The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscopic Studies
2.2. Axenic Cultures
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Sequence Alignment and Phylogenetic Analysis
2.5. The Analysis of Ecological and Biogeographical Traits
| Taxa | Voucher | Country | ITS | LSU | rpb2 | β-tub | Ref |
|---|---|---|---|---|---|---|---|
| Amphirosellinia fushanensis | HAST 91111209 HT | Taiwan | GU339496 | N/A | GQ848339 | GQ495950 | [31] |
| Annulohypoxylon annulatum | CBS 140775 ET | Texas | KY610418 | KY610418 | KY624263 | KX376353 | [10,32] |
| Annulohypoxylon atroroseum | ATCC 76081 | Thailand | AJ390397 | KY610422 | KY624233 | DQ840083 | [10,33] |
| Annulohypoxylon michelianum | CBS 119993 | Spain | KX376320 | KY610423 | KY624234 | KX271239 | [10,32] |
| Annulohypoxylon moriforme | CBS 123579 | France | KX376321 | KY610425 | KY624289 | KX271261 | [10,32] |
| Annulohypoxylon nitens | MFLUCC 12-0823 | Thailand | KJ934991 | KJ934992 | KJ934994 | KJ934993 | [34] |
| Annulohypoxylon stygium | MUCL 54601 | France | KY610409 | KY610475 | KY624292 | KX271263 | [10] |
| Annulohypoxylon truncatum | CBS 140778 ET | Texas | KY610419 | KY610419 | KY624277 | KX376352 | [10,32] |
| Astrocystis concavispora | MFLUCC 14-0174 | Italy | KP297404 | KP340545 | KP340532 | KP406615 | [34] |
| Biscogniauxia arima | WSP 122 IT | Mexico | EF026150 | N/A | GQ304736 | AY951672 | [31] |
| Biscogniauxia nummularia | MUCL 51395 ET | France | KY610382 | KY610427 | KY624236 | KX271241 | [10] |
| Brunneiperidium gracilentum | MFLUCC 14-0011 HT | Italy | KP297400 | KP340542 | KP340528 | KP406611 | [34] |
| Calceomyces lacunosus | CBS 633.88 HT | Japan | KY610397 | KY610476 | KY624293 | KX271265 | [10] |
| Camillea obularia | ATCC 28093 | Puerto Rico | KY610384 | KY610429 | KY624238 | KX271243 | [10] |
| Collodiscula fangjingshanensis | GZU H0109 HT | China | KR002590 | KR002591 | KR002592 | KR002589 | [35] |
| Creosphaeria sassafras | STMA 14087 | Argentina | KY610411 | KY610468 | KY624265 | KX271258 | [10] |
| Daldinia andina | CBS 114736 HT | Ecuador | AM749918 | KY610430 | KY624239 | KC977259 | [10,33,36] |
| Daldinia bambusicola | CBS 122872 HT | Thailand | KY610385 | KY610431 | KY624241 | AY951688 | [10,37] |
| Daldinia caldariorum | MUCL 49211 | France | AM749934 | KY610433 | KY624242 | KC977282 | [10,33,36] |
| Daldinia concentrica | CBS 113277 | Germany | AY616683 | KY610434 | KY624243 | KC977274 | [10,14,33] |
| Daldinia dennisii | CBS 114741 HT | Australia | JX658477 | KY610435 | KY624244 | KC977262 | [10,33,38] |
| Daldinia eschscholtzii | MUCL 45435 | Benin | JX658484 | KY610437 | KY624246 | KC977266 | [10,33,38] |
| Daldinia loculatoides | CBS 113279 ET | UK | AF176982 | KY610438 | KY624247 | KX271246 | [10,39] |
| Daldinia macaronesica | CBS 113040 PT | Spain | KY610398 | KY610477 | KY624294 | KX271266 | [10] |
| Daldinia petriniae | MUCL 49214 ET | Austria | AM749937 | KY610439 | KY624248 | KC977261 | [10,33,36] |
| Daldinia placentiformis | MUCL 47603 | Mexico | AM749921 | KY610440 | KY624249 | KC977278 | [10,33,36] |
| Daldinia pyrenaica | MUCL 53969 | France | KY610413 | KY610413 | KY624274 | KY624312 | [10] |
| Daldinia steglichii | MUCL 43512 PT | Papua New Guinea | KY610399 | KY610479 | KY624250 | KX271269 | [10] |
| Daldinia theissenii | CBS 113044 PT | Argentina | KY610388 | KY610441 | KY624251 | KX271247 | [10] |
| Daldinia vernicosa | CBS 119316 ET | Germany | KY610395 | KY610442 | KY624252 | KC977260 | [10,33] |
| Diatrype disciformis | CBS 197.49 | Netherlands | N/A | DQ470964 | DQ470915 | N/A | [40] |
| Entonaema cinnabarinum | agtS377 | Germany | AY616685 | N/A | N/A | N/A | [14] |
| Entonaema cinnabarinum | CNF 2/11046 | Croatia | OQ863621 | OQ863622 | OQ877102 | OQ877113 | This study |
| Entonaema cinnabarinum | CNF 2/11047 | Croatia | OQ863735 | OQ864983 | OQ877103 | OQ877114 | This study |
| Entonaema cinnabarinum | CNF 2/11052 | Croatia | OQ864984 | OQ865000 | OQ877104 | OQ877115 | This study |
| Entonaema cinnabarinum | CNF 2/11053 | Croatia | OQ869782 | OQ869785 | OQ877105 | OQ877116 | This study |
| Entonaema liquescens | ATCC 46302 | USA | KY610389 | KY610443 | KY624253 | KX271248 | [10] |
| Entonaema liquescens | agtS279 | Germany | AY616686 | N/A | N/A | N/A | [14] |
| Entonaema liquescens | CNF 2/11263 | USA | OQ869784 | OQ865124 | OQ877106 | OQ877117 | This study |
| Entonaema liquescens | S.D. Russell iNaturalist # 91210856 | USA | OM972573 | N/A | N/A | N/A | [41] |
| Entonaema pallida | PP92a | Peru | FJ884093 | FJ890379 | N/A | N/A | [42] |
| Entonaemasp. | JHGB08 1A | Peru | MH267933 | N/A | N/A | N/A | [43] |
| Entonaemasp. | AHB18 5B | Peru | MH267934 | N/A | N/A | N/A | [43] |
| Entonaemasp. | F5071 | Panama | KF746156 | N/A | N/A | N/A | [44] |
| Entonaema splendens | KA12-1283 | South Korea | KR673521 | N/A | N/A | N/A | [45] |
| Euepixylon sphaeriostomum | JDR 261 | USA | GU292821 | N/A | GQ844774 | GQ470224 | [31] |
| Graphostroma platystomum | CBS 270.87 HT | France | JX658535 | DQ836906 | KY624296 | HG934108 | [10,38,46,47] |
| Hypocreodendron sanguineum | JDR 169 | Mexico | GU322433 | N/A | GQ844819 | GQ487710 | [31] |
| Hypoxylon addis | MUCL 52797 HT | Ethiopia | KC968931 | N/A | N/A | KC977287 | [33] |
| Hypoxylonaff. rubiginosum | MUCL 57724 | Iran | MT214999 | MT214994 | MT212237 | MT212241 | [48] |
| Hypoxylonaff. rubiginosum | MUCL 57725 | Iran | MT215000 | MT214995 | MT212238 | MT212242 | [48] |
| Hypoxylon baihualingense | FCATAS 477 HT | China | MG490190 | N/A | N/A | MH790276 | [49] |
| Hypoxylon bellicolor | UCH 9543 | Panama | MN056425 | N/A | N/A | MK908139 | [50] |
| Hypoxylon carneum | MUCL 54177 | France | KY610400 | KY610480 | KY624297 | KX271270 | [10] |
| Hypoxylon cercidicola | CBS 119009 | France | KC968908 | KY610444 | KY624254 | KC977263 | [10,33] |
| Hypoxylon chrysalidosporum | FCATAS 2710 HT | China | OL467294 | OL615106 | OL584222 | OL584229 | [51] |
| Hypoxylon crocopeplum | CBS 119004 | France | KC968907 | KY610445 | KY624255 | KC977268 | [10,33] |
| Hypoxylon crocopeplum | CNF 2/11316 | Croatia | OQ865120 | OQ869786 | OQ877107 | OQ877118 | This study |
| Hypoxylon crocopeplum | CNF 2/11317 | Croatia | OQ865187 | OQ869787 | OQ877108 | OQ877119 | This study |
| Hypoxylon cyclobalanopsidis | FCATAS 2714 HT | China | OL467298 | OL615108 | OL584225 | OL584232 | [51] |
| Hypoxylon damuense | FCATAS 4207 HT | China | ON075427 | ON075433 | ON093251 | ON093245 | [52] |
| Hypoxylon damuense | FCATAS 4321 | China | ON075428 | ON075434 | ON093252 | ON093246 | [52] |
| Hypoxylon duranii | YMJ 85 | China | JN979414 | N/A | N/A | AY951714 | [37] |
| Hypoxylon eurasiaticum | MUCL 57720 HT | Iran | MW367851 | N/A | MW373852 | MW373861 | [53] |
| Hypoxylon fendleri | MUCL 54792 | France | KF234421 | KY610481 | KY624298 | KF300547 | [10,33] |
| Hypoxylon fragiforme | MUCL 51264 ET | Germany | KC477229 | KM186295 | KM186296 | KX271282 | [10,34,54] |
| Hypoxylon fraxinophilum | MUCL 54176 ET | France | KC968938 | N/A | N/A | KC977301 | [33] |
| Hypoxylon fuscum | CBS 113049 ET | France | KY610401 | KY610482 | KY624299 | KX271271 | [10] |
| Hypoxylon griseobrunneum | CBS 331.73 HT | India | KY610402 | KY610483 | KY624300 | KC977303 | [10,33] |
| Hypoxylon guilanense | MUCL 57726 HT | Iran | MT214997 | MT214992 | MT212235 | MT212239 | [48] |
| Hypoxylon haematostroma | MUCL 53301 ET | France | KC968911 | KY610484 | KY624301 | KC977291 | [10,33] |
| Hypoxylon howeanum | MUCL 47599 | Germany | AM749928 | KY610448 | KY624258 | KC977277 | [10,33,36] |
| Hypoxylon howeanum | CNF 2/11315 | Croatia | OQ865216 | OQ865215 | OQ877109 | OQ877120 | This study |
| Hypoxylon hypomiltum | MUCL 51845 | France | KY610403 | KY610449 | KY624302 | KX271249 | [10] |
| Hypoxylon investiens | CBS 118183 | Malaysia | KC968925 | KY610450 | KY624259 | KC977270 | [10,33] |
| Hypoxylon isabellinum | STMA10247 HT | France | KC968935 | N/A | N/A | KC977295 | [33] |
| Hypoxylon lateripigmentum | MUCL 53304 HT | France | KC968933 | KY610486 | KY624304 | KC977290 | [10,33] |
| Hypoxylon lenormandii | CBS 119003 | Ecuador | KC968943 | KY610452 | KY624261 | KC977273 | [10,33] |
| Hypoxylon liviae | CBS 115282 ET | Norway | NR155154 | N/A | N/A | KC977265 | [33] |
| Hypoxylon macrosporum | YMJ 47 | Canada | N/A | N/A | N/A | AY951736 | [37] |
| Hypoxylon monticulosum | MUCL 54604 ET | France | KY610404 | KY610487 | KY624305 | KX271273 | [10] |
| Hypoxylon musceum | MUCL 53765 | France | KC968926 | KY610488 | KY624306 | KC977280 | [10,33] |
| Hypoxylon notatum | YMJ 250 | USA | JQ009305 | N/A | N/A | AY951739 | [37] |
| Hypoxylon ochraceum | MUCL 54625 ET | France | KC968937 | N/A | KY624271 | KC977300 | [10,33] |
| Hypoxylon papillatum | ATCC 58729 HT | USA | KC968919 | KY610454 | KY624223 | KC977258 | [10,33] |
| Hypoxylon perforatum | CBS 115281 | France | KY610391 | KY610455 | KY624224 | KX271250 | [10] |
| Hypoxylon petriniae | CBS 114746 HT | France | NR155185 | KY610491 | KY624279 | KX271274 | [10,32] |
| Hypoxylon pilgerianum | STMA 13455 | France | KY610412 | KY610412 | KY624308 | KY624315 | [10] |
| Hypoxylon porphyreum | CBS 119022 | France | KC968921 | KY610456 | KY624225 | KC977264 | [10,33] |
| Hypoxylon pseudefendleri | MFLUCC 11-0639 HT | Thailand | KU940156 | KU863144 | N/A | N/A | [55] |
| Hypoxylon pseudofuscum | KR:0005879 HT | Germany | MW367857 | MW367848 | MW373858 | MW373867 | [53] |
| Hypoxylon pulicicidum | CBS 122622 HT | France | JX183075 | KY610492 | KY624280 | JX183072 | [10,56] |
| Hypoxylon rickii | MUCL 53309 ET | France | KC968932 | KY610416 | KY624281 | KC977288 | [10,33] |
| Hypoxylon rubiginosum | MUCL 52887 ET | Germany | KC477232 | KY610469 | KY624266 | KY624311 | [10,54] |
| Hypoxylon rubiginosum | MUCL 57727 | Iran | MT214998 | MT214993 | MT212236 | MT212240 | [48] |
| Hypoxylon samuelsii | MUCL 51843 ET | France | KC968916 | KY610466 | KY624269 | KC977286 | [10,33] |
| Hypoxylon sheariivar. minor | YMJ 29 | Mexico | EF026142 | N/A | N/A | AY951753 | [31,37] |
| Hypoxylon sporistriatatunicum | UCH 9542 HT | Panama | MN056426 | N/A | N/A | MK908140 | [50] |
| Hypoxylon submonticulosum | CBS 115280 | France | KC968923 | KY610457 | KY624226 | KC977267 | [10,33] |
| Hypoxylon texense | DSM 107933 HT | USA | MK287536 | MK287548 | MK287561 | MK287574 | [57] |
| Hypoxylon ticinense | CBS 115271 | France | JQ009317 | KY610471 | KY624272 | AY951757 | [10,37] |
| Hypoxylon ticinense | CNF 2/11314 | Croatia | OQ869783 | OQ865219 | OQ877110 | OQ877121 | This study |
| Hypoxylon trugodes | MUCL 54794 ET | Sri Lanka | KF234422 | KY610493 | KY624282 | KF300548 | [10,33] |
| Hypoxylon ulmophilum | YMJ 350 | Russia | JQ009320 | N/A | N/A | AY951760 | [37] |
| Hypoxylon vogesiacum | CBS 115273 | France | KC968920 | KY610417 | KY624283 | KX271275 | [10,32,33] |
| Hypoxylon wujianggense | GMBC0213 HT | China | MT568854 | MT568853 | MT585802 | MT572481 | [58] |
| Hypoxylon wuzhishanense | FCATAS 2708 HT | China | OL467292 | OL615104 | OL584220 | OL584227 | [51] |
| Hypoxylon wuzhishanense | FCATAS 2709 | China | OL467293 | OL615105 | OL584221 | OL584228 | [51] |
| Hypoxylon zangii | FCATAS 4029 HT | China | ON075423 | ON075429 | ON093247 | ON093241 | [52] |
| Hypoxylon zangii | FCATAS 4319 | China | ON075424 | ON075430 | ON093248 | ON093242 | [52] |
| Jackrogersella cohaerens | CBS 119126 | Germany | KY610396 | KY610497 | KY624270 | KY624314 | [10] |
| Jackrogersella minutella | CBS 119015 | Portugal | KY610381 | KY610424 | KY624235 | KX271240 | [10,32] |
| Jackrogersella multiformis | CBS 119016 ET | Germany | KC477234 | KY610473 | KY624290 | KX271262 | [10,32,33] |
| Kretzschmaria deusta | CBS 163.93 | Germany | KC477237 | KY610458 | KY624227 | KX271251 | [10,54] |
| Nemania bipapillata | HAST 90080610 | Taiwan | GU292818 | N/A | GQ844771 | GQ470221 | [31] |
| Nemania delonicis | MFLU 19-2124 | Thailand | MW240613 | MW240542 | MW342617 | MW775574 | [59] |
| Nemania primolutea | HAST 91102001 HT | Taiwan | EF026121 | N/A | GQ844767 | EF025607 | [31] |
| Obolarina dryophila | MUCL 49882 | France | GQ428316 | GQ428316 | KY624284 | GQ428322 | [10,60] |
| Podosordaria muli | WSP 167 HT | Mexico | GU324761 | N/A | GQ853038 | GQ844839 | [31] |
| Podosordariasp. | CNF 2/11073 | Croatia | OQ865223 | OQ865228 | OQ877111 | OQ877122 | This study |
| Poronia punctata | CBS 656.78 HT | Australia | KT281904 | KY610496 | KY624278 | KX271281 | [10,61] |
| Pyrenopolyporus hunteri | MUCL 52673 ET | Ivory Coast | KY610421 | KY610472 | KY624309 | KU159530 | [10,32] |
| Pyrenopolyporus laminosus | MUCL 53305 HT | France | KC968934 | KY610485 | KY624303 | KC977292 | [10,33] |
| Pyrenopolyporus nicaraguensis | CBS 117739 | Burkina Faso | AM749922 | KY610489 | KY624307 | KC977272 | [10,33,36] |
| Pyriformiascoma trilobatum | MFLUCC 14-0012 HT | Italy | KP297402 | KP340543 | KP340530 | KP406613 | [34] |
| Rhopalostroma angolense | CBS 126414 | Ivory Coast | KY610420 | KY610459 | KY624228 | KX271277 | [10] |
| Rosellinia aquila | MUCL 51703 | France | KY610392 | KY610460 | KY624285 | KX271253 | [10] |
| Rosellinia corticium | MUCL 51693 | France | KY610393 | KY610461 | KY624229 | KX271254 | [10] |
| Rosellinia necatrix | CBS 349.36 | Argentina | AY909001 | KF719204 | KY624275 | KY624310 | [10,62] |
| Rostrohypoxylon terebratum | JF-TH 06-04 HT | Thailand | DQ631943 | DQ840069 | DQ631954 | DQ840097 | [63] |
| Ruwenzoria pseudoannulata | MUCL 51394 HT | D. R. Congo | KY610406 | KY610494 | KY624286 | KX271278 | [10] |
| Sarcoxylon compunctum | CBS 359.61 | South Africa | KT281903 | KY610462 | KY624230 | KX271255 | [10,61] |
| Stilbohypoxylon elaeicola | YMJ 173 | France | EF026148 | N/A | GQ844826 | EF025616 | [31] |
| Stilbohypoxylon quisquiliarum | YMJ 172 | France | EF026119 | N/A | GQ853020 | EF025605 | [31] |
| Thamnomyces dendroidea | CBS 123578 HT | France | FN428831 | KY610467 | KY624232 | KY624313 | [10,64] |
| Xylaria bambusicola | WSP 205 HT | Taiwan | EF026123 | N/A | GQ844802 | AY951762 | [31] |
| Xylaria brunneovinosa | HAST 720 HT | France | EU179862 | N/A | GQ853023 | GQ502706 | [31,65] |
| Xylaria discolor | HAST 131023 ET | USA | JQ087405 | N/A | JQ087411 | JQ087414 | [66] |
| Xylaria hypoxylon | CBS 122620 ET | Sweden | KY610407 | KY610495 | KY624231 | KX271279 | [10,67] |
| Xylaria multiplex | HAST 580 | France | GU300098 | N/A | GQ844814 | GQ487705 | [31] |
| Xylaria polymorpha | MUCL 49884 | France | KY610408 | KY610464 | KY624288 | KX271280 | [10] |
| Xylaria sicula | CNF 2/11087 | Croatia | OQ865227 | OQ865230 | OQ877112 | OQ877123 | This study |
3. Results
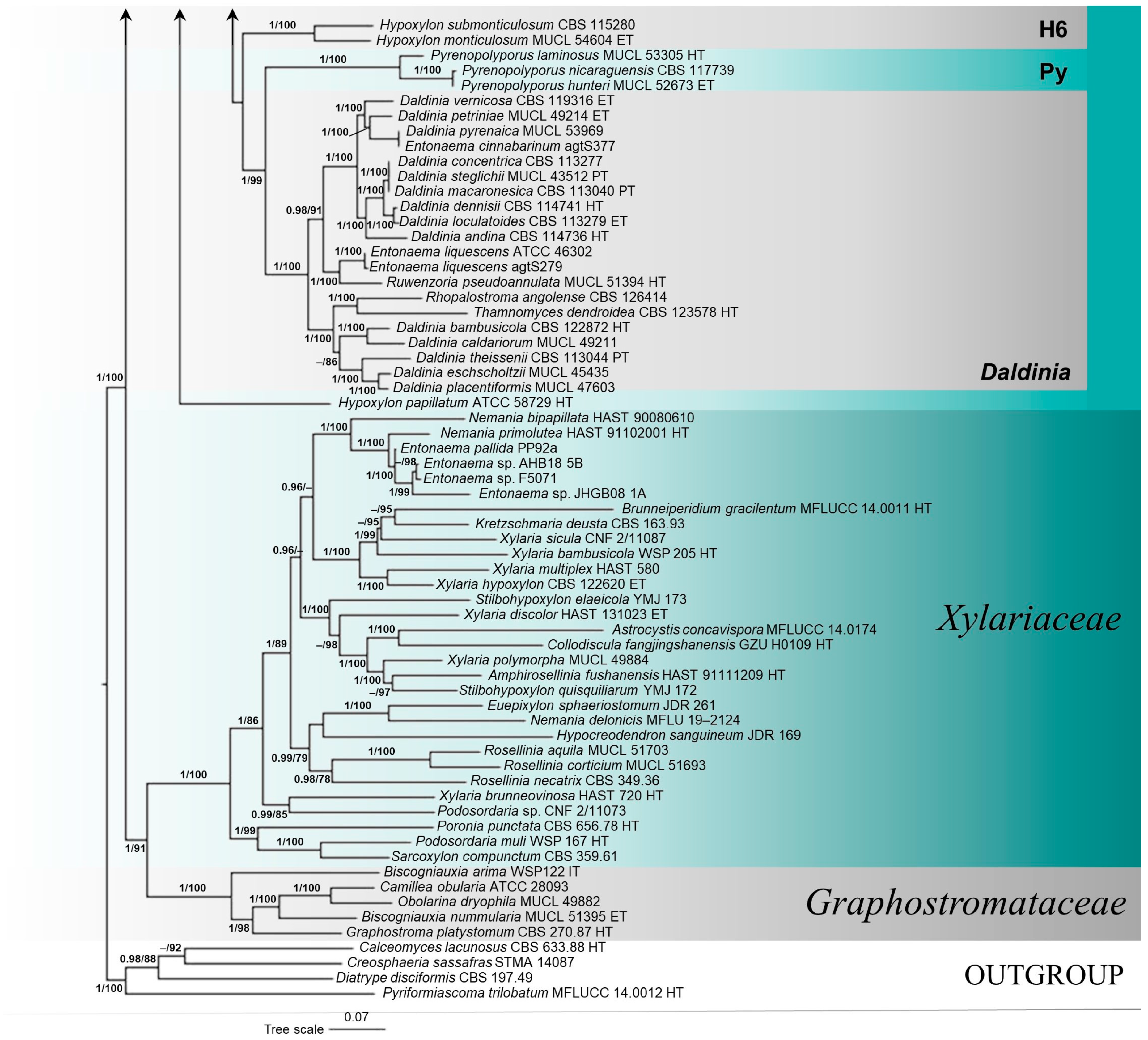
3.1. Molecular Phylogenetic Analyses
3.2. Taxonomy

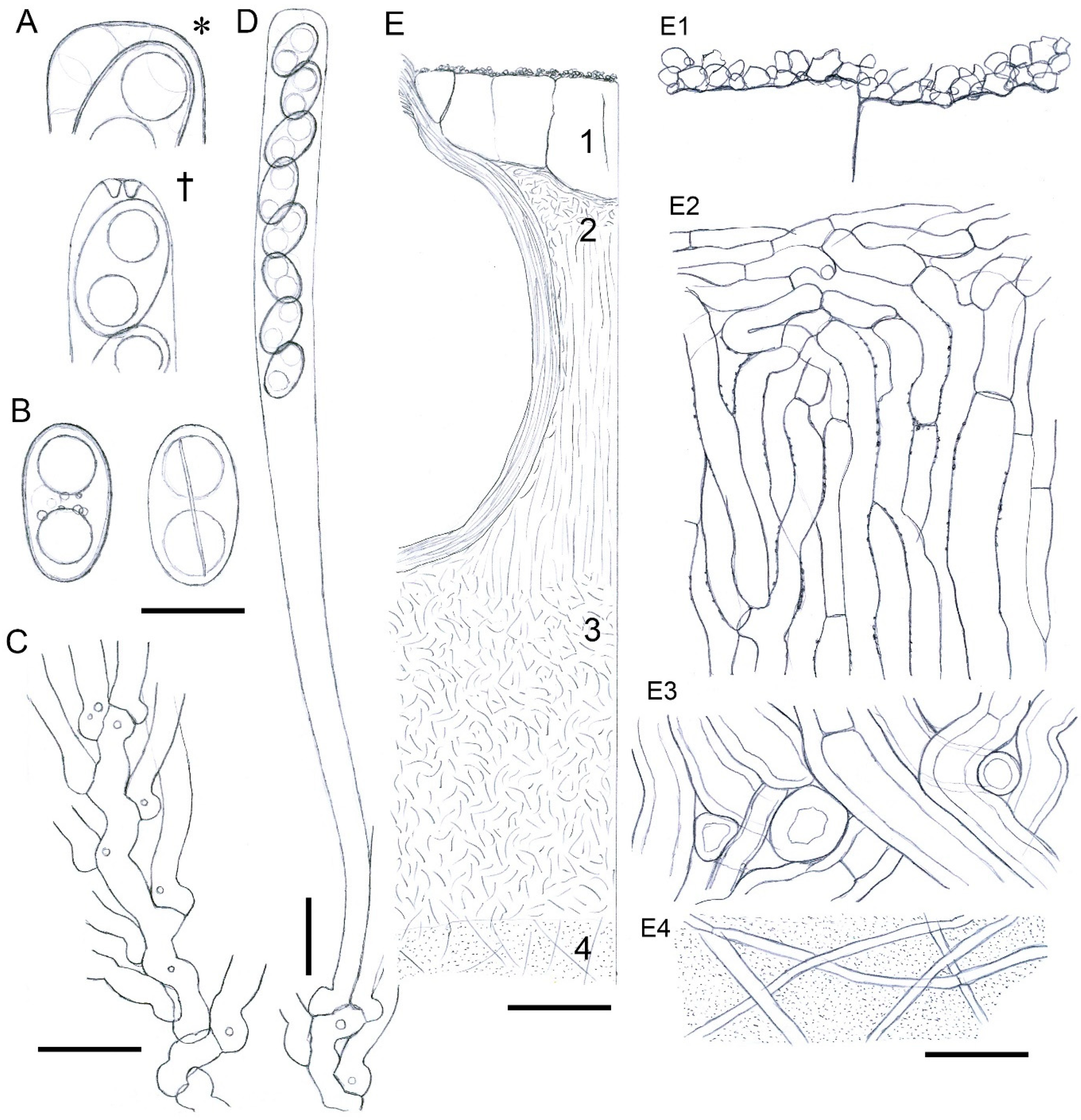
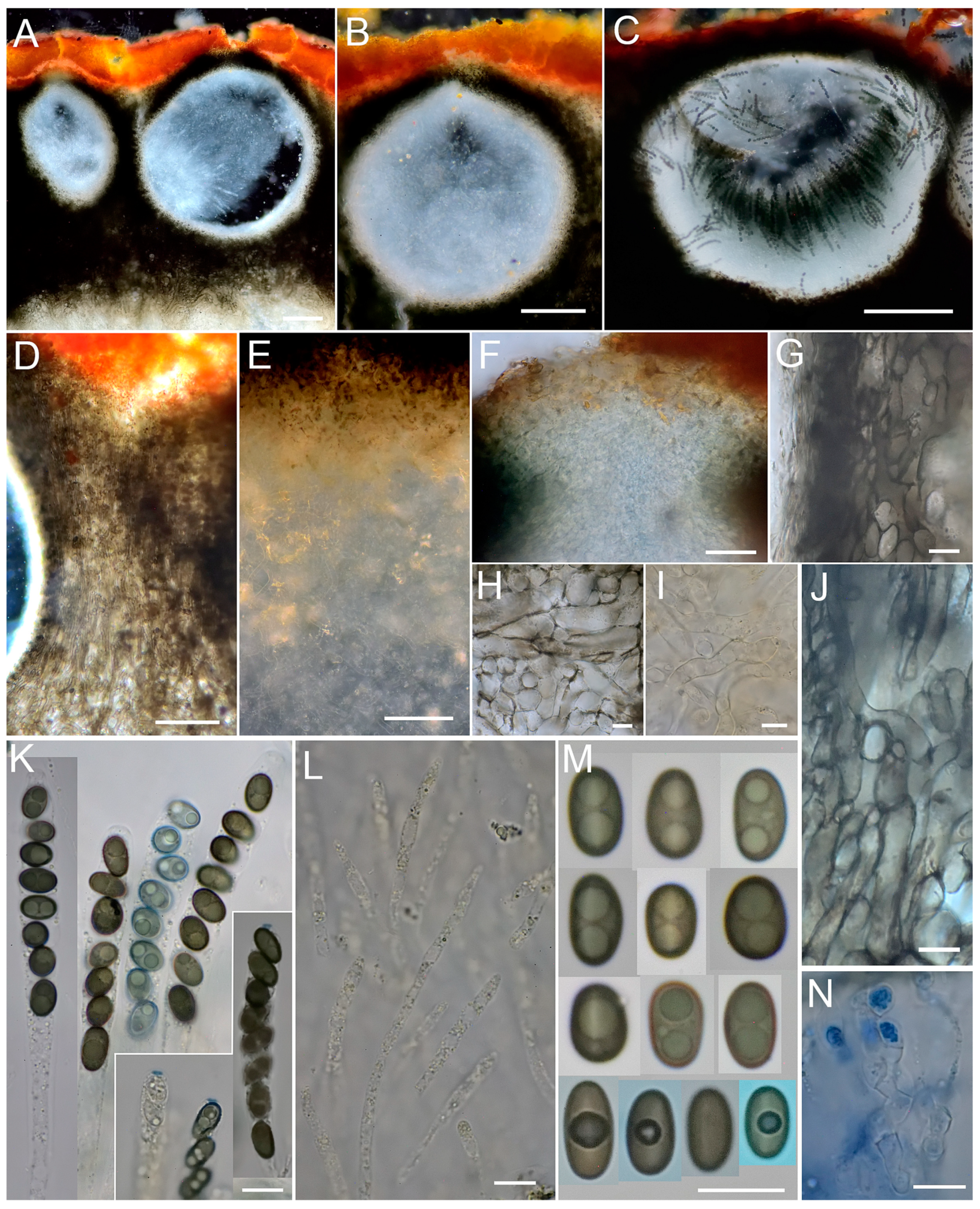
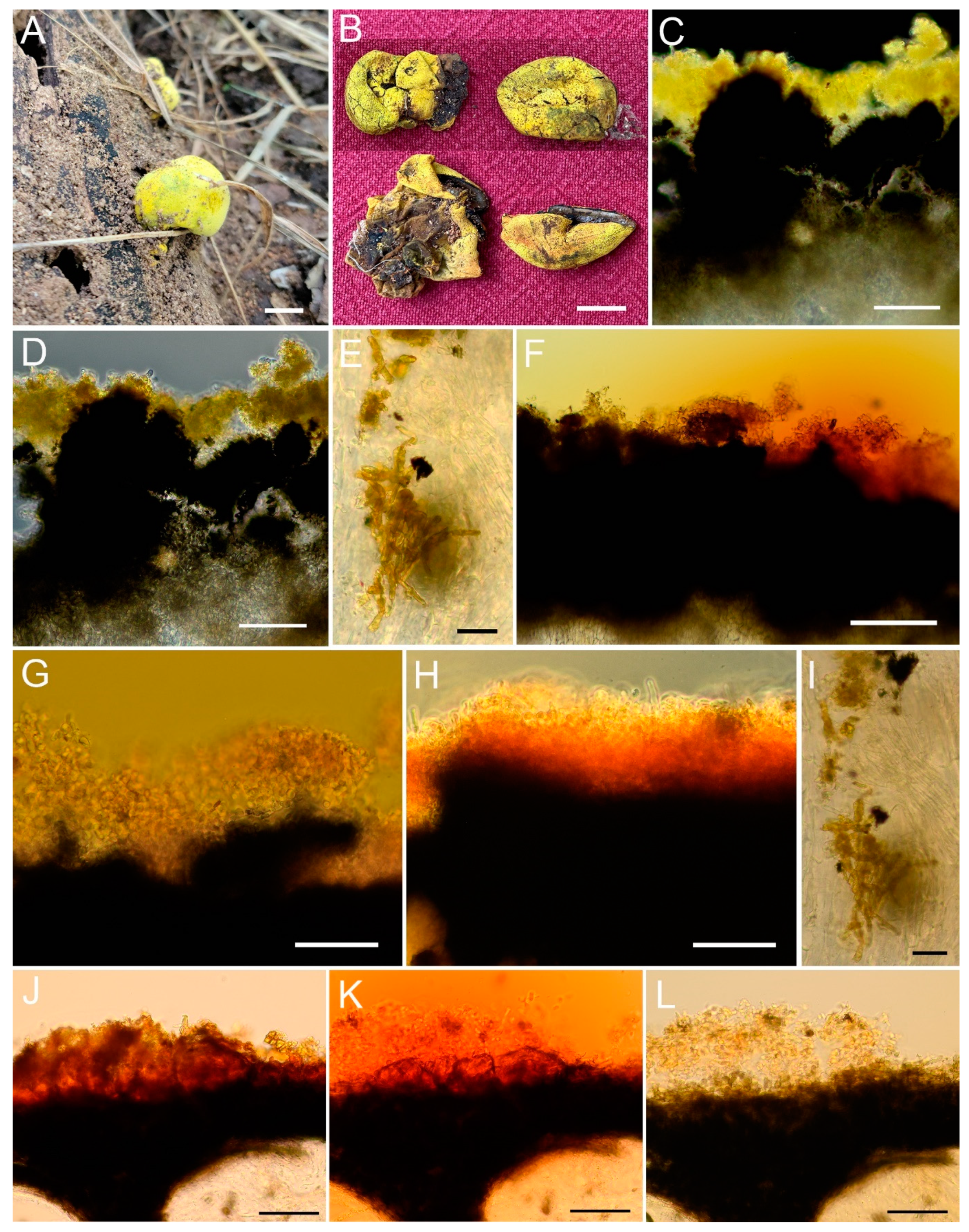
| (1) Stromatal extractable pigments greenish yellow in 10% KOH, but red in NH3 …...................................................................................................................................Xylaria p.p. |
| - Stromatal extractable pigments orange to brick red or entirely absent in 10% KOH, orange in NH3….....................................................................................................…Entonaema (2) |
| (2) Stromatal orange to red pigment granules present in the section immediately beneath the stromatal surface and around the perithecial ostioles, stromatal extractable pigments in 10% KOH brick red................................................................3 |
| - Stromatal pigment granules in the section of the stromatal cortex yellow, green, or absent, stromatal extractable pigments in 10% KOH yellowish-orange to orange, or absent...........................................................................................................................................................4 |
| (3) Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles rusty orange, ostioles papillate, perithecia 300–600 μm in diam., ascospores 9–13 μm long..........................................................E. cinnabarinum* |
| - Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles blood red, ostioles umbilicate, perithecia 100–300 μm in diam., ascospores 8–10 μm long..........................................................................................E. globosum |
| (4) Stromatal surface with olivaceous tint in maturity, perithecia 200–500 μm in diam., ascospores cylindrical with ± blunt ends, 8–13 × 3.5–6.5 μm; stromatal extractable pigments orange in 10% KOH, but not tested in E. siamensis...................................................5 |
| - Stromatal surface yellowish-tan or dark reddish-brown in maturity, perithecia 500–1000 μm in diam., ascospores ellipsoid with subacute ends, 13–18 × 6.5–8 μm, stromatal extractable pigments absent with 10% KOH…………………………….………......................6 |
| (5) Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles vividly yellow, ascospores 5–6.5 μm wide, asci 6–10 μm wide............................................................................................................E. liquescens* |
| - Stromatal pigment in the section immediately beneath the stromatal surface and around the perithecial ostioles consists of green granules, ascospores 3.5–5 μm wide, asci 5–6 μm wide........................................................................................................ E. siamensis |
| (6) Stromatal surface dark reddish-brown, perithecial ostioles prominently papillate, ascospores up to 15 μm long............................................................................................E. dengii |
| - Stromatal surface yellowish-tan with or without reddish-orange tinges, perithecial ostioles inconspicuous, punctate, ascospores always exceed 14.5 μm in length (up to 18 μm)..................................................................................................................................................7 |
| (7) Ascospores lemon-shaped with ± papillate ends, perispore indehiscent in 10% KOH ……………………….………………………………………………………….…E. moluccanum |
| - Ascospores ellipsoid with ± tapered ends, perispore dehiscent in 10% KOH………………..……………..E. moluccanum ss. Sánchez-Jácome & Guzmán-Dávalos |
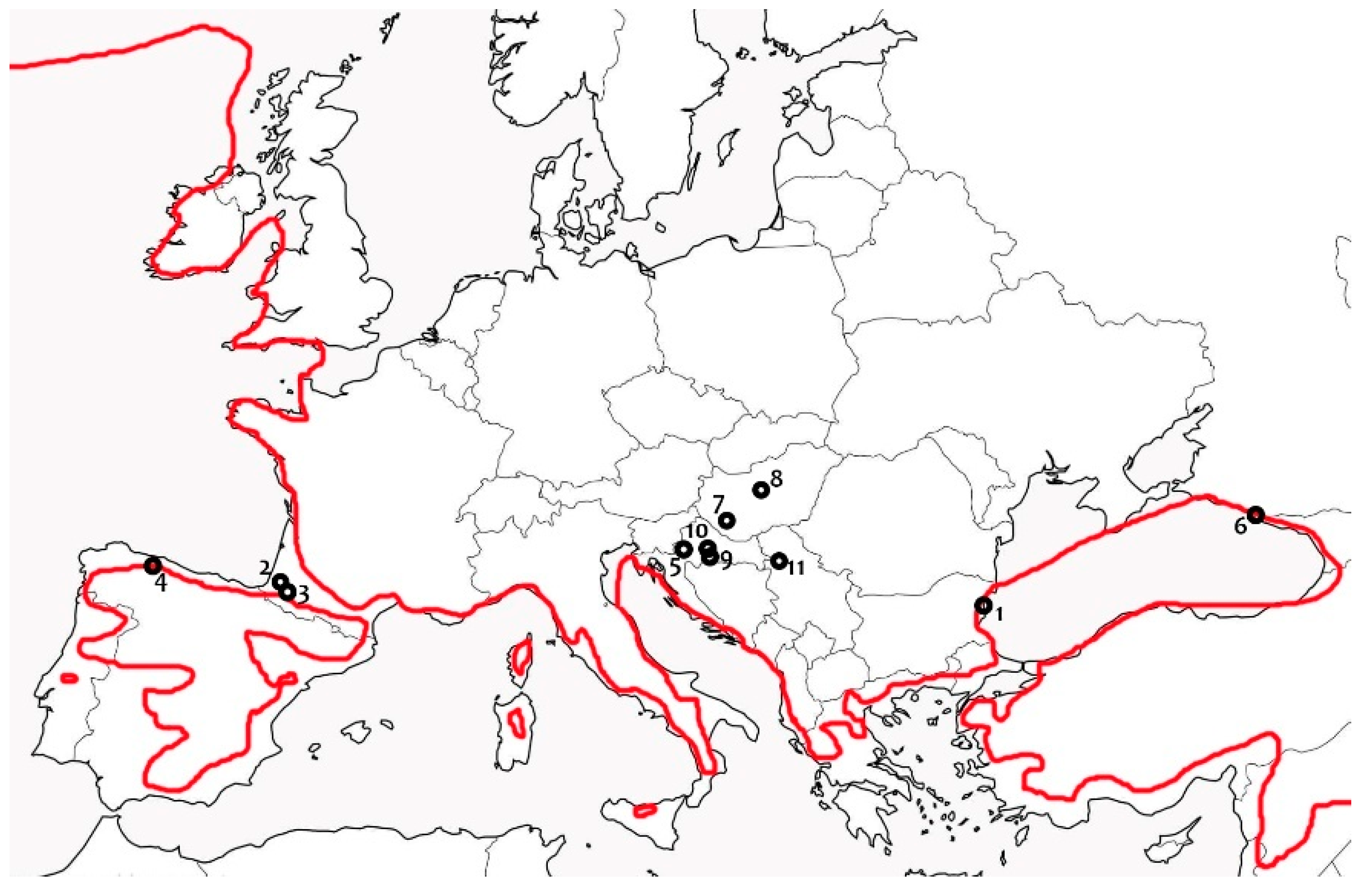
3.3. Ecology and Biogeography
4. Discussion
4.1. Taxonomic Implications
4.2. Molecular Misinterpretations
4.3. Distribution and Biogeography
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Möller, A. Phycomyceten Und Ascomyceten. Untersuchungen Aus Brasilien; Bot. Mitth. Tropen, Heft 9; G. Fischer: Jena, Germany, 1901. [Google Scholar]
- Stadler, M.; Fournier, J.; Læssøe, T.; Lechat, C.; Tichy, H.-V.; Piepenbring, M. Recognition of hypoxyloid and xylarioid Entonaema species and allied Xylaria species from a comparison of holomorphic morphology, HPLC profiles, and ribosomal DNA sequences. Mycol. Prog. 2008, 7, 53–73. [Google Scholar] [CrossRef]
- Lloyd, C.G. Mycological Notes, No. 69. Mycol. Writ. 1923, 7, 1185–1218. [Google Scholar]
- Martin, G.W. New or Noteworthy Fungi from Panama and Columbia. II. Mycologia 1938, 30, 431–441. [Google Scholar] [CrossRef]
- Rogers, J.D. Sarcoxylon and Entonaema (Xylariaceae). Mycologia 1981, 73, 26–61. [Google Scholar] [CrossRef]
- Rogers, J.D. Entonaema liquescens: Description of the Anamorph and Thoughts on Its Systematic Position. Mycotaxon 1982, 15, 500–506. [Google Scholar]
- Eriksson, O.E.; Hawksworth, D.L. Outline of the Ascomycetes—1993. Syst. Ascomycetum 1993, 12, 51–257. [Google Scholar]
- Whalley, A. The xylariaceous way of life. Mycol. Res. 1996, 100, 897–922. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Rogers, J.D. A Revision of the Genus Hypoxylon; APS Press: St Paul, MN, USA, 1996. [Google Scholar]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Cooke, M.C. Some Australian Fungi. Grevillea 1887, 15, 93–101. [Google Scholar]
- Heim, R. Quelques Ascomycétes Remarquables, II.—Le Genre Entonaema Möll. Au Mexique. Bull. Trimest. Société Mycol. De Fr. 1960, 76, 121–129. [Google Scholar]
- Sihanonth, P.; Thienhirun, S.; Whalley, A.J. Entonaema in Thailand. Mycol. Res. 1998, 102, 458–460. [Google Scholar] [CrossRef]
- Triebel, D.; Peršoh, D.; Wollweber, H.; Stadler, M. Phylogenetic relationships among Daldinia, Entonaema, and Hypoxylon as inferred from ITS nrDNA analyses of Xylariales. Nova Hedwig. 2005, 80, 25–43. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2020, 106, 7–28. [Google Scholar] [CrossRef]
- Baral, H.O. Vital versus Herbarium Taxonomy: Morphological Differences between Living and Dead Cells of Ascomycetes, and Their Taxonomic Implications. Mycologia 1992, 44, 333–390. [Google Scholar]
- Henriot, A.; Cheype, J.-L. Piximètre: La Mesure de Dimensions Sur Images. Version 5.10 R1541. Available online: http://ach.log.free.fr/Piximetre (accessed on 1 October 2020).
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Foodborne Fungi; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands; Delft, The Netherlands, 1996. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium are Nonorthologous. Mol. Phylogenetics Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Kazutaka, K.; Misakwa, K.; Kei-ichi, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-M.; Lin, C.-R.; Fang, M.-J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.-M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenetics Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Kuhnert, E.; Sir, E.B.; Lambert, C.; Hyde, K.D.; Hladki, A.I.; Romero, A.I.; Rohde, M.; Stadler, M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2016, 85, 1–43. [Google Scholar] [CrossRef]
- Kuhnert, E.; Fournier, J.; Peršoh, D.; Luangsa-Ard, J.J.D.; Stadler, M. New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers. 2013, 64, 181–203. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Li, Q.R.; Kang, J.C.; Hyde, K.D. Two new species of the genus Collodiscula (Xylariaceae) from China. Mycol. Prog. 2015, 14, 52. [Google Scholar] [CrossRef]
- Bitzer, J.; Læssøe, T.; Fournier, J.; Kummer, V.; Decock, C.; Tichy, H.-V.; Piepenbring, M.; Peršoh, D.; Stadler, M. Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol. Res. 2008, 112, 251–270. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Ju, Y.-M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, H.; Læssøe, T.; Stenlid, J. Molecular and morphological investigation of Daldinia in northern Europe. Mycol. Res. 2000, 104, 275–280. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Sung, G.-H.; Johnson, D.; Hesse, C.; O’rourke, B.; Serdani, M.; Spotts, R.; Lutzoni, F.; Hofstetter, V.; Miadlikowska, J.; et al. A five-gene phylogeny of Pezizomycotina. Mycologia 2006, 98, 1018–1028. [Google Scholar] [CrossRef]
- Entonaema Liquescens · INaturalist. Available online: https://www.inaturalist.org/taxa/350744-Entonaema-liquescens (accessed on 25 April 2023).
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Skaltsas, D.N.; Badotti, F.; Vaz, A.B.M.; da Silva, F.F.; Gazis, R.; Wurdack, K.; Castlebury, L.; Góes-Neto, A.; Chaverri, P. Exploration of stem endophytic communities revealed developmental stage as one of the drivers of fungal endophytic community assemblages in two Amazonian hardwood genera. Sci. Rep. 2019, 9, 12685. [Google Scholar] [CrossRef]
- Higginbotham, S.; Wong, W.R.; Linington, R.G.; Spadafora, C.; Iturrado, L.; Arnold, A.E. Sloth Hair as a Novel Source of Fungi with Potent Anti-Parasitic, Anti-Cancer and Anti-Bacterial Bioactivity. PLoS ONE 2014, 9, e84549. [Google Scholar] [CrossRef]
- Kim, C.S.; Jo, J.W.; Kwag, Y.-N.; Sung, G.-H.; Lee, S.-G.; Kim, S.-Y.; Shin, C.-H.; Han, S.-K. Mushroom Flora of Ulleung-gun and a Newly Recorded Bovista Species in the Republic of Korea. Mycobiology 2015, 43, 239–257. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Rossman, A.Y.; Rogers, J.D.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Koukol, O.; Kelnarová, I.; Černý, K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For. Pathol. 2014, 45, 21–27. [Google Scholar] [CrossRef]
- Pourmoghaddam, M.J.; Lambert, C.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stadler, M. Discovery of a new species of the Hypoxylon rubiginosum complex from Iran and antagonistic activities of Hypoxylon spp. against the Ash Dieback pathogen, Hymenoscyphus fraxineus, in dual culture. Mycokeys 2020, 66, 105–133. [Google Scholar] [CrossRef]
- Ma, H.-X.; Qiu, J.-Z.; Xu, B.; Li, Y. Two Hypoxylon species from Yunnan Province based on morphological and molecular characters. Phytotaxa 2018, 376, 27–36. [Google Scholar] [CrossRef]
- Cedeño–Sanchez, M. Three new species of Hypoxylon and new records of Xylariales from Panama. Mycosphere 2020, 11, 1457–1476. [Google Scholar] [CrossRef]
- Ma, H.; Song, Z.; Pan, X.; Li, Y.; Yang, Z.; Qu, Z. Multi-Gene Phylogeny and Taxonomy of Hypoxylon (Hypoxylaceae, Ascomycota) from China. Diversity 2022, 14, 37. [Google Scholar] [CrossRef]
- Song, Z.-K.; Zhu, A.-H.; Liu, Z.-D.; Qu, Z.; Li, Y.; Ma, H.-X. Three New Species of Hypoxylon (Xylariales, Ascomycota) on a Multigene Phylogeny from Medog in Southwest China. J. Fungi 2022, 8, 500. [Google Scholar] [CrossRef]
- Lambert, C.; Pourmoghaddam, M.J.; Cedeño-Sanchez, M.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stradal, T.E.B.; Stadler, M. Resolution of the Hypoxylon fuscum Complex (Hypoxylaceae, Xylariales) and Discovery and Biological Characterization of Two of Its Prominent Secondary Metabolites. J. Fungi 2021, 7, 131. [Google Scholar] [CrossRef]
- Stadler, M.; Kuhnert, E.; Peršoh, D.; Fournier, J. The Xylariaceae as Model Example for a Unified Nomenclature Following the “One Fungus-One Name” (1F1N) Concept. Mycology 2013, 4, 5–21. [Google Scholar] [CrossRef]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2016, 82, 1–105. [Google Scholar] [CrossRef]
- Bills, G.F.; González-Menéndez, V.; Martín, J.; Platas, G.; Fournier, J.; Peršoh, D.; Stadler, M. Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a Pantropical Insecticide-Producing Endophyte. PLoS ONE 2012, 7, e46687. [Google Scholar] [CrossRef]
- Sir, E.B.; Becker, K.; Lambert, C.; Bills, G.F.; Kuhnert, E. Observations on Texas hypoxylons, including two new Hypoxylon species and widespread environmental isolates of the H. croceum complex identified by a polyphasic approach. Mycologia 2019, 111, 832–856. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.-H.; Zhang, X.; Liu, L.-L.; Long, Q.-D.; Shen, X.-C.; Kang, Y.-Q.; Hyde, K.D.; Boonmee, S.; Kang, J.-C.; Li, Q.-R. Contributions to species of Xylariales in China—4. Hypoxylon wujiangensis sp. nov. Phytotaxa 2020, 455, 21–30. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Jones, E.B.G.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.-K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Pažoutová, S.; Šrůtka, P.; Holuša, J.; Chudíčková, M.; Kolařík, M. The phylogenetic position of Obolarina dryophila (Xylariales). Mycol. Prog. 2010, 9, 501–507. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Bhat, J.D.; Jones, E.B.G.; McKenzie, E.H.C.; Dai, D.Q.; Daranagama, D.A.; Dayarathne, M.C.; Goonasekara, I.D.; et al. Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers. 2015, 73, 73–144. [Google Scholar] [CrossRef]
- Peláez, F.; González, V.; Platas, G.; Sánchez-Ballesteros, J.; Rubio, V. Molecular Phylogenetic Studies within the Xylariaceae Based on Ribosomal DNA Sequences. Fungal Divers 2008, 31, 111–134. [Google Scholar]
- Tang, A.M.C.; Jeewon, R.; Hyde, K.D. A Re-Evaluation of the Evolutionary Relationships within the Xylariaceae Based on Ri-bosomal and Protein-Coding Gene Sequences. Fungal Divers 2009, 34, 127–155. [Google Scholar]
- Stadler, M.; Flessa, F.; Rambold, G.; Peršoh, D.; Fournier, J.; Læssøe, T.; Chlebicki, A.; Lechat, C. Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae). Mycoscience 2010, 51, 189–207. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Hsieh, H.-M. Xylaria species associated with nests of Odontotermes formosanus in Taiwan. Mycologia 2007, 99, 936–957. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Hsieh, H.-M.; Rogers, J.D.; Fournier, J.; Jaklitsch, W.M.; Courtecuisse, R. New and interesting penzigioid Xylaria species with small, soft stromata. Mycologia 2012, 104, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Sir, E.; Stadler, M. A new species of Daldinia (Xylariaceae) from the Argentine subtropical montane forest. Mycosphere 2016, 7, 1378–1388. [Google Scholar] [CrossRef]
- Fintha, G.; Benedek, L.; Orbán, S. New Macrofungial Record in Hungary: Entonaema cinnabarinum (Cooke & Massee) Lloyd. Acta Biol. Plant. Agriensis 2019, 7, 127–130. [Google Scholar] [CrossRef]
- Rogers, J.D.; San Martín, F.; Ju, Y.-M. Mexican Fungi: Xylaria entosulphurea Sp. Nov. and Neotypification of Entonaema globosum. Mycotaxon 1996, 58, 483–487. [Google Scholar]
- Stadler, M.; Ju, Y.-M.; Rogers, J.D. Chemotaxonomy of Entonaema, Rhopalostroma and other Xylariaceae. Mycol. Res. 2004, 108, 239–256. [Google Scholar] [CrossRef]
- Patouillard, N. Champignons de La Nouvelle-Calédonie. Bull. Trimest. Société Mycol. De Fr. 1911, 27, 329–333. [Google Scholar]
- Benkert, D. Kotlabaea macrospora Benkert nov. sp. und einige weitere bemerkenswerte Ascomyceten aus Bulgarien Mit einer Abbildung. Feddes Repert. 1993, 104, 547–549. [Google Scholar] [CrossRef]
- Fedosova, A.G. The New Record of Entonaema cinnabarinum (Xylariaceae, Ascomycota) in Europe. Vestnik of Saint Petersburg University. Series 3. Biology 2012, 1, 10–13. [Google Scholar]
- Harkevich, S.S. (Ed.) Флoра и Растительнoсть Уссурийскoгo Запoведника; Наука: Moscow, Russia, 1978. [Google Scholar]
- Azbukina, Z.M.; Baгdunov, L.V.; Bezdeleva, Т.А.; Bogacheva, А.V.; Bulakh, Е.М.; Vasilyeva, L.N.; Govorova, О.K.; Egorova, L.N.; Zhabyko, Е.V.; Nikulina, Т.V.; et al. Flora, Vegetation and Mycobiota of the Reserve “Ussuriysky”; Daljnauka: Vladivostok, Rasia, 2006. [Google Scholar]
- Sánchez-Jácome, M.; Guzmán-Dávalos, L. New Records of Ascomycetes from Jalisco, Mexico. Mycotaxon 2005, 92, 177–191. [Google Scholar]
- Quang, D.N.; Stadler, M.; Fournier, J.; Asakawa, Y. Carneic Acids A and B, Chemotaxonomically Significant Antimicrobial Agents from the Xylariaceous Ascomycete Hypoxylon carneum. J. Nat. Prod. 2006, 69, 1198–1202. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Zamora, J.C.; Svensson, M.; Kirschner, R.; Olariaga, I.; Ryman, S.; Parra, L.A.; Geml, J.; Rosling, A.; Adamčík, S.; Ahti, T.; et al. Considerations and consequences of allowing DNA sequence data as types of fungal taxa. IMA Fungus 2018, 9, 167–175. [Google Scholar] [CrossRef]
- Šrůtka, P.; Pažoutová, S.; Kolařík, M. Daldinia decipiens and Entonaema cinnabarina as fungal symbionts of Xiphydria wood wasps. Mycol. Res. 2007, 111, 224–231. [Google Scholar] [CrossRef]
- Lӕssøe, T. Entonaema cinnabarina-En Eksotisk Kernesvamp. Svampe 1997, 36, 21–22. [Google Scholar]
- Fintha, G.; Nagy, I.; Vitkó, T.; Benedek, L.; Baranyai, G. Az Ócsai Turjánvidék Natura 2000-es kijelölt területeinek nagygombái. J. Landscape Ecol. 2022, 20, 3–21. [Google Scholar] [CrossRef]
- Stanners, D.; Bourdeau, P. Europes’s Environment: The Dobřiš Assessment; European Environment Agency: Copenhagen, Denmark, 1995. [Google Scholar]
- Quijada, L.; Matočec, N.; Kušan, I.; Tanney, J.B.; Johnston, P.R.; Mešić, A.; Pfister, D.H. Apothecial Ancestry, Evolution, and Re-Evolution in Thelebolales (Leotiomycetes, Fungi). Biology 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Rogers, J.D.; San Martin, F. A Revision of the Genus Daldinia. Mycotaxon 1997, 61, 243–293. [Google Scholar]
- INaturalist Research-Grade Observations. Available online: https://www.inaturalist.org (accessed on 26 June 2023).
- Rubel, F.; Kottek, M. Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol. Z. 2010, 19, 135–141. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pošta, A.; Matočec, N.; Kušan, I.; Tkalčec, Z.; Mešić, A. The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology. Forests 2023, 14, 1764. https://doi.org/10.3390/f14091764
Pošta A, Matočec N, Kušan I, Tkalčec Z, Mešić A. The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology. Forests. 2023; 14(9):1764. https://doi.org/10.3390/f14091764
Chicago/Turabian StylePošta, Ana, Neven Matočec, Ivana Kušan, Zdenko Tkalčec, and Armin Mešić. 2023. "The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology" Forests 14, no. 9: 1764. https://doi.org/10.3390/f14091764
APA StylePošta, A., Matočec, N., Kušan, I., Tkalčec, Z., & Mešić, A. (2023). The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology. Forests, 14(9), 1764. https://doi.org/10.3390/f14091764









