Abstract
As an important aspect of plant water consumption, nocturnal water use (En) behavior provides reliable information on the effect of plantation carbon and water budgets at stand and regional scales. Therefore, quantifying En and its environmental and stomatal controlling mechanisms is urgent to establish adaptation strategies for plantation management in semiarid regions. With the help of the sap flow technique, our study investigated the seasonal variations in canopy transpiration and canopy conductance in a Caragana korshinskii Kom plantation. Environmental variables were measured concurrently during the growing seasons of 2020 and 2021. The results indicated that the average En values were 0.10 mm d−1 and 0.09 mm d−1, which accounted for 14% and 13% of daily water use, respectively, over two years. The proportions of nocturnal transpiration (Tn) to En were approximately 49.76% and 54.44%, while stem refilling (Re) accounted for 50.24% and 45.56% of En in 2020 and 2021, respectively, indicating that C. korshinskii was able to draw on stored stem water to support transpiration. En was predominantly affected by nocturnal canopy conductance (Gc–n), air temperature (Ta–n) and wind speed (u2-n). In contrast, Gc–n and Ta–n explained the highest variation in Tn and nocturnal vapor pressure (VPDn), and u2-n explained the highest variation in Re. Total effects of the five environmental and stomatal variables explained 50%, 36% and 32% of En, Tn and Re variation, respectively. These findings could enable a better understanding of nocturnal water use dynamics and their allocation patterns in C. korshinskii plantations on the Bashang Plateau. Moreover, our results reveal the water use strategies of artificial shrubs and highlight the importance of incorporating nocturnal water use processes into large-scale ecohydrological models in semiarid regions.
1. Introduction
Nocturnal water use is defined as the sap flux dynamics that occur at night with incomplete stomatal closure, and is different to diurnal water use in a single component. During the past two decades, nocturnal water use has been reported across a range of forest species and habitats; and the average contribution of nocturnal water use to daily water use is approximately 12% in diverse woody species, and reaches up to 30–60% in water-limited environments [1,2,3,4,5]. The occurrence, pattern and alteration of nocturnal water use play important roles in adjusting predawn disequilibrium between leaves and soil [6], reducing hydraulic redistribution within dry rhizosphere soils [7], avoiding the delay between carbon assimilation and stomatal opening in the early morning hours [8,9] and facilitating oxygen and nutrient uptake for tree growth [10,11]. Therefore, nocturnal water use is regarded as a special water use strategy that enables plants to adapt to different environments, and the accurate quantification of nocturnal water use and its contribution to total daily water use is important for understanding the carbon and water budgets of forest ecosystems [12]. However, there is limited research on the nocturnal water use behavior of planted shrubs in arid and semiarid regions, which limits our understanding of the water use strategy of shrubs, and of future changes in ecosystem function in these regions.
Nocturnal water use can be characterized as nocturnal transpiration from leaves and the water required for stem refilling, separately or synergistically [13,14,15]. Nocturnal transpiration is driven by leaf-to-air vapor pressure differences under the influence of nocturnal stomatal openness. It might help to promote nutrient absorption via the mass flow of water and carbon fixation in the early morning [16]. Stem refilling is an effective type of internal water storage, that functions to replenish the large amount of water consumed during diurnal transpiration. Water absorption by the roots during the night is needed to overcome xylem and stem water deficits that occur in the daytime. Precisely identifying nocturnal transpiration and stem refilling components from continuous nocturnal water use will contribute to developing a better understanding of physiological responses to external environmental changes. Several methods have been proposed to distinguish nocturnal transpiration and stem refilling from nocturnal water use, such as the Penman–Monteith model [17], a flow model based on a resistance network [18], a time series model with one or more exogenous inputs [19,20] and a forecasted refilling method regarding determination-based vapor pressure deficits (VPD) [13,21]. The forecasted refilling method, conducted by analyzing the slope of a daily sap flux curve, has been proven to be a simple and useful fraction method, and has been successfully applied on rainless nights in natural [1,2], planted [22,23] and urban forests [5].
The reported crucial environmental drivers of nocturnal water use include nocturnal VPD, temperature, wind speed and soil water content [7,22,24]. High nocturnal water use usually occurs when air temperatures and soil water content are high. Additionally, nocturnal water use in response to environmental factors is achieved through adjustments to nocturnal stomatal conductance. High nocturnal water use is regularly associated with high nocturnal stomatal conductance, which tracks VPD-induced variations in stomatal apertures [25]. The environmental and stomatal control mechanisms of nocturnal transpiration and stem refilling are generally different, according to prior studies. The concurrent occurrence of stomatal openness and vapor pressure differences between the atmosphere and leaf surface provide the necessary conditions for nocturnal transpiration [5]. Therefore, nocturnal transpiration is usually positively correlated with VPD and wind speed, while stem refilling is commonly correlated to biological and physiological variations, such as plant size, anatomical features, plant water status, etc. [26]. However, similar stem refilling proportion results were reported for plants of the same species but different individual sizes in planted forests in Mexico [27]. Therefore, the environmental and stomatal mechanism of nocturnal water use is still uncertain, and a more advanced understanding of the total effect of concurrent environmental and stomatal controls on nocturnal water use and its fractions is needed.
As a typical shrub type in arid and semiarid regions, Caragana korshinskii Kom has commonly been used since the implementation of the Beijing–Tianjin Sand Source Restoration Project in 2000 for establishing protective shrub plantations on the Bashang Plateau. Due to their strong drought tolerance, C. korshinskii plantations play an important role in windbreak and sand fixation, soil and water conservation, soil restoration and ecological protection. Major research has been conducted on C. korshinskii plantations to understand their daily water use and their capacity to adapt to water-limited environmental conditions [28,29,30]. However, the ecological importance of nocturnal water use dynamics in C. korshinskii plantations is poorly understood, and under soil water scarcity the responses of nocturnal water use, nocturnal transpiration and stem refilling to concurrent environmental and stomatal variables remain fully unknown. Therefore, we hypothesize that (1) nocturnal water use occurs in C. korshinskii plantations and has a great impact on daily water loss; (2) the environmental and stomatal controls of nocturnal water use and its fractions (nocturnal transpiration and stem refilling) may be subject to different influences. The objectives of this study are as follows: (1) to analyze the nocturnal water use dynamic and its contribution to the daily water use of C. korshinskii, (2) to determine the allocation of the nocturnal transpiration and stem refilling of C. korshinskii and (3) to explore the total effects of concurrent environmental and stomatal controls on nocturnal water use, nocturnal transpiration and stem refilling.
2. Materials and Methods
2.1. Site Description
The research area is situated at the northern Bashang Plateau, Kangbao County, which belongs to the northwest of the Beijing–Tianjin–Hebei Region of China (Figure 1). The climate of the region is arid and cold with mean annual precipitation of 330.0 mm and mean annual temperature of 2.3 °C (1980–2020). However, the potential evapotranspiration is over 850 mm. The mean annual wind speed is 3.15 m s−1. The field site located at the Kangbao pasture region (114°48′ E, 42°07′ N, altitude 1305 m) is composed of planted forests of pure C. korshinskii, which were planted according to the Beijing–Tianjin Sandstorm Source Sontrol Project. The C. korshinskii plantation is about 20 years old, with an average height of 179.28 ± 30.74 cm and stem basal diameter at stem base (10–15 cm above the ground) of 2.38 cm. The soil is mainly sandy loam with a high sand content of 52.89% and low clay content of 7.86% [31].
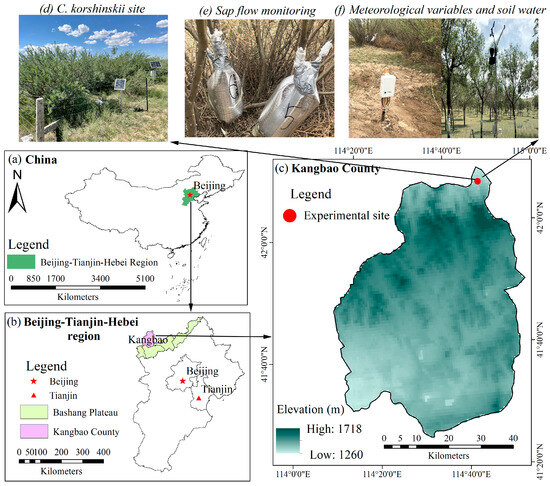
Figure 1.
Geographical research sites in this study, including (a–c) Beijing–Tianjin–Hebei region and the Bashang Plateau of Hebei province, (d) Caragana korshinskii Kom site in Kangbao County, (e,f) sap flow measurement and meteorological variables, soil water monitoring.
2.2. Materials and Experimental Design
2.2.1. Materials
We selected a sample site for the study and established a 20 m × 20 m fenced enclosure for purposes of monitoring. Based on the distribution of stem base diameter (SBD), nine sample stems (SDB = 2.68, 2.96, 3.38, 3.62, 3.66, 3.76, 4.14, 4.24 and 4.38 cm) of healthy and non-stressed C. korshinskii were selected for the 2020 and 2021 experimental periods. We assumed that the whole cross-section of these stems in C. korshinskii were conductive, sapwood area (As) which was then estimated as a stem basal cross-sectional area. The total sapwood area in the experimental plot was 6877.70 cm2.
2.2.2. Environmental Variables
Meteorological parameters were measuring at a height of 2 m in a nearby clear space with an Onset HOBO U21 automatic weather station (Onset Computer Corp., Bourne, MA, USA) and included the following: photosynthetically active radiation (PAR, µmol m−2 s−1), air temperature (Ta, °C), relative humidity (RH, %), wind speed (u2, m s−1) and precipitation (P, mm). Vapor pressure deficits (VPD, kPa) were calculated from Ta and RH according to Campbell and Norman [32]. These meteorological data were available as 10 min averages from 30 s samples and stored using CR1000 data loggers (Campbell Scientific, Logan, UT, USA).
The soil water content (SWC, m−3 m−3) was measured at five depths (i.e., 5, 15, 30, 50, 80 cm) with an EC-5TE sensor (Decagon, Inc. Pullman, WA, USA) and recorded at 30 min internals by CR1000 data loggers (Campbell Scientific, Logan, UT, USA).
Relative extractable soil water content (REW) was calculated using averaged SWC across 0–100 cm:
where SWCmin and SWCmax are the minimum and maximum daily average soil water content, respectively, during the two whole years.
2.2.3. Sap Flow Measurements
We measured xylem sap flux on stems using thermal dissipation probes (TDPs) during two growing seasons in 2020 and 2021 (1 May to 30 September). Nine pairs of Granier-type probes (TDP10, Dynamax Inc., Houston, TX, USA) consisting of a copper-constantan thermojunction were inserted at a depth of 10 mm into the xylem sapwood of stems of nine shrubs at a height of 40 cm above the ground on the northern side. In order to protect the probes to reduce solar heating and extraneous thermal gradients, an aluminum foil shield wrap was applied for each pair of Granier-type probes. Thermal insulation cotton and aluminum foil were placed on the ground around the probe to reduce the influence of ground temperature on its measurements. Additionally, for the homogenous and dense plantation stands, the influences of natural thermal gradients on sap flow measurements could be assumed to be negligible [22,33]. The detail procedure for measuring sap flow velocity can be found in [31]. The sap flow velocity (SF, mL cm−2 min−1) could be calculated from the measured temperature difference at 30 min intervals (CR1000, Campbell Scientific Inc., Logan, UT, USA) according to Granier [34]:
where ∆T (°C) is the temperature difference between the two probes at any given time, and ∆Tmax (°C) is the maximum temperature difference between sensors, which was determined as the maximum value of daily ∆Tmax over a 9-day period to avoid underestimation of the nighttime sap flow. (1) A linear regression of local maximal ∆Tmax determined by a 9-day moving window and time (day) was performed and local ∆Tmax values below the linear regression line were eliminated; (2) A new linear regression was then made based on the remaining ∆Tmax points; (3) finally the “real ∆Tmax” was recalculated by the new linear regressions [35,36]. As there was only one probe per sample stem, the azimuthal and radial variation in sap flow velocity were not taken into account in this study. We assume that these variations were low [23,37]. E is canopy transpiration per area of ground (mm h−1); is the average sap flow velocity of sampled shrubs (ml cm−2 min−1), Ag is the ground surface area of the studied plots (400 m2), and Asi is the total sapwood area in the studied plot.
Daily SF and E were further divided into diurnal SF/E (SFd/Ed) and nocturnal SF/E (SFn/En) based on the value of PAR. Diurnal SFd/Ed was defined as a PAR value greater than 5 µmol m−2 s−1, while nocturnal SFn/En was correspondingly defined as a PAR value less than 5 µmol m−2 s−1 or equal to 0. Therefore, nocturnal SFn/En ranged from 19:30 to 4:30 in May, 20:00 to 4:30 in June, 20:00 to 4:30 in July, 19:00 to 5:00 in August, 18:30 to 5:30 in September during the growing season in 2020 (Figure 2a). In 2021, nocturnal SFn/En ranged from 19:30 to 4:30 in May, 20:00 to 4:00 in June, 19:00 to 4:30 in July, 19:30 to 5:00 in August and 18:30 to 5:30 in September during the growing season (Figure 2b). To differentiate between the contributions of nocturnal transpiration (Tn) and stem refilling (Re) to SFn, the forecasted refilling method was used [2,21]. Fisher et al. [21] suggested that if no nocturnal water loss occurred, SFn would gradually continue to fall to zero flow via an exponential decay function. However, we found that SFn was greater than zero flow during the whole growing season in 2020 and 2021, and decreased at the beginning of the night but then increased until sunrise (Figure 2). It was indicated that the early sloped phase of SFn mostly consisted of Re and a nonzero linear phase representing Tn later [2,38]. Therefore, an exponential relationship between SFn and VPDn was conducted to fit the first 3 to 5 h:
where a and b are fitting parameters. The relationship between SFn and VPDn was determined, with R2 consistently > 0.96 (Figure A1) during the growing season in 2020. This approach was only applied for every clear night with low nocturnal VPD, so that: (1) the area below the forecasting curve could be considered as sap flow caused by Re, due to lack of strong atmospheric demand for nocturnal water loss; (2) the area above the curve could be considered as sap flow caused by Tn.
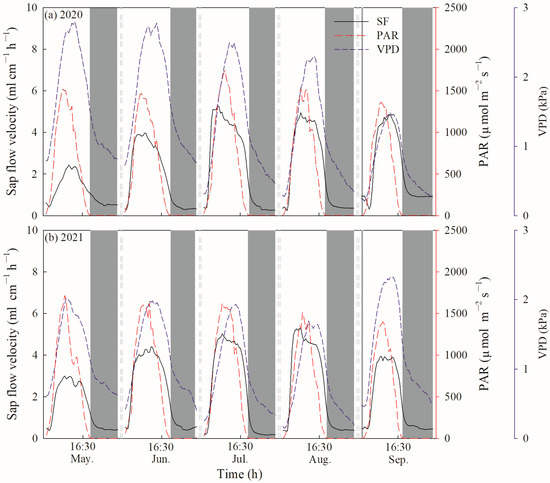
Figure 2.
Hourly variations in sap flow velocity of C. korshinskii, photosynthetically active radiation and vapor pressure deficits.
2.2.4. Canopy Conductance
Since the distribution of the C. korshinskii plantation was relative uniform and the canopy was not closed (the largest value of leaf area indices was 0.43 m2 m−2), the canopy was well-coupled to the atmosphere [31], The canopy conductance can be calculated by the simplified Penman–Monteith equation [39] using the following formula:
where Gc is canopy conductance (mm s−1), γ is the psychometric constant (kPa, °C−1), λ is latent heat of water vaporization (MJ kg−1), ρ is the air density (kg m−3) and Cp is the specific heat of the air (MJ kg−1 °C−1).
Nocturnal Gc (Gc–n) were also defined as stomatal opening occurring when a PAR value was less than 5 µmol m−2 s−1.
2.3. Data Analysis
The differences in the environmental and stomatal variables during the two growing seasons of 2020 and 2021 were tested using a paired-samples t-test. Significant differences in En, Tn and Re among different months during the growing season were tested using one-way ANOVA at a significance level of α = 0.05. Pearson’s correlation coefficient and a partial correlation coefficient were used to estimate associated pairwise relationships between En/Tn/Re and all environmental and stomatal variables. Then, linear, polynomial and nonlinear (i.e., exponential growth function, exponential threshold function) regression analyses were conducted to investigate the relationships between En/Tn/Re and major environmental and stomatal variables (i.e., VPDn, u2-n, Ta–n and Gc–n). However, these single-variable relationships above might be weak and showed a high degree of scatter, which was due to a strong effect of certain other factors. Therefore, an upper boundary line method was applied to determine these single-variable relationships of En/Tn/Re and VPDn, u2-n, Ta–n and Gc–n, without interference from other factors. The study divided VPDn, u2-n, Ta-n and Gc-n into several segments, with intervals of segments of VPDn, u2-n, Ta–n and Gc–n of 0.5 kPa, 1 m s−1, 5 °C and 0.5 mm s−1, respectively. Then, an upper boundary line was conducted as suitable linear and nonlinear functional forms based on the data of En/Tn/Re of at least one standard deviation greater than the mean En/Tn/Re at each VPDn, u2-n, Ta–n and Gc–n interval. All statistical analyses were conducted using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and all figures were created using Sigmaplot 11.0 software (Hearne Scientific Software Plc, Melbourne, Australia).
A path coefficient model was developed to quantify the direct and indirect effects of environmental and stomatal variables on En, Tn and Re, respectively. The initial model included all potential paths according to current knowledge and the above linear/nonlinear analyses. (1) VPDn, u2-n, Ta–n, REW and Gc–n were considered to directly affect En, Tn and Re, respectively. Daily REW was used to respond to En/Tn/Re since there was no significant difference between diurnal and nocturnal REW in this study. (2) u2-n may affect En/Tn/Re by altering VPDn, which may also affect En/Tn/Re by altering Gc–n. REW may affect En/Tn/Re by adjusting nocturnal canopy conductance. The standardized path coefficients were calculated through the maximum likelihood method. Path analysis was conducted using AMOS 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Environmental Conditions
The average diurnal photosynthetically active radiation (PAR) values were 681.16 ± 201.18 µmol m−2 s−1 and 744.18 ± 200.69 µmol m−2 s−1 during the growing season in 2020 and 2021, respectively. The averaged nocturnal weed speeds (u2-n) were 0.76 ± 0.67 m s−1 and 0.80 ± 0.75 m s−1 during the night in 2020 and 2021, respectively (Figure 3a). Nocturnal air temperature (Ta–n) displayed marked seasonal variations, with ranges of 2.04 to 22.40 °C and −1.89 to 22.61 °C, and mean values of 13.79 ± 4.38 °C and 13.93 ± 4.56 °C in 2020 and 2021, respectively. The average nocturnal vapor pressure deficits (VPDn) were 0.60 ± 0.34 kPa and 0.62 ± 0.31 kPa during the growing season in 2020 and 2021, respectively (Figure 3b). A significant difference was observed in REW between 2020 and 2021 (F = 22.299, p < 0.000). The daily REW ranged from 0.31 to 0.79 and 0.31 to 0.98, with mean values of 0.42 ± 0.11 and 0.46 ± 0.16, in 2020 and 2021, respectively (Figure 3c).
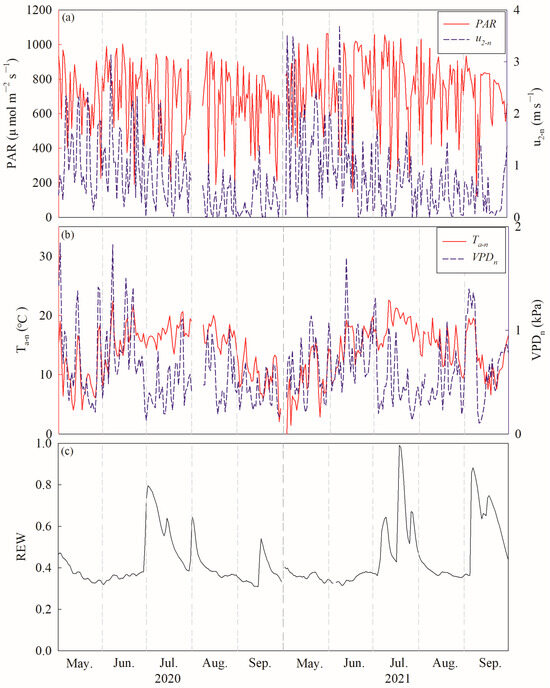
Figure 3.
Diurnal variations of (a) photosynthetically active radiation (PAR) and nocturnal wind speed at the height of 2 m (u2-n), (b) nocturnal air temperature (Ta–n) and nocturnal vapor pressure deficits and (VPDn) (c) soil water content at a depth 0–100 cm (REW) in 2020 and 2021.
3.2. Variations in Nocturnal Water Use
The annual average Ed values for C. korshinskii were 0.64 ± 0.24 mm d−1 and 0.69 ± 0.23 mm d−1; meanwhile, the accumulated Ed values over the growing season were 92.51 mm and 103.26 mm in 2020 and 2021, respectively. While the En values ranged from 0.01 to 0.61 mm d−1 in 2020, with a mean value of 0.10 ± 0.08 mm d−1, whereas in 2021, En ranged from 0.01 to 0.59 mm d−1 with a mean value of 0.09 ± 0.07 mm d−1. The accumulated En values over the growing season were 14.36 mm and 14.51 mm in 2020 and 2021, respectively, accounting for 15.48% and 14.04% of the accumulated diurnal water use and 13.40% and 12.31% of the accumulated daily water use over the same period. The annual average En:Ed values were 0.18 ± 0.15 and 0.17 ± 0.17, whereas the En:E values were 0.14 ± 0.09 and 0.13 ± 0.10 in 2020 and 2021, respectively (Figure 4a,b). No significant difference was observed for either En:Ed or En:E between 2020 and 2021; however, in both years, En:Ed and En:E were significantly higher in May and September than from June to August (p < 0.000, ANOVA).
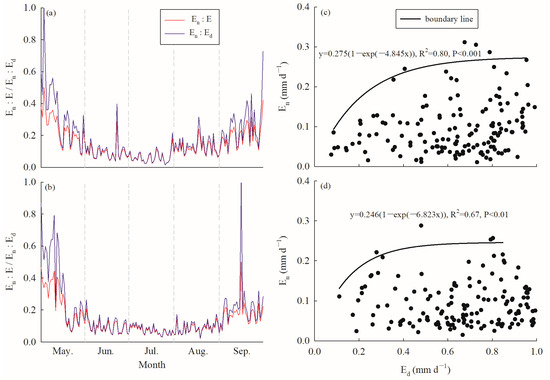
Figure 4.
Variation in the ratio of nocturnal to diurnal (En:Ed, red line) and nocturnal to daily water use (En:E, blue line) in (a) 2020 and (b) 2021; the relationship between En and Ed (black solid circle) and boundary line analysis (black line) in (c) 2020 and (d) 2021.
The saturated exponential analysis showed a significant positive relationship between En and Ed, but the goodness of fit was better in 2020 (R2 = 0.80) than in 2021 (R2 = 0.67) after conducting boundary line analysis (Figure 4c,d). En increased with increasing Ed, while it tended to be saturated when Ed reached approximately 0.4–0.5 mm d−1 in 2020 and 0.4 mm d−1 in 2021.
3.3. Components of Nocturnal Transpiration and Xylem Refilling
Tn was low and stable before mid-August, while it showed an obvious peak that appeared in mid-September; however, Re was relatively higher in May and remained stable between June and September in 2020 (p < 0.000, ANOVA) (Figure 5a and Table 1). In 2021, Tn was low and stable between July and August, but showed peaks in early May and mid-September; however, Re in May and July was significantly lower than that in June and August (p < 0.000, ANOVA) and showed a peak in early September (Figure 5b and Table 1). The annual average Tn values were 0.049 ± 0.073 and 0.052 ± 0.068 mm d−1, while the Re values were 0.050 ± 0.038 and 0.043 ± 0.030 mm d−1 in 2020 and 2021, respectively. The accumulated Tn values over the growing season were 7.14 and 7.90 mm in 2020 and 2021, respectively, accounting for 49.76% and 54.44% of En. Meanwhile, the sums of Re were 7.22 and 6.61 mm, accounting for 50.24% and 45.56% of En in 2020 and 2021, respectively.
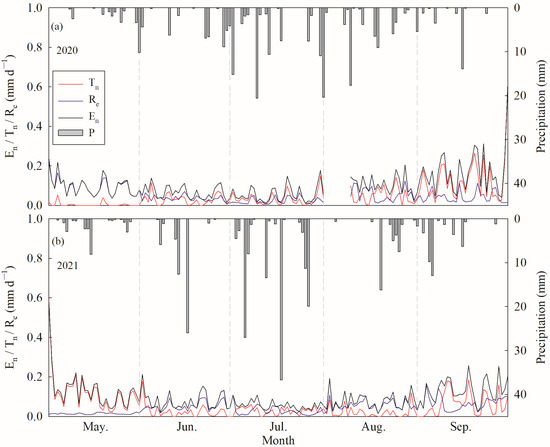
Figure 5.
Daily variations of nocturnal water use (En), nighttime canopy transpiration (Tn) and stem refilling (Re) and the distribution of precipitation during the growing season in (a) 2020 and (b) 2021.

Table 1.
Seasonal variation in nocturnal water use (En), nocturnal transpiration (Tn), stem refilling (Re) and contribution of Tn and Re to En (Tn:En and Re:En) during the growing season from May to September of 2020 and 2021 for Caragana korshinskii Kom.
3.4. Environmental and Stomatal Drivers of Nocturnal Water Use
The statistical analysis indicated that the significance effects of the environmental and stomatal variables on En had a ranking of Gc–n > u2-n > Ta–n > PAR > RHn in 2020 and Gc–n > Ta–n > RHn > PAR > u2-n in 2021. Moreover, Tn was positively correlated with Gc-n and PAR, but negatively correlated to Ta–n, u2-n and VPDn in 2020; while it was positively correlated with Gc–n, and negatively correlated to Ta–n, RHn and VPDn in 2021, when controlling for Re. However, Re was positively correlated with PAR and VPDn in both years, but negatively to RHn and Ta–n in 2020 and to RHn and u2-n in 2021, when controlling for Tn (Table 2).

Table 2.
Correlation and partial correlation coefficients between nocturnal water use (En, Tn and Re) and environmental, stomatal variables for C. korshinskii in 2020 and 2021.
Both En and Tn decreased linearly with increasing Ta–n, which explained 87% and 99% of the variation in En, and 60% and 84% of the variation in Tn, during the measurement periods of 2020 and 2021, respectively. Meanwhile, Re was stable and decreased slightly with increasing Ta–n in 2020, and increased linearly with increasing Ta–n in 2021 (Figure 6a–c). Both En and Tn displayed an exponential decay response to VPDn, but the goodness of fit was better (R2 = 0.72, 0.93) in 2020 than in 2021 (R2 = 0.78, 0.63). Meanwhile, Re exhibited a polynomial response to VPDn and tended to fall off when VPDn > 1.0 kPa in 2020, but increased linearly with increasing VPDn (Figure 6d–f). u2-n explained 90%, 87% and 91% of the variation in En, Tn and Re in 2020, and 47%, 32% and 93% in 2021, following exponential decay functions, respectively (Figure 6g–i). En exhibited an exponential saturation response to Gc–n both in 2020 and 2021, and En tended to level off at 1.5 mm s−1 in 2021. Tn increased linearly with increasing Gc–n in 2020, but had an exponential saturation response to Gc–n in 2021. Meanwhile, Re remained stable with different Gc–n values in 2020, but had an exponential decay response to Gc–n in 2021 (Figure 6j–l).
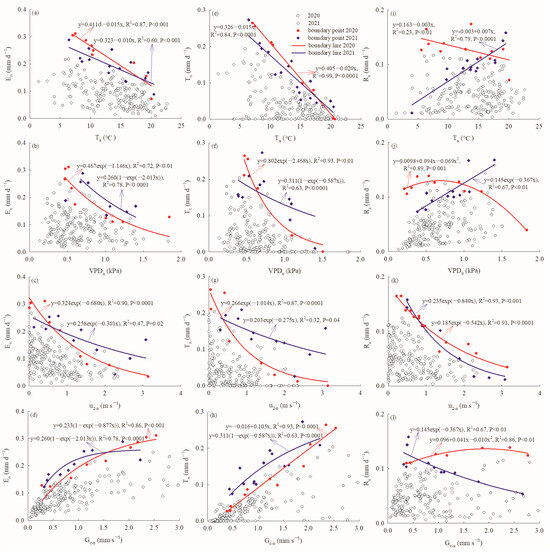
Figure 6.
Nocturnal water use (En) relationship with (a) nocturnal air temperature, (b) nocturnal vapor pressure deficits, (c) nocturnal weed speed, (d) nocturnal canopy conductance; nocturnal transpiration (Tn) relationship with (e) nocturnal air temperature, (f) nocturnal vapor pressure deficit, (g) nocturnal weed speed, (h) nocturnal canopy conductance; stem refilling (Re) relationship with (i) nocturnal air temperature, (j) nocturnal vapor pressure deficit, (k) nocturnal weed speed, (l) nocturnal canopy conductance.
Combined with the direct and indirect effects, VPDn positively affected En (0.146) and Re (0.476), while it negatively affected Tn (−0.135). u2-n decreased En (−0.157) and Re (−0.273) during two growing seasons. Finally, the total effect of these five environmental and stomatal variables explained 50%, 36% and 32% of the variation in En, Tn and Re, respectively (Figure 7).
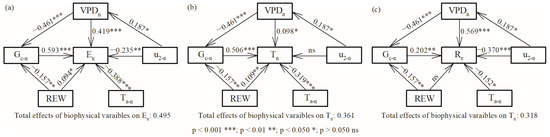
Figure 7.
Direct and indirect effects of environmental and stomatal variables on (a) nocturnal water use, (b) nocturnal transpiration, and (c) stem refilling in 2020 and 2021.
4. Discussion
4.1. The Application of Thermal Dissipation Probes for C. korshinskii Plantation
Sap flow was usually measured using the stem heat balance method for shrubs with short height and small diameter at breast height (DBH) [28]. However, in arid and semiarid regions, the influence of high wind and strong solar radiation brought great challenges to the complete sap flow velocity data continuity and stability. The thermal dissipation probes (TDP10) method has recently been used to measure the sap flow velocity for plants with small DBH, such as bamboo species [40], Populus adenopoda [41], Aegiceras corniculatum [42] and so on. Compared with an induced hydraulic pressure and sap flow changing device and a whole-culm pot weighing method, the 10 mm long TDP was proven to be a valid means of measuring the sap flow of Phyllostachys pubescens [43]. Additionally, an environmental temperature reduction from 25 to 0 °C did not alter the values of ∆Tmax between a heated probe and a reference probe when there was no sap flow, verifying that ΔT measured at night can be used as a reference in daytime [44]. The daily average temperature in our study was 15.14 ± 3.88 °C and 15.02 ± 4.28 °C during the growing season in 2020 and 2021, respectively (Figure 3b). Therefore, TDP10 could be verified to be a valid means of accurately quantifying the water use of C. korshinskii with SDB less than 5 cm in this study.
4.2. Nocturnal Water Use Behaviors of C. korshinskii
Increasing evidence from leaf microstructure and gas exchange experiments has suggested that stomata are partially open throughout the night, which provides the necessary conditions for nocturnal water use [5,38]. Like other tree and shrub species, such as P. euphratica, P. tabuliformis, A. truncatum, S. superba, Q. lancifolia, A. latifolia, A. jorullensis, S. psammophila, we observed substantial C. korshinskii En at night during the growing seasons in 2020 and 2021 (Figure 2). We found that the water stored in the stems, refilled mostly during the night from soil water uptake through the roots, is usually used for supply water consumption during the daytime [23,45]. However, the En of C. korshinskii was much lower than that reported in plant species in the past. This may be related to the smaller daily transpiration of C. korshinskii plantations and the lower soil water content (Figure 3c) on the Bashang Plateau [31]. The En:E values in this study were 14% and 13% in 2020 and 2021, respectively, which were similar to the values reported for P. tabuliformis and A. truncatum [22]. The sharp increase in En:E at the beginning and the end of the growing season in both 2020 and 2021 was similar to that found in birch [45] and a P. tomentosa plantation [23]. Nocturnal water loss was more important under drought conditions than in other conditions [15]. The sharp increase in sap flow during the early growth stage had a function of refilling xylem conduits that accumulated embolisms during winter in the previous years. Additionally, air temperature during winter ranged from −29 °C to −10 °C in 2020 and −30 °C to −5 °C in 2021 (Figure 3b), the relatively higher En:E was thus related to a minimized effect of freezing and drought on the hydraulic functioning of C. korshinskii at the beginning of the growing season [23]. Meanwhile, the sharp increase in soil water availability in September promoted an increase in En and En:E, which was used to refill the embolized xylem conduits at the end of growing season. However, the seasonal trend of En:Ed in our study was contrary to that reported by Zhao et al. [38]. Relatively smaller differences between En and Ed from June to August during the growing season resulted from smaller differences in diurnal air temperature and Ta–n (Figure 3b).
4.3. Nocturnal Transpiration and Stem Refilling Dynamics of C. korshinskii
The partitioning of En into nocturnal transpiration (Tn) and stem refilling (Re) is challenging because of their obvious temporal overlap. The forecasted refilling method has been successfully and widely used in previous studies on rain-free days under low levels of VPDn. However, Chen et al. [22] indicated that En could also be influenced by nocturnal wind speeds, soil water content and their interaction. The accuracy of the forecasted refilling method can only be guaranteed under a low value of VPD. On every rain-free night in this study (Figure 2 and Figure 3b), VPD was usually lower than that reported in A. truncatum [5] and P. euphratica [46]; we thus ignored the uncertainty of the forecasting method in this study. Our results indicate that, for C. korshinskii, Tn accounted for 49.76% and 54.44% of nocturnal water use, as the mean values of Re:En throughout the growing season were 50.24% and 45.56% in 2020 and 2021, respectively. The Re:En values were much higher than the 20%–25% observed for oak and pine growing in a humid montane region [27], and were similar to S. superba planted in a humid region in the dry season [20]; however, they were slightly lower than that found in P. tomentosa approximately 61% planted in a semiarid region [22], and much lower than that found in P. euphratica approximately 80% growing in an extremely arid environment [2] and A. truncatum (>85%) planted in an urban environment [5]. The higher value of Re:En in the water-limited environment indicated a greater reliance of plants on the water stored through stem refilling, which could help forests to maintain hydraulic support under higher transpiration demand [47,48]. This might be why canopy transpiration in C. korshinskii remained steady during the middle and end of the growing season (Figure listed in Zhang et al. [31]). Moreover, compared with that occurring in 2021, a relatively higher Re:En (50.24%) value was observed in 2020, influenced by the relatively lower REW (Figure 3c and Figure 5). Therefore, increasing Re:En could be an important drought adaptation strategy for forests to overcome seasonal water stress in the growing season.
4.4. Environmental and Stomatal Effects on Nocturnal Water Use
We found that nocturnal water use usually fluctuated with changes in the site’s environmental and stomatal variables. We found negative relationships between En and Ta–n, VPDn and u2-n, which was consistent with previous studies [12,23]. En was facilitated by VPDn when light was absent, especially for young species. However, associated with an increased risk of xylem cavitation and decreased hydraulic conductance within plant tissue, stomatal closure was also triggered by low SWC and atmospheric drought [49,50]. An obvious response of the Gc–n threshold (1.5 mm s−1) to En was observed in both 2020 and 2021 (Figure 6j). Variations in canopy stomatal conductance were proven to be strongly correlated with variations in site SWC and VPD [51]; nocturnal water use was thus significantly affected by soil drought, especially under high-VPDn conditions. En then declined in the face of high transpiration demands during the night (Figure 6d). Therefore, the negative relationship between En and VPDn might be related to the physiological effects of VPDn on stomata [22]. En decreased exponentially with increasing u2-n in this study, which was consistent with Gutiérrez et al. [52], but contrary to Zhao et al. [1]. The reasons for this could be as follows: (1) u2-n underwent an obvious change at night during the growing season at our study site (Figure 3a), and (2) u2-n mitigated Ta–n inversion and finally suppressed En by increasing VPDn [53]. Soil water availability is an important variable controlling nocturnal water use according to previous studies [9,12]. However, REW had a weak influence on En in our study (Figure 7). Because soil water availability was very low surrounding the root system (Figure 3a), En and Tn were minimal when soil water content was low [21]. The response of Tn to VPDn and Gc–n was similar to that of En, but different to that of Re (Figure 6). A positive relationship between Re and the VPD of previous day was reported for hybrid aspen coppice [6], indicating a high proportion of SFn in the refilling of dehydrated tissues. However, the driving force of stem refilling became weak with the decrease in water demand [54], especially under high levels of VPDn. The difference in the environmental and stomatal mechanisms between Tn and Re dynamics might be related to disequilibrium between leaf and soil water potentials and the whole tree’s hydraulic conductance [6]. Therefore, analysis of plant hydraulic capacity in planted forests should be undertaken in the future.
5. Conclusions
In this study, nocturnal water use dynamics and their environmental and stomatal control mechanism were explored for a C. korshinskii plantation on the Bashang Plateau. Nocturnal water use, transpiration and stem refilling in C. korshinskii accumulated to 14.36, 7.14 and 7.22 mm in 2020 and 14.51, 7.90 and 6.61 mm in 2021, respectively. Nocturnal transpiration accounted for 49.76% and 54.44% of total nocturnal water use, while stem refilling accounted for 50.24% and 45.56%, which indicates that C. korshinskii was able to draw on water stored in the stem to overcome seasonal drought. The sharp increase in the ratio of nocturnal to diurnal water use that appeared at the beginning and the end of the growing season might be an ecological strategy for recovering the hydraulic conductivity of the xylem conduits. Nocturnal water use was negatively correlated with all meteorological variables, but increased with increasing nocturnal canopy conductance, which indicates that nocturnal water use was sensitive to stomatal regulation at night. Specifically, nocturnal water use was predominantly affected by nocturnal canopy conductance, nocturnal air temperature and nocturnal wind speed. In contrast, canopy conductance, nocturnal air temperature, and nocturnal vapor pressure deficits explained the highest variation in nocturnal transpiration, and nocturnal vapor pressure deficits and nocturnal wind speed explained the highest variation in stem refilling. The total effects of the five environmental and stomatal variables explained 50%, 36% and 32% of the nocturnal water use, nocturnal transpiration and stem refilling variation, respectively. Our results provide a new understanding of water use strategies employed by plants in C. korshinskii plantations on the Bashang Plateau, and suggest that ecophysiological responses and adaptation to increasing drought severity and duration will occur under future climate changes.
Author Contributions
Conceptualization, W.L.; methodology, W.L. and Y.Z.; investigation, N.W. and Z.Q.; data curation, B.X. and C.L.; Supervision, J.C. and Y.Y.; Writing—original draft, W.L.; Writing—review and editing, W.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 42001027, 42101019, 42371048), Science and Technology Project of Hebei Education Department (BJK2022022), the Key Research and Development Plan Project of Hebei Province (22324202D), Natural Science Foundation of Hebei Province (D2021403023), the Key Research and Development Plan Project of Ningxia Hui Autonomous Region (2021BEG02008), Funding for the Science and Technology Innovation Team Project of Hebei GEO University (KJCXTD-2021-10), Innovation and Entrepreneurship Training Program for College Students (202210077015, S202210077021), and Hebei GEO University Student Science and Technology Fund (KAG202303).
Data Availability Statement
The data presented in this paper are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
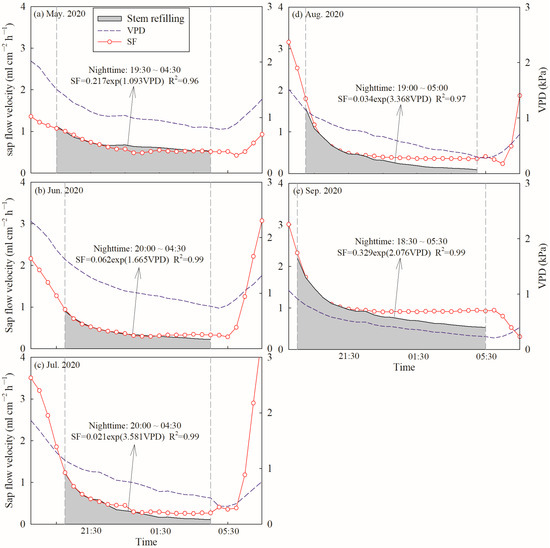
Figure A1.
Examples of the forecasted refilling method used to determine the percentage of nocturnal sap flow velocity attributable to nocturnal transpiration and stem refilling in C. korshinskii. Nighttime was defined between dashed gray vertical lines (PAR < 5 µmol m−2 s−1). Stem refilling is the shade proportion of nocturnal sap flow velocity, (a) May in 2020, (b) June in 2020, (c) July in 2020, (d) August in 2020 and (e) September in 2020.
References
- Zhao, C.; Si, J.; Feng, Q.; Yu, T.; Li, P.; Forster, M.A. Nighttime transpiration of Populus euphratica during different phenophases. J. For. Res. 2019, 30, 435–444. [Google Scholar] [CrossRef]
- Yu, T.; Feng, Q.; Si, J.; Mitchell, P.J.; Forster, M.A.; Zhang, X.; Zhao, C. Depressed hydraulic redistribution of roots more by stem refilling than by nocturnal transpiration for Populus euphratica Oliv. in situ measurement. Ecol. Evol. 2018, 8, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Dayer, S.; Herrera, J.C.; Dai, Z.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. Nighttime transpiration represents a negligible part of water loss and does not increase the risk of water stress in grapevine. Plant Cell Environ. 2021, 44, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, Z.; Cao, K.-F. Nocturnal transpiration in 18 broadleaf timber species under a tropical seasonal climate. For. Ecol. Manag. 2018, 418, 47–54. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Zhu, J.; Gong, L.; Xu, L.; Jin, G.; Li, J.; Hauer, R.; Xu, C. Nocturnal sap flow is mainly caused by stem refilling rather than nocturnal transpiration for Acer truncatum in urban environment. Urban Urban Gree. 2020, 56, 126800. [Google Scholar] [CrossRef]
- Kangur, O.; Tullus, A.; Sellin, A. Night-time transpiration, predawn hydraulic conductance and water potential disequilibrium in hybrid aspen coppice. Trees 2020, 34, 133–141. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Lewis, J.D.; Phillips, N.G.; Tissue, D.T. Consequences of nocturnal water loss: A synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol. 2014, 34, 1047–1055. [Google Scholar] [CrossRef]
- Dawson, T.E.; Burgess, S.S.O.; Tu, K.P.; Oliveira, R.S.; Santiago, L.S.; Fisher, J.B.; Simonin, K.A.; Ambrose, A.R. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol. 2007, 27, 561–575. [Google Scholar] [CrossRef]
- Hayat, M.; Iqbal, S.; Zha, T.; Jia, X.; Qian, D.; Bourque, C.P.A.; Khan, A.; Tian, Y.; Bai, Y.; Liu, P.; et al. Biophysical control on nighttime sap flow in Salix psammophila in a semiarid shrubland ecosystem. Agric. For. Meteorol. 2021, 300, 108329. [Google Scholar] [CrossRef]
- Lombardozzi, D.L.; Zeppel, M.J.B.; Fisher, R.A.; Tawfik, A. Representing nighttime and minimum conductance in CLM4.5: Global hydrology and carbon sensitivity analysis using observational constraints. Geosci. Model. Dev. 2017, 10, 321–331. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Chowdhury, F.I.; Granda, E.; Yao, Y.; Tissue, D.T. Assessing the potential functions of nocturnal stomatal conductance in C3 and C4 plants. New Phytol. 2019, 223, 1696–1706. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Zheng, X.; Li, X.; Wang, K.; Zhang, J.; Wang, G.; Sun, H. Water use dynamics of trees in a Pinus tabuliformis plantation in semiarid sandy regions, Northeast China. Agric. Water Manag. 2023, 275, 107995. [Google Scholar] [CrossRef]
- Phillips, N.G.; Lewis, J.D.; Logan, B.A.; Tissue, D.T. Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol. 2010, 30, 586–596. [Google Scholar] [CrossRef]
- Zeppel, M.; Tissue, D.; Taylor, D.; Macinnis-Ng, C.; Eamus, D. Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol. 2010, 30, 988–1000. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Anderegg, W.R.L.; Adams, H.D.; Hudson, P.; Cook, A.; Rumman, R.; Eamus, D.; Tissue, D.T.; Pacala, S.W. Embolism recovery strategies and nocturnal water loss across species influenced by biogeographic origin. Ecol. Evol. 2019, 9, 5348–5361. [Google Scholar] [CrossRef] [PubMed]
- de Dios, V.R.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci. Rep. 2015, 5, 10975. [Google Scholar] [CrossRef]
- Caspari, H.W.; Green, S.R.; Edwards, W.R.N. Transpiration of well-watered and water-stressed Asian pear trees as determined by lysimetry, heat-pulse, and estimated by a Penman-Monteith model. Agric. For. Meteorol. 1993, 67, 13–27. [Google Scholar] [CrossRef]
- Buckley, T.N.; Turnbull, T.L.; Pfautsch, S.; Adams, M.A. Nocturnal water loss in mature subalpine Eucalyptus delegatensis tall open forests and adjacent E. pauciflora woodlands. Ecol. Evol. 2011, 1, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.R.; Goranson, C.E.; Mitchell, R.J.; Will, R.E.; Teskey, R.O. Modeling canopy transpiration using time series analysis: A case study illustrating the effect of soil moisture deficit on Pinus taeda. Agric. For. Meteorol. 2005, 130, 163–175. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, P.; Zhu, L. Differentiating refilling and transpiration from night-time sap flux based on time series modelling. Trees 2022, 36, 1621–1632. [Google Scholar] [CrossRef]
- Fisher, J.B.; Baldocchi, D.D.; Misson, L.; Dawson, T.E.; Goldstein, A.H. What the towers don’t see at night: Nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiol. 2007, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Sun, G.; Chen, L.; Xu, H.; Chen, S. Biophysical controls on nocturnal sap flow in plantation forests in a semi-arid region of northern China. Agric. For. Meteorol. 2020, 284, 107904. [Google Scholar] [CrossRef]
- Di, N.; Xi, B.; Clothier, B.; Wang, Y.; Li, G.; Jia, L. Diurnal and nocturnal transpiration behaviors and their responses to groundwater-table fluctuations and meteorological factors of Populus tomentosa in the North China Plain. For. Ecol. Manag. 2019, 448, 445–456. [Google Scholar] [CrossRef]
- Chu, C.R.; Hsieh, C.-I.; Wu, S.-Y.; Phillips, N.G. Transient response of sap flow to wind speed. J. Exp. Bot. 2009, 60, 249–255. [Google Scholar] [CrossRef]
- Si, J.; Feng, Q.; Yu, T.; Zhao, C. Nighttime sap flow and its driving forces for Populus euphratica in a desert riparian forest, Northwest China. J. Arid. Land 2015, 7, 665–674. [Google Scholar] [CrossRef]
- Fuentes, S.; Mahadevan, M.; Bonada, M.; Skewes, M.A.; Cox, J.W. Night-time sap flow is parabolically linked to midday water potential for field-grown almond trees. Irrig. Sci. 2013, 31, 1265–1276. [Google Scholar] [CrossRef]
- Alvarado-Barrientos, M.S.; Holwerda, F.; Geissert, D.R.; Muñoz-Villers, L.E.; Gotsch, S.G.; Asbjornsen, H.; Dawson, T.E. Nighttime transpiration in a seasonally dry tropical montane cloud forest environment. Trees 2015, 29, 259–274. [Google Scholar] [CrossRef]
- Fang, W.; Lu, N.; Liu, J.; Jiao, L.; Zhang, Y.; Wang, M.; Fu, B. Canopy transpiration and stand water balance between two contrasting hydrological years in three typical shrub communities on the semiarid Loess Plateau of China. Ecohydrology 2019, 12, e2064. [Google Scholar] [CrossRef]
- Jian, S.; Zhao, C.; Fang, S.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese Loess Plateau. Agric. For. Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Li, B.-B.; Li, P.-P.; Zhang, W.-T.; Ji, J.-Y.; Liu, G.-B.; Xu, M.-X. Deep soil moisture limits the sustainable vegetation restoration in arid and semi-arid Loess Plateau. Geoderma 2021, 399, 115122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Yan, H.; Xie, B.; Zhao, J.; Wang, N.; Wang, X. Canopy Transpiration and Stomatal Conductance Dynamics of Ulmus pumila L. and Caragana korshinskii Kom. Plantations on the Bashang Plateau, China. Forests 2022, 13, 1081. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Lubczynski, M.W.; Chavarro-Rincon, D.; Roy, J. Novel, cyclic heat dissipation method for the correction of natural temperature gradients in sap flow measurements. Part 1. Theory and application. Tree Physiol. 2012, 32, 894–912. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Urban, L.; Zhao, P. Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: Theory and practice. Acta Bot. Sin. 2004, 46, 631–646. [Google Scholar]
- Rabbel, I.; Diekkrüger, B.; Voigt, H.; Neuwirth, B. Comparing ∆Tmax Determination Approaches for Granier-Based Sapflow Estimations. Sensors 2016, 16, 2042. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhu, J.; Zhang, T.; Wang, K.; Wang, G.; Liu, J. Higher canopy transpiration rates induced dieback in poplar (Populus × xiaozhuanica) plantations in a semiarid sandy region of Northeast China. Agric. Water Manag. 2021, 243, 106414. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Yu, T.F.; Li, P.D. Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Igarashi, Y.; Kumagai, T.o.; Yoshifuji, N.; Sato, T.; Tanaka, N.; Tanaka, K.; Suzuki, M.; Tantasirin, C. Environmental control of canopy stomatal conductance in a tropical deciduous forest in northern Thailand. Agric. For. Meteorol. 2015, 202, 1–10. [Google Scholar] [CrossRef]
- Mei, T.; Fang, D.; Röll, A.; Niu, F.; Hendrayanto; Hölscher, D. Water Use Patterns of Four Tropical Bamboo Species Assessed with Sap Flux Measurements. Front. Plant Sci. 2016, 6, 1202. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Yi, R.; Zhong, F.; Zhang, Y. Sap flow and plant water sources for typical vegetation in a subtropical humid karst area of southwest China. Hydrol. Process. 2021, 35, e14090. [Google Scholar] [CrossRef]
- Wu, S.; Gu, X.; Zheng, Y.; Chen, L. Nocturnal sap flow as compensation for water deficits: An implicit water-saving strategy used by mangroves in stressful environments. Front. Plant Sci. 2023, 14, 1118970. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-H.; Ping, Z.; Zhen, Z.-Z.; Li, Z.-W.; Jun, N.-F.; Guang, N.-Y.; Yan, H.-T.; Lei, O. Sap flow-based transpiration in Phyllostachys pubescens: Applicability of the TDP methodology, age effect and rhizome role. Trees 2017, 31, 765–779. [Google Scholar] [CrossRef]
- Xie, J.; Wan, X. The accuracy of the thermal dissipation technique for estimating sap flow is affected by the radial distribution of conduit diameter and density. Acta Physiol. Plant. 2018, 40, 88. [Google Scholar] [CrossRef]
- Hölttä, T.; Dominguez Carrasco, M.D.R.; Salmon, Y.; Aalto, J.; Vanhatalo, A.; Bäck, J.; Lintunen, A. Water relations in silver birch during springtime: How is sap pressurised? Plant Biol. 2018, 20, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Feng, Q.; Si, J.; Pinkard, E.A. Coordination of stomatal control and stem water storage on plant water use in desert riparian trees. Trees 2019, 33, 787–801. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Johnson, D.M.; Meinzer, F.C.; Woodruff, D.R. The dynamic pipeline: Hydraulic capacitance and xylem hydraulic safety in four tall conifer species. Plant Cell Environ. 2014, 37, 1171–1183. [Google Scholar] [CrossRef]
- Yi, K.; Dragoni, D.; Phillips, R.P.; Roman, D.T.; Novick, K.A. Dynamics of stem water uptake among isohydric and anisohydric species experiencing a severe drought. Tree Physiol. 2017, 37, 1379–1392. [Google Scholar] [CrossRef]
- Cirelli, D.; Equiza, M.A.; Lieffers, V.J.; Tyree, M.T. Populus species from diverse habitats maintain high night-time conductance under drought. Tree Physiol. 2016, 36, 229–242. [Google Scholar] [CrossRef]
- Shen, Q.; Gao, G.; Fu, B.; Lü, Y. Responses of shelterbelt stand transpiration to drought and groundwater variations in an arid inland river basin of Northwest China. J. Hydrol. 2015, 531, 738–748. [Google Scholar] [CrossRef]
- Zha, T.; Barr, A.G.; van der Kamp, G.; Black, T.A.; McCaughey, J.H.; Flanagan, L.B. Interannual variation of evapotranspiration from forest and grassland ecosystems in western canada in relation to drought. Agric. For. Meteorol. 2010, 150, 1476–1484. [Google Scholar] [CrossRef]
- GutiÉRrez, M.V.; Meinzer, F.C.; Grantz, D.A. Regulation of transpiration in coffee hedgerows: Covariation of environmental variables and apparent responses of stomata to wind and humidity. Plant Cell Environ. 1994, 17, 1305–1313. [Google Scholar] [CrossRef]
- Green, S.R.; McNaughton, K.G.; Clothier, B.E. Observations of night-time water use in kiwifruit vines and apple trees. Agric. For. Meteorol. 1989, 48, 251–261. [Google Scholar] [CrossRef]
- Karpul, R.H.; West, A.G. Wind drives nocturnal, but not diurnal, transpiration in Leucospermum conocarpodendron trees: Implications for stilling on the Cape Peninsula. Tree Physiol. 2016, 36, 954–966. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).