Abstract
Cadmium (Cd) is one of the most common toxic heavy metal elements in soil pollution, which can be continuously enriched in the food chain and eventually threaten human health. Phytoremediation, which is using plants to transfer heavy metal elements from soils, is a promising solution for the remediation of heavy metal-contaminated soils. In this study, we evaluated whether Cunninghamia lanceolata (Lambert) Hooker (Chinese fir), a widely planted timber tree worldwide, had the potential to remediate Cd-contaminated soils through 90 days pot of experiments with different Cd concentration soils (0, 5, 10, 20, 50, 100 mg kg−1). C. lanceolata did not show obvious toxic symptoms in Cd-contaminated soils, although Cd inhibited plant growth and decreased net photosynthetic rate slightly. The activities of antioxidant enzymes increased significantly under Cd stress, indicating that C. lanceolata had a strong self-regulation ability and can tolerate Cd stress. The Cd bioconcentration factor (Cd concentration in plant divided by Cd concentration in soil) of C. lanceolata were greater than 1 at all Cd concentrations, indicating that C. lanceolata had a strong ability to absorb Cd, although Cd was mainly accumulated in roots. Our results indicated that C. lanceolata had a strong tolerance and phytostabilization ability of Cd. Considering the wide distribution worldwide, large biomass, and rapid growth of C. lanceolata, it could be a promising candidate for phytoremediation of Cd-contaminated soils.
1. Introduction
With the process of industrialization and urbanization, the problem of heavy metal contamination in soils is becoming more and more serious [1,2]. Cadmium (Cd), which has high toxicity, large solubility in water, high mobility in the soil replacement system, and can be continuously enriched in the food chain, is one of the most hazardous heavy metals in the environment [3,4]. Once consumed by human beings, Cd will lead to chronic toxicity and pose a serious threat to human health, such as disrupting calcium metabolism, leading to hypercalciuria and the formation of kidney stones [5,6,7]. Cadmium pollution has become a worldwide problem that affects resources for food and drinking water mainly in Asia and Africa.
Phytoremediation is the use of plants to extract, sequester, and/or detoxify heavy metal elements and is a promising solution for the remediation of heavy metal contaminated soils due to its low cost, environmental friendliness, ease of operation, and wide applicability [8,9]. Phytoremediation can be divided into two main types, phytoextraction and phytostabilization. Phytoextraction refers to the uptake of soil heavy metals through the below-ground of the plant and accumulation in the above-ground, which is then collected and disposed of as hazardous waste or incinerated to recover the metals [10]. While phytostabilization reduces the mobility and bioavailability of heavy metals in the soil by immobilizing the metals at the plant rhizosphere through chelation (binding and adsorption) processes. The purpose of phytostabilization is to stabilize heavy metals and reduce the risk to human health and the environment, rather than remove heavy metals from polluted soil [11].
Plants used in phytoextraction that can accumulate high concentrations of heavy metals in the aboveground parts are called hyperaccumulators [12]. So far, hundreds of heavy metal hyperaccumulators have been identified, but most of them are herbaceous plants [13,14]. Although herbaceous plant species can accumulate high concentration of heavy metals, the total amount of heavy metals they can accumulate is limited due to their low biomass [15]. Furthermore, their low economic value and ease of entry into the food chain by livestock and poultry limited their application [16,17]. Fast-growing woody plants, which have the advantages of rapid growth, large biomass, well-developed roots, high tolerance to heavy metal stress, extensive uptake of different heavy metals, and do not enter the food chain of human beings [18,19], have the potential for phytostabilization of contaminated soil. In recent years, fast-growing woody plants such as Populus deltoides W.Bartram ex Marshall [20] and Salix viminalis L. [21] have been reported to be used for phytostabilization of heavy metal contaminated soil, but compared with herbaceous hyperaccumulators, there are few woody plants have been identified and applied for phytostabilization, indicating an area with significant potential for further research and application.
Cunninghamia lanceolata (Lambert) Hooker (Chinese fir), which is an evergreen sub-tropical conifer species with high timber yield, excellent timber quality, and rapid growth, accounted for 6.1% of the total global plantation area by 2015 [22]. In China, Chinese fir is the most widely planted species, with an area of 9.87 million hectares, accounting for 1/4 of the total plantation area in China [23,24]. Previous studies have found that Chinese fir could grow in heavy metal contaminated sites and have a good ability to develop and adapt under these conditions [25,26]. Given the wide distribution, substantial biomass, and rapid growth exhibited by C. lanceolata, these properties have attracted considerable interest in the remediation of contaminated soils. However, the tolerance and accumulation of Cd in C. lanceolata have been scarcely publicly reported. The aims of this study are: (1) estimate the Cd tolerance of C. lanceolata; (2) measure the accumulation ability of Cd in different C. lanceolata organs; (3) evaluate the feasibility of C. lanceolata for the remediation of Cd contaminated soils. The results will provide some theoretical basis and data reference for the further application of phytoremediation in cadmium-contaminated areas.
2. Materials and Methods
2.1. Plant Management and Growth Conditions
The experiment was conducted at the teaching and research station of Sichuan Agricultural University. Its geographical coordinates are 103°51′29″ E and 30°42′18″ N.
C. lanceolata seedlings were collected from the State-owned Forest Farm in Hongya County, Sichuan Province of China. Healthy seedlings with similar size at 1 year were selected and transplanted into the pots filled with nutrient-rich soil (1 seedling per pot) for 60 days of acclimatization. Seedlings were irrigated every 2–3 days and soil moisture was monitored using a HH2 soil moisture meter (ML2x, GBR) to maintain field water holding capacity at 80% of the saturated soil water capacity. Add 4 g of NPK compound fertilizers (15% N, 15% P2O5, 15% K2O) to each pot every 20 days to promote seedling growth.
The planting substrate was yellow soil collected from the same forest farm. Its chemical properties (5 replicates) were pH 5.75 ± 0.32, total nitrogen 4.62 ± 0.24 g kg−1, total potassium 15.63 ± 0.75 g kg−1, total phosphorus 1.45 ± 0.17 g kg−1, cadmium 0.36 ± 0.04 mg kg−1. The planting container was a polyethylene plastic pot with four drainage holes drilled in the bottom, 25 cm diameter at the top, 20 cm diameter at the bottom, 23 cm high, and the dry weight of the substrate in each pot was 10 kg.
2.2. Experimental Design
0, 10, 20, 50, and 100 mg Cd kg−1 (supplied as CdCl2 2.5H2O aqueous solution, the mass was calculated based on the molecular weights and the dry weight of the soil, then add enough water to dissolve it completely) were added to the soils, respectively. After the addition of cadmium solution, the substrate was thoroughly mixed to ensure homogeneity and kept in equilibrium for 30 days. After 60 days of acclimatization, seedlings were transplanted into the soil with a Cd concentration gradient. Five replicates were established for each treatment, and all treated plants were placed in a greenhouse. Water management as above, stop fertilizer application. Place a plastic tray at the bottom of the pot to collect any cadmium solution that may have leaked and pour it back into the soil. Once every half a month, the positions of each treatment in the greenhouse were randomly exchanged.
Height and ground diameter of all seedlings were measured before Cd treatment, and index measurement was carried out 90 d after Cd application. LI-6800 portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA) was used to measure gas exchange parameters. Leaves from seedlings were picked, sealed, and labeled, then placed in ice boxes and brought back to the laboratory for determination of chlorophyll content, physiological, and biochemical properties. Thereafter, seedling height and diameter were measured, followed by the harvest of seedlings for determination of cadmium content in organs of leaves, stems, and roots.
2.3. Determination of Plant Physiological and Biochemical Indices
2.3.1. Growth Traits
Seedling height (0.01 cm) was determined using a tape, and basal diameter (0.01 mm) was determined with a vernier caliper. Leaves, stems, and roots of seedlings were harvested, washed, and then dried at 80 °C to constant weight. Dry weights were measured for each organ.
2.3.2. Photosynthetic Parameters
Photosynthetic pigments content: the sampled leaves were wiped clean, cut, and immersed in an extract of 80% (v/v) acetone to extract photosynthetic pigments, and the mixture was placed in the dark and left for more than 24 h until the leaves were completely white [27]. Light absorbance was measured at 663, 645, and 470 nm using a spectrophotometer (UV-4802H, UNICO Inc. Shanghai, China), and the chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid (car) contents were calculated as described by [28]:
where Ca, Cb, and Cc represent the contents of chlorophyll a, chlorophyll b, and carotenoid, respectively, and A663, A645, and A470 represent the absorbances of the corresponding wavelength.
Ca = 12.71 × A663 − 2.59 × A645
Cb = 22.88 × A645 − 4.67 × A663
Cc = (1000 × A470 − 3.27 × Ca − 104 × Cb)/229
Gas exchange parameters: the gas exchange parameters were measured using a LI-6800 portable photosynthesis system. The CO2 concentration was set at 400 μmol mol−1, the leaf chamber temperature at 30 °C, the relative humidity at 60%, and the photosynthetic active radiation (PAR) at 800 μmol m−2 s−1. The net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) of the middle part of the second fully expanded leaves were measured between 9:00 and 11:30 AM. Three plants were randomly selected for each treatment, and three leaves were determined for each plant.
Chlorophyll fluorescence: the same LI-6800 portable photosynthesis system was adopted for measurement, and the settings and leaf sampling were the same as above. The sampled leaves were dark adapted for 1 h and then the minimal fluorescence level (F0) and maximum fluorescence level (Fm) were measured. Afterward, the actinic light was turned on and photo-activated until the fluorescence parameter was stable, and then the steady-state fluorescence (Fs), minimum fluorescence level in a light-adapted state (F0’), and maximum fluorescence level in a light-adapted state (Fm’) were measured. The following parameters were also measured or calculated simultaneously, Fv/F0: the ratio of photochemical to non-photochemical processes in PSII, Fv/Fm: the maximum photochemical efficiency of PSⅡ, Fv’/Fm’: the effective quantum yield, ΦPSⅡ: the actual quantum yield, ETR: the electron transfer rate, qP: the photochemical quenching coefficient, and NPQ: the non-photochemical quenching coefficient [29,30].
2.3.3. Determination of Antioxidant Parameters
0.3 g of fresh leaves were washed, cut, and ground in a mortar thoroughly with liquid nitrogen. The ground leaves were then homogenized with 10 mL phosphate buffer, followed by centrifugation at 10,000 r/min for 15 min at 4 °C, the supernatant was collected as the test sample. SOD was tested by evaluating the inhibition of nitro blue tetrazolium reduction [31]. One unit of SOD activity was considered as the amount of SOD activity that inhibits 50% of nitro blue tetrazolium in the photoreduction reaction (at 560 nm). CAT was assessed by measuring the reduction in H2O2 [32], and one unit of CAT activity was defined as a reduction of 0.1 units per minute at 240 nm. POD was assayed by the oxidation of guaiacol under H2O2 (at 470 nm) [33]. All antioxidant enzymatic activities were presented as U g−1 fresh weight (FW). The H2O2 content was determined using a method based on the formation of colored [TiO(H2O2)]2+ complexes (specific absorption peak of 410 nm) by H2O2 and titanium ions [34]. Free proline (Pro) was extracted with sulfosalicylic acid and determined by acidic ninhydrin colorimetric assay [35]. Determination of malondialdehyde (MDA) and soluble sugar content (SS) by heated colorimetric method using thiobarbituric acid [36]. The content or activities of all the above parameters were measured according to kit instructions (Jiancheng Bioengineering, Nanjing, China).
2.3.4. Concentration of Cd
The dried plant and soil samples (0.3 g) were crushed, ground, and passed through 1 mm sieve. These samples were then digested with 10 mL mixture of HNO3/HClO4 (4:1, v/v) at 170 °C [37], and the volume was brought to 50 mL with deionized water. The Cd contents in plant and soil were determined using a NexION 1000G ICP-mass spectrometer (Perkin Elmer Inc. Waltham, MA, USA). For quality assurance/control, the measured Cd values were verified using the GBW07603 (GSV-2; plants twigs and leaves) and GBW07428 (GSS-14; Sichuan basin soil) from the Center of National Standard Reference Materials of China (CNSRMC).
2.3.5. Calculation Formula
The root/shoot ratio was calculated according to the equations [38]: root/shoot ratio = Broot/Bshoot, where Broot and Bshoot was the root biomass and shoot biomass. The phytoextraction ability was determined using the translocation factor (TF) and the bioconcentration factor (BCF) according to the equations [39]: TF = Cshoot/Croot, BCF = Cshoot/Csoil or Croot/Csoil, where Cshoot, Croot, and Csoil was Cd concentration (mg kg−1) in the shoot, root, and soil, respectively.
2.4. Statistical Analysis
Significant differences between groups were determined by one-way analysis of variance (ANOVA) with Duncan’s test, which was carried out using SPSS 27 (IBM Inc., Chicago, IL, USA), and means and standard errors were also calculated using SPSS 27. Using Origin 2022 (Origin Lab Inc., Northampton, MA, USA) for correlation analysis and graph drawing.
3. Results
3.1. The Growth and Cd Tolerance of C. lanceolata
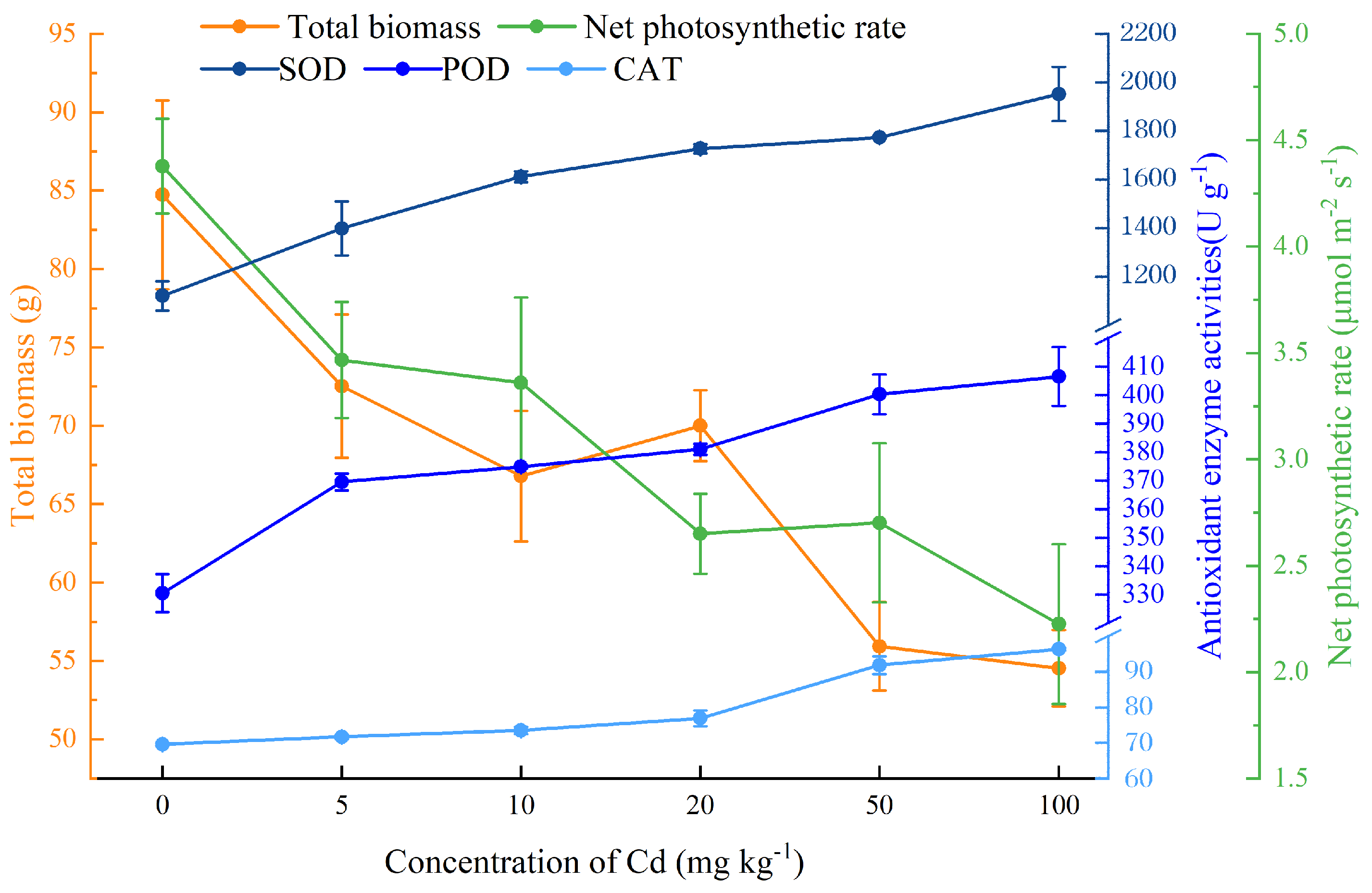
In this study, we found that the total biomass and Pn (net photosynthetic rate) of C. lanceolata decreased with the increase of Cd concentration in soil, while the activities of antioxidant enzymes SOD (superoxide dismutase), POD (peroxidase), and CAT (catalase) increased (Figure 1).
Figure 1.
Main tolerance parameters of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations. Error bars show the standard error of the mean values (n = 5).
Specifically, the addition of Cd significantly inhibited the height, basal diameter, and biomass of C. lanceolata (p < 0.05), while the effect was slight when Cd concentration was low (Table 1). For example, plant height was not significantly affected when the concentration of Cd was less than 10 mg kg−1. Cd had no significant effect on the growth of basal diameter when the concentration of Cd was less than 50 mg kg−1. Both root and shoot biomass were reduced by Cd stress, but the biomass of any given organs was not significantly influenced when the Cd concentration was less than 20 mg kg−1, which indicated that low concentrations of Cd had a slight effect on the growth of C. lanceolata.

Table 1.
Growth of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations.
Chlorophyll content (chlorophyll a, chlorophyll b) and Pn decreased significantly with the increase of Cd (Table 2). When the Cd concentration of soil was more than 50 mg kg−1, the contents of chlorophyll a, chlorophyll b, and total chlorophyll were significantly reduced. Gs, Tr, and ΦPSII also decreased significantly with the increase of Cd, while Ci, Fv/Fm, and Fv’/Fm’ did not change significantly (Table 2).

Table 2.
Photosynthetic parameters of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations.
H2O2 increased significantly with the increase of soil Cd concentration (p < 0.05), and H2O2 content was 2.18 times higher than that of the control when soil Cd concentration was 100 mg kg−1 (Table 3). As a product of lipid peroxidation, the content of MDA increased slightly with the aggravation of Cd stress, however, there was no significant effect on the content of MDA when the soil Cd content was lower than 50 mg kg−1. Similarly, to the antioxidant enzymes (SOD, POD, and CAT), the contents of proline and soluble sugar were also increased with the increase of Cd concentrations (Table 3).

Table 3.
The oxidative stress markers and antioxidant enzyme activities of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations.
3.2. Cd Uptake and Accumulation Characteristics of C. lanceolata
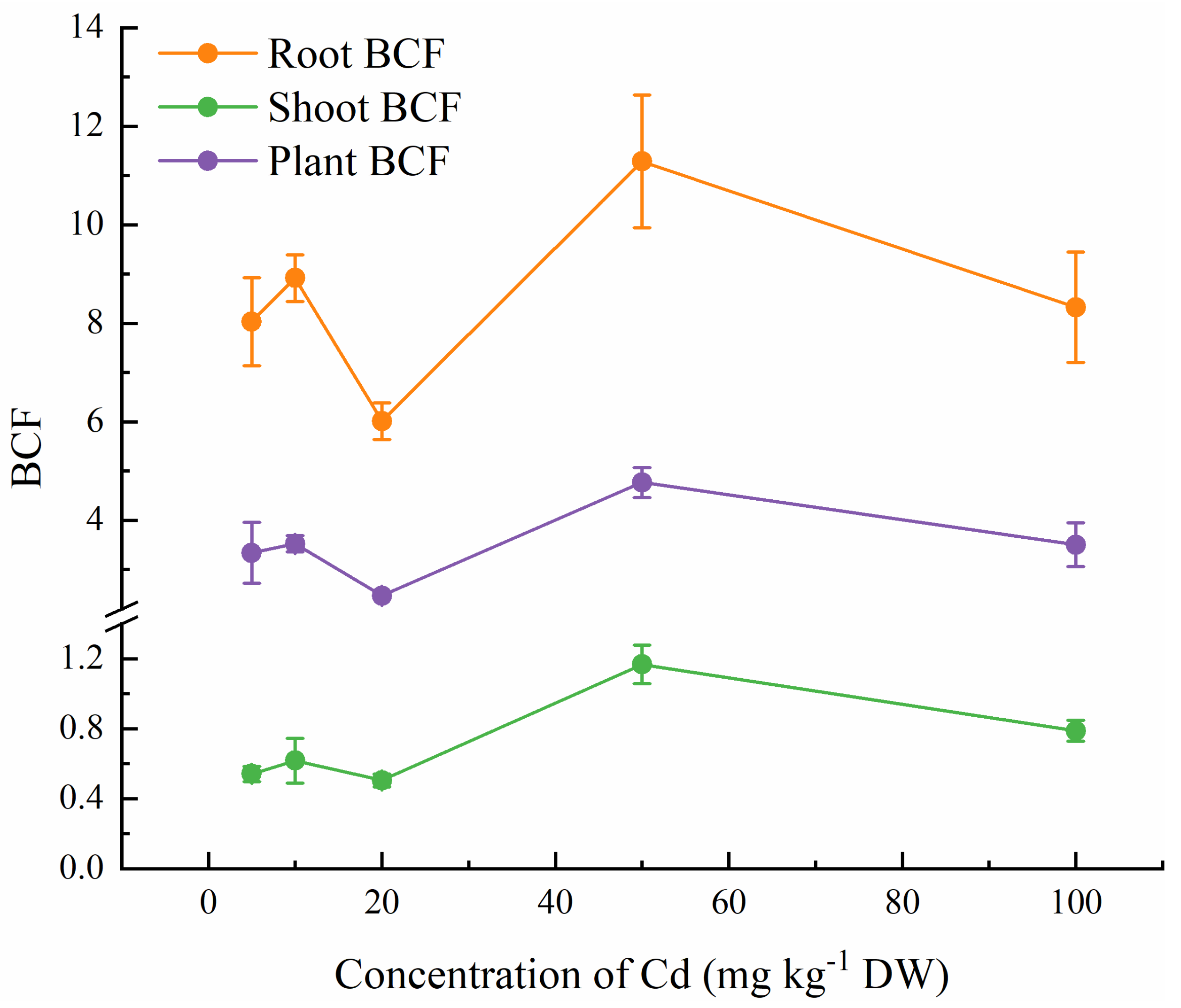
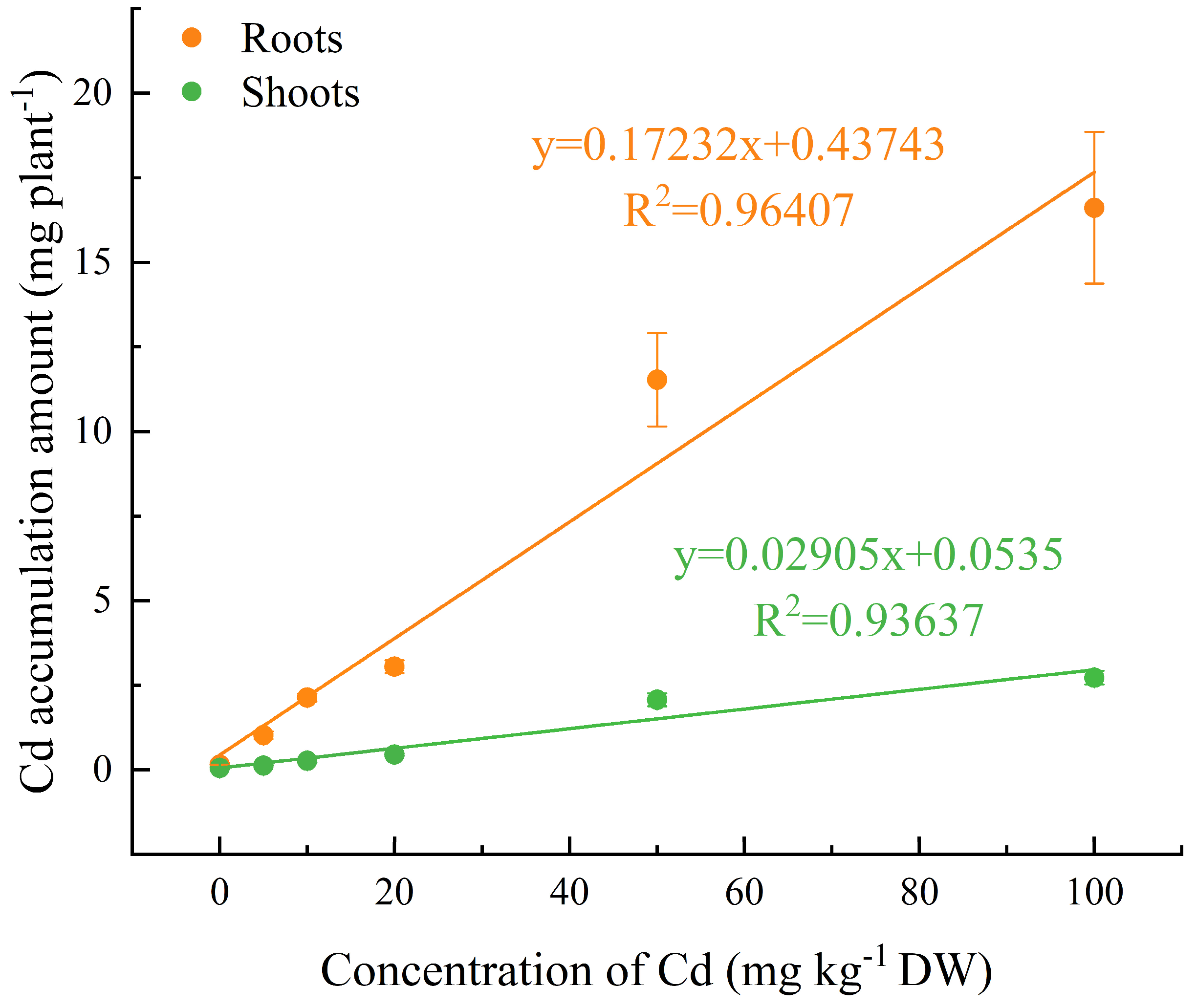
The Cd contents in stems, leaves, roots, and shoots of C. lanceolata seedlings increased significantly with the increase of soil Cd content, particularly when exceeding 20 mg kg−1. The Cd contents in roots were much higher than those in other organs and the bioconcentration factor (BCF) of C. lanceolata roots was 6.01–11.29 (Table 4, Figure 2). Under different soil Cd concentrations, the bioconcentration factor (BCF) of C. lanceolata plants was lager than 1 (2.47–4.77) and showed slight variation (Figure 2). The Cd accumulation amounts in shoots and roots of C. lanceolata seedlings increased linearly with the increase of Cd concentration in soil (r2 for shoots = 0.9364, r2 for roots = 0.9641; Figure 3). When the soil Cd concentration reached 100 mg kg−1, the total Cd accumulation amount in shoots and roots were 2.72 mg plant−1 and 19.34 mg plant−1, respectively (Figure 3). This study also found that the translocation factor (TF) of C. lanceolata did not vary significantly with the increase of Cd in soils (Table 4).

Table 4.
Cadmium accumulation characteristics on dry base of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations.
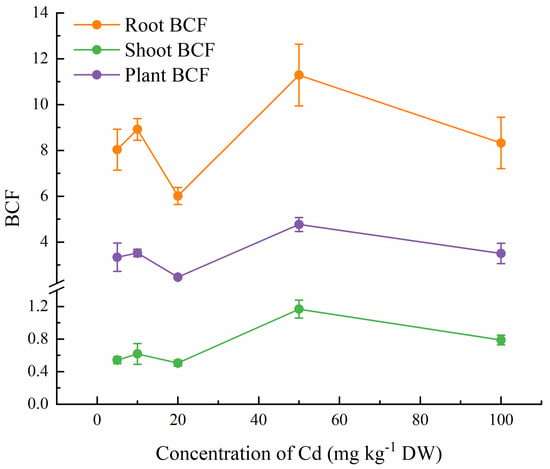
Figure 2.
BCF of C. lanceolata seedlings at different soil Cd concentrations. Error bars show the standard error of the mean values (n = 5).
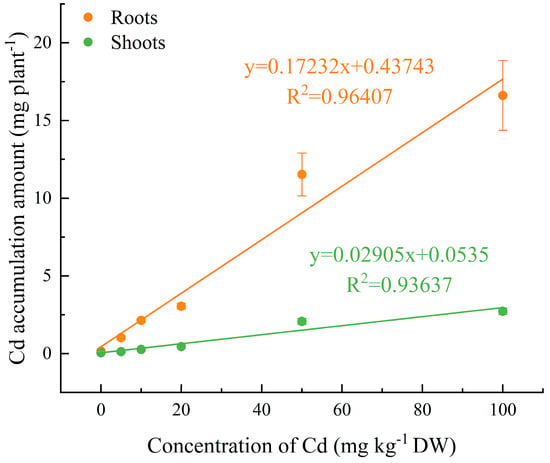
Figure 3.
Cd accumulation amount in shoots and roots of Cunninghamia lanceolata (Lambert) Hooker seedlings at different soil Cd concentrations. Error bars show the standard error of the mean values (n = 5).
4. Discussion
Phytoremediation, the use of plants for environmental cleanup, has been accepted as a cost-effective and environmentally friendly remediation strategy [40]. Cd is highly toxic to plants and animals and is one of the most common metal elements that has contaminated large areas of soils [4]. When the concentration of Cd in the soil exceeds 8 mg kg−1, growth retardation and other toxic symptoms are easily observed in most plant species [41]. When the concentration of Cd in soil reached 25 mg kg−1, the growth of most Cd accumulators and hyperaccumulators decreased significantly [34,42], due to Cd destroying plant roots, reducing the absorption of water and nutrients [43,44]. In this study, we found that although the growth of C. lanceolata seedlings decreased under Cd stress, the seedlings did not show obvious toxic symptoms during the experimental period (Supplementary Figure S1). In addition, the root and shoot biomass showed some variations at soil Cd concentrations from 0–20 mg kg−1, but not significantly, indicating that low concentrations of soil Cd did not inhibit the growth of C. lanceolata too much.
The growth and development of plants are mainly modulated by photosynthesis in plants [35]. Many studies have shown that Cd would accelerate the degradation of chlorophyll, reduce stomatal opening, decrease electron transfer rate, and finally affect photosynthetic capacity [9,34,45]. Although chlorophyll a, chlorophyll b, and Pn in C. lanceolata leaves gradually decreased with the increase of soil Cd concentrations, the Pn did not decrease significantly (Table 2) when the soil Cd concentrations were lower than 10 mg kg−1. In this study, the Gs, Tr, and Ci of C. lanceolata decreased significantly, and the decrease of Gs, Tr, and Ci were other possible reasons that limited Pn (called stomatal factor limitation) [46]. Similar results were found in Morus alba L. [34], Prosopis juliflora (Swartz) DC. [47], and Sassafras tzumu (Hemsl.) Hemsl. [48]. The values of Fv/Fm in most plant species are usually between 0.75 and 0.85 [49,50], which indicates a high photochemical activity of PSⅡ in C. lanceolata, Fv/Fm decreased slightly under Cd stress, but were all greater than 0.80 and did not differ significantly between treatments. The results revealed that Cd stress had a slight influence on photosynthetic apparatus and supported that the stomatal factor was the main reason for the decrease in Pn. ΦPSⅡ is the proportion of absorbed light energy actually used for photochemistry, and qP indicates the proportion of PSⅡ reaction centers that are open [51]. The results showed that ΦPSⅡ decreased significantly with the increase of Cd concentration, indicating that less and less absorbed light energy was used for photochemistry at the reaction center of PSII.
H2O2 is a potent signaling molecule with multiple roles in plant antioxidant defense, such as causing systemic acquired resistance (SAR) and hypersensitive resistance (HR) to improve their immunity [52]. In this study, the H2O2 content increased gradually with the increase of Cd concentration, indicating that C. lanceolata seedlings improved their resistance to Cd stress by inducing the production of H2O2. MDA is a representative index to measure lipid peroxidation induced by oxidative stress [53]. Cd stress caused a gradual increase of MDA content in C. lanceolata seedlings, but it was significantly higher than the control only when the Cd concentration was 100 mg kg−1, indicating that low concentrations of Cd did not cause oxidative stress on C. lanceolata seedlings.
Heavy metal stress will lead to the increase of ROS content such as superoxide anion (O2−), which will cause oxidative damage to biomolecules like lipids, proteins, and nucleic acids. Antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), can be improved by excess ROS and facilitate the removal of ROS [54]. Among these enzymes, SOD serves as the first line of defense, which converts O2− into H2O2 and O2; subsequently, H2O2 decomposes into H2O by the action of CAT, the main H2O2 scavenging enzyme [55,56]. POD is considered to be the last line of defense for H2O2 removal [57]. SOD, CAT, and POD activity in C. lanceolata leaves increased gradually with the increase of Cd concentration. Similar results were found in Salix matsudana Koidz. [58], Populus × euramericana (Dode) Guinier [59], and Populus yunnanensis Dode [60]. The content of proline and soluble sugar also increased significantly under Cd stress. Proline and soluble sugar are important compatible solutes in osmoregulation, which enhances plant tolerance to heavy metal stress through different mechanisms such as cellular osmotic adjustment, membrane integrity protection, quenching singlet oxygen atoms, metal chelation in the cytoplasm, and protection of enzymes against denaturation [61,62]. The increases in proline and soluble sugar indicated that Cd stress induced the synthesis of osmolytes in C. lanceolata leaves to maintain osmotic balance and enhanced resistance to stress.
Except tolerance, absorption, and translocation abilities of metal elements are other criteria for determine whether a plant species can be used in phytoremediation. BCF, which describes the ability of plants for heavy elemental accumulation from the substrate, is an important parameter for judging the phytoremediation potential of a given plant [63,64]. In our study, the BCF values of C. lanceolata were around 4, indicating that C. lanceolata has a strong capacity for Cd uptake and accumulation, as previously reported Cd hyperaccumulators usually had a value of BCF from 1.5–5, such as Populus deltoides [20] is 3.0, Youngia japonica (L.) DC. [65] is 5.2, Juncus bufonius L. [38] is 3.9. TF, which assesses the ability of plants to transfer heavy metals from roots to shoots, is another commonly used parameter to evaluate the phytoremediation potential of a given plant [66,67]. In C. lanceolata, the TF values under Cd stress ranged from 0.07–0.11, which implied that C. lanceolata has a weak ability to transfer Cd from roots to shoots. However, lower TF values imply a low shoots Cd content, which can prevent wildlife exposure and surface contamination, which is one of the candidate plant criteria for phytostabilization [68]. Besides, Fan [69] and Taamalli [70] hypothesized that plants restricted the translocation of Cd to the aboveground parts to avoid damage to the aboveground parts and maintain normal plant growth. Yoon et al. [71] found that most plants growing on a contaminated site in North Florida had high BCF values but low TF values, but suggested that these plants still had high potential for phytoremediation and could be considered as potential phytoremediation plants. Considering the high BCF and rapid growth of C. lanceolata, it still has great potential for Cd phytoremediation.
The soil Cd concentrations in most polluted areas generally do not exceed 50 mg kg−1 [72,73]. When the soil Cd concentration was below 50 mg kg−1, C. lanceolata seedlings did not show significant biomass reduction or visible symptoms of toxicity as well as high physiological and biochemical tolerance, allowed C. lanceolata to produce large amounts of biomass and grow rapidly enough to establish vegetative cover in time at a specific location. In the field, the huge canopy of C. lanceolata helps to reduce eolian dispersion, while the dense and deep root system helps to prevent water erosion and leaching, which creates a vegetative cap to achieving long-term stabilization of Cd-contaminated soil [23,68]. These findings indicated that C. lanceolata is a promising candidate for phytostabilization of Cd-contaminated soils, especially in non-severely contaminated soils with Cd concentrations below 50 mg kg−1.
Our findings offer compelling evidence of Cd tolerance and accumulation in C. lanceolata, suggesting its potential as a candidate for remediating Cd contaminated soils. However, it is important to note some limitations in our study. Our study was carried out on pot experiments, this can diminish susceptibility to environmental factors and experimental errors, thereby enabling the acquisition of objectively reliable data that can be replicated and compared. However, pot experiments may not fully replicate the intricate interactions and dynamic factors present in natural flora environments. Consequently, future research endeavors should extend beyond pot experiments to field studies in natural areas. Furthermore, a more in-depth investigation into the physiological and genetic mechanisms underlying the tolerance to and accumulation of Cd in C. lanceolata is warranted.
5. Conclusions
Cunninghamia lanceolata had high tolerance to Cd at physiological (e.g., photosynthesis characteristics and chlorophyll fluorescence parameters) and biochemical levels (e.g., oxidative stress markers and antioxidant enzyme), with strong Cd accumulation (predominance in roots). Considering the wide distribution, fast growth, large biomass, and high economic value, we suggested that C. lanceolata could be a promising candidate for the phytostabilization of Cd contaminated soils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15010115/s1.
Author Contributions
This idea was developed by H.H. and D.D. led the writing of the manuscript, with critical input from J.W., H.C., G.C. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Breeding Research Project of Sichuan Province during the 13th Five-year Plan period (2016 NZ0098-10), Key Project of Sichuan Education Department (13ZA0246).
Data Availability Statement
Data presented in this study are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Cd, cadmium; BCF, bioconcentration factor; CAT, catalase; Car, carotenoids; Chl, chlorophyll; Pn, net photosynthetic rate; Gs, stomatal conductance; Tr, transpiration rate; Ci, intercellular CO2 concentration; Fv/Fm, maximal quantum yield of PSII; Fv’/Fm’, photochemical efficiency of PSII; ΦPSII, Actual photochemical efficiency of PSII; H2O2, hydrogen peroxide; MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; TF, translocation factor.
References
- Chileshe, M.N.; Syampungani, S.; Festin, E.S.; Tigabu, M.; Daneshvar, A.; Odén, P.C. Physico-Chemical Characteristics and Heavy Metal Concentrations of Copper Mine Wastes in Zambia: Implications for Pollution Risk and Restoration. J. For. Res. 2020, 31, 1283–1293. [Google Scholar] [CrossRef]
- Chai, L.; Li, H.; Yang, Z.; Min, X.; Liao, Q.; Liu, Y.; Men, S.; Yan, Y.; Xu, J. Heavy Metals and Metalloids in the Surface Sediments of the Xiangjiang River, Hunan, China: Distribution, Contamination, and Ecological Risk Assessment. Environ. Sci. Pollut. Res. 2017, 24, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128148655. [Google Scholar]
- Rizwan, M.; Meunier, J.D.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon Alleviates Cd Stress of Wheat Seedlings (Triticum turgidum L. Cv. Claudio) Grown in Hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Abdelkrim, S.; Jebara, S.H.; Jebara, M. Antioxidant Systems Responses and the Compatible Solutes as Contributing Factors to Lead Accumulation and Tolerance in Lathyrus sativus Inoculated by Plant Growth Promoting Rhizobacteria. Ecotoxicol. Environ. Saf. 2018, 166, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiang, J.; Gao, J.; Zhang, J.; Zeng, L.; Cai, M.; Zhang, J. Evaluation of Cadmium Hyperaccumulation and Tolerance Potential of Myriophyllum aquaticum. Ecotoxicol. Environ. Saf. 2020, 195, 110502. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil-Plant Interface: Phytoavailability, Translocation, and Phytoremediation—A Review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Cao, X.; Xiao, X.; Liu, Y.; Shi, L. Phytostabilization Potential of Ornamental Plants Grown in Soil Contaminated with Cadmium. Int. J. Phytoremediat. 2018, 20, 311–320. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-up of Polluted Environment? Front. Plant Sci. 2018, 871, 1476. [Google Scholar] [CrossRef]
- Radziemska, M.; Koda, E.; Bilgin, A.; Vaverková, M.D. Concept of Aided Phytostabilization of Contaminated Soils in Postindustrial Areas. Int. J. Environ. Res. Public Health 2018, 15, 24. [Google Scholar] [CrossRef]
- Forte, J.; Mutiti, S. Phytoremediation Potential of Helianthus annuus and Hydrangea paniculata in Copper and Lead-Contaminated Soil. Water Air Soil Pollut. 2017, 228, 77. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal Tolerance and Hyperaccumulation: Costs and Trade-Offs between Traits and Environment. Environ. Exp. Bot. 2010, 68, 1–13. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of Metal and Metalloid Trace Elements: Facts and Fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- January, M.C.; Cutright, T.J.; Keulen, H.; Van Wei, R. Hydroponic Phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus Hyperaccumulate Multiple Heavy Metals? Chemosphere 2008, 70, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Z.Z. Phytoremediation of Soil Co-Contaminated with Heavy Metals and Deca-BDE by Co-Planting of Sedum Alfredii with Tall Fescue Associated with Bacillus cereus JP12. Plant Soil 2014, 382, 89–102. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Xia, X.; Chu, J.; Wei, Y.; Shi, S.; Chang, E.; Yin, W.; Jiang, Z. The Evaluation of Heavy Metal Accumulation and Application of a Comprehensive Bio-Concentration Index for Woody Species on Contaminated Sites in Hunan, China. Environ. Sci. Pollut. Res. 2014, 21, 5076–5085. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction Potential of Pteris vittata L. Co-Planted with Woody Species for As, Cd, Pb and Zn in Contaminated Soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef]
- Suo, Y.; Tang, N.; Li, H.; Corti, G.; Jiang, L.; Huang, Z.; Zhang, Z.; Huang, J.; Wu, Z.; Feng, C.; et al. Long-Term Effects of Phytoextraction by a Poplar Clone on the Concentration, Fractionation, and Transportation of Heavy Metals in Mine Tailings. Environ. Sci. Pollut. Res. 2021, 28, 47528–47539. [Google Scholar] [CrossRef]
- Tőzsér, D.; Magura, T.; Simon, E. Heavy Metal Uptake by Plant Parts of Willow Species: A Meta-Analysis. J. Hazard. Mater. 2017, 336, 101–109. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Daryanto, S.; Guo, S.; Huang, Z.; Wang, Z.; Wang, L.; Ma, X. Responses of Chinese Fir and Schima superba Seedlings to Light Gradients: Implications for the Restoration of Mixed Broadleaf-Conifer Forests from Chinese Fir Monocultures. For. Ecol. Manag. 2018, 419–420, 51–57. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Gilani, M.M.; Zou, X.H.; Wu, P.F. Chinese Fir (Cunninghamia lanceolata) a Green Gold of China with Continues Decline in Its Productivity over the Successive Rotations: A Review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- State Forestry Administration. General Situation of Forest Resources in China—The 9th National Forest Inventory; State Forestry Administration: Beijing, China, 2019.
- Sun, L.; Liao, X.; Yan, X.; Zhu, G.; Ma, D. Evaluation of Heavy Metal and Polycyclic Aromatic Hydrocarbons Accumulation in Plants from Typical Industrial Sites: Potential Candidate in Phytoremediation for Co-Contamination. Environ. Sci. Pollut. Res. 2014, 21, 12494–12504. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lei, L.; Yang, Q. Pb, Cu Botanogeochemical Anomalies and Toxic Effects on Plant Cells in Pb-Zn (Sn) Ore Fields, Northeast Guangxi Autonomous Region, China. Chin. J. Geochem. 2007, 26, 329–332. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, D.C.; Ma, Y.F.; Yin, Y.; Shen, S.D. Antioxidative Defense Response of Ulva prolifera under High or Low-Temperature Stimulus. Algal Res. 2019, 44, 101703. [Google Scholar] [CrossRef]
- Bayçu, G.; Moustaka, J.; Gevrek, N.; Moustakas, M. Chlorophyll Fluorescence Imaging Analysis for Elucidating the Mechanism of Photosystem II Acclimation to Cadmium Exposure in the Hyperaccumulating Plant Noccaea caerulescens. Materials 2018, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of Antioxidants to Paraquat in Pea Leaves: Relationships to Resistance. Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef]
- Beers, R., Jr.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Merey, H.A.; Ramadan, N.K.; Diab, S.S.; Moustafa, A.A. Validated UPLC Method for the Determination of Guaiphenesin, Oxeladin Citrate, Diphenhydramine, and Sodium Benzoate in Their Quaternary Mixture Used in Treatment of Cough, in the Presence of Guaiphenesin-Related Substance (Guaiacol). Chem. Pap. 2018, 72, 2247–2254. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Xu, Z.; Wang, Y.; Teng, Z.; An, M.; Zhang, Y.; Zhu, W.; Xu, N.; Sun, G. Toxic Effects of Heavy Metals Pb and Cd on Mulberry (Morus alba L.) Seedling Leaves: Photosynthetic Function and Reactive Oxygen Species (ROS) Metabolism Responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Alam, P.; Kohli, S.K.; Al Balawi, T.; Altalayan, F.H.; Alam, P.; Ashraf, M.; Bhardwaj, R.; Ahmad, P. Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity. Plants 2020, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Guo, Z.; Li, R.; Ali, A.; Guo, D.; Lahori, A.H.; Wang, P.; Liu, X.; Wang, X.; Zhang, Z. Screening of Chinese Mustard (Brassica juncea L.) Cultivars for the Phytoremediation of Cd and Zn Based on the Plant Physiological Mechanisms. Environ. Pollut. 2020, 261, 114213. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, J.; Wan, H.; Zhuang, X.; Li, H.; Qin, S.; Lyu, D. Rootstock–Scion Interaction Affects Cadmium Accumulation and Tolerance of Malus. Front. Plant Sci. 2020, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Chang Kee, J.; Gonzales, M.J.; Ponce, O.; Ramírez, L.; León, V.; Torres, A.; Corpus, M.; Loayza-Muro, R. Accumulation of Heavy Metals in Native Andean Plants: Potential Tools for Soil Phytoremediation in Ancash (Peru). Environ. Sci. Pollut. Res. 2018, 25, 33957–33966. [Google Scholar] [CrossRef] [PubMed]
- Tanhan, P.; Kruatrachue, M.; Pokethitiyook, P.; Chaiyarat, R. Uptake and Accumulation of Cadmium, Lead and Zinc by Siam Weed [Chromolaena odorata (L.) King & Robinson]. Chemosphere 2007, 68, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- He, S.; He, Z.; Yang, X.; Stoffella, P.J.; Baligar, V.C. Soil Biogeochemistry, Plant Physiology, and Phytoremediation of Cadmium-Contaminated Soils; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 134, ISBN 9780128033234. [Google Scholar]
- Xu, P.; Wang, Z. Physiological Mechanism of Hypertolerance of Cadmium in Kentucky Bluegrass and Tall Fescue: Chemical Forms and Tissue Distribution. Environ. Exp. Bot. 2013, 96, 35–42. [Google Scholar] [CrossRef]
- Filek, M.; Gzyl-Malcher, B.; Zembala, M.; Bednarska, E.; Laggner, P.; Kriechbaum, M. Effect of Selenium on Characteristics of Rape Chloroplasts Modified by Cadmium. J. Plant Physiol. 2010, 167, 28–33. [Google Scholar] [CrossRef]
- Younes, N.A.; Dawood, M.F.A.; Wardany, A.A. Biosafety Assessment of Graphene Nanosheets on Leaf Ultrastructure, Physiological and Yield Traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 2019, 228, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, T.Q.; Jin, X.F.; Yang, X.E.; Islam, E.; Mahmood, Q. Lead Induced Changes in the Growth and Antioxidant Metabolism of the Lead Accumulating and Non-Accumulating Ecotypes of Sedum alfredii. J. Integr. Plant Biol. 2008, 50, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at High Dose Perturbs Growth, Photosynthesis and Nitrogen Metabolism While at Low Dose It up Regulates Sulfur Assimilation and Antioxidant Machinery in Garden Cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Michel-López, C.Y.; Espadas y Gil, F.; Fuentes Ortíz, G.; Santamaría, J.M.; González-Mendoza, D.; Ceceña-Duran, C.; Grimaldo Juarez, O. Bioaccumulation and Effect of Cadmium in the Photosynthetic Apparatus of Prosopis juliflora. Chem. Speciat. Bioavailab. 2016, 28, 1–6. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of Cadmium Stress on Growth and Physiological Characteristics of Sassafras Seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Jakl, T.; Bolhar-Nordenkampf, H.R. Energy Conversion Efficiency and Energy Partitioning of White Lupins (Lupinus albus L.). Bioresour. Technol. 1991, 36, 193–197. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Petrov, V.D.; Van Breusegem, F. Hydrogen Peroxide-a Central Hub for Information Flow in Plant Cells. AoB Plants 2012, 12, pls014. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil Contamination by Phthalate Esters in Chinese Intensive Vegetable Production Systems with Different Modes of Use of Plastic Film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef]
- Terzi, R.; Güler, N.S.; Güven, F.G.; Kadioglu, A. Alpha Lipoic Acid Treatment Induces the Antioxidant System and Ameliorates Lipid Peroxidation in Maize Seedlings under Osmotic Stress. Arch. Biol. Sci. 2018, 70, 503–511. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Chen, W.; Yuan, F.; Yan, K.; Tao, D. Accumulation and Tolerance Characteristics of Cadmium in a Potential Hyperaccumulator-Lonicera japonica Thunb. J. Hazard. Mater. 2009, 169, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, T.H.; Choi, B.C.; Lee, B.S.; Lee, J.H. Effect of CO, NOx and SO2 on ROS Production, Photosynthesis and Ascorbate-Glutathione Pathway to Induce Fragaria×annasa as a Hyperaccumulator. Redox Biol. 2014, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jin, Q.; Liu, Y.; Ning, B.; Liao, M.; Luo, L. Screening of a New Cadmium Hyperaccumulator, Galinsoga parviflora, from Winter Farmland Weeds Using the Artificially High Soil Cadmium Concentration Method. Environ. Toxicol. Chem. 2014, 33, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, R.; Hu, X.; Song, J.; Li, B.; Ou, D.; Hu, X.; Zhao, Y. Exogenous Spermidine Elevating Cadmium Tolerance in Salix Matsudana Involves Cadmium Detoxification and Antioxidant Defense. Int. J. Phytoremediat. 2019, 21, 305–315. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Chen, J.; Zhang, S.; Xu, J.; Han, X.; Feng, Y.; Chen, Y.; Zhang, X.; Dong, G.; et al. Xylem Development, Cadmium Bioconcentration, and Antioxidant Defense in Populus × euramericana Stems under Combined Conditions of Nitrogen and Cadmium. Environ. Exp. Bot. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen Nutrient Status Induces Sexual Differences in Responses to Cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.H.; Verslues, P.E. Dynamic Proline Metabolism: Importance and Regulation in Water Limited Environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in Modulating Proline Metabolism in Plants for Salt and Drought Stress Tolerance: Phytohormones, Mineral Nutrients and Transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N.; Singh, R.P.; Singh, D.P. Rhizoremediation Potential of Spontaneously Grown Typha Latifolia on Fly Ash Basins: Study from the Field. Ecol. Eng. 2014, 71, 722–727. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Insights into the Tolerance and Phytoremediation Potential of Coronopus didymus L. (Sm) Grown under Zinc Stress. Chemosphere 2020, 244, 125350. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Peng, Y.; Xu, J.; Qin, D.; Gao, T.; Zhu, H.; Zuo, S.; Song, H.; Dong, J. Phytoremediation Potential of Youngia japonica (L.) DC: A Newly Discovered Cadmium Hyperaccumulator. Environ. Sci. Pollut. Res. 2020, 28, 6044–6057. [Google Scholar] [CrossRef] [PubMed]
- Stiles, A.R.; Bautista, D.; Atalay, E.; Babaoǧlu, M.; Terry, N. Mechanisms of Boron Tolerance and Accumulation in Plants: A Physiological Comparison of the Extremely Boron-Tolerant Plant Species, Puccinellia distans, with the Moderately Boron-Tolerant Gypsophila arrostil. Environ. Sci. Technol. 2010, 44, 7089–7095. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and Hyperaccumulation of Cadmium by a Wild, Unpalatable Herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Maier, R.M. Phytoremediation of Mine Tailings in Temperate and Arid Environments. Rev. Environ. Sci. Biotechnol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Fan, K.C.; Hsi, H.C.; Chen, C.W.; Lee, H.L.; Hseu, Z.Y. Cadmium Accumulation and Tolerance of Mahogany (Swietenia macrophylla) Seedlings for Phytoextraction Applications. J. Environ. Manag. 2011, 92, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, M.; Ghabriche, R.; Amari, T.; Mnasri, M.; Zolla, L.; Lutts, S.; Abdely, C.; Ghnaya, T. Comparative Study of Cd Tolerance and Accumulation Potential between Cakile maritima L. (Halophyte) and Brassica juncea L. Ecol. Eng. 2014, 71, 623–627. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Florida Site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in Plants on Polluted Soils: Effects of Soil Factors, Hyperaccumulation, and Amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A Review of Soil Cadmium Contamination in China Including a Health Risk Assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).