Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Collection and Anatomical Measurements
2.3. Hydraulic Properties
2.4. Conduit Tapering Ratio (T)
2.5. Soil Water Measurement
2.6. Data Analyses
3. Results
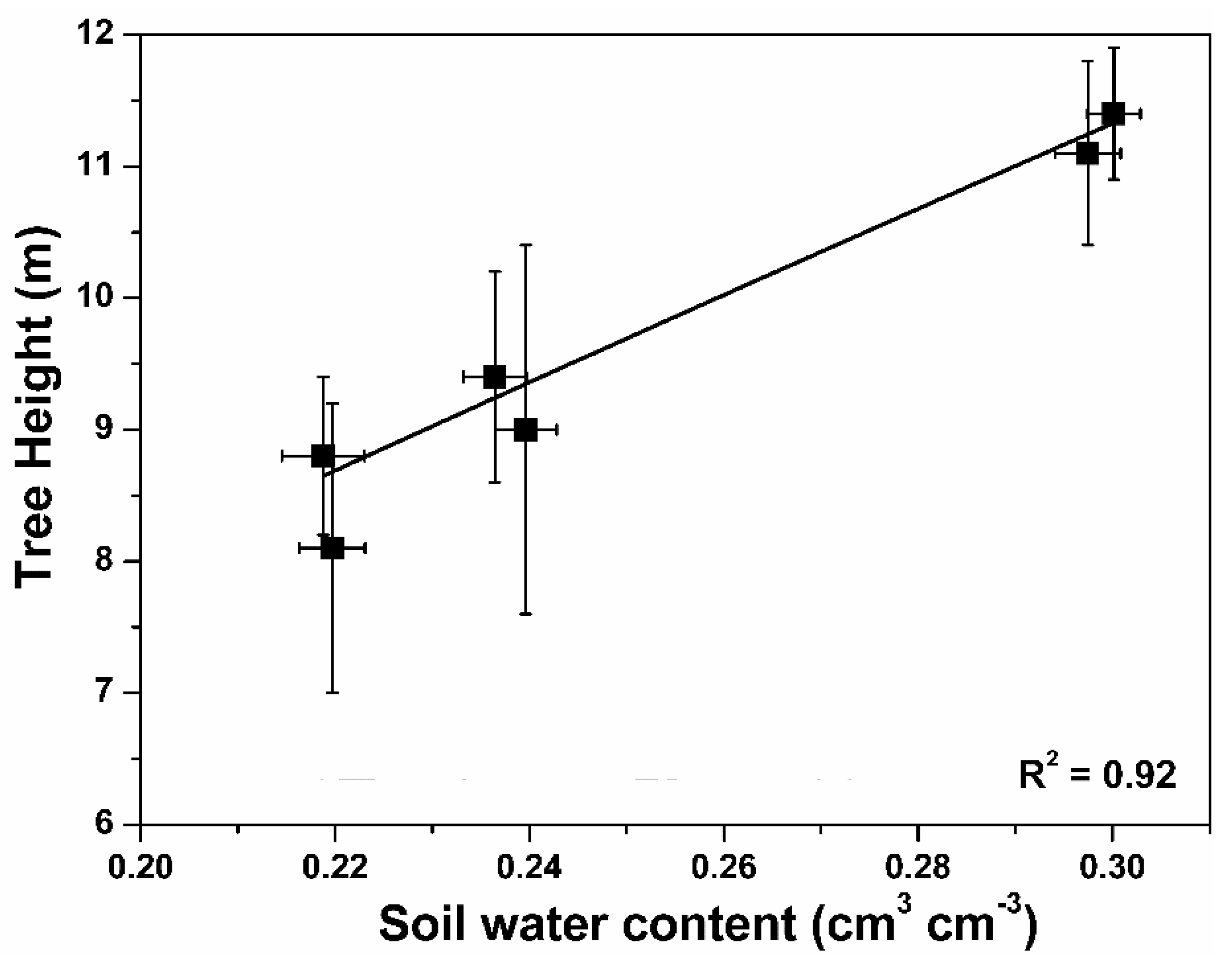
3.1. Soil Moisture Characteristics
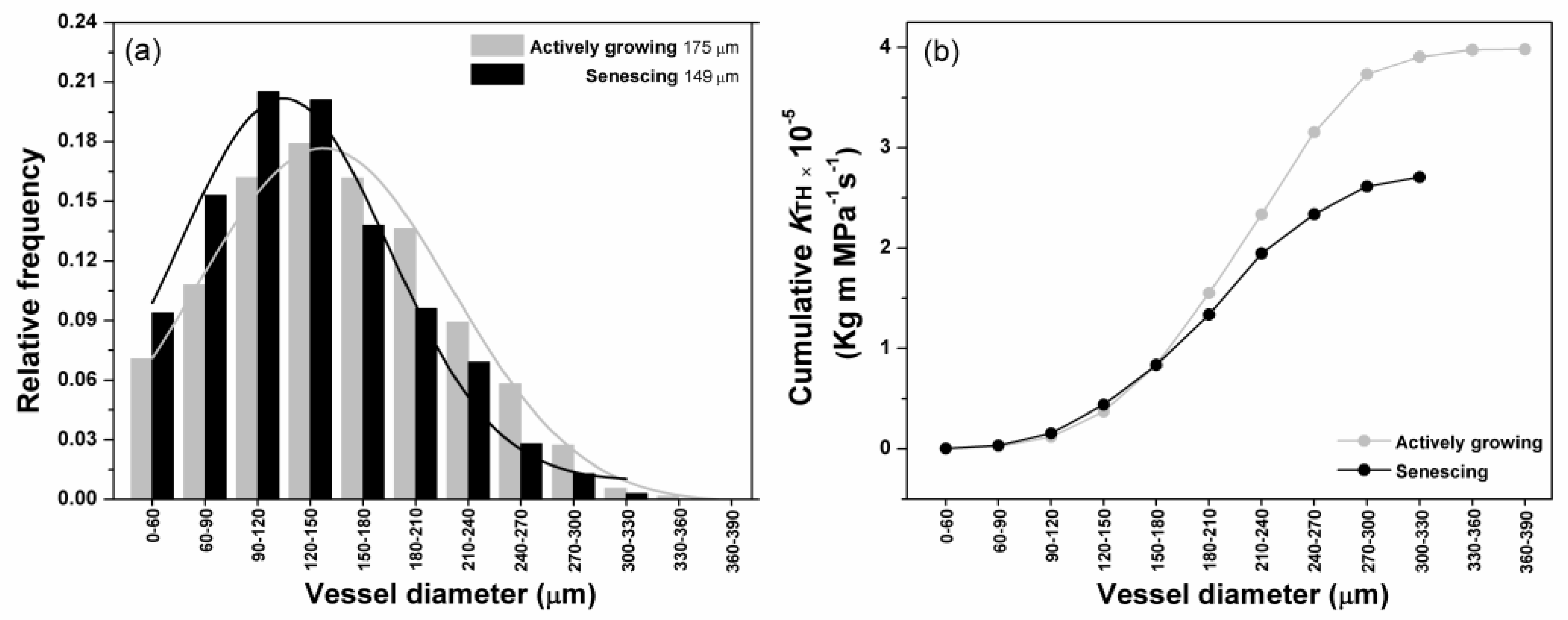
3.2. Xylem Conduit Diameter and Distribution
3.3. Tapering Ratio (T)
3.4. Conduit Diameter, Stem Diameter, and Stem Length
| SLtree-SD + Status | Dh0-SD + Status | Dh0-SL + Status | DhN-1-SL + Status | Dh0-SL + Site | |
|---|---|---|---|---|---|
| R2 | 0.42 | 0.75 | 0.86 | 0.54 | 0.74 |
| Slope (95% CI) | 0.52 (0.31, 0.73) | 0.40 (0.30, 0.50) | 0.48 (0.40, 0.56) | 0.29 (0.21, 0.37) | 0.39 (0.24, 0.55) |
| Intercept | 0.61 (0.44, 0.78) | 2.06 (1.98, 2.15) | 1.91 (1.83, 1.99) | 1.76 (1.68, 1.84) | 1.89 (1.78, 1.99) |
| Model fit | F(2,33) = 13.66 *** | F(2,33) =54.12 *** | F(2,33) = 111.60 *** | F(2,33) = 44.13 *** | F(3,32) = 49.83 *** |
| Equality of slopes | p = 0.622 | p = 0.768 | p = 0.421 | p = 0.992 | p = 0.631 |
| Equality of intercepts | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Actively growing trees | R2 = 0.80 *** | R2 = 0.79 *** | R2 = 0.9 *** | R2 = 0.81 *** | - |
| Intercept | 0.46 (0.29, 0.59) | 1.95 (1.86, 2.05) | 1.83 (1.72, 1.89) | 1.72 (1.65, 1.79) | - |
| Declining trees | R2 = 0.24 * | R2 = 0.57 *** | R2 = 0.76 *** | R2 = 0.52 *** | - |
| Intercept | 0.44 (0.11, 0.88) | 1.79 (1.59, 1.99) | 1.81 (1.72, 1.96) | 1.70 (1.58, 1.82) | - |
| Upper-slope intercept | - | - | - | - | 1.81 (1.59, 2.02) |
| Middle-slope intercept | - | - | - | - | 1.91 (1.72, 2.07) |
| Bottom-slope intercept | - | - | - | - | 2.00 (1.81, 2.20) |
| Figure | Figure 5a | Figure 5b | Figure 5c | Figure 5d | Figure 6 |
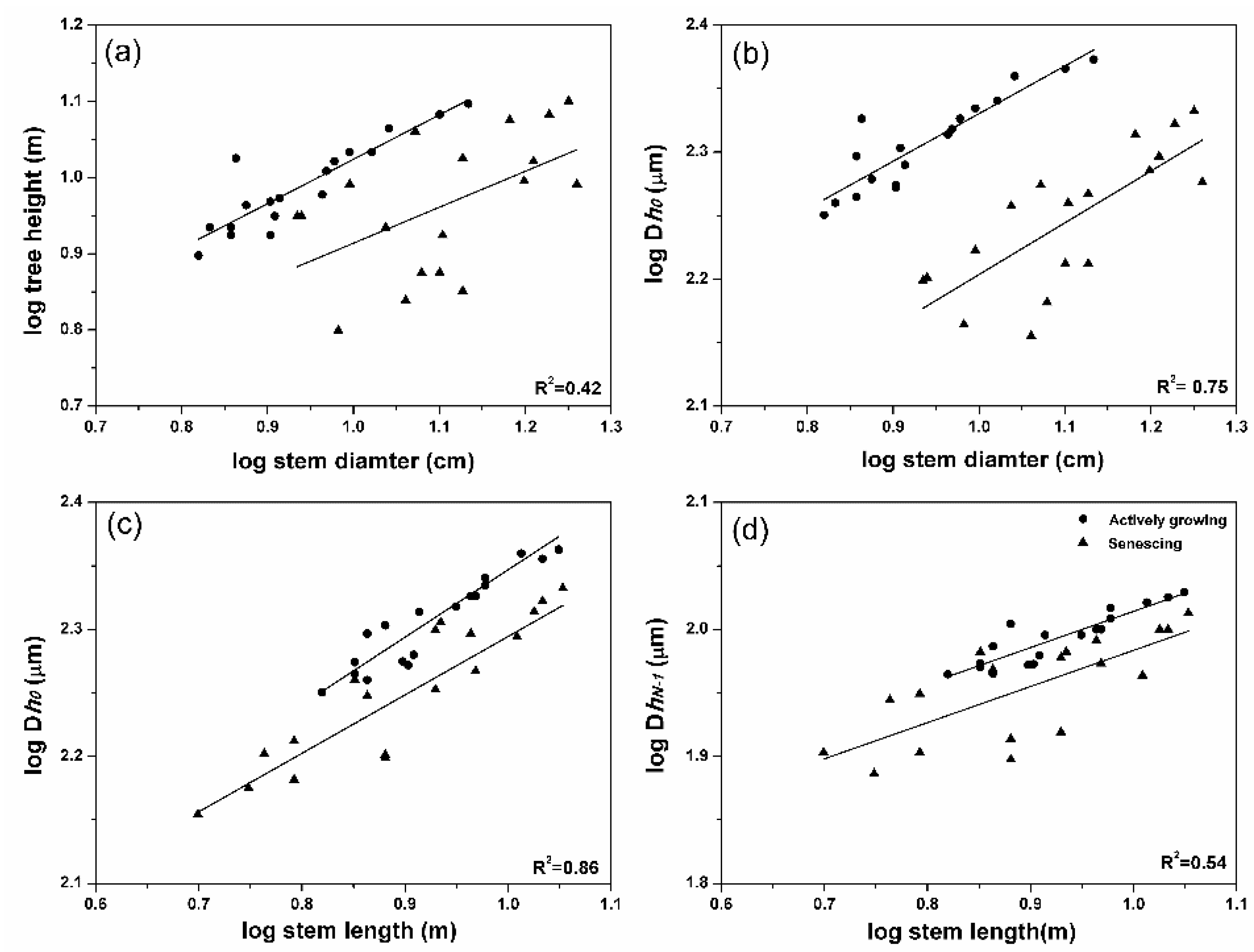
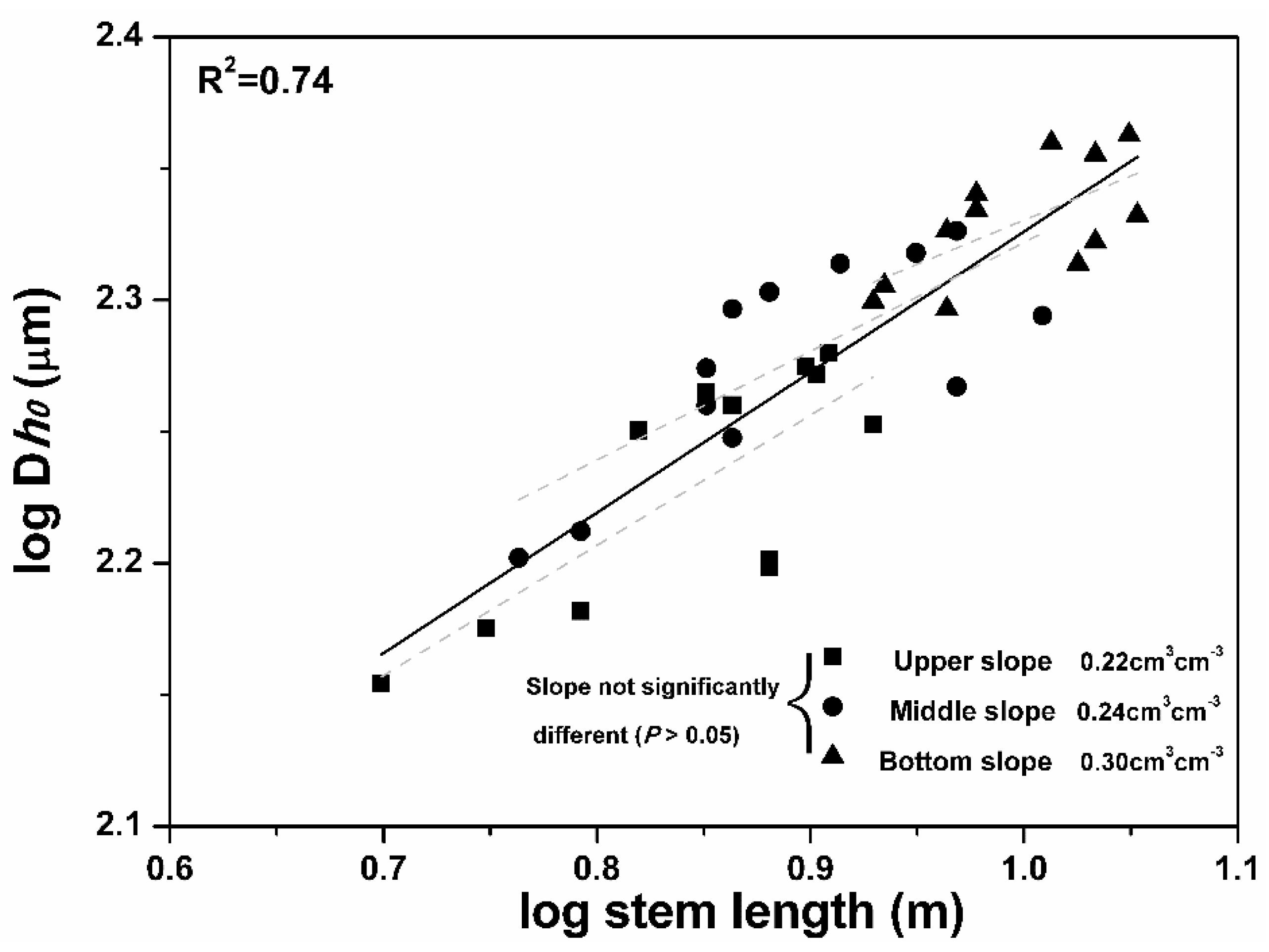
3.5. Soil Water Effect on Conduit Diameter–Stem Length Relationship
4. Discussion
4.1. Conduit Diameter and Distribution
4.2. Tapering Ratio
4.3. Conduit Diameter, Stem Diameter, and Stem Length
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree Mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sanguesa-Barreda, G.; Oliva, J.; Vicenta-Serrano, S.M. To die or not to die early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Rice, K.J.; Matzner, S.L.; Byer, W.; Brown, J.R. Patterns of tree dieback in Queensland, Australia: The importance of drought stress and the role of resistance to cavitation. Oecologia 2004, 139, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Berry, J.A.; Field, C.B. Loss of whole-tree hydraulic conductance during severe drought and multi-year forest die-off. Oecologia 2014, 175, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.A.; Yepez, E.A.; Hill, J.; Pangle, R.; Sperry, J.S.; Pockman, W.T.; McDowell, N.G. Hydraulic limits preceding mortality in a pinon-juniper woodland under experimental drought. Plant Cell Environ. 2012, 35, 1601–1617. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hoffmann, W.A.; Marchin, R.M.; Abit, P.; Lau, O.L. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Chang. Biol. 2011, 17, 2731–2742. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: New York, NY, USA, 1983. [Google Scholar]
- Schuldt, B.; Leuschner, C.; Brock, N.; Horna, V. Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol. 2013, 33, 161–174. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Zach, A.; Schuldt, B.; Brix, S.; Horna, V.; Culmsee, H.; Leuschner, C. Vessel diameter and xylem hydraulic conductivity increase with tree height in tropical rainforest trees in Sulawesi, Indonesia. Flora 2010, 205, 506–512. [Google Scholar] [CrossRef]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef]
- Magnani, F.; Mencuccini, M.; Grace, J. Age-related decline in stand productivity: The role of structural acclimation under hydraulic constraints. Plant Cell Environ. 2000, 23, 251–263. [Google Scholar] [CrossRef]
- McDowell, N.G.; Phillips, N.; Lunch, C.; Bond, B.J.; Ryan, M.G. An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol. 2002, 22, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Enquist, B.J.; West, G.B.; Charnov, E.L.; Brown, J.H. Allometric scaling of production and life-history variation in vascular plants. Nature 1999, 401, 907–911. [Google Scholar] [CrossRef]
- Petit, G.; Anfodillo, T.; De Zan, C. Degree of tapering of xylem conduits in stems and roots of small Pinus cembra and Larix decidua trees. Botany 2009, 87, 501–508. [Google Scholar] [CrossRef]
- Becker, P.; Gribben, R.J.; Lim, C.M. Tapered conduits can buffer hydraulic conductance from path-length effects. Tree Physiol. 2000, 20, 965–967. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Cao, K.-F.; Becker, P. Axial and radial variations in xylem anatomy of angiosperm and conifer trees in Yunnan, China. Iawa J. 2009, 30, 1–13. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Sperry, J.S. Patterns in hydraulic architecture and their implications for transport efficiency. Tree Physiol. 2005, 25, 257–267. [Google Scholar] [CrossRef]
- Olson, M.E.; Anfodillo, T.; Rosell, J.A.; Petit, G.; Crivellaro, A.; Isnard, S.; Leon-Gomez, C.; Alvarado-Cardenas, L.O.; Castorena, M. Universal hydraulics of the flowering plants: Vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol. Lett. 2014, 17, 988–997. [Google Scholar] [CrossRef]
- Olson, M.E.; Rosell, J.A. Vessel diameterstem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol. 2013, 197, 1204–1213. [Google Scholar] [CrossRef]
- Enquist, B.J. Universal scaling in tree and vascular plant allometry: Toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef]
- Anfodillo, T.; Deslauriers, A.; Menardi, R.; Tedoldi, L.; Petit, G.; Rossi, S. Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. J. Exp. Bot. 2012, 63, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Anfodillo, T.; Mencuccini, M. Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol. 2008, 177, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Pfautsch, S.; Harbusch, M.; Wesolowski, A.; Smith, R.; Macfarlane, C.; Tjoelker, M.G.; Reich, P.B.; Adams, M.A. Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecol. Lett. 2016, 19, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z.; Lü, Y.; Zeng, Y.; Li, Y.; Jiang, X.; et al. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Chang. 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Shi, H.; Shao, M.G. Soil and water loss from the Loess Plateau in China. J. Arid. Environ. 2000, 45, 9–20. [Google Scholar] [CrossRef]
- Wang, S.; Fu, B.; Piao, S.; Lü, Y.; Ciais, P.; Feng, X.; Wang, Y. Reduced sediment transport in the Yellow River due to anthropogenic changes. Nat. Geosci. 2015, 9, 38–41. [Google Scholar] [CrossRef]
- Lu, Y.H.; Fu, B.J.; Feng, X.M.; Zeng, Y.; Liu, Y.; Chang, R.Y.; Sun, G.; Wu, B.F. A policy-driven large scale ecological restoration: Quantifying ecosystem eservices changes in the Loess Plateau of China. PLoS ONE 2012, 7, e31782. [Google Scholar]
- Liang, W.; Bai, D.; Wang, F.; Fu, B.; Yan, J.; Wang, S.; Yang, Y.; Long, D.; Feng, M. Quantifying the impacts of climate change and ecological restoration on streamflow changes based on a Budyko hydrological model in China’s Loess Plateau. Water Resour. Res. 2015, 51, 6500–6519. [Google Scholar] [CrossRef]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental controls on sap flow in black locust forest in Loess Plateau, China. Sci. Rep. 2017, 7, 13160. [Google Scholar] [CrossRef]
- Xu, M.; Liu, G. The characteristics and evolution of soil nutrient in artificial black locust (Robinia pseudoacacia) forest-land in the hilly Loess Plateau. Plant Nutr. Fertil. Sci. 2004, 10, 40–46. [Google Scholar]
- Du, S.; Wang, Y.-L.; Kume, T.; Zhang, J.-G.; Otsuki, K.; Yamanaka, N.; Liu, G.-B. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Tanaka-Oda, A.; Kenzo, T.; Koretsune, S.; Sasaki, H.; Fukuda, K. Ontogenetic changes in water-use efficiency (δ13C) and leaf traits differ among tree species growing in a semiarid region of the Loess Plateau, China. Forest Ecol. Manag. 2010, 259, 953–957. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.D.; Wang, S.Q.; Hou, Q.C. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. Forest Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.P.; Horton, R.; Shao, M.A. Effects of vegetation and slope aspect on water budget in the hill and gully region of the Loess Plateau of China. Catena 2011, 87, 90–100. [Google Scholar] [CrossRef]
- Macfarlane, C.; Arndt, S.K.; Livesley, S.J.; Edgar, A.C.; White, D.A.; Adams, M.A.; Eamus, D. Estimation of leaf area index in eucalypt forest with vertical foliage, using cover and fullframe fisheye photography. Forest Ecol. Manag. 2007, 242, 756–763. [Google Scholar] [CrossRef]
- Arbellay, E.; Fonti, P.; Stoffel, M. Duration and extension of anatomical changes in wood structure after cambial injury. J. Exp. Bot. 2012, 63, 3271–3277. [Google Scholar] [CrossRef]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef]
- Jia, X.X.; Shao, M.A.; Zhu, Y.J.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Wang, M.-C.; Wang, J.-X.; Shi, Q.-H.; Zhang, J.-S. Photosynthesis and water use efficiency of Platycladus orientalis and Robinia pseudoacacia saplings under steady soil water stress during different stages of their annual growth period. J. Integr. Plant Biol. 2007, 49, 1470–1477. [Google Scholar] [CrossRef]
- McElrone, A.J.; Pockman, W.T.; Martinez-Vilalta, J.; Jackson, R.B. Variation in xylem structure and function in stems and roots of trees to 20 m depth. New Phytol. 2004, 163, 507–517. [Google Scholar] [CrossRef]
- Cruiziat, P.; Cochard, H.; Ameglio, T. Hydraulic architecture of trees: Main concepts and results. Ann. Forest Sci. 2002, 59, 723–752. [Google Scholar] [CrossRef]
- Borja, I.; Svetlik, J.; Nadezhdin, V.; Cermak, J.; Rosner, S.; Nadezhdina, N. Sap flux—A real time assessment of health status in Norway spruce. Scand. J. Forest Res. 2016, 31, 450–457. [Google Scholar] [CrossRef]
- Savage, V.M.; Bentley, L.P.; Enquist, B.J.; Sperry, J.S.; Smith, D.D.; Reich, P.B.; von Allmen, E.I. Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants. Proc. Natl. Acad. Sci. USA 2010, 107, 22722–22727. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Clearwater, M.J.; Goldstein, G. Water transport in trees: Current perspectives, new insights and some controversies. Environ. Exp. Bot. 2001, 45, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, E.A.; Baas, P.; Rodgers, S. Variations in dieot wood anatomy: A global analysis based on the insidewood database. Iawa J. 2007, 28, 229–258. [Google Scholar] [CrossRef]
- Barij, N.; Stokes, A.; Bogaard, T.; Van Beek, R. Does growing on a slope affect tree xylem structure and water relations? Tree Physiol. 2007, 27, 757–764. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy, 2nd ed.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 689–701. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hoang, N. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants a basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Meinzer, F.C. Functional convergence in plant responses to the environment. Oecologia 2003, 134, 1–11. [Google Scholar] [CrossRef]

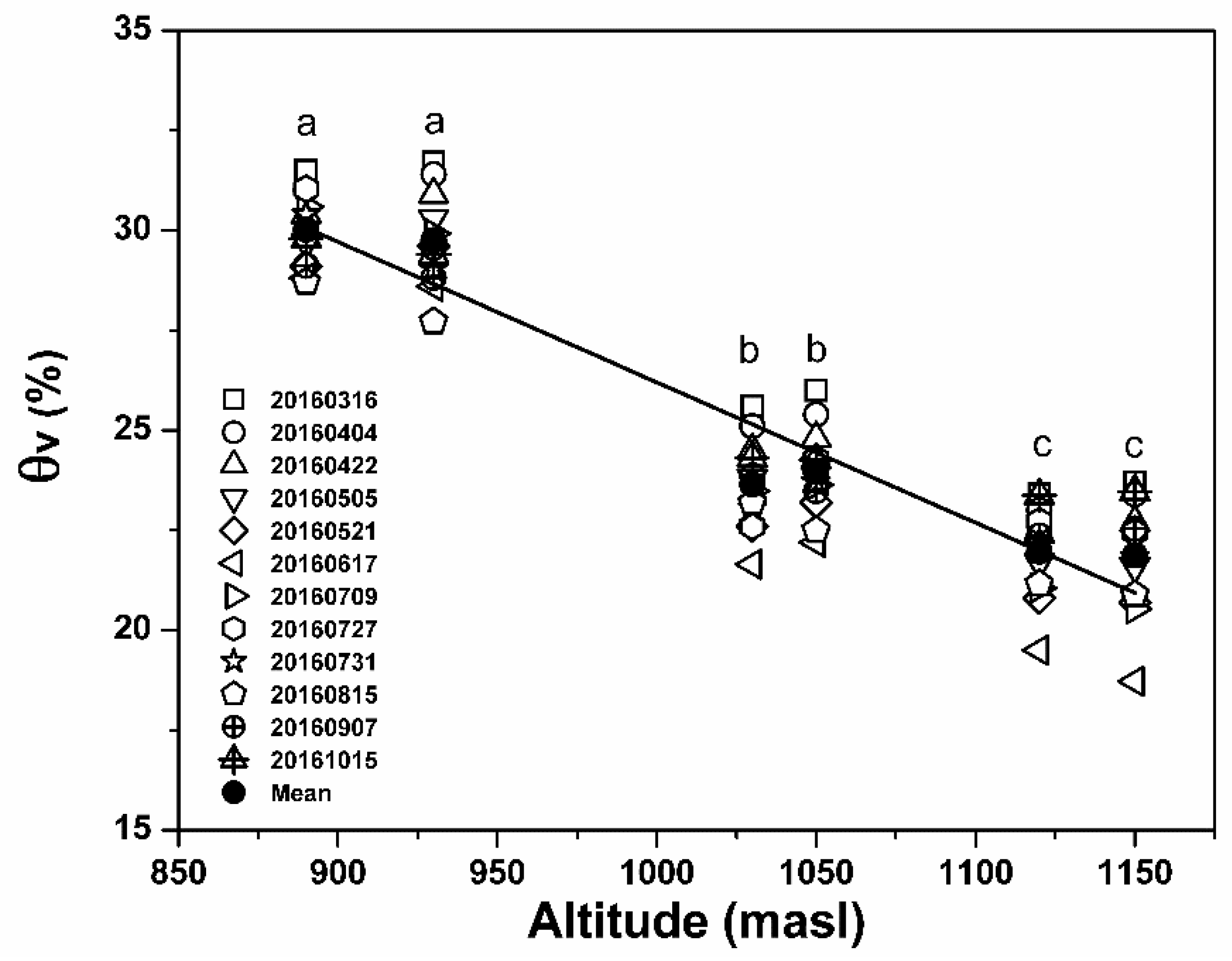

| Growth Status | Actively Growing | Senescing | ||||
|---|---|---|---|---|---|---|
| Stand ID | S1 | S2 | S3 | S4 | S5 | S6 |
| Altitude (m) | 1150 | 1030 | 890 | 1120 | 1050 | 930 |
| Density (tree ha−1) | 2300 | 2400 | 2400 | 800 | 900 | 700 |
| AL/AS (m2 cm−2) | 0.13 | 0.11 | 0.08 | 0.07 | 0.05 | 0.04 |
| Tree samples | 6 | 6 | 6 | 6 | 6 | 6 |
| Mean height of samples (m) | 8.8 (0.2) | 9.4 (0.4) | 11.4 (0.3) | 8.1 (0.6) | 9.0 (0.7) | 11.1 (0.5) |
| Mean diameter of samples (cm) | 7.4 (0.3) | 8.2 (0.4) | 11.2 (0.3) | 10.1 (0.6) | 12.5 (0.4) | 16.7 (0.5) |
| 95% CI | |||||
|---|---|---|---|---|---|
| Model | Slope | Intercept | R2 | Slope | Intercept |
| All | 0.212 | 0.111 | 0.75 | 0.172–0.252 | 0.074–0.148 |
| Actively growing trees | 0.244 | 0.089 | 0.89 | 0.201–0.287 | 0.049–0.129 |
| Senescing trees | 0.175 | 0.136 | 0.85 | 0.146–0.198 | 0.110–0.172 |
| Explained Variable | R2 | p |
|---|---|---|
| Explained variations in Dh0, not taking stem length into account. | 0.51 | <0.001 |
| Explained variations in Dh0, taking stem length into account. (Dh0 -stem length + site residuals) | 0.049 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, X.; Yao, Q.; Zhou, B.; Wang, Q.; Shao, M. Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China. Forests 2024, 15, 116. https://doi.org/10.3390/f15010116
Ma C, Zhang X, Yao Q, Zhou B, Wang Q, Shao M. Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China. Forests. 2024; 15(1):116. https://doi.org/10.3390/f15010116
Chicago/Turabian StyleMa, Changkun, Xi Zhang, Qian Yao, Beibei Zhou, Quanjiu Wang, and Mingan Shao. 2024. "Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China" Forests 15, no. 1: 116. https://doi.org/10.3390/f15010116
APA StyleMa, C., Zhang, X., Yao, Q., Zhou, B., Wang, Q., & Shao, M. (2024). Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China. Forests, 15(1), 116. https://doi.org/10.3390/f15010116






