Abstract
The lignicolous saprotrophic genus Entonaema contains six formally accepted species: E. liquescens (type species), E. cinnabarinum, E. globosum, E. dengii, E. moluccanum, and E. siamensis. Its stromatic ascomata develop on the surface of dead wood remnants; they are rather large, globose to irregularly shaped, and vividly coloured. The fresh stroma interior is filled with a liquid matter. In early studies, the genus was considered to have a preference for tropical habitats, while in more recent field research, numerous collections have been added from warm, temperate areas of Europe, North America, and Asia. Our taxonomic and phylogenetic studies were based on freshly collected E. cinnabarinum from Croatia and E. liquescens from the USA. A phylogenetic study of the sequence alignment of four concatenated gene regions (ITS, LSU, rpb2, and β-tub) revealed the true taxonomic position of Entonaema within Hypoxylaceae (Xylariales), a sister to Hypoxylon carneum. Detailed macroscopic and microscopic descriptions of E. cinnabarinum are accompanied by drawings and colour photographs, while the study of E. liquescens is focused on stromatal microchemical reaction. With new information, the worldwide identification key to the putative species of Entonaema is proposed. Ecological data and biogeographical patterns were studied using all available and reliable sources of recorded data. Climatic preferences of the two most widespread Entonaema species, E. liquescens and E. cinnabarinum, are discussed in detail.
1. Introduction
The genus Entonaema Möller was established in 1901 [1], represented by E. liquescens Möller as a type species and E. mesentericum Möller as an additional species of the genus. The latter species was later justifiably removed from the genus [2]. The genus was retained as a member of Xylariaceae Tul. & C. Tul. in the same study, a taxonomic view supported by the majority of taxonomists (e.g., [3,4,5,6,7,8,9]). Recently, after detailed molecular phylogenetic study with a sufficient amount of xylarialean DNA sequences, Entonaema was assigned to the newly resurrected and emended family Hypoxylaceae DC [10].
As with the majority of xylarialean fungi, the genus is characterised by its lignicolous way of life [8], developing rather large, globose to irregularly shaped, vividly coloured stromata on the surface of dead wood remnants; the intact interior of the stroma is filled with a liquid matter, while the perithecial layer is subgelatinous when fresh but coriaceous and hard when dried. The perithecia are inserted into the stromatal cortex; they are monostichous and carbonaceous, and they contain cylindrical, pedicellate asci with an amyloid apical ring. Ascospores are one-celled, brownish, and equipped with a longitudinal germ slit exceeding one-half of the spore’s length. Besides E. liquescens, five more species are accepted in the genus today: E. cinnabarinum (Cooke & Massee) Lloyd [11], E. dengii J.D. Rogers [5], E. moluccanum J.D. Rogers [5], E. globosum R. Heim [12], and E. siamensis Sihan., Thienh. & Whalley [13]. Only two out of the six species currently belonging to the genus are biogeographically widespread, viz., E. liquescens and E. cinnabarinum. To date, a true phylogenetic position of the genus was, however, a matter of uncertainty [10,14,15]. Our study was significantly concentrated on the phylogenetic analyses as well as the stromatal pigments and anatomy of the newly collected, living material of the two aforementioned typical and most widespread Entonaema species in order to reveal the true affinities of the genus. The analysis of their ecological–biogeographical traits was conducted on the basis of all accessible and verifiable records worldwide, and their climatic preferences are discussed in detail. The worldwide identification key to the putative species of Entonaema is proposed.
2. Materials and Methods
2.1. Microscopic Studies
Microscopic characteristics based on living cells and tissues (*) were recorded using vital taxonomy methods [16], while those based on dead cells and tissues (†) were obtained from fixed fresh and dry materials. All described microscopic elements were observed in tap water (H2O), and cytochemical and histochemical data were additionally observed in Lugol’s solution (IKI), Brilliant Cresyl Blue (CRB), potassium hydroxide (5% and 10% KOH), and Cotton Blue (CB). Microscopic elements were studied with a Zeiss Axioskop 40 FL (Carl Zeiss AG, Oberkochen, Germany) transmission light microscope (bright-field, phase-contrast, and dark-field techniques) under magnifications up to 1000×. Drawings were made freehand to scale, and microphotographs were taken with Nikon D750 and Nikon Z6 (Nikon Corporation, Tokyo, Japan) cameras mounted on the camera adapter T2-T2 SLR 2.5× (Carl Zeiss AG, Oberkochen, Germany) attached to the microscope’s trinocular tube. Characters of stromatal and hymenial elements were based on a minimum of three stromata from each collection. Spore measurements were based on samples of 150 fully mature, normally developed, living, and randomly selected ascospores. Measurements were taken directly using an ocular micrometre and from microphotographs using PIXIMÈTRE software ver. 5.10 [17] to an accuracy of 0.1 μm. Length, width, and length/width ratio (“Q” value) were given as follows: (min.) stat. min.—stat. mode—stat. max. (max.), where “min.” = minimum (lowest measured value), “stat. min.” = statistical minimum (arithmetic mean minus two times standard deviation), “stat. mode” = statistical mode, “stat. max.” = statistical maximum (arithmetic mean plus two times standard deviation), and “max.” = maximum (highest measured value). The dried material was deposited at the Croatian National Fungarium (CNF), Zagreb, Croatia.
2.2. Axenic Cultures
Ascospore germination of E. cinnabarinum was tested by inoculation of freshly ejected ascospores on potato dextrose agar (PDA, HiMedia Laboratories Pvt. Ltd., Mumbai, India), malt extract agar (MEA, HiMedia Laboratories Pvt. Ltd., Mumbai, India), and oatmeal agar (OA, after Samson et al. [18]), both with and without pretreatment with hydrochloric acid (pH = 3.3; 0.0005 M HCl) for two hours at 25 °C. Petri dishes were kept at 24 °C in the dark for 7 days.
2.3. DNA Extraction, PCR Amplification, and Sequencing
Genomic DNA was extracted from fresh tissue of stromata using an EZNA® HP Fungal DNA Kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s protocol, with time adjustment of lysis incubation to 1 h. Four gene regions, ITS (internal transcribed spacer region), LSU (28S large subunit of ribosomal DNA), rpb2 (second largest subunit of the DNA-directed RNA polymerase II), and β-tub (beta-tubulin), were amplified using primer pairs ITS1F/ITS4 [19,20], LR0R/LR7 [21], RPB2-5F/RPB2-7cR [22], and T1/T2, T11/T22 [23], respectively. The 25 µL PCR mixtures contained 9.5 µL of ddH2O, 12.5 µL of GoTaq® G2 Green Master Mix (Promega, Madison, WI, USA), 1 µL of DNA template, and 1 µL of each forward and reverse primer with a final concentration of 0.2 µM, respectively. The PCR amplification for ITS and LSU gene regions was performed using a touchdown program: initial denaturation at 95 °C for 2 min; followed by 5 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 45 s (add −1 °C per cycle), extension at 72 °C for 1.5 min; 30 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 45 s, extension at 72 °C for 1.5 min; and a final extension at 72 °C for 5 min. The PCR amplification of rpb2 was set up as described by Liu et al. [22], and of β-tub as described by O’Donnell and Cigelnik [23]. Successful PCR products were purified using ExoSAP-IT™ (Thermo Fisher Scientific, Waltham, MA, USA) cleanup reagent following the manufacturer’s protocol and sent to Macrogen Europe (Amsterdam, The Netherlands) for bidirectional Sanger sequencing.
2.4. Sequence Alignment and Phylogenetic Analysis
Sequence reads were assembled and edited using Geneious Prime 2023.0.4. (https://www.geneious.com, Biomatters, Auckland, New Zealand, accessed on 18 October 2022). Assembled sequences were deposited at the National Center for Biotechnology Information (NCBI) GenBank database.
A phylogenetic dataset comprised of 501 sequences of four gene regions (ITS, LSU, rpb2, β-tub) from 142 taxa was selected for further analyses (Table 1). The listed species of Hypoxylaceae, Xylariaceae and Graphostromataceae M.E. Barr, J.D. Rogers & Y.M. Ju in Table 1 originated from previously published studies. All published sequences from NCBI Nucleotide database resulting with ‘Entonema’ as the genus name were also included in phylogenetic analyses. Sequences were aligned by each gene region using MAFFT v7.450 [24,25] available as a Geneious Prime plugin. After being aligned and trimmed, concatenation of ITS, LSU, rpb2 and β-tub alignments was accomplished using Geneious Prime 2023.0.4. Concatenated alignment contained 5794 characters positions including gaps, with 974 character positions for ITS, 1778 characters positions for LSU, 1065 characters positions for rpb2, and 1977 characters positions for β-tub. Pyriformiascoma trilobatum Daranag., Camporesi & K.D. Hyde, Diatrype disciformis (Hoffm.) Fr., Creosphaeria sassafra Y.M. Ju, F. San Martín & J.D. Rogers, and Calceomyces lacunosus Udagawa & S. Ueda were selected as the outgroup for phylogenetic analyses following Wendt et al. [10].
Phylogenetic analyses were conducted using Maximum Likelihood (ML) analysis in IQTREE v1.6.12 [26,27] and a Bayesian Inference (BI) analysis in MrBayes 3.2.6 (Geneious plugin, [28]). The best model was selected by ModelFinder implemented in IQ-TREE separately considering the corrected Akaike, and Bayesian Information Criterion (cAIC, BIC). TIM2+F+I+G4 was selected as best model for both phylogenetic datasets. ML analysis was executed by applying the ultrafast bootstrap approximation with 1000 replicates. BI analysis was executed for 5,000,000 generations, sampling trees and other parameters every 1000 generations. The default numbers of chains (four) and heating parameters were used. Posterior probabilities (BPP) were calculated after burning the first 25% of the posterior sample. Phylogenetic trees were visualized and annotated using iTOL v6.5.4 [29] and FigTree 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 22 October 2023).
2.5. The Analysis of Ecological and Biogeographical Traits
A thorough inspection of currently accessible information on Entonaema species record data revealed a huge potential because each of the previous studies has always treated only a very limited amount of collections. Record data were taken from all available scientific publications known to the authors and from the online database GBIF (www.gbif.org, accessed on 26 June 2023), as well as the data derived from our own research and that of our colleagues, as a source for better understanding of the species ecology and biogeography. Only those records that were identified by specialists or were accompanied with high quality macrophotographs showing certain Entonaema species and stromatal ontogeny were accepted for the analysis. Additionally, the record should have had a precise locality name or assigned geographic coordinates, accompanied with a record date to ascertain species phenology. This was a prerequisite for obtaining ecological data (habitat type, vicinity of water bodies, elevation, etc.) for each record, using Google Earth Pro 7.3.6.9345 (Google LLC, Mountain View, CA, USA) software.
The same software was used to visualize ‘Global_1986-2010_KG_5m.kmz’ digital layer downloaded from https://koeppen-geiger.vu-wien.ac.at/present.htm (accessed on 26 June 2023) in order to ascribe the current Köppen–Geiger climate type to every Entonaema record for climatic characterisation. Obtained data were also compared to an older worldwide Köppen–Geiger climate classification made by Kottek et al. [30]. Abbreviations of climatic types follow the same reference.

Table 1.
Species included in this study, associated voucher numbers, countries of origin, and GenBank accession numbers. Newly generated sequences are marked in bold. Abbreviations: HT = holotype, ET = epitype, IT = isotype, PT = paratype.
Table 1.
Species included in this study, associated voucher numbers, countries of origin, and GenBank accession numbers. Newly generated sequences are marked in bold. Abbreviations: HT = holotype, ET = epitype, IT = isotype, PT = paratype.
| Taxa | Voucher | Country | ITS | LSU | rpb2 | β-tub | Ref |
|---|---|---|---|---|---|---|---|
| Amphirosellinia fushanensis | HAST 91111209 HT | Taiwan | GU339496 | N/A | GQ848339 | GQ495950 | [31] |
| Annulohypoxylon annulatum | CBS 140775 ET | Texas | KY610418 | KY610418 | KY624263 | KX376353 | [10,32] |
| Annulohypoxylon atroroseum | ATCC 76081 | Thailand | AJ390397 | KY610422 | KY624233 | DQ840083 | [10,33] |
| Annulohypoxylon michelianum | CBS 119993 | Spain | KX376320 | KY610423 | KY624234 | KX271239 | [10,32] |
| Annulohypoxylon moriforme | CBS 123579 | France | KX376321 | KY610425 | KY624289 | KX271261 | [10,32] |
| Annulohypoxylon nitens | MFLUCC 12-0823 | Thailand | KJ934991 | KJ934992 | KJ934994 | KJ934993 | [34] |
| Annulohypoxylon stygium | MUCL 54601 | France | KY610409 | KY610475 | KY624292 | KX271263 | [10] |
| Annulohypoxylon truncatum | CBS 140778 ET | Texas | KY610419 | KY610419 | KY624277 | KX376352 | [10,32] |
| Astrocystis concavispora | MFLUCC 14-0174 | Italy | KP297404 | KP340545 | KP340532 | KP406615 | [34] |
| Biscogniauxia arima | WSP 122 IT | Mexico | EF026150 | N/A | GQ304736 | AY951672 | [31] |
| Biscogniauxia nummularia | MUCL 51395 ET | France | KY610382 | KY610427 | KY624236 | KX271241 | [10] |
| Brunneiperidium gracilentum | MFLUCC 14-0011 HT | Italy | KP297400 | KP340542 | KP340528 | KP406611 | [34] |
| Calceomyces lacunosus | CBS 633.88 HT | Japan | KY610397 | KY610476 | KY624293 | KX271265 | [10] |
| Camillea obularia | ATCC 28093 | Puerto Rico | KY610384 | KY610429 | KY624238 | KX271243 | [10] |
| Collodiscula fangjingshanensis | GZU H0109 HT | China | KR002590 | KR002591 | KR002592 | KR002589 | [35] |
| Creosphaeria sassafras | STMA 14087 | Argentina | KY610411 | KY610468 | KY624265 | KX271258 | [10] |
| Daldinia andina | CBS 114736 HT | Ecuador | AM749918 | KY610430 | KY624239 | KC977259 | [10,33,36] |
| Daldinia bambusicola | CBS 122872 HT | Thailand | KY610385 | KY610431 | KY624241 | AY951688 | [10,37] |
| Daldinia caldariorum | MUCL 49211 | France | AM749934 | KY610433 | KY624242 | KC977282 | [10,33,36] |
| Daldinia concentrica | CBS 113277 | Germany | AY616683 | KY610434 | KY624243 | KC977274 | [10,14,33] |
| Daldinia dennisii | CBS 114741 HT | Australia | JX658477 | KY610435 | KY624244 | KC977262 | [10,33,38] |
| Daldinia eschscholtzii | MUCL 45435 | Benin | JX658484 | KY610437 | KY624246 | KC977266 | [10,33,38] |
| Daldinia loculatoides | CBS 113279 ET | UK | AF176982 | KY610438 | KY624247 | KX271246 | [10,39] |
| Daldinia macaronesica | CBS 113040 PT | Spain | KY610398 | KY610477 | KY624294 | KX271266 | [10] |
| Daldinia petriniae | MUCL 49214 ET | Austria | AM749937 | KY610439 | KY624248 | KC977261 | [10,33,36] |
| Daldinia placentiformis | MUCL 47603 | Mexico | AM749921 | KY610440 | KY624249 | KC977278 | [10,33,36] |
| Daldinia pyrenaica | MUCL 53969 | France | KY610413 | KY610413 | KY624274 | KY624312 | [10] |
| Daldinia steglichii | MUCL 43512 PT | Papua New Guinea | KY610399 | KY610479 | KY624250 | KX271269 | [10] |
| Daldinia theissenii | CBS 113044 PT | Argentina | KY610388 | KY610441 | KY624251 | KX271247 | [10] |
| Daldinia vernicosa | CBS 119316 ET | Germany | KY610395 | KY610442 | KY624252 | KC977260 | [10,33] |
| Diatrype disciformis | CBS 197.49 | Netherlands | N/A | DQ470964 | DQ470915 | N/A | [40] |
| Entonaema cinnabarinum | agtS377 | Germany | AY616685 | N/A | N/A | N/A | [14] |
| Entonaema cinnabarinum | CNF 2/11046 | Croatia | OQ863621 | OQ863622 | OQ877102 | OQ877113 | This study |
| Entonaema cinnabarinum | CNF 2/11047 | Croatia | OQ863735 | OQ864983 | OQ877103 | OQ877114 | This study |
| Entonaema cinnabarinum | CNF 2/11052 | Croatia | OQ864984 | OQ865000 | OQ877104 | OQ877115 | This study |
| Entonaema cinnabarinum | CNF 2/11053 | Croatia | OQ869782 | OQ869785 | OQ877105 | OQ877116 | This study |
| Entonaema liquescens | ATCC 46302 | USA | KY610389 | KY610443 | KY624253 | KX271248 | [10] |
| Entonaema liquescens | agtS279 | Germany | AY616686 | N/A | N/A | N/A | [14] |
| Entonaema liquescens | CNF 2/11263 | USA | OQ869784 | OQ865124 | OQ877106 | OQ877117 | This study |
| Entonaema liquescens | S.D. Russell iNaturalist # 91210856 | USA | OM972573 | N/A | N/A | N/A | [41] |
| Entonaema pallida | PP92a | Peru | FJ884093 | FJ890379 | N/A | N/A | [42] |
| Entonaemasp. | JHGB08 1A | Peru | MH267933 | N/A | N/A | N/A | [43] |
| Entonaemasp. | AHB18 5B | Peru | MH267934 | N/A | N/A | N/A | [43] |
| Entonaemasp. | F5071 | Panama | KF746156 | N/A | N/A | N/A | [44] |
| Entonaema splendens | KA12-1283 | South Korea | KR673521 | N/A | N/A | N/A | [45] |
| Euepixylon sphaeriostomum | JDR 261 | USA | GU292821 | N/A | GQ844774 | GQ470224 | [31] |
| Graphostroma platystomum | CBS 270.87 HT | France | JX658535 | DQ836906 | KY624296 | HG934108 | [10,38,46,47] |
| Hypocreodendron sanguineum | JDR 169 | Mexico | GU322433 | N/A | GQ844819 | GQ487710 | [31] |
| Hypoxylon addis | MUCL 52797 HT | Ethiopia | KC968931 | N/A | N/A | KC977287 | [33] |
| Hypoxylonaff. rubiginosum | MUCL 57724 | Iran | MT214999 | MT214994 | MT212237 | MT212241 | [48] |
| Hypoxylonaff. rubiginosum | MUCL 57725 | Iran | MT215000 | MT214995 | MT212238 | MT212242 | [48] |
| Hypoxylon baihualingense | FCATAS 477 HT | China | MG490190 | N/A | N/A | MH790276 | [49] |
| Hypoxylon bellicolor | UCH 9543 | Panama | MN056425 | N/A | N/A | MK908139 | [50] |
| Hypoxylon carneum | MUCL 54177 | France | KY610400 | KY610480 | KY624297 | KX271270 | [10] |
| Hypoxylon cercidicola | CBS 119009 | France | KC968908 | KY610444 | KY624254 | KC977263 | [10,33] |
| Hypoxylon chrysalidosporum | FCATAS 2710 HT | China | OL467294 | OL615106 | OL584222 | OL584229 | [51] |
| Hypoxylon crocopeplum | CBS 119004 | France | KC968907 | KY610445 | KY624255 | KC977268 | [10,33] |
| Hypoxylon crocopeplum | CNF 2/11316 | Croatia | OQ865120 | OQ869786 | OQ877107 | OQ877118 | This study |
| Hypoxylon crocopeplum | CNF 2/11317 | Croatia | OQ865187 | OQ869787 | OQ877108 | OQ877119 | This study |
| Hypoxylon cyclobalanopsidis | FCATAS 2714 HT | China | OL467298 | OL615108 | OL584225 | OL584232 | [51] |
| Hypoxylon damuense | FCATAS 4207 HT | China | ON075427 | ON075433 | ON093251 | ON093245 | [52] |
| Hypoxylon damuense | FCATAS 4321 | China | ON075428 | ON075434 | ON093252 | ON093246 | [52] |
| Hypoxylon duranii | YMJ 85 | China | JN979414 | N/A | N/A | AY951714 | [37] |
| Hypoxylon eurasiaticum | MUCL 57720 HT | Iran | MW367851 | N/A | MW373852 | MW373861 | [53] |
| Hypoxylon fendleri | MUCL 54792 | France | KF234421 | KY610481 | KY624298 | KF300547 | [10,33] |
| Hypoxylon fragiforme | MUCL 51264 ET | Germany | KC477229 | KM186295 | KM186296 | KX271282 | [10,34,54] |
| Hypoxylon fraxinophilum | MUCL 54176 ET | France | KC968938 | N/A | N/A | KC977301 | [33] |
| Hypoxylon fuscum | CBS 113049 ET | France | KY610401 | KY610482 | KY624299 | KX271271 | [10] |
| Hypoxylon griseobrunneum | CBS 331.73 HT | India | KY610402 | KY610483 | KY624300 | KC977303 | [10,33] |
| Hypoxylon guilanense | MUCL 57726 HT | Iran | MT214997 | MT214992 | MT212235 | MT212239 | [48] |
| Hypoxylon haematostroma | MUCL 53301 ET | France | KC968911 | KY610484 | KY624301 | KC977291 | [10,33] |
| Hypoxylon howeanum | MUCL 47599 | Germany | AM749928 | KY610448 | KY624258 | KC977277 | [10,33,36] |
| Hypoxylon howeanum | CNF 2/11315 | Croatia | OQ865216 | OQ865215 | OQ877109 | OQ877120 | This study |
| Hypoxylon hypomiltum | MUCL 51845 | France | KY610403 | KY610449 | KY624302 | KX271249 | [10] |
| Hypoxylon investiens | CBS 118183 | Malaysia | KC968925 | KY610450 | KY624259 | KC977270 | [10,33] |
| Hypoxylon isabellinum | STMA10247 HT | France | KC968935 | N/A | N/A | KC977295 | [33] |
| Hypoxylon lateripigmentum | MUCL 53304 HT | France | KC968933 | KY610486 | KY624304 | KC977290 | [10,33] |
| Hypoxylon lenormandii | CBS 119003 | Ecuador | KC968943 | KY610452 | KY624261 | KC977273 | [10,33] |
| Hypoxylon liviae | CBS 115282 ET | Norway | NR155154 | N/A | N/A | KC977265 | [33] |
| Hypoxylon macrosporum | YMJ 47 | Canada | N/A | N/A | N/A | AY951736 | [37] |
| Hypoxylon monticulosum | MUCL 54604 ET | France | KY610404 | KY610487 | KY624305 | KX271273 | [10] |
| Hypoxylon musceum | MUCL 53765 | France | KC968926 | KY610488 | KY624306 | KC977280 | [10,33] |
| Hypoxylon notatum | YMJ 250 | USA | JQ009305 | N/A | N/A | AY951739 | [37] |
| Hypoxylon ochraceum | MUCL 54625 ET | France | KC968937 | N/A | KY624271 | KC977300 | [10,33] |
| Hypoxylon papillatum | ATCC 58729 HT | USA | KC968919 | KY610454 | KY624223 | KC977258 | [10,33] |
| Hypoxylon perforatum | CBS 115281 | France | KY610391 | KY610455 | KY624224 | KX271250 | [10] |
| Hypoxylon petriniae | CBS 114746 HT | France | NR155185 | KY610491 | KY624279 | KX271274 | [10,32] |
| Hypoxylon pilgerianum | STMA 13455 | France | KY610412 | KY610412 | KY624308 | KY624315 | [10] |
| Hypoxylon porphyreum | CBS 119022 | France | KC968921 | KY610456 | KY624225 | KC977264 | [10,33] |
| Hypoxylon pseudefendleri | MFLUCC 11-0639 HT | Thailand | KU940156 | KU863144 | N/A | N/A | [55] |
| Hypoxylon pseudofuscum | KR:0005879 HT | Germany | MW367857 | MW367848 | MW373858 | MW373867 | [53] |
| Hypoxylon pulicicidum | CBS 122622 HT | France | JX183075 | KY610492 | KY624280 | JX183072 | [10,56] |
| Hypoxylon rickii | MUCL 53309 ET | France | KC968932 | KY610416 | KY624281 | KC977288 | [10,33] |
| Hypoxylon rubiginosum | MUCL 52887 ET | Germany | KC477232 | KY610469 | KY624266 | KY624311 | [10,54] |
| Hypoxylon rubiginosum | MUCL 57727 | Iran | MT214998 | MT214993 | MT212236 | MT212240 | [48] |
| Hypoxylon samuelsii | MUCL 51843 ET | France | KC968916 | KY610466 | KY624269 | KC977286 | [10,33] |
| Hypoxylon sheariivar. minor | YMJ 29 | Mexico | EF026142 | N/A | N/A | AY951753 | [31,37] |
| Hypoxylon sporistriatatunicum | UCH 9542 HT | Panama | MN056426 | N/A | N/A | MK908140 | [50] |
| Hypoxylon submonticulosum | CBS 115280 | France | KC968923 | KY610457 | KY624226 | KC977267 | [10,33] |
| Hypoxylon texense | DSM 107933 HT | USA | MK287536 | MK287548 | MK287561 | MK287574 | [57] |
| Hypoxylon ticinense | CBS 115271 | France | JQ009317 | KY610471 | KY624272 | AY951757 | [10,37] |
| Hypoxylon ticinense | CNF 2/11314 | Croatia | OQ869783 | OQ865219 | OQ877110 | OQ877121 | This study |
| Hypoxylon trugodes | MUCL 54794 ET | Sri Lanka | KF234422 | KY610493 | KY624282 | KF300548 | [10,33] |
| Hypoxylon ulmophilum | YMJ 350 | Russia | JQ009320 | N/A | N/A | AY951760 | [37] |
| Hypoxylon vogesiacum | CBS 115273 | France | KC968920 | KY610417 | KY624283 | KX271275 | [10,32,33] |
| Hypoxylon wujianggense | GMBC0213 HT | China | MT568854 | MT568853 | MT585802 | MT572481 | [58] |
| Hypoxylon wuzhishanense | FCATAS 2708 HT | China | OL467292 | OL615104 | OL584220 | OL584227 | [51] |
| Hypoxylon wuzhishanense | FCATAS 2709 | China | OL467293 | OL615105 | OL584221 | OL584228 | [51] |
| Hypoxylon zangii | FCATAS 4029 HT | China | ON075423 | ON075429 | ON093247 | ON093241 | [52] |
| Hypoxylon zangii | FCATAS 4319 | China | ON075424 | ON075430 | ON093248 | ON093242 | [52] |
| Jackrogersella cohaerens | CBS 119126 | Germany | KY610396 | KY610497 | KY624270 | KY624314 | [10] |
| Jackrogersella minutella | CBS 119015 | Portugal | KY610381 | KY610424 | KY624235 | KX271240 | [10,32] |
| Jackrogersella multiformis | CBS 119016 ET | Germany | KC477234 | KY610473 | KY624290 | KX271262 | [10,32,33] |
| Kretzschmaria deusta | CBS 163.93 | Germany | KC477237 | KY610458 | KY624227 | KX271251 | [10,54] |
| Nemania bipapillata | HAST 90080610 | Taiwan | GU292818 | N/A | GQ844771 | GQ470221 | [31] |
| Nemania delonicis | MFLU 19-2124 | Thailand | MW240613 | MW240542 | MW342617 | MW775574 | [59] |
| Nemania primolutea | HAST 91102001 HT | Taiwan | EF026121 | N/A | GQ844767 | EF025607 | [31] |
| Obolarina dryophila | MUCL 49882 | France | GQ428316 | GQ428316 | KY624284 | GQ428322 | [10,60] |
| Podosordaria muli | WSP 167 HT | Mexico | GU324761 | N/A | GQ853038 | GQ844839 | [31] |
| Podosordariasp. | CNF 2/11073 | Croatia | OQ865223 | OQ865228 | OQ877111 | OQ877122 | This study |
| Poronia punctata | CBS 656.78 HT | Australia | KT281904 | KY610496 | KY624278 | KX271281 | [10,61] |
| Pyrenopolyporus hunteri | MUCL 52673 ET | Ivory Coast | KY610421 | KY610472 | KY624309 | KU159530 | [10,32] |
| Pyrenopolyporus laminosus | MUCL 53305 HT | France | KC968934 | KY610485 | KY624303 | KC977292 | [10,33] |
| Pyrenopolyporus nicaraguensis | CBS 117739 | Burkina Faso | AM749922 | KY610489 | KY624307 | KC977272 | [10,33,36] |
| Pyriformiascoma trilobatum | MFLUCC 14-0012 HT | Italy | KP297402 | KP340543 | KP340530 | KP406613 | [34] |
| Rhopalostroma angolense | CBS 126414 | Ivory Coast | KY610420 | KY610459 | KY624228 | KX271277 | [10] |
| Rosellinia aquila | MUCL 51703 | France | KY610392 | KY610460 | KY624285 | KX271253 | [10] |
| Rosellinia corticium | MUCL 51693 | France | KY610393 | KY610461 | KY624229 | KX271254 | [10] |
| Rosellinia necatrix | CBS 349.36 | Argentina | AY909001 | KF719204 | KY624275 | KY624310 | [10,62] |
| Rostrohypoxylon terebratum | JF-TH 06-04 HT | Thailand | DQ631943 | DQ840069 | DQ631954 | DQ840097 | [63] |
| Ruwenzoria pseudoannulata | MUCL 51394 HT | D. R. Congo | KY610406 | KY610494 | KY624286 | KX271278 | [10] |
| Sarcoxylon compunctum | CBS 359.61 | South Africa | KT281903 | KY610462 | KY624230 | KX271255 | [10,61] |
| Stilbohypoxylon elaeicola | YMJ 173 | France | EF026148 | N/A | GQ844826 | EF025616 | [31] |
| Stilbohypoxylon quisquiliarum | YMJ 172 | France | EF026119 | N/A | GQ853020 | EF025605 | [31] |
| Thamnomyces dendroidea | CBS 123578 HT | France | FN428831 | KY610467 | KY624232 | KY624313 | [10,64] |
| Xylaria bambusicola | WSP 205 HT | Taiwan | EF026123 | N/A | GQ844802 | AY951762 | [31] |
| Xylaria brunneovinosa | HAST 720 HT | France | EU179862 | N/A | GQ853023 | GQ502706 | [31,65] |
| Xylaria discolor | HAST 131023 ET | USA | JQ087405 | N/A | JQ087411 | JQ087414 | [66] |
| Xylaria hypoxylon | CBS 122620 ET | Sweden | KY610407 | KY610495 | KY624231 | KX271279 | [10,67] |
| Xylaria multiplex | HAST 580 | France | GU300098 | N/A | GQ844814 | GQ487705 | [31] |
| Xylaria polymorpha | MUCL 49884 | France | KY610408 | KY610464 | KY624288 | KX271280 | [10] |
| Xylaria sicula | CNF 2/11087 | Croatia | OQ865227 | OQ865230 | OQ877112 | OQ877123 | This study |
3. Results
3.1. Molecular Phylogenetic Analyses
In this study, a total of 44 DNA sequences (11 ITS, 11 LSU, 11 rpb2, 11 β-tub) belonging to 11 fungal strains from the CNF were newly generated. Of the 11 fungal strains, four strains were identified as E. cinnabarinum (CNF 2/11046, 2/11047, 2/11052, 2/11053), two as Hypoxylon crocopeplum Berk. & M.A. Curtis (CNF 2/11316, 2/11317) and one each as E. liquescens (CNF 2/11263), Hypoxylon howeanum Peck (CNF 2/11315), Hypoxylon ticinense L.E. Petrini (CNF 2/11314), Podosordaria sp. (CNF 2/11073), and Xylaria sicula Pass. & Beltrani (CNF 2/11087). Associated accession numbers are marked in bold in Table 1.
The results of the phylogenetic analyses were similar to those previously published by Wendt et al. [10], Pourmoghaddam et al. [48], Song et al. [52] and Ma et al. [51]. Only significant branch support values of Bayesian posterior probability (BI-PP ≥ 0.95) and ultrafast bootstrap support (ML-BP ≥ 70%) are shown in the phylogram (Figure 1).
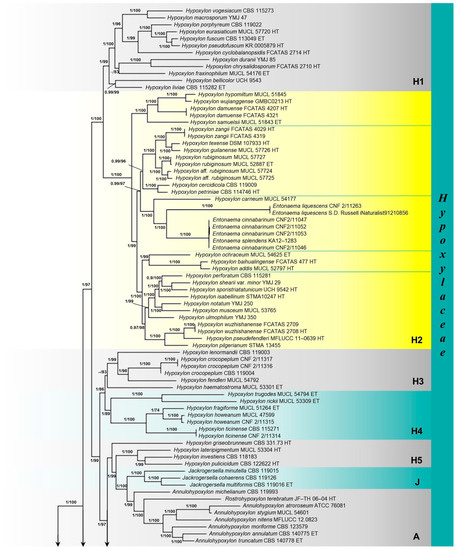
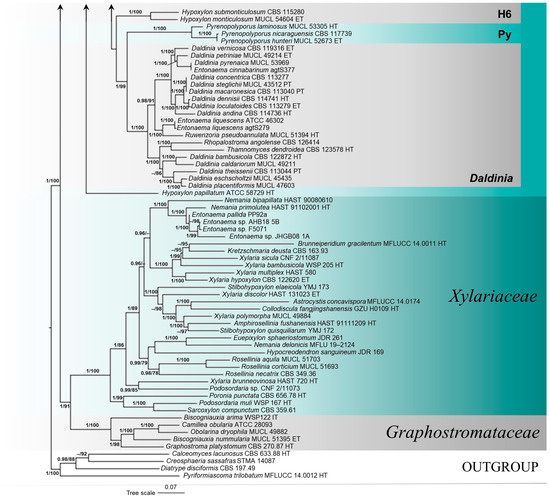
Figure 1.
Phylogenetic tree of family Hypoxylaceae, based on Bayesian Inference (BI) and Maximum Likelihood (ML) analyses of concatenated four-gene (ITS, LSU, rpb2, β-tub) sequence alignment. Significant branch support values, Bayesian posterior probability (BI-PP ≥ 0.95) and ultrafast bootstrap support (ML-BP ≥ 70%), are presented at the nodes. Abbreviations: HT = holotype, ET = epitype, IT = isotype, PT = paratype.
The four-gene phylogeny revealed three main clades belonging to the families Hypoxylaceae, Xylariaceae, and Graphostromataceae. The most represented genus of Hypoxylaceae in the phylogenetic analysis was Hypoxylon Bull. with 76 strains, followed by 14 strains of Daldinia Ces. & De Not. and Entonaema, nine strains of Annulohypoxylon Y.M. Ju, J.D. Rogers & H.M. Hsieh, three strains of Jackrogersella L. Wendt, Kuhnert & M. Stadler, and Pyrenopolyporus Lloyd, and representative strains of Rhopalostroma D. Hawksw., Thamnomyces Ehrenb., Ruwenzoria J. Fourn., M. Stadler, Læssøe & Decock, and Rostrohypoxylon J. Fourn. & M. Stadler. Phylogeny revealed paraphyly of the genus Hypoxylon (H1-H6) with the genera Annulohypoxylon (A), Daldinia, Entonaema, Jackrogersella (J), Pyrenopolyporus (Py), and Rhopalostroma, Thamnomyces, and Ruwenzoria (Daldinia clade) embedded in the Hypoxylaceae clade.
The Hypoxylon H2 clade consisted of five strongly supported groups. Newly sequenced strains of Entonaema from the CNF, along with E. splendens KA12-1283 and E. liquescens S.D. Russell iNaturalist91210856, were phylogenetically very well differentiated (BI-PP = 1, ML-BP = 100) from a single lineage of Hypoxylon carneum Petch, in a strongly supported (BI-PP = 1, ML-BP = 100) subclade of the H2 clade. Entonaema cinnabarinum (CNF 2/11046, 2/11047, 2/11052, and 2/11053) clustered with E. splendens KA12-1283 and formed a maximally supported monophyletic clade (BI-PP = 1, ML-BP = 100) with E. liquescens CNF 2/11263 and the sister strain E. liquescens S.D. Russell iNaturalist91210856.
However, the Entonaema strains from the GenBank (with the exception of E. splendens KA12-1283) were distributed among other clades in the phylogenetic tree. In the Daldinia clade of Hypoxylaceae, E. cinnabarinum agtS377 recovered in a strongly supported monophyletic clade (BI-PP = 1, ML-BP = 100) with the epitype of D. vernicosa Ces. & De Not. (CBS 119316), the epitype of D. petriniae Y.M. Ju, J.D. Rogers & F. San Martín (MUCL 49214), and D. pyrenaica M. Stadler & Wollw. (MUCL 53969). Also, E. liquescens ATCC 4630 and E. liquescens agtS279 formed a monophyletic group (BI-PP = 1, ML-BP = 100) with Ruwenzoria pseudoannulata J. Fourn., M. Stadler, Læssøe & Decock (MUCL 51394) in the Daldinia clade. Entonaema sp. F5071, E. pallida PP92a, Entonaema sp. AHB18 5B, and Entonaema sp. JHGB08 1A were recovered in a phylogenetically fully supported clade (BI-PP = 1, ML-BP = 100) with the holotype strain of Nemania primolutea Y.M. Ju, H.M. Hsieh & J.D. Rogers (HAST 91102001) and N. bipapillata (Berk. & M.A. Curtis) Pouzar (HAST 90080610) in the Xylariaceae (Figure 1).
3.2. Taxonomy
Entonaema Möller, Bot. Mitt. Trop. 9: 306 (1901)
Generic diagnostic characters: Stromata pulvinate, subglobose to globose, often becoming irregularly lobed and/or wrinkled, especially at the tapered base, arising from woody substrates, most often from coarse woody debris. During the development, the enlarging cavity becoming filled with watery liquid. Stroma entonaematoid: stromatal flesh gelatinous without any concentric zonation, with ±vividly coloured surface until reaching late sporulation phase, when gradually turn to a horny or hard consistence and becoming wrinkled and carbonaceus due to the deposition of melanin pigments. Perithecia monostichous, developing immediately below bright coloured outer cortex. Interperithecial tissue ± carbonaceous at least around perithecial walls and in subperithecial layer (between perithecial bases and gelatinous inner fleshy layer). Ostioles inconspicuous, punctiform, umbilicate to papillate. Asci cylindric with tapered base arising from croziers, apically with amyloid ring. Ascospores unicellular, walls with a shade of brown, with ±longitudinal ventral germ slit. Stromatal pigments of mitorubrin/rubiginosin type (azaphilones) are present in three species that are so far certain members of the genus confirmed either by phylogenetic (this paper) or HPLC analyses [2], viz. E. liquescens (type), E. cinnabarinum, and E. globosum. All three species also possess yellowish-orange to orange or rusty red extractable stromatal pigments and an indehiscent perispore in 10% KOH.
Anamorph: when cultivated, they often contain contamination, often of Daldinia spp., in need of thorough reinvestigation (see text below).
Notes: Three certain members of Entonaema (E. liquescens, E. cinnabarinum and E. globosum) differ from the most similar entonaematoid species from the genus Xylaria (X. mesenterica, X. telfairii (Berk.) Sacc. and allies) producing voluminous stromata with azonate and gelatinous to liquid interior, by orange to red KOH-extractable pigments vs. greenish-yellow ones, and by mitorubrin/rubiginosin-type metabolites, that lack in Xylaria. On the other hand, the three Entonaema spp. differ from the most phylogenetically related Hypoxylon carneum in having entonaematoid stromata with orange to red KOH-extractable pigments, while H. carneum has hypoxyloid, flat-pulvinate stromata with dark brown, thin (~200 µm thick), hard tissue below the perithecia and livid-violet KOH-extractable pigments, which are absent in aged material [48]. Contrary to Entonaema spp., azaphilone metabolites are not present in H. carneum.
Entonaema cinnabarinum (Cooke & Massee) Lloyd, Mycol. Writ. 7(69): 1203 (1923); Figure 2, Figure 3 and Figure 4 and Figure 5J–L.
Basionym: Xylaria cinnabarina Cooke & Massee, Grevillea 15(76): 101 (1887)
= Sarcoxylon aurantiacum Pat., Bull. Soc. mycol. Fr. 27(3): 331 (1911)
≡ Entonaema aurantiacum (Pat.) Lloyd, Mycol. Writ. 7(69): 1203 (1923)
Stromata: globose to irregularly convoluted, often constricted to the cerebriform at the base, *28–80 × 16–72 mm, when immature surface yellowish-cream to pale rosy, on smearing apricot orange, at maturity surface yellow-ochre to rosaceous-orange, fulvous, dull brick red, often cinnabar red around the ostioles, ostioles and some areas between perithecia dusted blackish due to the ejected spores, with age becoming brownish-orange to reddish-brown, surface finely pruinose in younger stages, becoming smooth and often cracked with age. Ostioles punctate to papillate, rounded. Interior hollow and filled with pale yellow translucent viscose liquid; in section outer cortex orange to cinnabar red, 0.1–0.2 mm thick, covered with dull yellow detachable pruinose matter, beneath is a tough layer, 0.8–1 mm thick, composed of whitish to pale grey interperithecial tissue blackening with age, and a thin carbonaceous layer (in which perithecia are embedded) continuing to form a black layer underlying perithecial bases; perithecia globose to ellipsoid, black when mature; below the perithecial layer there is 2–6 mm thick, pale yellow to olivaceous, gelatinous, elastic, semitranslucent layer. In 5% and 10% KOH, the cortex is strongly brick red, liquid slightly discolouring, flesh apricot.
Ascomatal structures: Perithecia ranging from globose through ellipsoid to cylindrical, (350–)430–690 µm high, 340–660 µm wide, ostioles *95–120(–150) µm wide, periphyses hyaline eguttulate, cylindrical, apically obtusely tapered to sublanceolate, simple, one to few celled, flexuous, *2.5–5.2 µm wide. Asci cylindrical to narrowly clavate, 8-spored, †100–136 × 7.3–9 µm, *11.4–12.8 µm wide, delicate walled, wall persistent, not evanescent, apex thin-walled, apical dome barely visible, when *mature subtruncate-obtuse, †rounded to obtuse tapered, in IKI moderately (2bb) euamyloid, reaction zone simple, 2–2.4 µm wide, 1–1.2 µm high, ascoplasm highly vacuolate in all developmental phases, arising from Xylaria-type croziers, cells with weakly refractive globules. Ascospores brownish- olive to brownish-grey, dorsiventrally almost radially symmetrical, in profile slightly inequilateral, ellipsoid to suboblong with blunt ends, 1-celled, (8.1–)8.5–9.8–11.5(–12.1) × (4.7–)5–5.9–6.8(–7.1) µm, Q = (1.34–)1.38–1.73–2.02(–2.16), (1)2(3)-guttulate, without sheath, germ slit ventral, longitudinal to ± oblique and almost straight, 2/3 to almost whole spore length, spore wall thin, ±0.2 µm thick, sporoplasm contain a deBary bubble in CB, perispore smooth, in 10% KOH indehiscent.
Stromatal tissue: Cortex (crust) covered with rusty red granules, mainly consisting of ± homogenous block of highly refractive resin-like 50–210 µm thick layer, interspersed with locally densely set, hyaline, thin-walled sparingly septate cylindrical sinuous hyphae with blunt apices, hyphae †2.4–6.5 µm wide. Interperithecial tissue *440–720 µm thick, mainly composed of vertically oriented textura prismatica with ± thin-walled cells encrusted with very fine minute blackish granules, cells *3–7.7 µm wide, walls 0.3–0.5 µm thick. Perithecial walls ca. *50–80 µm thick, mainly composed of textura angularis-prismatica, walls of the same type as in interperithecial tissue, cells *7.5–16.1 × 2.9–9.5 µm wide. Interperithecial tissue below perithecia gradually turn into subperithecial layer, 190–380 µm thick, which is composed of textura fascicularis-intricata, cells *7.5–16.5 µm wide, walls thickened and highly melanised, and a thin layer of textura oblita with intricate cell organization, cells *3.3–11 µm wide, walls hyaline, moderately refractive, gelified, up to 2.6 µm thick. Internal tissue of textura gelatinosa, hyphae widely spaced, hyaline, thin-walled, delicate, cells *3–8.8 µm wide, interspersed with rich gel.
Anamorph: not obtained on PDA, MEA, and OA.
Material examined:
CROATIA. Zagreb County: Malinje forest, Crna Mlaka near Jastrebarsko, 109 m a.s.l., 45.606817° N, 15.71695° E, on the lying trunk of Quercus robur in an old growth forest of Q. robur, Fraxinus angustifolia, Ulmus minor, Carpinus betulus, Tilia sp., 5 July 2009, M. Čerkez (CNF 2/8250); ibid. 45.605918° N, 15.716523° E, on the fallen branch of U. minor, 20 September 2009, N. Matočec & M. Čerkez (no voucher), ibid. 45.608199° N, 15.715229° E, on the fallen thick branch of Q. robur, 29 October 2020, I. Kušan & N. Matočec (CNF 2/11052), ibid. 45.608048° N, 15.715265° E, on the fallen thick branch of Q. robur, 29 October 2020, I. Kušan & N. Matočec (CNF 2/11053). Sisak-Moslavina County: Lonjsko Polje Nature Park, Opeke area near Kraljeva Velika, 95 m a.s.l., 45.370119° N, 16.820806° E, on the fallen semi-decorticated thin branch of C. betulus in a forest of Q. robur, F. angustifolia and C. betulus, 3 October 2020, J. Marković (CNF 2/11046); ibid. 45.373779° N, 16.821250° E, on the lying trunk of F. angustifolia, 16 October 2021, I. Kušan & N. Matočec, (no voucher); Trebeški đol area near Kraljeva Velika, 95 m a.s.l., 45.369202° N, 16.780788° E, on the fallen corticated thin branch of Salix sp. in a forest of Q. robur, C. betulus with Salix sp., 17 October 2020, M. Josipović, (CNF 2/11047); Tena’s walking path near Ilova, 99 m a.s.l., 45.433639° N, 16.823863° E, on the lying trunk of Fraxinus angustifolia in an old growth forest of Q. robur, F. angustifolia and Ulmus minor, 28 September 2021, J. Marković, I. Kušan & N. Matočec, (CNF 2/11267). This species was here recorded for the first time for Croatia.
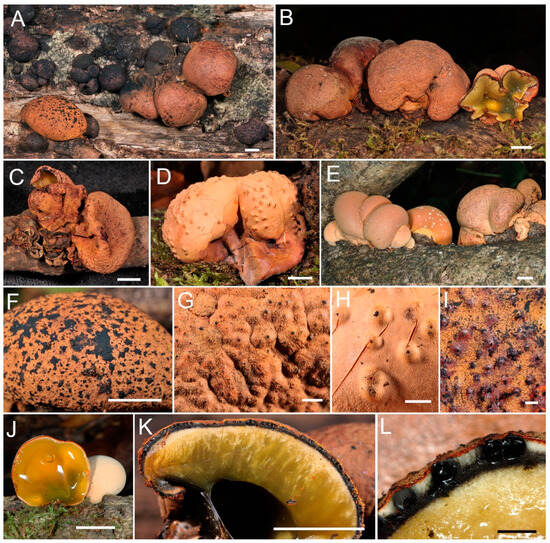
Figure 2.
Entonaema cinnabarinum. (A) Stromata associated with Daldinia childiae J.D. Rogers & Y.M. Ju. (B–D) Mature stromata. (E) Young stromata. (F) Surface of the stroma dusted with ascospores. (G–I) Perithecial mounds with ostioles. (J) Section through young stroma. (K,L) Section through mature stroma. (A,F,G,K) CNF 2/11046; (B) (Croatia, 16.10.2021., no voucher); (C) CNF 2/11047; (D,H) CNF 2/11052; (E,J) (Croatia, 20.9.2009., no voucher); (I,L) CNF 2/8250. Bars: (A–F,J,K) = 1 cm; (G–I,L) = 1 mm. Photo: N. Matočec & I. Kušan.
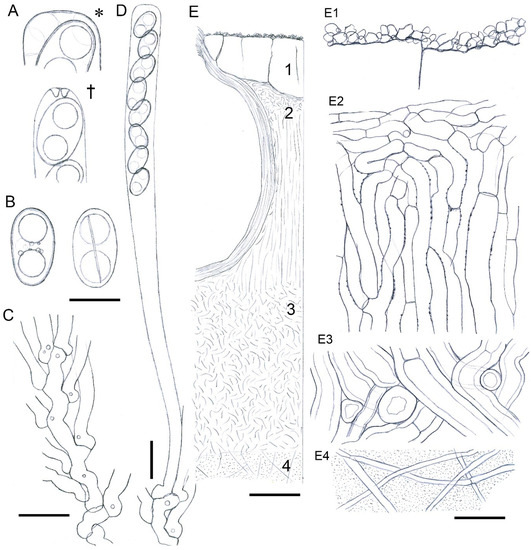
Figure 3.
Entonaema cinnabarinum (CNF 2/8250). (A) Living (*) and dead (†) ascal apices. (B) Ascospores in frontal and dorsiventral view with a visible germ slit. (C) Ascogenous system. (D) Ascus. (E) Stromatal section ((E1) stromatal surface, (E2) interperithecial tissue, (E3) lower part of subperithecial layer, (E4) internal tissue). Bars: (A,B) = 5 µm; (C,D,E1–E4) = 10 µm; (E) = 100 µm. Del. N. Matočec.
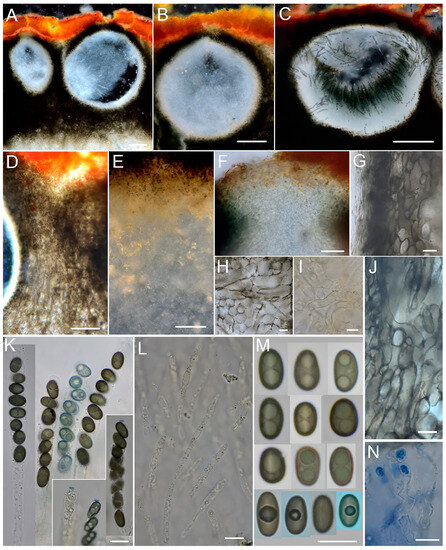
Figure 4.
Entonaema cinnabarinum. (A–C) Sections through perithecia. (D) Interperithecial tissue. (E) Subperithecial layer (upper part) and internal tissue (lower part). (F) Ostiole. (G) Perithecial wall. (H) Cells of the subperithecial layer. (I) Cells of the internal tissue. (J) Cells in the interperithecial tissue. (K) Asci in H2O and IKI. (L) Periphyses. (M). Ascospores in H2O and CB (last row). (N) Croziers in CRB. (A,B,F,K–M) CNF 2/11046; (C,N) CNF 2/11047; (D,E,G–J) CNF 2/11053. Bars: (A–E) = 100 µm; (F) = 20 µm (G–N) = 10 µm. Photo: N. Matočec & I. Kušan.
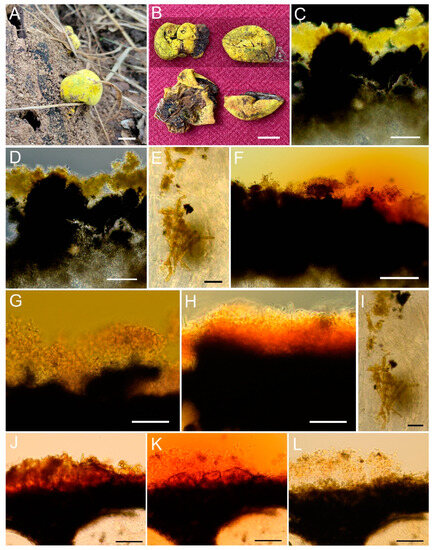
Figure 5.
(A–I) Entonaema liquescens (CNF 2/11263), (J–L) E. cinnabarinum (CNF 2/11267). (A) Stromata in situ. (B) Stromata ex situ. (C,D) Lemon yellow pigments on the stromatal surface in H2O (dark field, phase contrast). (E) Crystalloid pigments embedded inside interperithecial tissue (H2O). (F) Pigment reaction upon adding 10% KOH. (G) Pigment soluble phase extracted in 10% KOH. (H) Pigment insoluble phase becoming rusty orange in 10% KOH. (I) Crystalloid pigments embedded inside interperithecial tissue in 10% KOH. (J) Rusty orange pigment granules on the stromatal surface in H2O. (K) Pigment reaction upon adding 10% KOH. (L) Hyphal cover completely left without pigments after 10% KOH treatment. Bars: (A,B) = 1 cm; (C,D,F–H,J–L) = 50 µm; (E,I) = 10 µm. Photo: (A) T. Gonzales; (B) B. Bunyard; (C–L) N. Matočec & I. Kušan.
Entonaema liquescens Möller, Bot. Mitt. Trop. 9: 307 (1901); Figure 5A–I
= Xylaria splendens Berk. & M.A. Curtis, J. Linn. Soc., Bot. 10(46): 382 (1868)
≡ Entonaema splendens (Berk. & M.A. Curtis) Lloyd, Mycol. Writ. (Cincinnati) 7(69): 1202 (1923)
≡ Glaziella splendens (Berk. & M.A. Curtis) Berk., in Glaziou, Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn 80: 31 (1879)
NOTE: The studied collection of E. liquescens consisted of two stromata, both immature. Perithecia and ascal structures were found only in an immature stage, without any traces of developed ascospores. It was stromata globose to slightly cerebriform, 22–29 × 17–20 mm, lemon yellow, and hollow. Pigments on the stromatal surface were lemon yellow in H2O (best visible in dark field, Figure 5C). After adding 5% KOH and 10% KOH, they extract a yellowish-orange to rusty orange colour to the medium, while the remaining pigments that are fixed in the cortex turn rusty orange (Figure 5H). The crystalloid pigments embedded in interperithecial tissue remain unchanged (Figure 5E,I). Since E. liquescens and E. cinnabarinum have very similar microscopical characters usually used in species distinctions, the two species differ sharply in their microchemical traits. In contrast to E. liquescens, E. cinnabarinum lacks an insoluble pigment phase whereby the cortical layer remains subhyaline after KOH treatment (Figure 5L). Additionally, the two species differ by the pigment granules colour in *H2O, which is lemon yellow in E. liquescens (Figure 5C,D) and rusty red in E. cinnabarinum (Figure 5J).
Material examined: USA. Kansas: Morris Co., Council Grove Reservoir, on the fallen decorticated branch of a Quercus sp. in a forest of Quercus stellata and Q. marilandica, 18 September 2021, T. Gonzales, (CNF 2/11263).
Worldwide identification key to the putative species of Entonaema
| (1) Stromatal extractable pigments greenish yellow in 10% KOH, but red in NH3 …...................................................................................................................................Xylaria p.p. |
| - Stromatal extractable pigments orange to brick red or entirely absent in 10% KOH, orange in NH3….....................................................................................................…Entonaema (2) |
| (2) Stromatal orange to red pigment granules present in the section immediately beneath the stromatal surface and around the perithecial ostioles, stromatal extractable pigments in 10% KOH brick red................................................................3 |
| - Stromatal pigment granules in the section of the stromatal cortex yellow, green, or absent, stromatal extractable pigments in 10% KOH yellowish-orange to orange, or absent...........................................................................................................................................................4 |
| (3) Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles rusty orange, ostioles papillate, perithecia 300–600 μm in diam., ascospores 9–13 μm long..........................................................E. cinnabarinum* |
| - Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles blood red, ostioles umbilicate, perithecia 100–300 μm in diam., ascospores 8–10 μm long..........................................................................................E. globosum |
| (4) Stromatal surface with olivaceous tint in maturity, perithecia 200–500 μm in diam., ascospores cylindrical with ± blunt ends, 8–13 × 3.5–6.5 μm; stromatal extractable pigments orange in 10% KOH, but not tested in E. siamensis...................................................5 |
| - Stromatal surface yellowish-tan or dark reddish-brown in maturity, perithecia 500–1000 μm in diam., ascospores ellipsoid with subacute ends, 13–18 × 6.5–8 μm, stromatal extractable pigments absent with 10% KOH…………………………….………......................6 |
| (5) Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles vividly yellow, ascospores 5–6.5 μm wide, asci 6–10 μm wide............................................................................................................E. liquescens* |
| - Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles consists of green granules, ascospores 3.5–5 μm wide, asci 5–6 μm wide........................................................................................................ E. siamensis |
| (6) Stromatal surface dark reddish-brown, perithecial ostioles prominently papillate, ascospores up to 15 μm long............................................................................................E. dengii |
| - Stromatal surface yellowish-tan with or without reddish-orange tinges, perithecial ostioles inconspicuous, punctate, ascospores always exceed 14.5 μm in length (up to 18 μm)..................................................................................................................................................7 |
| (7) Ascospores lemon-shaped with ± papillate ends, perispore indehiscent in 10% KOH ……………………….………………………………………………………….…E. moluccanum |
| - Ascospores ellipsoid with ± tapered ends, perispore dehiscent in 10% KOH………………..……………..E. moluccanum ss. Sánchez-Jácome & Guzmán-Dávalos |
An asterisk (*) denotes species belonging to Entonaema confirmed by phylogenetic analysis.
3.3. Ecology and Biogeography
All scientific publications accessible to the authors and the GBIF database (an extensive high quality online database on worldwide occurrences of biological species; www.gbif.org, accessed on 26 June 2023) were searched for verifiable Entonaema records in an attempt to provide better understanding of the ecology and biogeography of Entonaema species. A detailed analysis of a total of the here-accepted 266 worldwide Entonaema records that were attributable to a species (209 finds of E. liquescens, 51 of E. cinnabarinum, two of E. globosum, one of each E. dengii, E. mollucanum, E. ‘mollucanum’, and E. siamensis), accompanied with precise localities, revealed much more information on biogeographic traits on the two most frequent species (E. liquescens and E. cinnabarinum) than in any of the previous studies. According to all here-accepted records of Entonaema spp. with known record dates, two phenological fructification patterns were recognized: (a) Mature stromata may be found all year round in tropical rainforest zones (Af) or depending on rainy period in monsoon (Am) and savannah areas (Aw); and (b) immature stromata were found during May and June, and mature ones from July to October (November) in four-season warm temperate northern zones (Cfa, Cfb, Csa, Cwa), see Figure 6. In the areas under the warm temperate climatic regime, immature stromata may also be found during summer and the autumn along with the mature ones, and quite often the maturation of perithecia/asci may completely fail in certain years in some localities ([2,68], GBIF data, our study), or only a few perithecia manage to ripe per stroma. Consequently, this unpredictability posed difficulties when re-studying fungaria material. A number of valuable collections consist only of sterile stromata (cf. [2,4,5,69]). According to all accepted records accompanied either with ecological data (substrate, habitat) or precise geographic coordinates, Entonaema species were mainly found in various forest ecosystems (including managed ones), or sometimes in city parks, on widely diverse angiosperm tree hosts, very often on coarse woody debris. Nearly all records found in warm temperate areas were in the near vicinity of freshwater bodies (rivers, rivulets, creeks, lakes, etc.).
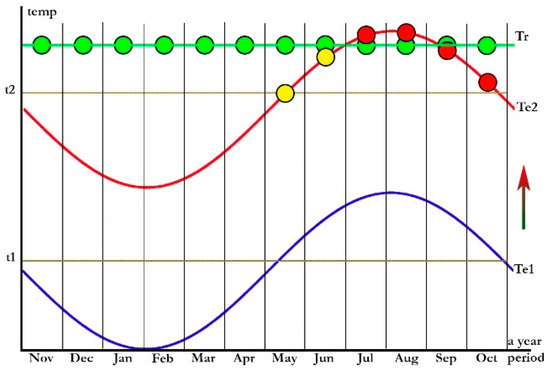
Figure 6.
Two Entonaema fructification patterns: tropical pattern (Tr) represented by green circles as potential records of ripe stromata during all year (Af climate type) or depending on rainy season (Aw and Am climate types); warm temperate zone pattern (Te2) represented by yellow circles corresponding to immature stromata and red circles to ripe stromata, that occur during the warmest months. Areas with cool temperate climate (Te1) are devoid of Entonaema records. Legend: t1 = cardinal minimum temperature that would enable ascospore overwintering; t2 = minimal temperature needed for stromatic development; right arrow denotes climatic shift from Dfa (cool temperate climatic type) to Cfa (warm temperate climatic type).
According to 266 records accepted by us, E. liquescens is by far the most-frequently recorded species (209 records). This species has transcontinental distribution in humid tropical (Am and Aw) areas of Africa (Kenya, Mayotte Island, South Africa, Uganda—[3,5], Zaire—[5]), the Americas (Brazil, Colombia—[4], Costa Rica, Cuba—[3], El Salvador, Honduras, Mexico—[5], Panama—[2], Trinidad and Tobago, Venezuela), Asia (China—[5], Thailand—[13]), and in humid warm temperate (Cfa, Cfb, Csa, Cwa) areas of Asia (India, Japan—[3]) and the Americas (Argentina—[5], Brazil—[1,2,5], Ecuador, Mexico, USA—[5,70]). There are also a few records of this species in cool temperate areas near the edge of the areas of warm temperate climatic regimes: the Russian Far East (Dwb) and northern USA (Dfa).
Entonaema cinnabarinum is another relatively frequently recorded species, with 51 records accepted here. This species also has transcontinental distribution in humid tropical (Af and Am) areas of Africa (Congo—[5], Sierra Leone, Uganda—[5]), the Americas (Costa Rica—[5]), Asia (India—[3], Philippines—[70], Sri Lanka—[3]), Oceania (Australia—[11], Nova Caledonia—[71]), and in humid warm temperate (Cfa, Csa, Cwa, Cwb) areas of Asia (Iran—[2], Japan—[70], Nepal), the Americas (Mexico—[2], USA), and Europe (Bulgaria—[72], Croatia—this paper, France—[2], Hungary—[68], Russia—[73], Serbia, Spain). There are also a few records of this species in cool temperate regions near the edge of the areas of warm temperate climatic regimes: the Russian Far East (Dwb—[74,75]).
This study did not bring any new distributional or taxonomic data for other species, because all records are already treated in earlier publications. The following four species are apparently so far confined to a tropical climatic areas. Entonaema globosum is so far known only from two Mexican localities under the influence of tropical rainforest (Af) and savannah (Aw) climatic regimes [12,69]. Entonaema siamensis is apparently known only from a type locality in Thailand [13], while E. dengii is from a type locality on Hainan Island, China [5], both situated in the areas of tropical savannah climate (Aw). Entonaema moluccanum is known only from a type locality on Halmahera Island, Indonesia [5], under influence of a tropical rainforest climate (Af). We agree with Stadler et al. [2] that, according to perispore dehiscence and different ascospore morphology, material published by Sánchez-Jácome and Guzmán-Dávalos [76] does not represent E. moluccanum, but some undescribed species. Also, it is hitherto known from a single locality under a warm temperate climatic regime (Cwa), outside of tropics. The first record from each locality in published papers are cited above. The other country records were mainly covered solely by GBIF.
4. Discussion
4.1. Taxonomic Implications
A data matrix for DNA sequence alignment was constructed to re-analyse the phylogenetic position of three entonaemoid species, viz. E. liquescens—a type species, E. cinnabarinum, and Xylaria mesenterica (Möller) M. Stadler, Læssøe & J. Fourn.—a former member of Entonaema (=E. mesentericum Möller, E. pallidum G.W. Martin). With the emphasis on a wide selection of hypoxyloid species groups previous chemotaxonomic research revealed that three Entonaema species (E. liquescens, E. cinnabarinum, and E. globosum) share the same stromatic HPLC profiles [2], i.e., mitorubrins and rubiginosins (group of azaphilone metabolites), that are characteristic to members of Hypoxylaceae, but not to Xylariaceae. Consequently, we included a wide spectrum of hypoxyloid species in our phylogenetic analysis. The sequences of E. liquescens and E. cinnabarinum obtained from perithecial elements were newly generated for that matter. In the previous studies [10,48,51], the ITS-LSU-rpb2-β-tub phylogeny confirmed Hypoxylon as a paraphyletic genus in the Hypoxylaceae that was recovered in at least six independent clades. Only one Entonaema strain (E. liquescens ATCC46302, [10]) was included in the study and was placed between the two Daldinia clades as a sister species to Ruwenzoria pseudoannulata MUCL 51394. Hypoxylon carneum was recovered as a single lineage with H. cercidicola (Berk. & M.A. Curtis ex Peck) Y.M. Ju & J.D. Rogers, H. petriniae M. Stadler & J. Fourn., and H. rubiginosum (Pers.) Fr. as the phylogenetically closest species, but distant from the type species H. fragiforme (Pers.) J. Kickx f.
In the present study, the phylogeny based on concatenated analysis of ITS, LSU, rpb2 and β-tub gene regions supported previous studies, but nested two analysed species, E. liquescens and E. cinnabarinum, in the H2 subclade of Hypoxylaceae, next to H. carneum as a sister lineage and distant from the strain E. liquescens ATCC46302 in the Daldinia clade. On the chemotaxonomic level, H. carneum differs from Entonaema species in non-azaphilone metabolites (carneic acids) that accumulate in the stromata [77,78]. Additionally, H. carneum differs from true Entonaema spp. (E. liquescens, E. cinnabarinum, and E. globosum) in flat-pulvinate stromata with only very thin, hard, dark brown tissue below the perithecia and livid-violet KOH-extractable pigments, which are absent in aged material (see Section 3.2 above). Moreover, in addition to the CNF collections of Entonaema, nine other Entonaema strains were phylogenetically analysed in this study. Two strains (E. liquescens S.D. Russell iNaturalist91210856 [41] and E. splendens KA12-1283) were recovered with CNF collections in the H2 subclade of Hypoxylaceae as ‘true’ Entonaema. The phylogenetic placement of E. pallidum in the Xylariaceae in our study supports the research of Stadler et al. [2], who recognised similarities between E. palidum and Xylaria spp. in their morphology, 5.8S/ITS nrDNA sequences, and HPLC profiles. Strains named Entonaema sp. JHGB08 1A, Entonaema sp. AHB18 5B [43], and Entonaema sp. [44] recovered alongside E. pallidum in the Xylaria clade, and consequently cannot be considered members of Entonaema. Therefore, our phylogenetic results agree with Lücking et al. [79] that comparison of sequences with GenBank blastn alone [42,43,44] is insufficient for accurate taxonomic characterisation because the reference databases used for molecular identifications are still incomplete and often contain erroneously named sequences. Furthermore, in our opinion only the integrative approach is acceptable if we seek for stabilised and operable taxonomy [80].
Our phylogenetic placement of true Entonaema spp., viz. the type species E. liquescens and E. cinnabarinum in Hypoxylaceae, and on the other hand ‘Entonaema’ pallidum in Xylariaceae is now in correlation with earlier stromatal chemotaxonomic characterisation of the two separate groups, where E. liquescens and E. cinnabarinum have mitorubrinoid/rubiginosinoid HPLC profiles, characteristic for Hypoxylaceae, whereas ‘Entonaema’ pallidum posesses xylaralic HPLC profile characteristic for Xylariaceae [2]. Clear overlapping of genetic and phenetic traits in a small Entonaema clade led us to retain its current generic concept, which would contain only several species around E. liquescens characterised by vividly coloured, large, vesiculose hollow stroma with elastic gelatinous flesh that contain liquid matter inside its cavity, possessing yellow, orange to red stromatic KOH extractable pigments, and mitorubrine/rubiginosine HPLC profile. This would necessitate the erection of several more small genera inside the H2 clade of Hypoxylon s.l. [10]. In such a concept, only a few species would thus clearly belong to Entonaema, viz. E. liquscens (type), E. cinnabarinum, and, relying on specific morphological–chemotaxonomical traits, also E. globosum and E. moluccanum ss. Sánchez-Jácome & Guzmán-Dávalos. The true affinity of E. dengii, E. moluccanum ss. str., and E. siamensis is yet to be ascertained.
4.2. Molecular Misinterpretations
The strains E. liquescens ATCC46302 [10,15], E. liquescens agtS279 [14], and E. cinnabarinum agtS377 [14] were recovered in the Daldinia clade of Hypoxylaceae, similar to previous studies [10,14,48,51]. The phylogenetic position of Entonaema in the Dalidinia clade based on the ITS region was not well supported [14], but maximally supported when the phylogeny was based on four gene regions (including protein gene regions), as presented by Wendt et al. [10] and Wibberg et al. [15]. In these studies, Entonema species were not described by thorough macro- and micromorphological examination of fruiting bodies, or by chemotaxonomical characterization, and DNA for sequencing was obtained from culture collections. The close phylogenetic relationship of Entonaema and Daldinia species was supported by the naphthalene and chromone derivatives produced by both [70]. On the other hand, the presence of mitorubrin-type azaphilones in the ascomata of E. cinnabarinum and E. liquescens [70] and the absence of binaphthalenes (which are ubiquitous in Daldinia) [2] clearly distinguishes Entonaema from Daldinia at the chemotaxonomic level.
Our Entonaema finds were often associated with Daldinia spp. (Figure 2A), including D. childiae, whose stromata have been found on the same dead wood fragments together with the stromata of E. cinnabarinum (cf. [38]). All our efforts to establish E. cinnabarinum in axenic culture failed, where freshly ejected ascospores did not germinate on any of the three tested nutrient media (PDA, MEA and OA), regardless of the varied procedure. In cases where stromata of Entonaema were dusted by spores originated from neighbouring Daldinia stromata, the agar plates were occupied by rapid mycelial growth and conidial development of Daldinia contamination. It seems that Daldinia may often take over the plates because E. cinnabarinum is hardly culturable, if at all, while Daldina propagules germinate very rapidly and its mycelia are very fast growing. Therefore, it is not surprising that the same happened with two critical attempts to obtain culture of E. liquescens—ATCC 46302 strain, KANSAS, USA [6], and of E. cinnabarinum—CBS 113034, Pyrénées Atlantiques, FRANCE [14,70] in previous Entonaema studies. As a consequence, all sequences derived from this reference cultures actually belong to some Daldinia species, but not to Entonaema (E. liquescens nor E. cinnabarina), what Wibberg et al. [15] already justifiably suspected for E. liquescens. This further led to erroneous molecular identification of E. cinnabarium in the study of symbiotic relationships between saproxylic Xiphydria wasps and fungi from the genera Daldinia and Entonaema [81], which turned out to be true only for the fungi of the former genus (cf. [38]). Consequently, the anamorph of Entonaema itself remains vague.
4.3. Distribution and Biogeography
Outside America’s tropical areas, E. liquescens was, until the 1980s, known only from the most southeastern USA with its very humid and warm temperate climate (Cfa), starting with Florida—1939, Louisiana—1956, Alabama—1965, Georgia—1978, and Mississippi—1986 (Figure 7), including few finds in the warmest areas of Kansas along the Missouri River (1979). The species was not found in northern states on the verge of the Cfa climatic area and cooler temperate climatic area (Dfa) (Nebraska, Indiana, Ohio, and Iowa), as well as inside the Dfa area before 2016. Since this time, E. liquescens occurrences in those northern areas has become quite regular—as much as in the southern states—and spreading along the watercourses of the Mississippi, Missouri, Kansas, Platte, Illinois, Ohio, and Tennessee rivers, and their tributaries, as well as along the warm Atlantic Coast and the Potomac River (cf. www.gbif.org, accessed on 26 June 2023).
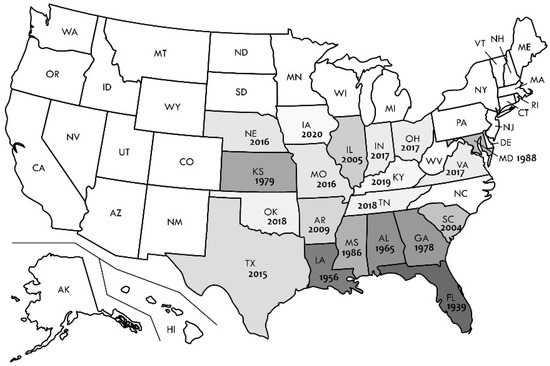
Figure 7.
Map of USA with year of first record of Entonaema liquescens per federal state. Shading intensity reflects the time order in records, where the darkest shade represents the oldest known record.
Virtually all precisely recorded localities revealed (with the aid of Google Earth Pro) that the species’ habitats are either flood plains or alluvial forests, developed along the watercourses, at lake banks, or under the dams of hydroaccumulation reservoirs. The species is mostly distributed in the area of the warm temperate Cfa climate type [30], especially in the USA and Japan. Entonaema liquescens is not known in Europe so far.
Apart from a single Bulgarian longose forest (Mediterranean flood alluvial forest) [72,82], the presence of E. cinnabarinum in Europe (being one of the best explored areas in general, in mycological terms) is recorded only from the turn of the 21st century onwards [68,70,73,83]. Longoses represent subtropical oases in the otherwise relatively dry Mediterranean, and in temperate zones in general, because soil humidity is much prolonged by canopy coverage and by riverine inundation, and therefore not entirely dependent on precipitation. On the other hand, the habitat’s ground substrates are well protected from heavy frosts and dry freezing under dense canopy coverage and thermic marine influence. Therefore, knowing the thermophilic species’ preference, we could assume that this species might have been inhabiting such habitat types long before it was first recorded in 1987 in Bulgaria. Outside Bulgarian longose, the species was first found in the warm temperate area of the Pyrénées Atlantiques in France (1999), then in the Asturian rivulet valley of Spain (2006), Sochi in Russian Federation (2011), and with repetitive records in the Pannonian Plain in Croatia (since 2009), Hungary (2018), and Serbia (2022), see Figure 8.
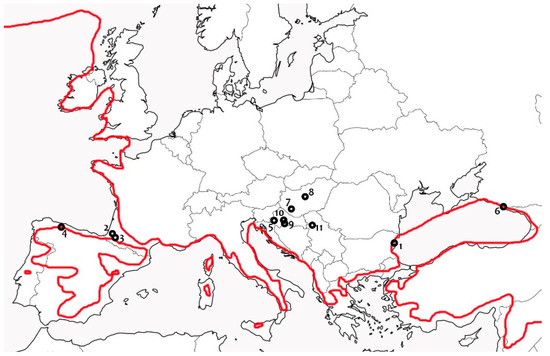
Figure 8.
Known localities of Entonaema cinnabarinum in Europe, arranged according to the year of first record: 1—Kamchiya longose, Bulgaria (1987), 2—Auterrive and 3—Oloron-Ste. Marie, both Pyrénées Atlantiques, France (1999), 4—Belmonte, Asturias, Spain (2006), 5—Crna Mlaka, Zagreb County, Croatia (2009), 6—Agurskye waterfalls near Sochi, Russian Federation (2011), 7—Tókaj forest park, near Kaposvár, Hungary (2018), 8—Selyemrét Nature Trail, Ócsa, Pest County, Hungary (2019), 9—Opeke area near Kraljeva Velika, Sisak-Moslavina County, Croatia (2020), 10—Piljenice area near Ilova, Sisak-Moslavina County, Croatia (2021), and 11—north from Divoš, South Bačka District, Vojvodina, Serbia. Red line represents January isotherm of +5 °C mean air temperature after Stanners & Bourdeau [84]. In cases of two or more too close localities, some are omitted with regard of a map scale.
It is surprising that a fungal species with such a large stromata could be left unrecorded in the second half of the 20th century in mycologically well-explored regions and countries (e.g., USA, Japan, Europe). Croatian mycologist Milica Tortić paid special attention to lignicolous fungi without any Entonaema records during her long and diligent fieldwork, spanning the 1960s to the end of 20th century. The second author conducted a series of detailed fieldwork sessions during 1990s exactly in the same area and the habitat type (oak-ash flood forest, Crna Mlaka) where E. cinnabarinum was first recorded only later in 2009, also without noticing it. Therefore, on the basis of rather abundant recent records of E. cinnabarinum in Europe, and the ascertained global image of the species’ ecological traits, we can assume that this species has been spreading from its most humid-thermophilic European strongholds into new western and southeastern European areas on the account of global warming effects [83].
Owing to a series of eight mild winters (2015/2016–2022/2023) in the above-mentioned European areas for E. cinnabarinum, and in the midwestern USA along main watercourses for E. liquescens (Britt Bunyard pers. comm.), this species could be able to overwinter. This was followed by successful stromatal development during unusually warm summer and autumnal months (Figure 6), after which the species was capable of conquering new available substrates in the forests of other areas via sporulation. The species’ capability to actively spread, overwinter, and to withstand drying conditions and direct UV radiation could be significantly enhanced by its forcible discharge of ascospores via turgor of the living asci, as well as by the small volume of ascospores and the wall, equipped with melanins, that are also richly developed in the stromatal crust and perithecial walls (cf. [85]). This capability is also enhanced by the species’ preference for coarse woody debris as a substrate (significant water containers) and the humid forest habitat types, as well as by a development of large stromata equipped with voluminous gelatinous interior capable of accumulating and preserving water transported from the substrate. This enables the fungus to use this water against drying and to keep full turgor in the ascogenous system during the warmest periods when the evaporation is highest, and at the same time, when the organism could only develop ascospores in temperate areas (Figure 6). This internal stromatal liquor, similar as in Xylaria mesenterica, could be homologous in origin and/or the function as the internal stromatal tissue of Daldinia spp. [86].
Finally, the authors herein wish to draw attention to the emergence of erroneous data in the GBIF (www.gbif.org, accessed on 26 June 2023) portal about the occurrence of E. cinnabarinum in eastern Croatia. The actual material (CNF 2/11267) discussed was collected from a completely different geographic area and was uploaded to iNaturalist (www.inaturalist.org, accessed on 26 June 2023 [87]) by someone else who was unfamiliar with the material’s true origin. When compiling an overview on the distribution of a given fungal species, one should take great caution with regard to adopting electronic data since those not accompanied by sufficient evidence about the origin and species identity could include uncontrollable errors. Erroneous data about Entonaema species distribution were earlier discussed by Rogers [5].
5. Conclusions
Six species of Entonaema have been formally accepted to date, the type species E. liquescens, E. cinnabarinum, E. dengii, E. moluccanum, E. globosum, and E. siamensis. Prior to this study, the ITS sequence was available for only one true Entonaema (E. liquescens S.D. Russell iNaturalist # 91210856), but had not been published or phylogenetically analysed. In this study, four gene regions (ITS, LSU, rpb2, β-tub) of the four Croatian collections (CNF 2/11046, 2/11047, 2/11052, 2/11053) of E. cinnabarinum and one (American) collection of E. liquescens (CNF 2/11263) were sequenced for the first time. The results of this study reveal a true phylogenetic position of the genus Entonaema within the Hypoxylaceae.
Judging from available data, dominant tree hosts in Europe for E. cinnabarinum are Fraxinus species (F. angustifolia in the Pannonian area and in Bulgarian longose, F. excelsior in the French Pyrénées Atlantiques), as well as Quercus robur in the Pannonian area. Being the (co-)dominant tree species in suitable flood and alluvial forests, those tree species may have served as the species’ ‘host bridge’ for recent colonisation of the European humid warm temperate areas. In the midwestern USA, the most frequently mentioned hosts were Quercus spp., which could have represented the ‘host bridge’ for E. liquescens in the species’ spreading from the most southeastern USA species’ strongholds towards the north via the Mississippi River and its tributaries. However, the analysed worldwide data prove that the two most frequent Entonaema species (E. liquescens and E. cinnabarinum) are plurivorous lignicolous saprotrophs. Therefore, we could expect the fungal adaptation to some other tree species growing under adequate climatic regime in suitable humid forest habitats in the future.
As for the rest of the Entonaema species, both E. liquescens and E. cinnabarinum were often considered subtropical–tropical species in the earliest papers (compare [68,73]). They are present in the areas under several types of warm temperate climate: Cfa—USA, Europe, Japan, Brazil; Cfb—Brazil, Ecuador; Csa—Spain; Cwa—Argentina, India, Mexico; Cwb—Mexico, Nepal. However, it is evident that both species were regularly recorded in the temperate zones of USA and Europe beginning only in the 21st century, on the well explored areas where those species have never been recorded before. Moreover, the apparent spread of these two Entonaema species during the last decade into the areas under (formerly) cooler temperate climate is in line with predicted climatic shift in Europe and North America [88] when we compare the current distribution of Köppen–Geiger climatic types in Europe, based on a period 1951–2000 [30], with the area that these climatic types would presumably cover during the period 2076–2100 [88]. Therefore, both E. liquescens and E. cinnabarinum could be good candidates for bioindicator species of climate change, especially because both are easily visible and recognizable by trained observers. Their appearance in the areas where they did not previously appear, could point to biologically effective climatic shifting along the border of a given cooler climatic type into a warmer, but the otherwise similar climatic type, e.g., Dfa or Cfb into Cfa (northwest Croatia and Hungary); Dfb (Nebraska, Iowa, northern Illinois and Indiana in the USA) or Dwb (Russian Far East) into a Cfa (Figure 6).
Author Contributions
Conceptualization, A.P., N.M. and I.K.; methodology, A.P., I.K. and N.M.; formal analysis, A.P., N.M. and I.K.; investigation, A.P., N.M., I.K., Z.T. and A.M.; resources, N.M., I.K., Z.T. and A.M.; data curation, A.P., N.M. and I.K.; writing—original draft preparation, A.P., N.M. and I.K.; writing—review and editing, A.P., N.M., I.K., Z.T. and A.M.; visualization, A.P., N.M. and I.K.; supervision, I.K., N.M., A.M. and Z.T.; project administration, A.M.; funding acquisition, A.M., I.K., N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was fully supported by the Croatian Science Foundation under the project grants ForFungiDNA HRZZ-IP-2018-01-1736 (to A.P., I.K., N.M., A.M., Z.T.), and HRZZ-DOK-2018-09-7081 (to A.P.).
Data Availability Statement
All sequences used in this study are available in GenBank. Alignments and phylogenetic trees generated in this study are available at Zenodo (DOI: 10.5281/zenodo.8302699).
Acknowledgments
We thank Milan Čerkez, Juraj Marković and Matija Josipović for their fieldwork effort and providing the collections of Entonaema cinnabarinum. Trent Gonzales and Britt Bunyard are appreciated for sampling and delivery of E. liquescens from the USA. The authors are grateful to Britt Bunyard for making improvements to the English text.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Möller, A. Phycomyceten Und Ascomyceten. Untersuchungen Aus Brasilien; Bot. Mitth. Tropen, Heft 9; G. Fischer: Jena, Germany, 1901. [Google Scholar]
- Stadler, M.; Fournier, J.; Læssøe, T.; Lechat, C.; Tichy, H.-V.; Piepenbring, M. Recognition of hypoxyloid and xylarioid Entonaema species and allied Xylaria species from a comparison of holomorphic morphology, HPLC profiles, and ribosomal DNA sequences. Mycol. Prog. 2008, 7, 53–73. [Google Scholar] [CrossRef]
- Lloyd, C.G. Mycological Notes, No. 69. Mycol. Writ. 1923, 7, 1185–1218. [Google Scholar]
- Martin, G.W. New or Noteworthy Fungi from Panama and Columbia. II. Mycologia 1938, 30, 431–441. [Google Scholar] [CrossRef]
- Rogers, J.D. Sarcoxylon and Entonaema (Xylariaceae). Mycologia 1981, 73, 26–61. [Google Scholar] [CrossRef]
- Rogers, J.D. Entonaema liquescens: Description of the Anamorph and Thoughts on Its Systematic Position. Mycotaxon 1982, 15, 500–506. [Google Scholar]
- Eriksson, O.E.; Hawksworth, D.L. Outline of the Ascomycetes—1993. Syst. Ascomycetum 1993, 12, 51–257. [Google Scholar]
- Whalley, A. The xylariaceous way of life. Mycol. Res. 1996, 100, 897–922. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Rogers, J.D. A Revision of the Genus Hypoxylon; APS Press: St Paul, MN, USA, 1996. [Google Scholar]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Cooke, M.C. Some Australian Fungi. Grevillea 1887, 15, 93–101. [Google Scholar]
- Heim, R. Quelques Ascomycétes Remarquables, II.—Le Genre Entonaema Möll. Au Mexique. Bull. Trimest. Société Mycol. De Fr. 1960, 76, 121–129. [Google Scholar]
- Sihanonth, P.; Thienhirun, S.; Whalley, A.J. Entonaema in Thailand. Mycol. Res. 1998, 102, 458–460. [Google Scholar] [CrossRef]
- Triebel, D.; Peršoh, D.; Wollweber, H.; Stadler, M. Phylogenetic relationships among Daldinia, Entonaema, and Hypoxylon as inferred from ITS nrDNA analyses of Xylariales. Nova Hedwig. 2005, 80, 25–43. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2020, 106, 7–28. [Google Scholar] [CrossRef]
- Baral, H.O. Vital versus Herbarium Taxonomy: Morphological Differences between Living and Dead Cells of Ascomycetes, and Their Taxonomic Implications. Mycologia 1992, 44, 333–390. [Google Scholar]
- Henriot, A.; Cheype, J.-L. Piximètre: La Mesure de Dimensions Sur Images. Version 5.10 R1541. Available online: http://ach.log.free.fr/Piximetre (accessed on 1 October 2020).
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Foodborne Fungi; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands; Delft, The Netherlands, 1996. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium are Nonorthologous. Mol. Phylogenetics Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Kazutaka, K.; Misakwa, K.; Kei-ichi, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-M.; Lin, C.-R.; Fang, M.-J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.-M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenetics Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Kuhnert, E.; Sir, E.B.; Lambert, C.; Hyde, K.D.; Hladki, A.I.; Romero, A.I.; Rohde, M.; Stadler, M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2016, 85, 1–43. [Google Scholar] [CrossRef]
- Kuhnert, E.; Fournier, J.; Peršoh, D.; Luangsa-Ard, J.J.D.; Stadler, M. New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers. 2013, 64, 181–203. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Li, Q.R.; Kang, J.C.; Hyde, K.D. Two new species of the genus Collodiscula (Xylariaceae) from China. Mycol. Prog. 2015, 14, 52. [Google Scholar] [CrossRef]
- Bitzer, J.; Læssøe, T.; Fournier, J.; Kummer, V.; Decock, C.; Tichy, H.-V.; Piepenbring, M.; Peršoh, D.; Stadler, M. Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol. Res. 2008, 112, 251–270. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Ju, Y.-M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, H.; Læssøe, T.; Stenlid, J. Molecular and morphological investigation of Daldinia in northern Europe. Mycol. Res. 2000, 104, 275–280. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Sung, G.-H.; Johnson, D.; Hesse, C.; O’rourke, B.; Serdani, M.; Spotts, R.; Lutzoni, F.; Hofstetter, V.; Miadlikowska, J.; et al. A five-gene phylogeny of Pezizomycotina. Mycologia 2006, 98, 1018–1028. [Google Scholar] [CrossRef]
- Entonaema Liquescens · INaturalist. Available online: https://www.inaturalist.org/taxa/350744-Entonaema-liquescens (accessed on 25 April 2023).
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Skaltsas, D.N.; Badotti, F.; Vaz, A.B.M.; da Silva, F.F.; Gazis, R.; Wurdack, K.; Castlebury, L.; Góes-Neto, A.; Chaverri, P. Exploration of stem endophytic communities revealed developmental stage as one of the drivers of fungal endophytic community assemblages in two Amazonian hardwood genera. Sci. Rep. 2019, 9, 12685. [Google Scholar] [CrossRef]
- Higginbotham, S.; Wong, W.R.; Linington, R.G.; Spadafora, C.; Iturrado, L.; Arnold, A.E. Sloth Hair as a Novel Source of Fungi with Potent Anti-Parasitic, Anti-Cancer and Anti-Bacterial Bioactivity. PLoS ONE 2014, 9, e84549. [Google Scholar] [CrossRef]
- Kim, C.S.; Jo, J.W.; Kwag, Y.-N.; Sung, G.-H.; Lee, S.-G.; Kim, S.-Y.; Shin, C.-H.; Han, S.-K. Mushroom Flora of Ulleung-gun and a Newly Recorded Bovista Species in the Republic of Korea. Mycobiology 2015, 43, 239–257. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Rossman, A.Y.; Rogers, J.D.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Koukol, O.; Kelnarová, I.; Černý, K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For. Pathol. 2014, 45, 21–27. [Google Scholar] [CrossRef]
- Pourmoghaddam, M.J.; Lambert, C.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stadler, M. Discovery of a new species of the Hypoxylon rubiginosum complex from Iran and antagonistic activities of Hypoxylon spp. against the Ash Dieback pathogen, Hymenoscyphus fraxineus, in dual culture. Mycokeys 2020, 66, 105–133. [Google Scholar] [CrossRef]
- Ma, H.-X.; Qiu, J.-Z.; Xu, B.; Li, Y. Two Hypoxylon species from Yunnan Province based on morphological and molecular characters. Phytotaxa 2018, 376, 27–36. [Google Scholar] [CrossRef]
- Cedeño–Sanchez, M. Three new species of Hypoxylon and new records of Xylariales from Panama. Mycosphere 2020, 11, 1457–1476. [Google Scholar] [CrossRef]
- Ma, H.; Song, Z.; Pan, X.; Li, Y.; Yang, Z.; Qu, Z. Multi-Gene Phylogeny and Taxonomy of Hypoxylon (Hypoxylaceae, Ascomycota) from China. Diversity 2022, 14, 37. [Google Scholar] [CrossRef]
- Song, Z.-K.; Zhu, A.-H.; Liu, Z.-D.; Qu, Z.; Li, Y.; Ma, H.-X. Three New Species of Hypoxylon (Xylariales, Ascomycota) on a Multigene Phylogeny from Medog in Southwest China. J. Fungi 2022, 8, 500. [Google Scholar] [CrossRef]
- Lambert, C.; Pourmoghaddam, M.J.; Cedeño-Sanchez, M.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stradal, T.E.B.; Stadler, M. Resolution of the Hypoxylon fuscum Complex (Hypoxylaceae, Xylariales) and Discovery and Biological Characterization of Two of Its Prominent Secondary Metabolites. J. Fungi 2021, 7, 131. [Google Scholar] [CrossRef]
- Stadler, M.; Kuhnert, E.; Peršoh, D.; Fournier, J. The Xylariaceae as Model Example for a Unified Nomenclature Following the “One Fungus-One Name” (1F1N) Concept. Mycology 2013, 4, 5–21. [Google Scholar] [CrossRef]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2016, 82, 1–105. [Google Scholar] [CrossRef]
- Bills, G.F.; González-Menéndez, V.; Martín, J.; Platas, G.; Fournier, J.; Peršoh, D.; Stadler, M. Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a Pantropical Insecticide-Producing Endophyte. PLoS ONE 2012, 7, e46687. [Google Scholar] [CrossRef]
- Sir, E.B.; Becker, K.; Lambert, C.; Bills, G.F.; Kuhnert, E. Observations on Texas hypoxylons, including two new Hypoxylon species and widespread environmental isolates of the H. croceum complex identified by a polyphasic approach. Mycologia 2019, 111, 832–856. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.-H.; Zhang, X.; Liu, L.-L.; Long, Q.-D.; Shen, X.-C.; Kang, Y.-Q.; Hyde, K.D.; Boonmee, S.; Kang, J.-C.; Li, Q.-R. Contributions to species of Xylariales in China—4. Hypoxylon wujiangensis sp. nov. Phytotaxa 2020, 455, 21–30. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Jones, E.B.G.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.-K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Pažoutová, S.; Šrůtka, P.; Holuša, J.; Chudíčková, M.; Kolařík, M. The phylogenetic position of Obolarina dryophila (Xylariales). Mycol. Prog. 2010, 9, 501–507. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Bhat, J.D.; Jones, E.B.G.; McKenzie, E.H.C.; Dai, D.Q.; Daranagama, D.A.; Dayarathne, M.C.; Goonasekara, I.D.; et al. Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers. 2015, 73, 73–144. [Google Scholar] [CrossRef]
- Peláez, F.; González, V.; Platas, G.; Sánchez-Ballesteros, J.; Rubio, V. Molecular Phylogenetic Studies within the Xylariaceae Based on Ribosomal DNA Sequences. Fungal Divers 2008, 31, 111–134. [Google Scholar]
- Tang, A.M.C.; Jeewon, R.; Hyde, K.D. A Re-Evaluation of the Evolutionary Relationships within the Xylariaceae Based on Ri-bosomal and Protein-Coding Gene Sequences. Fungal Divers 2009, 34, 127–155. [Google Scholar]
- Stadler, M.; Flessa, F.; Rambold, G.; Peršoh, D.; Fournier, J.; Læssøe, T.; Chlebicki, A.; Lechat, C. Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae). Mycoscience 2010, 51, 189–207. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Hsieh, H.-M. Xylaria species associated with nests of Odontotermes formosanus in Taiwan. Mycologia 2007, 99, 936–957. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Hsieh, H.-M.; Rogers, J.D.; Fournier, J.; Jaklitsch, W.M.; Courtecuisse, R. New and interesting penzigioid Xylaria species with small, soft stromata. Mycologia 2012, 104, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Sir, E.; Stadler, M. A new species of Daldinia (Xylariaceae) from the Argentine subtropical montane forest. Mycosphere 2016, 7, 1378–1388. [Google Scholar] [CrossRef]
- Fintha, G.; Benedek, L.; Orbán, S. New Macrofungial Record in Hungary: Entonaema cinnabarinum (Cooke & Massee) Lloyd. Acta Biol. Plant. Agriensis 2019, 7, 127–130. [Google Scholar] [CrossRef]
- Rogers, J.D.; San Martín, F.; Ju, Y.-M. Mexican Fungi: Xylaria entosulphurea Sp. Nov. and Neotypification of Entonaema globosum. Mycotaxon 1996, 58, 483–487. [Google Scholar]
- Stadler, M.; Ju, Y.-M.; Rogers, J.D. Chemotaxonomy of Entonaema, Rhopalostroma and other Xylariaceae. Mycol. Res. 2004, 108, 239–256. [Google Scholar] [CrossRef]
- Patouillard, N. Champignons de La Nouvelle-Calédonie. Bull. Trimest. Société Mycol. De Fr. 1911, 27, 329–333. [Google Scholar]
- Benkert, D. Kotlabaea macrospora Benkert nov. sp. und einige weitere bemerkenswerte Ascomyceten aus Bulgarien Mit einer Abbildung. Feddes Repert. 1993, 104, 547–549. [Google Scholar] [CrossRef]
- Fedosova, A.G. The New Record of Entonaema cinnabarinum (Xylariaceae, Ascomycota) in Europe. Vestnik of Saint Petersburg University. Series 3. Biology 2012, 1, 10–13. [Google Scholar]
- Harkevich, S.S. (Ed.) Флoра и Растительнoсть Уссурийскoгo Запoведника; Наука: Moscow, Russia, 1978. [Google Scholar]
- Azbukina, Z.M.; Baгdunov, L.V.; Bezdeleva, Т.А.; Bogacheva, А.V.; Bulakh, Е.М.; Vasilyeva, L.N.; Govorova, О.K.; Egorova, L.N.; Zhabyko, Е.V.; Nikulina, Т.V.; et al. Flora, Vegetation and Mycobiota of the Reserve “Ussuriysky”; Daljnauka: Vladivostok, Rasia, 2006. [Google Scholar]
- Sánchez-Jácome, M.; Guzmán-Dávalos, L. New Records of Ascomycetes from Jalisco, Mexico. Mycotaxon 2005, 92, 177–191. [Google Scholar]
- Quang, D.N.; Stadler, M.; Fournier, J.; Asakawa, Y. Carneic Acids A and B, Chemotaxonomically Significant Antimicrobial Agents from the Xylariaceous Ascomycete Hypoxylon carneum. J. Nat. Prod. 2006, 69, 1198–1202. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Zamora, J.C.; Svensson, M.; Kirschner, R.; Olariaga, I.; Ryman, S.; Parra, L.A.; Geml, J.; Rosling, A.; Adamčík, S.; Ahti, T.; et al. Considerations and consequences of allowing DNA sequence data as types of fungal taxa. IMA Fungus 2018, 9, 167–175. [Google Scholar] [CrossRef]
- Šrůtka, P.; Pažoutová, S.; Kolařík, M. Daldinia decipiens and Entonaema cinnabarina as fungal symbionts of Xiphydria wood wasps. Mycol. Res. 2007, 111, 224–231. [Google Scholar] [CrossRef]
- Lӕssøe, T. Entonaema cinnabarina-En Eksotisk Kernesvamp. Svampe 1997, 36, 21–22. [Google Scholar]
- Fintha, G.; Nagy, I.; Vitkó, T.; Benedek, L.; Baranyai, G. Az Ócsai Turjánvidék Natura 2000-es kijelölt területeinek nagygombái. J. Landscape Ecol. 2022, 20, 3–21. [Google Scholar] [CrossRef]
- Stanners, D.; Bourdeau, P. Europes’s Environment: The Dobřiš Assessment; European Environment Agency: Copenhagen, Denmark, 1995. [Google Scholar]
- Quijada, L.; Matočec, N.; Kušan, I.; Tanney, J.B.; Johnston, P.R.; Mešić, A.; Pfister, D.H. Apothecial Ancestry, Evolution, and Re-Evolution in Thelebolales (Leotiomycetes, Fungi). Biology 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Rogers, J.D.; San Martin, F. A Revision of the Genus Daldinia. Mycotaxon 1997, 61, 243–293. [Google Scholar]
- INaturalist Research-Grade Observations. Available online: https://www.inaturalist.org (accessed on 26 June 2023).
- Rubel, F.; Kottek, M. Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol. Z. 2010, 19, 135–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).