Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia
Abstract
:1. Introduction
2. Materials and Methods
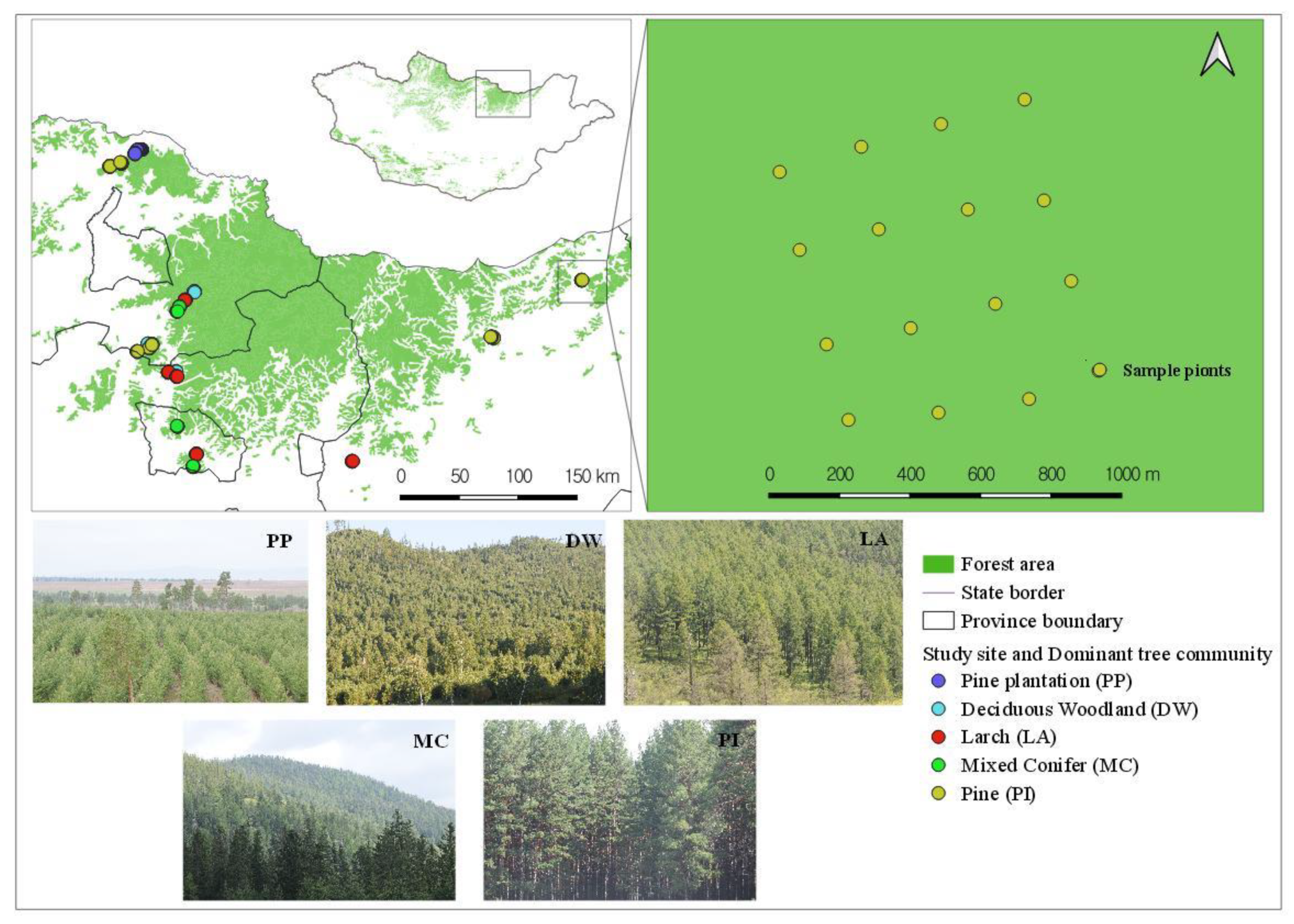
2.1. Study Sites
2.2. Sample Collection and Analyses
3. Results
3.1. Bird Assemblage Composition
3.2. Correlations between Bird Assemblage and Habitat Types
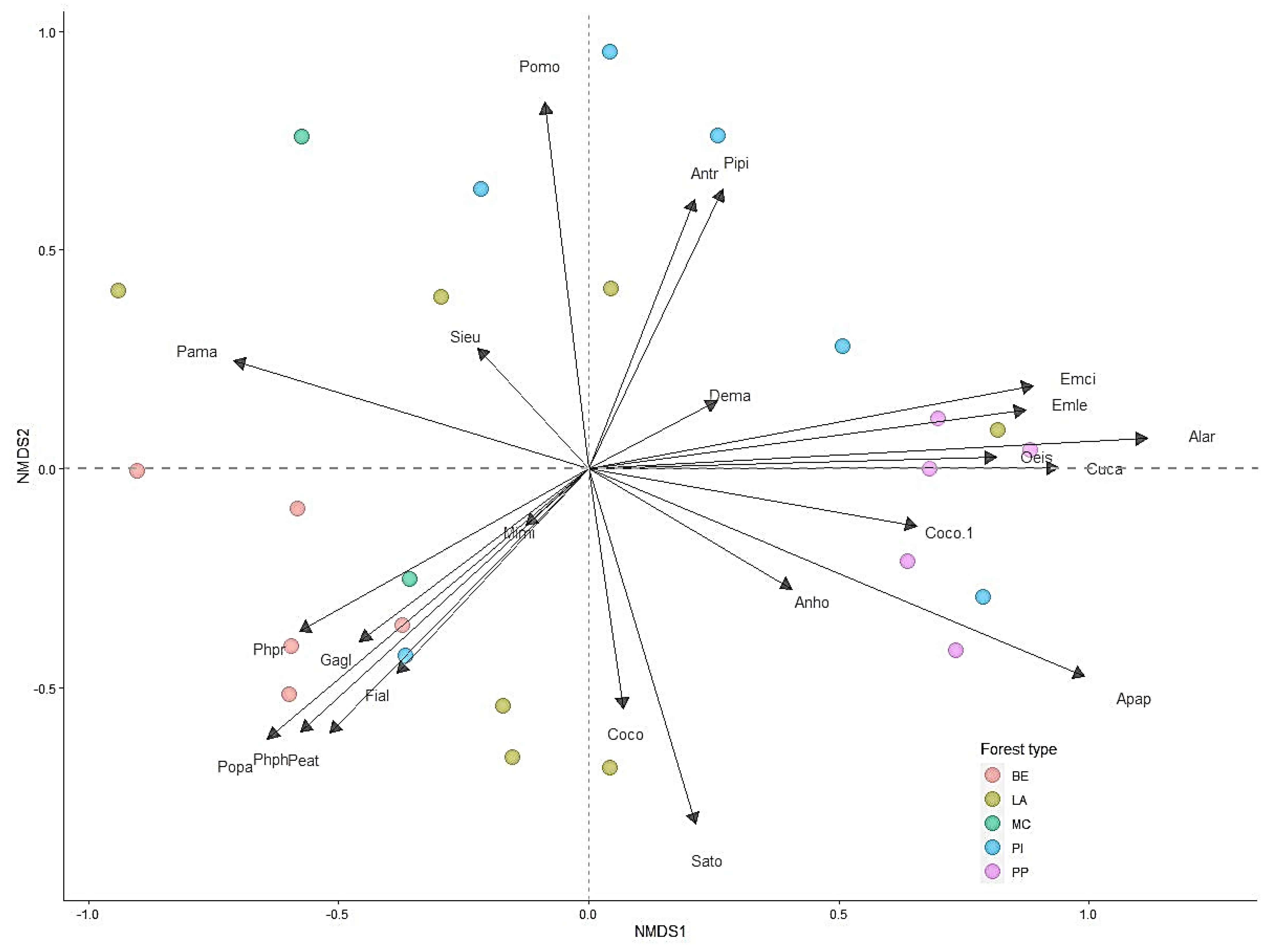
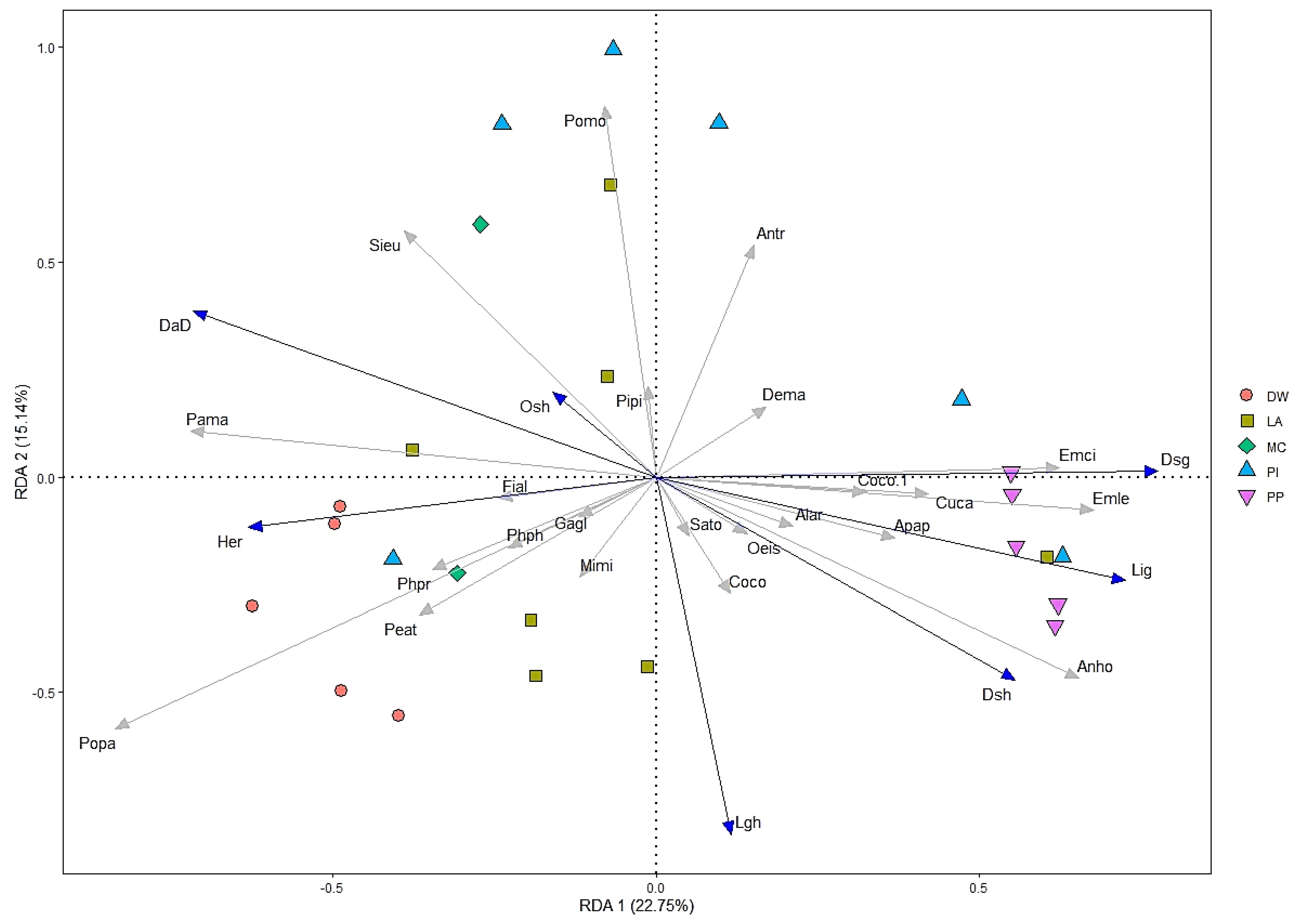
3.3. Relationships between Bird Assemblages and Environmental Variables
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Roberge, J.M.; Virkkala, R.; Monkkonen, M. Boreal 6 r Forest Bird Assemblages and Their Conservation. Ecol. Conserv. For. Birds 2018, 29, 183. [Google Scholar] [CrossRef]
- Dugarjav, C.H. Larch Forests of Mongolia; Bembi San: Ulan Bator, Mongolia, 2006; p. 317. (In Mongolian) [Google Scholar]
- FAO. Mongolia—Global Forest Resources Assessment 2015—Country Report; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2014; p. 97. [Google Scholar]
- Batkhuu, N.O.; Lee, D.K.; Tsogtbaatar, J. Forest and forestry research and education in Mongolia. J. Sustain. For. 2011, 30, 600–617. [Google Scholar] [CrossRef]
- Government of Mongolia. Mongolia’s Forest Reference Level Submission to the United Nations Framework Convention on Climate Change; UN-REDD Mongolia National Programme, Ministry of Environment and Tourism: Ulaanbaatar, Mongolia, 2018; p. 62. [Google Scholar]
- Tsogtbaatar, J. Deforestation and reforestation of degraded forestland in Mongolia. In the Mongolian Ecosystem Network; Springer: Tokyo, Japan, 2013; pp. 83–98. [Google Scholar] [CrossRef]
- Sainnemekh, S.; Isabel, C.B.; Bulgamaa, D.; Brandon, B.; Ása, L.A. Rangeland degradation in Mongolia: A systematic review of the evidence. J. Arid. Environ. 2022, 196, 104654. [Google Scholar] [CrossRef]
- Erdenechuluun, T. Wood Supply in Mongolia: The Legal and Illegal Economies. In Mongolia Discussion Papers, East Asia and Pacific Environment and Social Development Department; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Wildlife Science and Conservation Center, Institute of Biology and BirdLife International. Directory of Important Bird Areas in Mongolia: Key Sites for Conservation; Nyambayar, B., Tseveenmyadag, N., Eds.; Wildlife Science and Conservation Center, Institute of Biology and BirdLife International: Ulaanbaatar, Mongolia, 2009. [Google Scholar]
- Collar, N.J.; Crosby, M.J.; Stattersfield, A.J. Birdlife International. Birds to Watch 2: The World List of Threatened Birds: The Official Source for Birds on the Iucn Red List; BirdLife International: Cambridge, UK, 1994. [Google Scholar]
- Balestrieri, R.; Basile, M.; Posillico, M.; Altea, T.; De Cinti, B.; Matteucci, G. A guild-based approach to assessing the influence of beech forest structure on bird communities. For. Ecol. Manag. 2015, 356, 216–223. [Google Scholar] [CrossRef]
- Kamp, J.; Oppel, S.; Heldbjerg, H.; Nyegaard, T.; Donald, P.F. Unstructured citizen science data fail to detect long-term population declines of common birds in Denmark. Divers. Distrib. 2016, 22, 1024–1035. [Google Scholar] [CrossRef]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultiral intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Brinkert, A.; Hölzel, N.; Sidorova, T.V.; Kamp, J. Spontaneous steppe restoration on abandoned cropland in Kazakhstan: Grazing affects successional pathways. Biodivers. Conserv. 2016, 25, 2543–2561. [Google Scholar] [CrossRef]
- Kwok, H.K.; Corlett, R.T. The bird communities of a natural secondary forest and a Lophostemon confertus plantation in Hong Kong, South China. For. Ecol. Manag. 2000, 130, 227–234. [Google Scholar] [CrossRef]
- Lott, C.A.; Akresh, M.E.; Costanzo, B.E.; D’Amato, A.W.; Duan, S.; Fiss, C.J.; Fraser, J.S.; He, H.S.; King, D.I.; McNeil, D.J.; et al. Do Review Papers on Bird–Vegetation Relationships Provide Actionable Information to Forest Managers in the Eastern United States. Forests 2021, 12, 990. [Google Scholar] [CrossRef]
- Thompson, P.S.; Greenwood, J.D.; Greenaway, K. Birds in European gardens in the winter and spring of 1988–89. Bird Stud. 1993, 40, 120–134. [Google Scholar] [CrossRef]
- Tu, H.M.; Fan, M.W.; Ko, J.C. Different habitat types affect bird richness and evenness. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, W.; Seol, A.; Chung, O.-S.; Sagong, J.; Lee, J.K. Avian Reporting Rates in Chugcheongnam Province, South Korea Depend on Distance from Forest Edge, Size of Trees, and Size of Forest Fragments. Forests 2019, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Gombobaatar, S.; Monks, E.M.; Seidler, R.; Sumiya, D.; Tseveenmyadag, N.; Bayarkhuu, S.; Baillie, J.E.; Boldbaatar, S.; Uuganbayar, C. Regional Red List Series Vol. 7. Birds; Zoological Society of London, National University of Mongolia, and Mongolian Ornithological Society: Ulaanbaatar, Mongolia, 2011; (In English and Mongolian). [Google Scholar]
- Bold, A. Mongolian Birds. In Mongolia Today Science, Culture, Environment and Development; Badarch, D., Zilinskas, R.A., Balint, P., Eds.; RoutledgeCurzon: London, UK, 2003; pp. 143–171. [Google Scholar]
- Bold, A. Mongolian Birds study of past 5 years. Sci. Proceed. Inst. Biol. Mong. Acad. Sci. 1973, 7, 143–171. (In Mongolian) [Google Scholar]
- Bold, A. Birds of Hentii mountain region. Sci. Proc. Inst. Biol. Mong. Acad. Sci. 1969, 3, 4–26. (In Mongolian) [Google Scholar]
- Bold, A. Birds of Hentii Mountain Region and Their Practical Importance. Ph.D. Dissertation, Department of Biology, National University of Mongolia, Ulaanbaatar, Mongolia, 1977. (In Russian). [Google Scholar]
- Bold, A. Census result of game birds in Hentii. Sci. Proc. Inst. Biol. Mong. Acad. Sci. 1970, 4, 19–27. (In Mongolian) [Google Scholar]
- Jambaajamts, A. Climate Brief Overview of the Republic of Mongolia; National Press Office: Ulaanbaatar, Mongolia, 1989. (In Mongolian) [Google Scholar]
- Tsegmid, S. Physical Geography of Mongolia; Mongolian Academy of Sciences, Institute of Geography and Permafrost, National publishing: Ulaanbaatar, Mongolia, 1969; p. 405. (In Mongolian) [Google Scholar]
- Sato, T.; Kimura, F.; Kitoh, A. Projection of global warming onto regional precipitation over Mongolia using a regional climate model. J. Hydrol. 2007, 333, 144–154. [Google Scholar] [CrossRef]
- Tsegmid, C. Some results of studies on microclimate and soil humidity of microassociations in mossy Larix forest of the eastern Khentey. Tesisi Docl Nauchnoi Konf. Posveshennie Vopr. Vozobnov. Resur. Lesa MNR 1989, 170–176. [Google Scholar]
- Ermakov, N.; Cherosov, M.; Gogoleva, P. Classification of ultracontinental boreal forests in central Yakutia. Folia Geobot. 2002, 37, 419–440. [Google Scholar] [CrossRef]
- Dulamsuren, C. Floristische Diversität, Vegetation und Standortbedingungen in der Gebirgstaiga des Westkhentej, Nordmongolei. Ber Forsch. Wald. A 2004, 191, 1–290. [Google Scholar]
- Mühlenberg, M.; Appelfelder, J.; Hoffmann, H.; Ayush, E.; Wilson, K.J. Structure of the montane taiga forests of West Khentii, Northern Mongolia. J. For. Sci. 2012, 58, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Dulamsuren, C.; Hauck, M.; Mühlenberg, M. Vegetation at the taiga forest-steppe borderline in the western Khentej Mountains, northern Mongolia. Ann. Bot. Fenn. 2005, 42, 411–426. [Google Scholar]
- Bowman, J. Tujiin Nars: A Story of the Forest. Independent Study Project (ISP) Collection. 2012. Available online: https://digitalcollections.sit.edu/isp_collection/1453 (accessed on 20 June 2021).
- Bibby, C.J.; Burgess, N.D.; Hill, D.A. Bird Census Techniques; Academic Press: London, UK, 1992. [Google Scholar]
- Bibby, C.J.; Jones, M.; Marsden, S. Bird Surveys; Expedition Advisory Centre: London, UK, 1998. [Google Scholar]
- Hanni, D.J.; White, C.M.; van Lanen, N.J.; Birek, J.J.; Berven, J.M.; McLaren, M.F. Integrated Monitoring in Bird Conservation Regions (Imbcr): Field Protocol for Spatially-Balanced Sampling of Landbird Populations, Unpublished Report; Bird Conservancy of the Rockies: Brighton, CO, USA, 2016. [Google Scholar]
- Blakesley, J.A.; Hanni, D.J. Monitoring Colorado’s Birds, 2008. Technical Report M-MCB08-01; Rocky Mountain Bird Observatory: Brighton, CO, USA, 2009. [Google Scholar]
- QGIS. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: https://qgis.org/en/site/ (accessed on 20 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 15 September 2021).
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2019. Available online: https://cran.r-project.org/package=vegan (accessed on 5 January 2022).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Stenzel, T.; Stubbe, M.; Samjaa, R.; Gombobaatar, S. Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia; Erforschung biologischer Ressourcen der Mongolei/Exploration into the Biological Resources of Mongolia: Ulaanbaatar, Mongolia, 2005; p. 127. ISSN 0440-1298. [Google Scholar]
- Stokland, J.N. Representativeness and Efficiency of Bird and Insect Conservation in Norwegian Boreal Forest Reserves: Representatividad y Eficiencia en la Conservación de Aves e Insectos en las Reservas de Bosque Boreal de Noruega. Conserv. Biol. 1997, 11, 101–111. [Google Scholar] [CrossRef]
- Felton, A.; Andersson, E.; Ventorp, D.; Lindbladh, M. A comparison of avian diversity in spruce monocultures and spruce-birch polycultures in Southern Sweden. Silva Fenn. 2011, 45, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Duco, R.A.; Fidelino, J.S.; Duya, M.V.; Ledesma, M.M.; Ong, P.S.; Duya, M.R. Bird Assemblage and Diversity along Different Habitat Types in a Karst Forest Area in Bulacan, Luzon Island, Philippines. Philipp. J. Sci. 2020, 15, 399–414. [Google Scholar]
- Berg, Å. Diversity and abundance of birds in relation to forest fragmentation, habitat quality and heterogeneity. Bird Study 1997, 44, 355–366. [Google Scholar] [CrossRef]
- Bersier, L.F.; Meyer, D.R. Bird assemblages in mosaic forests: The relative importance of vegetation structure and floristic composition along the successional gradient. Acta Oecologica 1994, 15, 561–576. [Google Scholar]
- Estades, C.F.; Temple, S.A. Deciduous-forest bird communities in a fragmented landscape dominated by exotic pine plantations. Ecol. Appl. 1999, 9, 573–585. [Google Scholar] [CrossRef]
- Mansor, M.S.; Sah, S.A. The influence of habitat structure on bird species composition in lowland malaysian rain forests. Trop. Life Sci. Res. 2012, 23, 1–14. [Google Scholar] [PubMed]
- Deconchat, M.; Balent, G. Vegetation and bird community dynamics in fragmented coppice forests. Forestry 2001, 74, 105–118. [Google Scholar] [CrossRef]
- Haapanen, A. Bird fauna of the Finnish forests in relation to forest succession. In I. InAnnales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 1965; Volume 2, pp. 153–196. [Google Scholar]
- Bergner, A.; Avcı, M.; Eryiğit, H.; Jansson, N.; Niklasson, M.; Westerberg, L.; Milberg, P. Influences of forest type and habitat structure on bird assemblages of oak (Quercus spp.) and pine (Pinus spp.) stands in southwestern Turkey. For. Ecol. Manag. 2015, 336, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Nikolov, S.C. Effect of stand age on bird communities in late successional Macedonian pine forests in Bulgaria. For. Ecol. Manag. 2009, 257, 580–587. [Google Scholar] [CrossRef]
- Morozov, N.S. Breeding forest birds in the Valdai Uplands, north-west Russia: Assemblage composition, interspecific associations and habitat amplitudes. In InAnnales Zoologici Fennici; Finnish Zoological Publishing Board, formed by the Finnish Academy of Sciences, Societas Biologica Fennica Vanamo, Societas pro Fauna et Flora Fennica, and Societas Scientiarum Fennica: Helsinki, Finland, 1992; pp. 7–28. [Google Scholar]
- Enoksson, B.; Angelstam, P.; Larsson, K. Deciduous forest and resident birds: The problem of fragmentation within a coniferous forest landscape. Landsc. Ecol. 1995, 10, 267–275. [Google Scholar] [CrossRef]
- Laurance, S.G.; Stouffer, P.C.; Laurance, W.F. Effects of road clearings on movement patterns of understory rainforest birds in central Amazonia. Conserv. Biol. 2004, 18, 1099–1109. [Google Scholar] [CrossRef]
- Tvardíková, K. Bird abundances in primary and secondary growths in Papua New Guinea: A preliminary assessment. Trop. Conserv. Sci. 2010, 3, 373–388. [Google Scholar] [CrossRef]
- Şekercioḡlu, Ç.H.; Ehrlich, P.R.; Daily, G.C.; Aygen, D.; Goehring, D.; Sandí, R.F. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl. Acad. Sci. USA 2002, 99, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Scientific Name | English Name | Bird Individuals in Different Habitat Types | Frequency of Occurrence (%) | Relative Abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Status | DW | LA | PI | PP | MC | ||||
| Accipitriformes-Accipitridae | ||||||||||
| Accipiter gentilis | Northern Goshawk | Carn | M | 1 | 0.25 | 0.06 | ||||
| Aquila nepalensis | Steppe Eagle | Carn | M | 1 | 0.25 | 0.06 | ||||
| Buteo buteo | Eurasian Buzzard | Carn | M | 3 | 1 | 2 | 1.25 | 0.35 | ||
| Hieraaetus pennatus | Booted Eagle | Carn | M | 1 | 1 | 0.5 | 0.12 | |||
| Milvus migrans | Black Kite | Carn | M | 12 | 20 | 7 | 11 | 3 | 9 | 3.06 |
| Aegypius monachus | Cinereous Vulture | Carn | R | 1 | 0.25 | 0.06 | ||||
| Anseriformes-Anatidae | ||||||||||
| Tadorna ferruginea | Ruddy Shelduck | Omni | M | 9 | 1.5 | 0.52 | ||||
| Bucerotiformes-Upupidae | ||||||||||
| Upupo epops | Eurasian Hoopoe | Inse | M | 1 | 3 | 1 | 1 | 0.29 | ||
| Caprimulgiformes-Apodidae | ||||||||||
| Apus apus | Common Swift | Inse | M | 5 | 21 | 1.25 | 1.50 | |||
| Ciconiiformes-Ciconiidae | ||||||||||
| Ciconia nigra | Black Stork | Carn | M | 1 | 1 | 0.5 | 0.12 | |||
| Columbiformes-Columbidae | ||||||||||
| Streptopelia orientalis | Oriental Turtle Dove | Gran | M | 15 | 2.5 | 0.87 | ||||
| Cuculiformes-Cuculidae | ||||||||||
| Cuculus canorus | Common Cuckoo | Inse | M | 5 | 26 | 6.75 | 1.79 | |||
| Cuculus saturatus | Oriental Cuckoo | Inse | M | 1 | 0.25 | 0.06 | ||||
| Falconiformes-Falconidae | ||||||||||
| Falco cherrug | Saker Falcon | Carn | M | 1 | 1 | 0.5 | 0.12 | |||
| Falco tinnunculus | Common Kestrel | Carn | M | 1 | 4 | 0.75 | 0.29 | |||
| Falco amurensis | Amur Falcon | Carn | M | 4 | 0.75 | 0.23 | ||||
| Falco columbarius | Merlin | Carn | M | 3 | 0.5 | 0.17 | ||||
| Galliformes-Phasianidae | ||||||||||
| Lyrurus tetrix | Black Grouse | Inse | R | 12 | 1.5 | 0.69 | ||||
| Gruiformes-Gruidae | ||||||||||
| Anthropoides virgo | Demoiselle Crane | Omni | M | 2 | 5 | 1.25 | 0.40 | |||
| Antigone vipio | White-naped Crane | Omni | M | 3 | 0.25 | 0.17 | ||||
| Passeriformes-Aegithalidae | ||||||||||
| Aegithalos caudatus | Long tailed Tit | Inse | R | 14 | 1 | 2 | 1.75 | 0.98 | ||
| Alaudidae | ||||||||||
| Alauda arvensis | Eurasian Skylark | Inse | M | 28 | 1.75 | 1.62 | ||||
| Corvidae | ||||||||||
| Corvus dauuricus | Daurian Jackdaw | Omni | M | 6 | 1 | 0.35 | ||||
| Corvus corax | Northern Raven | Omni | R | 4 | 5 | 5 | 14 | 2 | 4 | 1.73 |
| Corvus corone | Carrion Crow | Omni | R | 11 | 12 | 9 | 18 | 9 | 10 | 3.41 |
| Cyanopica cyanus | Azure-winged Magpie | Omni | R | 14 | 0.75 | 0.81 | ||||
| Garrulus glandarius | Eurasian Jay | Omni | R | 9 | 3 | 5 | 10 | 3 | 1.56 | |
| Pica pica | Eurasian Magpie | Omni | R | 3 | 9 | 15 | 2.5 | 1.56 | ||
| Pyrrhocorax pyrrhocorax | Red-billed Chough | Omni | R | 1 | 0.25 | 0.06 | ||||
| Emberizidae | ||||||||||
| Emberiza cioides | Meadow Bunting | Inse | M | 18 | 26 | 25 | 8.75 | 3.99 | ||
| Emberiza leucocephalos | Pine Bunting | Inse | M | 14 | 24 | 67 | 10.25 | 6.07 | ||
| Emberiza pusilla | Little Bunting | Inse | M | 7 | 0.75 | 0.40 | ||||
| Emberiza pallasi | Pallas’s Bunting | Inse | M | 2 | 0.5 | 0.12 | ||||
| Fringillidae | ||||||||||
| Fringilla montifringilla | Brambling | Omni | M | 1 | 0.25 | 0.06 | ||||
| Carpodacus erythrinus | Common Rosefinch | Gran | M | 10 | 1 | 0.58 | ||||
| Carpodacus roseus | Pallas’s Rosefinch | Gran | R | 1 | 0.25 | 0.06 | ||||
| Coccothrraustes coccothrraustes | Hawfinch | Gran | R | 6 | 1.25 | 0.35 | ||||
| Laniidae | ||||||||||
| Lanius cristatus | Brown Shrike | Inse | M | 12 | 2 | 2.5 | 0.81 | |||
| Motacillidae | ||||||||||
| Anthus hodgsoni | Olive Backed Pipit | Inse | M | 15 | 62 | 41 | 94 | 8 | 26.25 | 12.72 |
| Anthus richardi | Richard’s Pipit | Inse | M | 1 | 0.25 | 0.06 | ||||
| Anthus trivialis | Tree Pipit | Inse | M | 10 | 1 | 27 | 28 | 8.5 | 3.82 | |
| Motacilla alba | White Wagtail | Inse | M | 1 | 3 | 1 | 0.23 | |||
| Muscicapidae | ||||||||||
| Ficedula albicilla | Taiga Flycatcher | Inse | M | 8 | 16 | 4 | 3.5 | 1.62 | ||
| Muscicapa sibirica | Dark-sided Flycatcher | Inse | M | 13 | 2 | 0.75 | ||||
| Oenanthe oenanthe | Northern Wheatear | Inse | M | 7 | 1 | 0.40 | ||||
| Phoenicurus phoenicurus | Common Redstart | Inse | M | 15 | 10 | 3 | 2 | 5 | 1.73 | |
| Saxicola torquatus | Common Stonechat | Inse | M | 2 | 10 | 4 | 2 | 2 | 1.04 | |
| Muscicapa dauurica | Asian Brown Flycatcher | Inse | M | 1 | 8 | 1 | 0.52 | |||
| Oenanthe pleschanka | Inse | M | 2 | 0.25 | 0.12 | |||||
| Oenanthe isabellina | Isabelline Wheatear | Inse | M | 2 | 4 | 23 | 2.5 | 1.68 | ||
| Phoenicurus auroreus | Daurian Redstart | Inse | M | 9 | 1 | 6 | 2.5 | 0.92 | ||
| Phoenicurus erythrogastrus | White winged Radstart | Inse | R | 1 | 0.25 | 0.06 | ||||
| Paridae | ||||||||||
| Cyanistes cyanus | Azure Tit | Inse | R | 14 | 1.25 | 0.81 | ||||
| Parus major | Great Tit | Inse | R | 50 | 27 | 32 | 4 | 31 | 16.75 | 8.32 |
| Periparus ater | Coal Tit | Inse | R | 26 | 9 | 1 | 7 | 6.75 | 2.49 | |
| Poecile montanus | Willow Tit | Gran | R | 9 | 32 | 42 | 1 | 14 | 9.75 | 5.66 |
| Poecile palustris | Marsh Tit | Gran | R | 72 | 31 | 20 | 4 | 15.25 | 7.34 | |
| Passeridae | ||||||||||
| Passer domesticus | House Sparrow | Inse | R | 5 | 0.5 | 0.29 | ||||
| Passer montanus | Eurasian Tree Sparrow | Omni | R | 9 | 8 | 1 | 0.98 | |||
| Phylloscopidae | ||||||||||
| Phylloscopus borealis | Arctic Warbler | Inse | M | 1 | 0.25 | 0.06 | ||||
| Phylloscopus fuscatus | Dusky Warbler | Inse | M | 5 | 0.75 | 0.29 | ||||
| Phylloscopus proregulus | Palla’s leaf Warbler | Inse | M | 26 | 5 | 5 | 1 | 4.5 | 2.14 | |
| Sittidae | ||||||||||
| Sitta europaea | Wood Nuthach | Inse | R | 11 | 31 | 66 | 11 | 13.25 | 6.88 | |
| Turdidae | ||||||||||
| Turdus naumanni | Naumanns Thrush | Omni | M | 5 | 0.5 | 0.29 | ||||
| Turdus ruficollis | Red Throated Trush | Omni | M | 5 | 0.75 | 0.29 | ||||
| Piciformes-Picidae | ||||||||||
| Dendrocopos leucotos | White backed Woodpecker | Inse | R | 5 | 2 | 1.5 | 0.40 | |||
| Dendrocopos major | Great spotted Woodpecker | Inse | R | 5 | 3 | 2 | 11 | 3 | 4.75 | 1.39 |
| Dryobates minor | Lesser spotted Woodpecker | Inse | R | 7 | 7 | 2 | 3.25 | 0.92 | ||
| Dryocopus martius | Black Woodpecker | Inse | R | 1 | 1 | 0.5 | 0.12 | |||
| Strigiformes-Strigidae | ||||||||||
| Aegolius funereus | Boreal Owl | Carn | R | 2 | 0.25 | 0.12 | ||||
| Bubo bubo | Eurasian Eagle Owl | Carn | R | 1 | 1 | 0.5 | 0.12 | |||
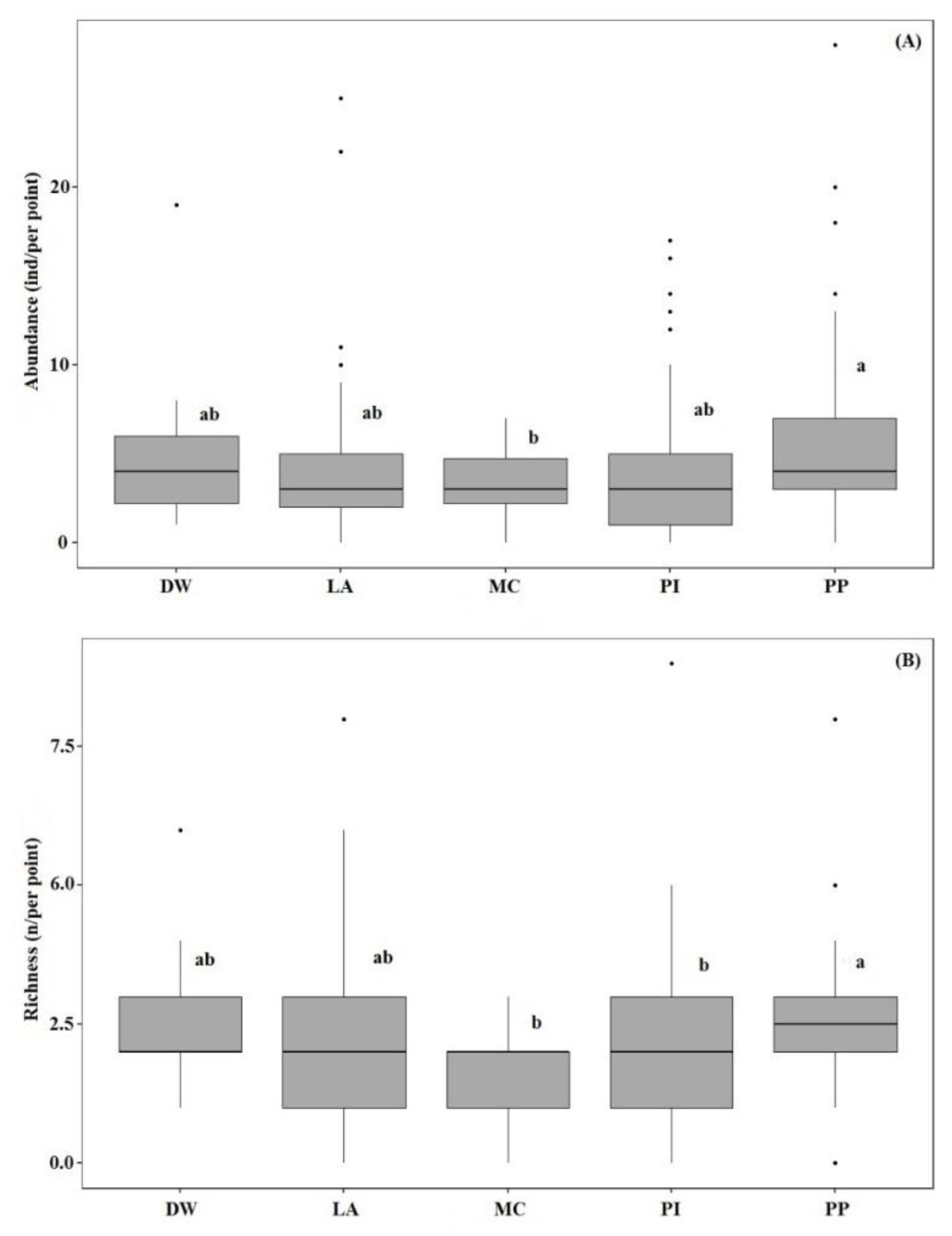
| Abundance | 344 | 434 | 401 | 442 | 109 | - | - | |||
| Species richness | 31 | 46 | 36 | 28 | 16 | - | - | |||
| Structural Variables | Code | Description |
|---|---|---|
| Altitude (m) | Alt | Point elevation (m.a.s.l) |
| Number of snags | Sna | Counted the numbers of snags (≥15 cm dbh, trees that are completely dead) and stems those are ≥3 m high and within a 20 m radius of the center survey point. |
| Number of stem | Ste | |
| Dbh (cm) | Dbh | Measured the dominant tree’s average diameter at breast height (dbh) of overstore trees within a number of ≥10 stem. |
| Over story cover (%) | Osc | Estimated the total percent coverage and dominant tree’s average height of all overstore trees within a 50 m radius. |
| Over story height (m) | Osh | |
| Understory cover (%) | Usc | Estimated the percent cover and species makeup of any woody vegetation (including seedling trees) that is ≥0.5 m high and <3.0 m high of the understory layer. |
| Understory height (m) | Ush | |
| Bare/Litter cover (%) | Bal | The percentage of the ground surface covered by shrubs 0–0.5 m high, litter, down wood, forbs, grasses, and moss was estimated visually within 50 m radius plots (total 100%). |
| Dead and down (%) | Dad | |
| Dead standing grass cover (%) | Dsg | |
| Herbaceous cover (%) | Her | |
| Live grass cover (%) | Lig | |
| Moss cover (%) | Mos | |
| Dead standing grass height (cm) | Dsh | |
| Live grass&herb. height (cm) | Lgh | |
| Deciduous Woodland | DW | Forested habitat type of dominant tree species with ≥50% present in the overstore. The overstore cover should be ≥10% trees within a 50 m radius. |
| Larch | LA | |
| Pine | PI | |
| Plantation Pine | PP | |
| Mixed Conifer | MC |
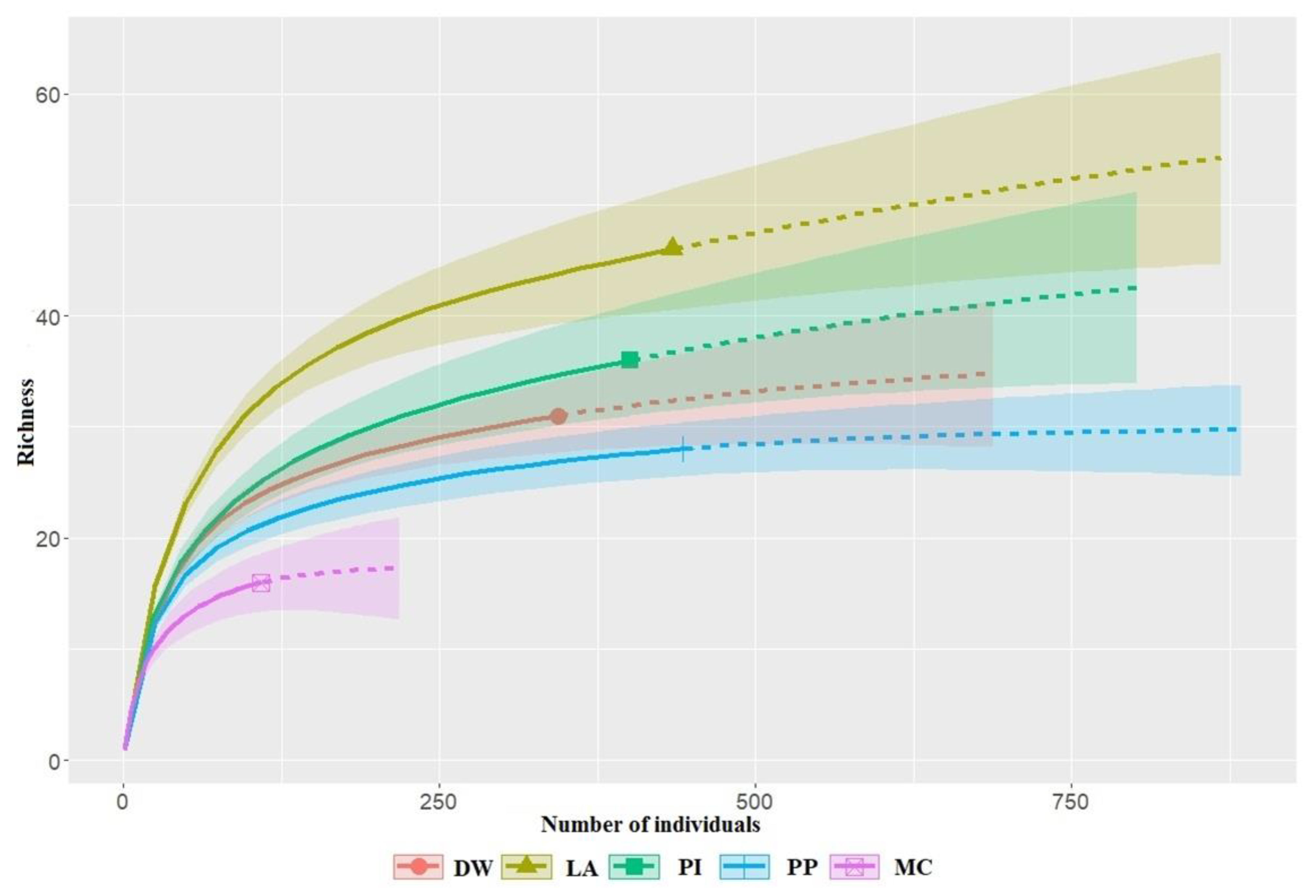
| Habitat Type | Observed Richness | Sample Coverage | S.LCL | CI |
|---|---|---|---|---|
| Table 1 Deciduous Woodland | 31 | 0.983 | 27.4 | 32–64.5 |
| Larch | 46 | 0.977 | 41.0 | 51–168.9 |
| Pine | 36 | 0.978 | 31.7 | 38.8–98.7 |
| Pine Plantation | 28 | 0.991 | 25.2 | 28.2–42.3 |
| Mixed Conifer | 16 | 0.973 | 13.4 | 16.1–28.7 |
| Species | Av. Dissim | Contrib. % | Cumulative % | Mean DW | Mean LA | Mean MC | Mean PI | Mean PP |
|---|---|---|---|---|---|---|---|---|
| Anthus hodgsoni | 10.95 | 11.70 | 11.70 | 0.18 | 0.63 | 0.28 | 0.47 | 1.25 |
| Parus major | 8.28 | 8.85 | 20.55 | 0.61 | 0.28 | 1.07 | 0.36 | 0.05 |
| Poecile palustris | 7.32 | 7.82 | 28.37 | 0.88 | 0.32 | 0.14 | 0.23 | - |
| Sitta europaea | 5.65 | 6.04 | 34.41 | 0.13 | 0.32 | 0.38 | 0.75 | - |
| Emberiza leucocephalos | 5.23 | 5.59 | 40.00 | - | 0.14 | - | 0.27 | 0.89 |
| Poecile montanus | 5.08 | 5.43 | 45.42 | 0.11 | 0.33 | 0.48 | 0.48 | 0.01 |
| Emberiza cioides | 3.96 | 4.23 | 49.66 | - | 0.18 | - | 0.30 | 0.33 |
| Corvus corone | 3.88 | 4.15 | 53.80 | 0.13 | 0.12 | 0.31 | 0.10 | 0.24 |
| Anthus trivialis | 3.45 | 3.69 | 57.49 | 0.12 | 0.01 | - | 0.31 | 0.37 |
| Milvus migrans | 2.80 | 2.99 | 60.49 | 0.15 | 0.20 | 0.10 | 0.08 | 0.15 |
| Periparus ater | 2.57 | 2.75 | 63.24 | 0.32 | 0.09 | 0.24 | 0.01 | - |
| Phylloscopus proregulus | 1.98 | 2.12 | 65.35 | 0.32 | 0.05 | 0.03 | 0.06 | - |
| Phoenicurus phoenicurus | 1.97 | 2.10 | 67.46 | 0.18 | 0.10 | 0.07 | 0.03 | - |
| Cuculus canorus | 1.91 | 2.04 | 69.49 | - | 0.05 | - | - | 0.35 |
| Ficedula albicilla | 1.71 | 1.82 | 71.32 | 0.10 | 0.16 | - | 0.05 | - |
| Dendrocopos major | 1.66 | 1.77 | 73.09 | 0.06 | 0.03 | 0.10 | 0.02 | 0.15 |
| Garrulus glandarius | 1.60 | 1.71 | 74.80 | 0.11 | 0.03 | 0.35 | 0.06 | - |
| Corvus corax | 1.42 | 1.51 | 76.32 | 0.05 | 0.05 | 0.07 | 0.06 | 0.19 |
| Pica pica | 1.16 | 1.24 | 77.56 | 0.04 | 0.09 | - | 0.17 | - |
| Apus apus | 1.12 | 1.19 | 78.75 | - | - | - | 0.06 | 0.28 |
| Alauda arvensis | 1.04 | 1.11 | 79.87 | - | - | - | - | 0.37 |
| Oenanthe isabellina | 1.04 | 1.11 | 80.97 | 0.02 | 0.04 | - | - | 0.31 |
| Phoenicurus auroreus | 1.03 | 1.11 | 82.08 | - | 0.09 | - | 0.01 | 0.08 |
| Saxicola torquatus | 1.01 | 1.08 | 83.15 | 0.02 | 0.10 | - | 0.05 | 0.03 |
| Habitat Types | Overstory Level | Understory Level | ||||||
|---|---|---|---|---|---|---|---|---|
| Alt (m) | Sna (n) | Dbh (cm) | Ste (n) | Osh (m) | Osc (%) | Usc (%) | Ush (m) | |
| DW | 1139.4 ± 119.9 c | 4.6 ± 4.3 a | 15.4 ± 6.9 a | 46.8 ± 46.2 b | 7.7 ± 3.4 b | 25.8 ± 20.7 c | 32.5 ± 15.5 a | 2.3 ± 1 a |
| LA | 1374.7 ± 267.9 b | 3.8 ± 3.8 a | 20.5 ± 8.7 a | 66.5 ± 69.4 ab | 10.8 ± 4 a | 32.5 ± 25.5 bc | 12.4 ± 10.5 b | 1.4 ± 0.8 bc |
| MC | 1688.6 ± 204.6 a | 3.3 ± 3.1 ab | 20.5 ± 8 a | 86.2 ± 52.3 a | 12.0 ± 4.8 a | 53.1 ± 28.1 a | 9.8 ± 7 b | 1.8 ± 0.6 ab |
| PI | 956.7 ± 205.29 d | 2.4 ± 2.8 b | 18.7 ± 8.7 b | 63.5 ± 62.6 ab | 10.5 ± 4.3 a | 39.7 ± 29 ab | 13.4 ± 12.7 b | 1.8 ± 1.1 c |
| PP | 701.7 ± 18.3 e | 1.6 ± 1.6 b | 13.0 ± 4.6 b | 88.1 ± 85.9 a | 7.2 ± 2.6 b | 36.9 ± 31.1 bc | 16.7 ± 16.9 b | 2.2 ± 0.6 a |
| Ground level | ||||||||
| DaD (%) | Her (%) | Bal (%) | Lig (%) | Mos (%) | Lgh (cm) | |||
| DW | 7.8 ± 6 a | 31.7 ± 11.3 a | 19.9 ± 13 a | 32.1 ± 14.2 bc | 1.3 ± 2.3 c | 27.8 ± 8.9 a | ||
| LA | 5.5 ± 6 b | 31.3 ± 16.8 a | 14.0 ± 10.6 a | 38.220.5 b | 3.8 ± 9.8 bc | 21.0 ± 8.3 b | ||
| MC | 8.6 ± 7.3 a | 23.3 ± 17.7 b | 12.9 ± 8.5 b | 26.1 ± 16.2 c | 26.6 ± 28.2 a | 16.0 ± 7.5 c | ||
| PI | 5.5 ± 6.5 b | 16.8 ± 12.7 b | 19.3 ± 13.5 b | 39.0 ± 21.1 b | 7.0 ± 11.3 b | 16.3 ± 9.8 c | ||
| PP | 0.5 ± 1.1 c | 6.9 ± 6 c | 5.4 ± 2.9 c | 63.5 ± 5.8 a | 0.0 c | 28.2 ± 10 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purevdorj, Z.; Munkhbayar, M.; Paek, W.K.; Ganbold, O.; Jargalsaikhan, A.; Purevee, E.; Amartuvshin, T.; Genenjamba, U.; Nyam, B.; Lee, J.W. Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia. Forests 2022, 13, 1037. https://doi.org/10.3390/f13071037
Purevdorj Z, Munkhbayar M, Paek WK, Ganbold O, Jargalsaikhan A, Purevee E, Amartuvshin T, Genenjamba U, Nyam B, Lee JW. Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia. Forests. 2022; 13(7):1037. https://doi.org/10.3390/f13071037
Chicago/Turabian StylePurevdorj, Zoljargal, Munkhbaatar Munkhbayar, Woon Kee Paek, Onolragchaa Ganbold, Ariunbold Jargalsaikhan, Erdenetushig Purevee, Tuvshinlkhagva Amartuvshin, Uranchimeg Genenjamba, Batbayar Nyam, and Joon Woo Lee. 2022. "Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia" Forests 13, no. 7: 1037. https://doi.org/10.3390/f13071037
APA StylePurevdorj, Z., Munkhbayar, M., Paek, W. K., Ganbold, O., Jargalsaikhan, A., Purevee, E., Amartuvshin, T., Genenjamba, U., Nyam, B., & Lee, J. W. (2022). Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia. Forests, 13(7), 1037. https://doi.org/10.3390/f13071037






