments. Han et al. [39] also revealed increased sensitivity to metal (Pb) in both sexes of Populus cathayana under the reduced watering regime, but this was more the case in females. The occurrence of drought usually causes an increase in the sensitivity of plants to toxic elements, depending on the type of element [39,40]. In the case of poplar, a sister genus to a willow (Salicaceae), such observations were described by Xu et al. [41], and female individuals showed greater sensitivity to drought compared to male plants, which was primarily related to lower root sensitivity and their greater biomass in the case of males. In the present study, an induction of root growth was observed in females in response to applied treatments, contributing to their greater tolerance.
The differences in metal uptake at the sexual level were described earlier by Lin et al. [42], who indicated a clear differentiation of Cd uptake and distribution in Populus deltoides. Male plants contained higher amounts of Cd in leaves than in roots, while the opposite situation was described for females. In our study, both Zn and Cu accumulated mainly in S. × fragilis roots and Zn to a greater extent than Cu. Diverse metal accumulation at equimolar doses can be attributed to its characteristics but also to the ability of S. × fragilis to activate defense mechanisms aimed at limiting the metal uptake and translocation to avoid toxicity [43]. Moreover, males of S. × fragilis accumulated Zn in roots and Cu in leaves more efficiently than females, while Zn was translocated to leaves at a higher ratio by females. In another study [44], males of Populus cathayana were also characterized by a higher accumulation of Cu in leaves than females; however, this was accompanied by a higher tolerance to the metal.
The differentiation of metal uptake depending on water status is a very complex issue, especially in dioecious plant species [45,46]. According to Yang et al. [47], uptake ability is closely related to the organ and biomass of the plant, and in turn, to the amount of water available due to the transpiration rate [48]. Under isolated drought, the effective translocation of Cu and Zn from rod deposits both in female and male individuals was noted and indicated their higher demand for transition metals for the activation of enzymatic antioxidants to counteract the water stress [49,50]. Under metal or metal and drought co-treatments, the highest contents of Cu and Zn were found in roots, which clearly indicated the restricted transport of the metals to aerial plant parts [51,52]; however, this was modified by limited water availability. Drought limited Zn accumulation, mainly in roots by both sexes, and elevated its translocation to leaves. Surprisingly, in the case of Cu, limited water availability led to increased metal accumulation in roots > leaves > rod, predominantly in the case of females, showing considerably higher tolerance to the metal.
Among investigated mineral nutrients, potassium is characterized by high mobility and is easily transported to leaves. It is involved in the electrical neutralization of inorganic and organic anions and macromolecules, pH homeostasis, control of membrane electrical potential, activation of enzymes, protein synthesis, cell metabolism, and photosynthesis [53]. Potassium regulates the cell osmotic pressure and along with the accompanying anion, comprises the major, osmotically active solutes in the guard cells of open stomata [54]. In the present study, drought simulation caused a marked increase in the K content in the leaves of male individuals, which corresponded to their lower tolerance and influenced the accumulation and translocation of other minerals [55,56,57]. In contrast to males, K translocation was restricted by drought in females, and its deficiency-related symptoms were noted, including brown scorching and the curling of leaf tips and chlorosis [58]. In contrast to K, sodium (Na) is unessential for most plants, and its high concentrations in the cytoplasm result in deleterious effects on cell metabolism. In the investigated S. × fragilis, the intense transport of Na from rod deposits to leaves was noted for both sexes, probably to serve the function of osmoticum when compartmentalized outside the cytoplasm and accumulated at high concentrations in vacuoles [53].
The exposure of the plant to stress conditions activates an array of molecular and physiological mechanisms to reduce the negative effect of the stressor [59,60]. Among bioactive metabolites, phenolic compounds play an essential role in the protection, restoration, and degradation processes in plants challenged with toxic chemicals as adaptative or defensive mechanisms [61]. Phenolic compounds and both acids and flavonoids are well known for metal-chelating and radical-scavenging abilities, resulting from the presence of hydroxyl and carboxyl groups in their structures [62,63]. Our previous studies reported an increased biosynthesis of phenolics in tree species subjected to metal stress [64,65]; however, the variation between plants depending on sex has not been yet investigated. In the present study, phenolic compounds were quantified in S. × fragilis at higher contents and with a more diverse profile in photosynthetic tissue than in roots. The findings agree with other studies confirming higher phenolic content in leaves compared to roots or stems of a vast majority of higher plants [66]. As presented by Budny et al. [67], young shoots of different willow cultivars showed a high level and diversity of metabolites belonging to phenolic acids, flavonoids, and salicylates, which varied between cultivars planted within a studied area. The highest contents among phenolic metabolites determined in the investigated S. × fragilis were found for salicylic acid and reached 105 µg g−1 FW in the leaf tissue. Surprisingly, only female plants intensively accumulated the metabolite, and higher contents were noted for Cu-containing treatments compared to Zn. The Cu-triggered induction was confirmed by a high correlation coefficient for salicylic acid and Cu content in leaves (higher for females). This may reflect a higher tolerance toward Cu compared to male plants, despite the increased bioaccumulation of Cu under co-action with drought. The use of exogenic salicylic acid was found to be an efficacious strategy to improve the phytoremediation of heavy metals and plant growth under stress conditions, particularly under water deficiency. As previously documented, a combined treatment of plant-growth-promoting bacteria (PGPR) and salicylic acid increased metal accumulation in Helianthus annus by 21, 11, and 8% for Cu, Co, and Zn, respectively [13]. Chlorogenic acid was the second major compound determined in leaves of S. × fragilis. The metabolite is a well-known antioxidant with a crucial role in plant defense reactions [68,69,70]. A significant increase in its content was noted for female specimens under all treatments and for male specimens excluding isolated Zn addition. In the leaves of males, chlorogenic acid was positively correlated with Cu content; however, a lower Pearson’s coefficient value was found compared to salicylic acid, proving the effect of other stressors (Zn, drought) on its accumulation.
Along with antioxidant activity, phenolic acids are structural components of polymers building the cell wall, and the lignification process requires the induction of phenolic metabolism [71,72]. Gallic acid is a component of suberin, lignin, and polyphenolic barriers, while ferulic, p-coumaric, vanillic, and trans-cinnamic acids are part of monolignol, the building block of lignin polymers [71,72]. Ferulic acid also serves as a precursor for various chemically diverse phenylpropanoid derivatives and flavonoids [68,69]. The enhanced accumulation of lignin and flavonoid precursors was confirmed predominantly in leaves of males under drought, Cu, and combined drought and Cu treatments, and was correlated with Cu accumulation. In contrast to males, the content of the mentioned acids was not related or negatively correlated with Cu accumulation in the leaves of females. In the roots, the majority of phenolics were negatively correlated with Zn or Cu content, except for vanillic acid and Zn in females, proving a high affinity toward this metal.
Drought simulation also induced the biosynthesis of particular phenolics in leaves of S. × fragilis. According to Hura et al. [73], the increase in cell wall-bound phenolics under drought stress was an indicator of drought tolerance in plants. In the present study, drought and combined drought and metal treatments caused an increase mainly in flavonoid content and predominantly in male plants, and the accumulation of rutin was highly induced in the leaves of both sexes. As previously documented, drought increased the content of flavonoids in different plants [74,75], and stimulated flavonoid synthesis was a mediator in the homeostasis of reactive oxygen species [76]. In contrast to our results, in the bark of S. daphnoides Vill. and S. purpurea L. cultivated in pots and treated with three irrigation levels, particular flavonoids and phenolic acid derivatives were lowered under severe drought [77]. Similarly, in micropropagated plantlets of S. myrsinifolia and its interspecific hybrids, drought stress reduced the total content of salicylates and phenolic acids in a pure species, while in hybrids, only phenolic acids were decreased [78]. According to the authors, drought stress was clone-specific, and consequently, climatic changes may affect the genetic composition of forest habitats.
The second investigated group of metabolites, organic acids, act as intermediates in the energy cycle and are involved in element transport from roots to shoots and leaves [79,80]. Their metabolism is one of the key elements of plant tolerance to elevated concentrations of metals, including hazardous ones [81,82,83]; adaptation to nutrient deficiency; and response to other abiotic stressors, including the increasingly common drought stress [84,85]. In some species, the increased content of succinic, formic, oxalic, citric, or malic acids increased the ability of plants to adapt to increased levels of metals (Pb, Cd, and Ni) in soil [81,83,86], while malic, succinic, or citric acids increased plant tolerance to drought [87,88].
Magdziak et al. [22] described the content of organic acids in the roots and leaves of nine willow taxa, including the dioecious S. × smithiana. The examined taxa were cultivated in the control area (unpolluted) and at metal (Cu, Zn, and Pb)-contaminated sites. Roots of female S. × smithiana contained citric acid as a dominant acid, and its content significantly decreased at polluted sites. For female S. × fragilis, citric acid was also dominant; however, a significant increase in its content was noted for Cu- or Zn-treated plants and perpetuated by co-treatment with drought. The opposite pattern was found for males. Further, both sexes of S. × smithiana were characterized by increased content of fumaric acid in roots at the contaminated site. An increased content of the acid was observed for male S. × fragilis, while a decrease in its content was found for females treated with Zn or Cu. Additional drought stress contributed to a decrease in organic acid content in the roots of males and an increase in females, which corresponded with increased Cu uptake. The opposite was previously found for S. × smithiana, i.e., males accumulated Cu more efficiently than females, accompanied by increased contents of citric and fumaric acids [22]. In the present study, considering all treatments, correlation analysis revealed weak (p > 0.05) negative relations between Zn or Cu accumulation and the content of particular organic acids in roots, pointing to the unspecific formation of organic acids in the response of S. × fragilis to applied stressors.
In the leaf profile of S. × fragilis, oxalic, malonic, lactic, malic, citric, fumaric, and succinic acids were found regardless of the plant sex, and similar profiles were previously determined for other willow taxa [22]. Citric, malic, succinic, and fumaric acids were found in leaves of both sexes, and the occurrence of other acids depended significantly on the plant sex and the applied treatment. In previous studies, the content of citric acid increased in leaves of female S. × smithiana and decreased in males growing at polluted sites, while for S. × fragilis, the opposite pattern was found for metal-treated specimens. Considering correlation analysis, most of the profiled organic acids were strongly correlated with Cu content in the leaves of male plants, while for female plants, negative relations were found. Malic acid was correlated with the leaf Zn level in plants of both sexes, and oxalic acid was correlated with both Zn and Cu in the case of males.
Drought may also significantly affect the content of organic acids in plant organs [89]. Levi et al. [90] found that the accumulation of certain organic acids, including citric acid, may contribute to an increase in drought tolerance of some cotton genotypes. The assumptions presented for tobacco are partly valid for investigated S. × fragilis [84,90]. In the present study, the content of citric acid increased in the presence of metals, but a significant increase under drought stress was also noted. Further, the accumulation of acetic acid was elevated mainly under drought simulation and for metal and drought co-treatments.
A different profile for both sexes most likely resulted from individual differences in response to the given stress and different adaptations to the cultivation conditions, confirmed by differences in metal accumulation and tolerance [85]. The mechanisms of metal accumulation and detoxification have not been clearly explained, but the synthesis of organic acids may be a key factor for plant resistance [91]. Based on the recent results on willows, we can hypothesize that organic acids can serve as effective chelators of Zn and Cu [91]. Apart from the chelation and transporting role of organic acids, they represent important intermediates of the tricarboxylic acid (TCA) cycle, and their enhanced accumulation may reflect the elevated energy demand of S. × fragilis due to metal or water stress.
5. Conclusions
S. × fragilis showed a marked sensitivity to drought or a joint drought and metal presence. Differences in tolerance index, bioconcentration, and translocation factors for Zn and Cu indicated that males and females of S. × fragilis could be employed for different purposes in phytoremediation programs. Females are promising candidates for the phytostabilization of Cu under drought conditions, whereas males could be used for the phytostabilization of Zn under unlimited water supply, though under drought conditions, they may show phytoextractor properties toward Zn. Besides changes in Zn and Cu uptake, drought and applied metals led to a mineral nutrient imbalance affecting plant tolerance. Response to drought and applied metals shared both primary and secondary metabolic pathways; however, reaction patterns were stressor-specific and sex-dependent. For males, Cu accumulation in leaves was correlated with the majority of profiled metabolites, while for both sexes, kaempferol and salicylic acid were strongly correlated, proving their role in S. × fragilis tolerance against the metal. The described response of S. × fragilis in this study fits perfectly into the issue of transforming the utilization form of inactive flotation sediment reservoirs and remediating disturbed industrial sites. The ground rock, on which soil-forming processes occur successively, requires plants capable of uninterrupted growth in the case of drought as well as periodic inundation. The presence of numerous trace elements, including toxic heavy metals, with demonstrated varied tolerance among individuals of both genders, suggests the applied nature of the research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15030562/s1, Figure S1: Exemplary images presenting toxicity symptoms of leaves and roots of Salix × fragilis L. treated with zinc (“Zn”), copper (“Cu”), simulated drought (“d”) and under combined treatments (“Zn + d”, “Cu + d”) (“c”—control); Table S1: Metal content in in leaves and roots of male (M) and female (F) Salix × fragilis L. specimens cultivated hydroponically in a sand-stabilized system with the addition of zinc (Zn) or copper (Cu) and under simulated drought conditions (d) subjected to isolated and combined treatments (c—control; data presented as mean ±standard deviation, n = 3; p—empirical level of significance for “sex × treatment” fixed effect, identical superscripts denote no significant differences between means according to a post-hoc Tukey’s HSD test following two-way ANOVA at α = 95%); Table S2: Parameters of metal uptake by Salix × fragilis male (M) and female (F) specimens cultivated hydroponically in a sand-stabilized system with the addition of zinc (Zn) or copper (Cu) and under simulated drought conditions (d) subjected to isolated and combined treatments (c—control; data presented as mean ±standard deviation, n = 3; “-”—not calculated for variants without addition of particular metal; p—empirical level of significance for (i) “sex × treatment” fixed effect (identical superscript lettering indicates non-significant differences between mean values according to a post-hoc Tukey’s HSD test following a two-factor ANOVA analysis significant at α = 0.05, (ii) for non-significant ANOVAs, a Dunnett’s test was performed to compare the results with control for each sex separately, and p was presented as *** (<0.001), ** (<0.01) or * (<0.05)); Table S3: Profiling of phenolic compounds and low-molecular-weight organic acids [µg g−1 FW] in leaves and roots of male (M) and female (F) Salix × fragilis L. specimens cultivated hydroponically in a sand-stabilized system with the addition of zinc (Zn) or copper (Cu) and under simulated drought conditions subjected to isolated and combined treatments.
Author Contributions
Conceptualization, K.D.; methodology, K.D., M.G., Z.M. and P.N.; validation, K.D. and M.M.; formal analysis, K.D. and M.R.; investigation, K.D., M.G., Z.M. and S.B.; resources, K.D., P.N. and P.R.; data curation, K.D. and M.R.; writing—original draft preparation, K.D., M.G., Z.M., S.B. and M.R.; writing—review and editing, K.D.; visualization, K.D. and M.R.; supervision, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Müller, L.M.; Bahn, M. Drought legacies and ecosystem responses to subsequent drought. Glob. Chang. Biol. 2022, 28, 5086–5103. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fu, B.; Liu, Y.; Li, Y.; Wang, S.; Zhan, T.; Wang, Y.; Gao, D. Evaluation of ecosystem resilience to drought based on drought intensity and recovery time. Agric. For. Meteorol. 2022, 314, 108809. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.; Chen, D.; Cao, M.; Luo, J. Influence of drought stress and post-drought rewatering on phytoremediation effect of Arabidopsis thaliana. Bull. Environ. Contam. Toxicol. 2022, 108, 594–599. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Gor, B.K.; Desai, N.S.; Nikam, T.D.; Suprasanna, P. Sesuvium portulacastrum, a plant for drought, salt stress, sand fixation, food and phytoremediation. A review. Agron. Sustain. Dev. 2013, 33, 329–348. [Google Scholar] [CrossRef]
- Marchenko, A.M.; Kuzovkina, Y.A. Notes on the nomenclature and taxonomy of Salix fragilis (Salicaceae). Taxon 2022, 71, 721–732. [Google Scholar] [CrossRef]
- Clive, S. A S. × fragilis. In New Flora of the British Isles, 4th ed.; C & M Floristics: Stowmarket, UK, 2019; p. 348. [Google Scholar]
- Bita-Nicolae, C. Distribution of the riparian Salix communities in and around Romanian Carpathians. Diversity 2023, 15, 397. [Google Scholar] [CrossRef]
- Abdikaimova, T.; Dogan, I. Evaluation of heavy metal pollution along Alamedin River in Bishkek/Kyrgyzstan using woody species, Populus nigra (black poplar) and Salix fragilis (crack willow). 2022; Preprint. [Google Scholar] [CrossRef]
- Sharma, R.C.; Singh, N.B.; Tripathi, D.; Anil, S. Status and distribution of willow mortality in Lahaul and Spiti cold desert of Himachal Pradesh. Indian. For. 2011, 137, 196–204. [Google Scholar]
- Sharma, R.K.; Sharma, D. Drying willow (Salix fragilis L.) population under agroforestry system in cold desert region of Trans-Himalaya: A possible consequence of repeated vegetative propagation. Int. J. Ecol. Environ. Sci. 2022, 48, 119–125. [Google Scholar] [CrossRef]
- Henderson, L. Alien weeds and invasive plants. In Plant Protection Research Institute Handbook No 12; Agricultural Research Council: Pretoria, South Africa, 2001. [Google Scholar]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds; CAB International: Wallingford, UK, 2003; p. 548. [Google Scholar]
- Magdziak, Z.; Mleczek, M.; Rutkowski, P.; Goliński, P. Diversity of low-molecular weight organic acids synthesized by Salix growing in soils characterized by different Cu, Pb and Zn concentrations. Acta Physiol. Plant 2017, 39, 137. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and nickel co-treatment alters metal uptake and stress parameters of Salix purpurea × viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, B.A.; Budimir-Hussey, M.T.; Macfie, S.M. Production of organic acids and adsorption of Cd on roots of durum wheat (Triticum turgidum L. var. durum). Acta Physiol. Plant 2010, 32, 1063–1072. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Sidoli, C.M.D.; Reeves, R.D. The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour. Conserv. Recycl. 1994, 11, 41–49. [Google Scholar] [CrossRef]
- Weatherley, P.E.; Slatyer, R.O. Relationship between relative turgidity and diffusion pressure deficit in leaves. Nature 1957, 179, 1085–1086. [Google Scholar] [CrossRef]
- Baker, A.J. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Magdziak, Z.; Gąsecka, M.; Budka, A.; Goliński, P.; Mleczek, M. Profile and concentration of the low molecular weight organic acids and phenolic compounds created by two-year-old Acer platanoides seedlings growing under different As forms. J. Hazard. Mater. 2020, 392, 122280. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Gąsecka, M.; Magdziak, Z.; Budzyńska, S.; Szostek, M.; Niedzielski, P.; Budka, A.; Roszyk, E.; Doczekalska, B.; Górska, M.; et al. The possibility of using Paulownia elongata SY Hu × Paulownia fortunei hybrid for phytoextraction of toxic elements from post-industrial wastes with biochar. Plants 2021, 10, 2049. [Google Scholar] [CrossRef]
- Wickham, H. Elegant Graphics for Data Analysis: Ggplot2, Applied Spatial Data Analysis with R, Use R; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- McElrone, A.J.; Choat, B.; Gambetta, G.A.; Brodersen, C.R. Water uptake and transport in vascular plants. Nat. Educ. Knowl. 2013, 4, 6. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Atta, K.; Pal, A.K.; Jana, K. Effects of salinity, drought and heavy metal stress during seed germination stage in ricebean [Vigna umbellata (Thunb.) Ohwi and Ohashi]. Plant Physiol. Rep. 2021, 26, 109–115. [Google Scholar] [CrossRef]
- Disante, K.B.; Fuentes, D.; Cortina, J. Response to drought of Zn-stressed Quercus suber L. seedlings. Environ. Exp. Bot. 2011, 70, 96–103. [Google Scholar] [CrossRef]
- Konatowska, M.; Rutkowski, P.; Budka, A.; Goliński, P.; Szentner, K.; Mleczek, M. The interactions between habitat, sex, biomass and leaf traits of different willow (Salix) genotypes. Int. J. Environ. Res. 2021, 15, 395–412. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Zhang, X.; Korpelainen, H.; Li, C. Sexual differences in photosynthetic activity, ultrastructure and phytoremediation potential of Populus cathayana exposed to lead and drought. Tree Physiol. 2013, 33, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Liu, G.; Huang, G.; Dong, T.; Liao, Y.; Xu, X. Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environ. Exp. Bot. 2018, 146, 68–76. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.A.N.; Xiao, X.; Zhang, S.; Korpelainen, H.; Li, C. Sex-specific responses of Populus cathayana to drought and elevated temperatures. Plant Cell Environ. 2008, 31, 850–860. [Google Scholar] [CrossRef]
- Lin, T.; Tang, J.; He, F.; Chen, G.; Shi, Y.; Wang, X.; Han, S.; Li, S.; Zhu, T.; Chen, L. Sexual differences in above-and belowground herbivore resistance between male and female poplars as affected by soil cadmium stress. Sci. Total Environ. 2022, 803, 150081. [Google Scholar] [CrossRef] [PubMed]
- Utmazian, M.N.D.S.; Wieshammer, G.; Vega, R.; Wenzel, W.W. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ. Pollut. 2007, 148, 155–165. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Chen, F.; Korpelainen, H.; Li, C. The effects of exogenous putrescine on sex-specific responses of Populus cathayana to copper stress. Ecotoxicol. Environ. Saf. 2013, 97, 94–102. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Loganathan, P.; Sivakumaran, S.; Anderson, C.W.N.; McLaren, R.G. Bioavailability of copper and zinc to poplar and microorganisms in a biosolids-amended soil. Soil. Res. 2010, 48, 459–469. [Google Scholar] [CrossRef]
- Zimmer, D.; Kruse, J.; Baum, C.; Borca, C.; Laue, M.; Hause, G.; Meissner, R.; Leinweber, P. Spatial distribution of arsenic and heavy metals in willow roots from a contaminated floodplain soil measured by X-ray fluorescence spectroscopy. Sci. Total Environ. 2011, 409, 4094–4100. [Google Scholar] [CrossRef]
- Yang, W.D.; Wang, Y.Y.; Zhao, F.L.; Ding, Z.L.; Zhang, X.C.; Zhu, Z.Q.; Yang, X.E. Variation in copper and zinc tolerance and accumulation in 12 willow clones: Implications for phytoextraction. J. Zhejiang Univ. Sci. B 2014, 15, 788–800. [Google Scholar] [CrossRef]
- Santana, K.B.; de Almeida, A.-A.F.; Souza, V.L.; Mangabeira, P.A.O.; Silva, D.d.C.; Gomes, F.P.; Dutruch, L.; Loguercio, L.L. Physiological analyses of Genipa americana L. reveals a tree with ability as phytostabilizer and rhizofilterer of chromium ions for phytoremediation of polluted watersheds. Environ. Exp. Bot. 2012, 80, 35–42. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. In Water Stress and Crop Plants: A Sustainable Approach, 2 Volume Set; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 649–668. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Sun, H.; Chen, Y.; Pan, H.; Yang, X.; Rafiq, T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and transport of sodium and potassium in plants. The alkali metal ions: Their role for life. Met. Ions Life Sci. 2016, 16, 291–324. [Google Scholar] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Grabařová, S.; Martinková, M. Changes in mineral nutrition of Norway spruce (Picea abies [L.] Karst.) under the impact of drought. Ekologia 2001, 20, 46–60. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil. Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J.; Ogaya, R. Drought’s impact on Ca, Fe, Mg, Mo and S concentration and accumulation patters in the plants and soil a Mediterranean evergreen Quercus ilex forest. Biogeochemistry 2008, 87, 49–69. [Google Scholar] [CrossRef]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Ali, N.; Hadi, F. Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ. Sci. Pollut. Res. 2015, 22, 13305–13318. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef] [PubMed]
- Almughraby, E.; Kalimullin, M.I.; Timofeeva, O.A. Phytochemical composition and antioxidant activity in Brassica oleracea var. Sabellica under the effect of plant growth regulators. Eurasia J. Biosci. 2019, 13, 1037–1043. [Google Scholar]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Andrea Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, K.; Piechalak, A.; Goliński, P.; Gąsecka, M.; Magdziak, Z.; Szostek, M.; Budzyńska, S.; Niedzielski, P.; Mleczek, M. Differences of Acer platanoides L. and Tilia cordata Mill. response patterns/survival strategies during cultivation in extremely polluted mining sludge—A Pot Trial. Chemosphere 2019, 229, 589–601. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Gąsecka, M.; Budzyńska, S.; Drzewiecka, K.; Magdziak, Z.; Rutkowski, P.; Goliński, P.; Niedzielski, P. Copper, lead and zinc interactions during phytoextraction using Acer platanoides L.—A pot trial. Environ. Sci. Pollut. Res. 2022, 30, 27191–27207. [Google Scholar] [CrossRef]
- Uraguchi, S.; Watanabe, I.; Yoshitomi, A.; Kiyono, M.; Kuno, K. Characteristics of cadmium accumulation and tolerance in novel Cd-accumulating crops, Avena strigosa and Crotalaria juncea. J. Exp. Bot. 2006, 57, 2955–2965. [Google Scholar] [CrossRef]
- Budny, M.; Zalewski, K.; Stolarski, M.J.; Wiczkowski, W.; Okorski, A.; Stryiński, R. The phenolic compounds in the young shoots of selected willow cultivars as a determinant of the plants’ attractiveness to cervids (Cervidae, Mammalia). Biology 2021, 10, 612. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Facchini, P.J.; Hagel, J.; Zulak, K.G. Hydroxycinnamic acid amide metabolism: Physiology and biochemistry. Can. J. Bot. 2002, 80, 577–589. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Zoń, J. Significance of phenols in cadmium and nickel uptake. J. Plant Physiol. 2011, 168, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Lavid, N.; Schwartz, A.; Lewinsohn, E.; Tel-Or, E. Phenols and phenol oxidases are involved in cadmium accumulation in the water plants Nymphoides peltata (Menyanthaceae) and Nymphaeae (Nymphaeaceae). Planta 2001, 214, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mishra, S.; Bisen, K.; Singh, S.; Sarma, B.K.; Singh, H.B. Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway. Microbiol. Res. 2018, 207, 100–107. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Grzesiak, M.; Dziurka, K. The cell wall-bound phenolics as a biochemical indicator of soil drought resistance in winter triticale. Plant Soil. Environ. 2013, 59, 189–195. [Google Scholar] [CrossRef]
- Ballizany, W.L.; Hofmann, R.W.; Jahufer, M.Z.; Barrett, B.A. Multivariate associations of flavonoid and biomass accumulation in white clover (Trifolium repens) under drought. Funct. Plant Biol. 2012, 39, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, P.; Mayoral, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant 2016, 38, 9. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Förster, N.; Zander, M.; Ulrichs, C. Compound-specific responses of phenolic metabolites in the bark of drought-stressed Salix daphnoides and Salix purpurea. Plant Physiol. Biochem. 2020, 155, 311–320. [Google Scholar] [CrossRef]
- Turtola, S.; Rousi, M.; Pusenius, J.; Yamaji, K.; Heiska, S.; Tirkkonen, V.; Meier, B.H.; Julkunen-Tiitto, R. Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Glob. Chang. Biol. 2005, 11, 1655–1663. [Google Scholar] [CrossRef]
- Bhatia, N.P.; Walsh, K.B.; Baker, A.J. Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey. J. Exp. Bot. 2005, 56, 1343–1349. [Google Scholar] [CrossRef]
- Callahan, D.L.; Baker, A.J.; Kolev, S.D.; Wedd, A.G. Metal ion ligands in hyperaccumulating plants. J. Biol. Inorg. Chem. 2006, 11, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Zacchini, M.; Iori, V.; Scarascia Mugnozza, G.; Pietrini, F.; Massacci, A. Cadmium accumulation and tolerance in Populus nigra and Salix alba. Biol. Plant 2011, 55, 383–386. [Google Scholar] [CrossRef]
- Pietrini, F.; Iori, V.; Cheremisina, A.; Shevyakova, N.I.; Radyukina, N.; Kuznetsov, V.V.; Zacchini, M. Evaluation of nickel tolerance in Amaranthus paniculatus L. plants by measuring photosynthesis, oxidative status, antioxidative response and metal-binding molecule content. Environ. Sci. Pollut. Res. 2015, 22, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Han, H.; Cai, L.; Liu, A.; Ma, X.; Zhou, C.; Wang, G.; Meng, F. Pb stress effects on leaf chlorophyll fluorescence, antioxidative enzyme activities, and organic acid contents of Pogonatherum crinitum seedlings. Flora 2018, 240, 82–88. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Li, H.; Sivasithamparam, K.; Jones, M.G.; Du, X.; Ren, Y.; Wylie, S.J. Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress. Environ. Exp. Bot. 2017, 143, 59–71. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.; Babar, A. Role of sugars amino acids organic acids in improving plant abiotic stress tolerance Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.; Wang, D.; Sun, H.; Chen, Y.; Liu, J.; Jiang, Z. Woody species Rhus chinensis Mill. seedlings tolerance to Pb: Physiological and biochemical response. J. Environ. Sci. 2019, 78, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Naya, L.; Ladrera, R.; Ramos, J.; González, E.M.; Arrese-Igor, C.; Minchin, F.R.; Becana, M. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol. 2007, 144, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Yüce, M.; Neslihan Öztürk Gökçe, Z.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Paterson, A.H.; Cakmak, I.; Saranga, Y. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits. Physiol. Plant 2011, 141, 265–275. [Google Scholar] [CrossRef]
- Goliński, P.; Rutkowski, P.; Waliszewska, B.; Stolarski, M.; Czapiewski, G.; Szentner, K.; Gąsecka, M.; Magdziak, Z.; Mleczek, M. Poplars and Willows. In Cultivation, Applications and Environmental Benefits. Chapter 2: Salix: Properties and Practical Purposes; Nova Publishers: New York, NY, USA, 2016; pp. 29–73. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).