Abstract
Elucidating the influence mechanisms of seed germination and seedling growth is important for revealing the natural regeneration of forest plantations. We collected the seeds from 58-year-old Quercus acutissima Carruth. forest, and the seeds were further divided into three classes: large, medium, and small, and sown under the forest gaps (I, 197.82 m2; II, 91.85 m2, III, understory) to observe seed germination and early seedling growth. Precipitation in the study area and soil moisture content in the forest gaps were also observed during the trial period. The results showed that the precipitation in 2019 was similar to that in 2020; both were significantly lower than the precipitation in 2021. The difference in soil water content between gaps I and II was not significant, and both were significantly lower than III. The order of seedling emergence rate in gaps was II > III > I, but the minimum was almost close to zero in I. Large and medium seeds showed significantly greater emergence rate than small seeds. The seedlings of II had higher seedling height, ground diameter, ground diameter relative growth rate, seedling biomass, root surface area, and root volume than those of III. Large seeds had the highest ground diameter, ground diameter relative growth rate, biomass, root mass ratio, root shoot ratio, and root surface area. Correlation analysis showed that seedling biomass was significantly and positively correlated with root surface area and root volume, and significantly and negatively correlated with specific root length and specific root surface area. The regulation of soil moisture in the gap and the adaptability related to seed size were two key factors influencing the seed germination and early seedling growth of Q. acutissima.
1. Introduction
In recent decades, facing the pattern of global natural forest reduction and total forest resources decline, countries all over the world are rapidly developing forest plantations [1]. At present, China has the largest area of planted forests in the world, which plays an important role in greening the national territory, improving forest quality, improving the environment, and promoting the sustainable development of society. However, for a long time, the main purpose was to increase the speed of afforestation and the manufacture of timber products, ignoring the law of natural succession of forests. Current studies have shown that the natural regeneration of planted forests is extremely difficult to achieve [2,3]. In Austria, more than 2/3 of the forests required artificial measures to assist in natural regeneration. Seed germination and seedling establishment are critical stages of forest natural regeneration [4,5]. Many studies suggest that these stages are closely related to seed quality [5], seed dispersal [6], climatic factors [7,8,9], forest environment [8,10,11], and management modes [11,12,13], etc. Some scholars found that there could be a large density of seedlings under the forest canopy, but saplings were very scarce [3,10]. Therefore, the limiting factors for seed germination and seedling growth are not identical. An in-depth study of the influence mechanisms of limiting seed germination and seedling establishment is of great significance to solve the difficulties of natural regeneration in forest plantations.
Forest gap, an important factor affecting seed germination and seedling establishment of tree species, causes significant changes in environmental conditions in the forest, such as light intensity, air temperature, humidity, and soil characteristics [14]. With the increase in gap size, light intensity and air temperature increase significantly, and soil water content changes in a complex way [14]. Some have suggested that small and medium forest gaps have a small effect on seed germination, but large forest gaps are not conducive to seed germination [11], which is related to low soil water content and high temperature [3]. However, Bílek et al. [15] believed that seed germination was not a factor limiting seedling establishment as long as there was appropriate soil water content. Rozas [16] also thoughts that seed germination is not the limiting factor of oak seed germination, and this process is not affected by light. Forest gaps show a significant promotion effect on seedling growth under the forest canopy [17,18], but large forest gaps can inhibit their growth [11]. In addition, the promotion of understory seedling growth in forest gaps is also related to the shade tolerance of the tree species [12,19] and the age of the seedlings [20]. Forest gap promotes the growth of early seedlings in the forest understory [21], but few seedlings emerge in newly formed gaps [22,23]. The formation of new forest gaps provides more favorable conditions for the growth of light-demanding species [24]. Studies on oak species have shown that their natural regeneration was good in large forest gaps [16,25,26]. However, Grogan et al. [23] and Holladay et al. [27] concluded that seedling regeneration was best in medium-sized forest gaps, and excessive forest gaps can inhibit seedling regeneration. Therefore, the effect of forest gaps on seedling establishment is very complex.
Seed size is another key factor affecting seedling establishment. Numerous studies have shown that seed size is closely related to their germination and seedling growth. Large seeds have faster germination rates and seedling survival than small seeds, which has been consistently found in both inter-species and intra-species correlations [28,29,30,31]. This may be related to the fact that large seeds themselves store more nutrients available for early seedling growth [5]. Large seeds produce large seedlings with a highly competitive survival capacity [23,31,32]. Seedlings from large seeds show a lower relative growth rate than seedlings from small seeds [31,33]. However, Quero et al. [34] partially support this view. It has been found that large seeds improved the adaptive capacity of plants under adversities [30,33]. However, some studies have suggested that this advantage was not always available [31,35]. One of the studies on the germination of Pinus thunbergii. Parl. seeds under sand burial reported that the advantage of large seeds was shown under non-stressful stress [5]. Moreover, the advantage of large seeds gradually disappear with seedling growth [35,36]. Therefore, revealing the influence of seed size and its interaction with habitat on the seedling establishment is of great significance to the natural regeneration of the forest.
Quercus acutissima Carruth. is one of the main species of forest vegetation in warm temperate and subtropical regions of China. It is a pioneer tree species in barren hills and land and an excellent soil and water conservation species. It has high ecological, economic, and landscape value [37]. Field investigations found that, in highly shady forests, Q. acutissima seedlings died in large numbers and were poorly regenerated. Field investigation found that a Q. acutissima plantation has a sufficient and stable seedling bank [38]. The number of Q. acutissima seedlings in the gap was significantly higher than that in the forest understory, but the number of seedlings under the too-large gap decreased. The research on the growth of Q. acutissima seedlings under different light intensities found that it exhibited some shade tolerance, but it grew best under full light [39]. Therefore, Q. acutissima has a natural regeneration ability, but its regeneration requires higher light intensity. However, there are few studies on the natural regeneration limitation mechanisms of Q. acutissima plantations. In this study, the effects of forest gap and seed size on seed germination and early seedling growth of Q. acutissima plantations, and the key restriction mechanism in the early stage of the seedling establishment, was revealed.
2. Materials and Methods
2.1. Study Area
The study site is located in Yaoxiang Forest Farm, Shandong Province (117°06′48.2″ N, 36°20′05.3″ E), with an average altitude of more than 700 m. It has a warm temperate continental monsoon climate. The average annual temperature is 13.5 °C, and the maximal and minimal temperatures are 41.5 °C and −20 °C, respectively. The frost-free period is 196 days. The average annual precipitation is 758 mm, with precipitation mainly concentrated in summer. Most of the forests at this farm were planted between the 1950s and the 1960s, with a total forest area of 9490 hm2 and a forest coverage rate of 81.5%. The main tree species are Q. acutissima Carruth., Juglans regia L., Robinia pseudoacacia L., Pinus armandii Franch., Castanea mollissima Bl., Platycladus orientalis (L.) Franco, Pinus thunbergii Parl, and Pinus densiflora Sieb. et Zucc. The main shrubs are Lespedeza bicolor Turcz, Spiraea fritschiana Schneid., and Vitis amurensis Rupr. The vines mainly include Celastrus orbiculatus Thunb., Rubus corchorifolius L.f., and Ziziphus mauritiana Lam. The herbs mainly include Artemisia lavandulaefolia D.C., Oplismenus undulatifolius (Arduino) Beauv., Bothriochloa ischaemum (L.) Keng., and Zoysia japonica Steud.
2.2. Seed Collection
Seeds with full appearance and no obvious moth were collected by hand in the Q. acutissima plantation on 15 October 2018. The forest was planted in 1960 using one-year-old seedlings. Its density is 675 trees per hectare, the average tree height is 16.9 m, and the average diameter at breast height (DBH) is 21.2 cm. The collected seeds were first soaked in water to remove the floating seeds, then the remaining seeds were sorted by hand, and healthy seeds without pests and diseases were selected. According to the 1000-seed weight of Q. acutissima seeds, the seeds were divided into three seed size categories: large (1951.67 ± 66.90 g), medium (1634.00 ± 27.85 g), and small (826.23 ± 10.80 g).
2.3. Seed Germination and Seedling Growth Test
On 21 December 2018, according to the investigation results of the gap size of a Q. acutissima plantation in the forest farm, two forest gaps (I, with an area of 197.82 m2; II, with an area of 91.85 m2) and understory (III, 45 m2) with the same slope, slope position, and slope aspect in the seed-harvesting Q. acutissima planted forest were selected for different sizes of seed germination tests. The large, medium, and small seeds (LS, MS, SS) were sown in each forest gap in four 50 cm × 50 cm plots with 40 seeds in each plot; thus, there were a total of 36 small sample plots. We removed the litter, loosened the topsoil slightly, then planted the seeds one by one, and finally re-covered the seeds with the litter to an approximately 3 cm depth. In August 2020, the number of existing seedlings was counted and the emergence rate (ER, number of seedlings/number of seeds sown × 100%) was calculated. One seedling was carefully excavated from each plot, and a total of 36 biennial seedlings were taken back to the laboratory. Then, another three to five seedlings of similar size were selected and tagged from each plot. The height and diameter of the selected seedlings were measured monthly from September 2020 to October 2021. Among these tagged seedlings, one three-year-old seedling was selected from each plot and carefully dug up. A total of 36 seedlings were taken back to the laboratory. Because the seedling emergence rate in gap I was too low and only a few seedlings from large seeds were found, they were not analyzed in the seedling growth test. The relative height growth rate (RGRH, cm cm−1 d−1) and relative ground diameter growth rate (RGRD, mm mm−1 d−1) for each month were calculated with Equations (1) and (2), respectively.
RGRH = (lnH2 − lnH1)/(t2 − t1)
RGRD = (lnD2 − lnD1)/(t2 − t1)
H is the seedling height (cm) and D is the seedling ground diameter (mm). Subscripts refer to the first month (1) and the next month (2), and t2 − t1 is the number of days between two adjacent months.
In the laboratory, the seedlings were divided into root, stem, and leaf. The area of each leaf of each seedling was determined using a CI-202 portable laser leaf area meter (CID Inc., Washington, DC, USA). The soil on the root system was first rinsed off with water, and after scanning the root system with an HP Scanjet 8200 scanner, the scanned images were analyzed with Delta-T Area Meter Type AMB2 root parameter analysis software to obtain root length (RL), root surface area (RSA), and root volume (RV). After scanning, the roots, stems and leaves were placed in an envelope and put inside an oven at 120 °C for 30 min to inactivate, and then the temperature was adjusted to dry the moisture at 80 °C to a constant weight, and the dry weight was weighed. Seedling biomass (SB, root dry weight + stem dry weight + leaf dry weight), specific leaf area (SLA, leaf area/leaf dry weight), leaf area ratio (LAR, leaf area/biomass), root mass ratio (RMR, root dry weight/seedling biomass), stem mass ratio (SMR, stem dry weight/seedling biomass), leaf mass ratio (LMR, leaf dry weight/seedling biomass), root/shoot ratio (RS, root dry weight)/(leaf dry weight + stem dry weight)), photosynthetic tissues/non-photosynthetic tissues (P/NP, leaf mass/(stem + root mass)), Specific root length (SRL, root length/root dry weight) and specific root surface area (SRA, root surface area/root dry weight) were calculated.
2.4. Measurement of Precipitation and Soil Water Content in the Forest
The precipitation at 1.5 m in the forest was measured from January 2019 to October 2021 using a CR3000 automatic weather station (Campbell Scientific, Logan, UT, USA), which automatically recorded daily data every 10 min. Data were collected every month.
Soil water content in the forest gaps and in the understory was measured monthly from September 2020 to October 2021. Three soil samples were randomly taken from each forest gap monthly with a soil auger at a depth of 0–10 cm and were then placed in an aluminum box and brought back to the laboratory to measure the fresh weight of the soil (m) with an electronic analytical balance. The weighed soil samples were placed in an oven at 105 °C, dried for 8 h, and then taken out to measure the mass of the dried soil samples (ms), and the soil water content (SWC) was measured according to the formula:
SWC (%) = (m − ms)/ms × 100%
2.5. Statistical Analysis
The precipitation effect was tested by a two-way ANOVA, with year and month as the sources of variations. The soil water content was tested by a two-way ANOVA, with gap size and month as the sources of variation. The indexes of seedling growth (height, ground diameter, relative height growth rate, and relative ground diameter growth rate) were tested by a three-way ANOVA, with gap size, seed size, and month as the sources of variation. The biomass and morphological parameters of seedlings were also analyzed by three-factor ANOVA, with gap size, seed size, and age as the sources of variation. Before the analysis of variance, all the data passed the test of normality and homogeneity of variance. When ANOVA indicated a significant overall treatment effect, a multiple comparison test (post-hoc Least Significant Difference test) was carried out to compare the parameter means. The correlation analysis of seedling biomass, biomass allocation, and root morphological indicators was carried out. All the seedling indices were analyzed by principal component analysis (PCA) to identify the main factors relevant to seedling biomass. The statistically significant level was set at p = 0.05. All statistics were conducted with SPSS for Windows 26.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Monthly Dynamics of Precipitation and Soil Water Content
3.1.1. Precipitation
It was shown that the month (F = 5.07, p < 0.01) and the year (F = 6.42, p < 0.01) had significant effects on precipitation. As the month increases, precipitation first increased and then decreased, with the highest values appearing between July and September (Figure 1). The difference in precipitation was not significant between in 2019 and in 2020, and both were significantly lower than that in 2021. The precipitation distribution differed between the three years. The precipitation in 2019 was mainly concentrated in July and August, with high rainfall from April to July in 2020, and concentrated precipitation from June to October in 2021 (Figure 1).
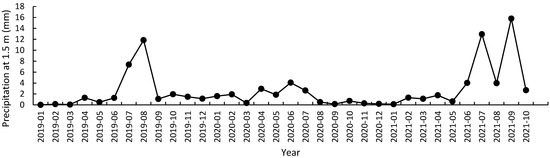
Figure 1.
Change of daily average precipitation at 1.5 m in Quercus acutissima forest from January 2019 to October 2021.
3.1.2. Soil Water Content of Different Gap Sizes
The month (F = 30.74, p < 0.01) and the forest gap size (F = 37.97, p < 0.01) showed significant effects on soil water content. As the month increased, the soil water content fluctuated, with less variation from September 2020 to June 2021, and it varied greatly from July 2021 to September 2021, with the highest values of soil water content in September 2021, followed July 2021 (Figure 2). Among the forest gaps, the difference between the soil water content of gap I and gap II was not significant (p > 0.05), and both were significantly lower than that of understory III (p < 0.01). Significant interaction effects between the month and gap size were found (F = 10.15, p < 0.01). From November 2020 to April 2021, the rank of soil water content was gap I < II < III (Figure 2).
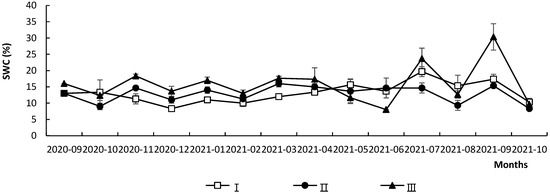
Figure 2.
Monthly dynamic changes of soil water content under different gap sizes in Quercus acutissima forest from September 2020 to October 2021.
3.2. Effects of Forest Gap and Seed Size on Seedling Germination Rate
The results showed that gap size had a significant effect on ER (F = 21.53, p < 0.01), and seed size also had a significant effect (F = 5.29, p < 0.05). The interaction between the two factors was not significant (F = 2.57, p > 0.05). With the decreasing forest gap size, ER first increased and then decreased (Figure 3), ranked as II > III > I (p < 0.05). ER in gap I was extremely low, and was only 2.92 ± 4.74%. Between seed sizes, ER did not show significant differences between LS and MS (p > 0.05), and both were significantly higher than that of SS (p < 0.05).
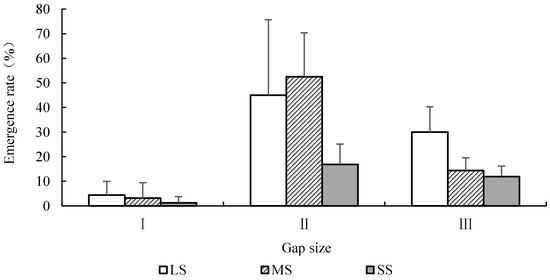
Figure 3.
The emergence rate of seeds with different sizes of Quercus acutissima with the decrease of forest gap. LS: large seed; MS: medium seed; SS: Small seed.
3.3. Effects of Forest Gap and Seed Size on Seedling Growth Dynamics
3.3.1. Seedling Height and Ground Diameter
The results showed that the month had a highly significant effect on SH and GD (Table 1). SH and GD increased significantly with the month (Figure 4). The seed size did not have a significant effect on SH but had a significant effect on GD (Table 1). The difference in GD between LS and MS was not significant (p > 0.05) and both were significantly larger than the GD of SS (p < 0.01). The forest gap showed a significant effect on SH and GD (Table 1), and GD of gap II was significantly larger than that of understory III. The interaction between the month and forest gap exhibited a highly significant effect on SH and GD (Table 1). SH and GD increased significantly more between April 2021 and October 2021 than those between September 2020 and March 2021 (Figure 4). The interaction of seed size and forest gap had a highly significant effect on SH and GD (Table 1). Seedlings of different sizes in gap II were close in SH and GD, but seedlings from LS in understory III had the highest SH and GD, while the seedlings from SS were close to LS in SH and close to MS in GD (Figure 4).

Table 1.
Three-factor analysis of variance of time, seed size, and gap size on seed germination and seedling indexes of Quercus acutissima. ER: emergence rate; SH: seedling height; GD: ground diameter; RGRH: relative height growth rate; RGRD: relative ground diameter growth rate.
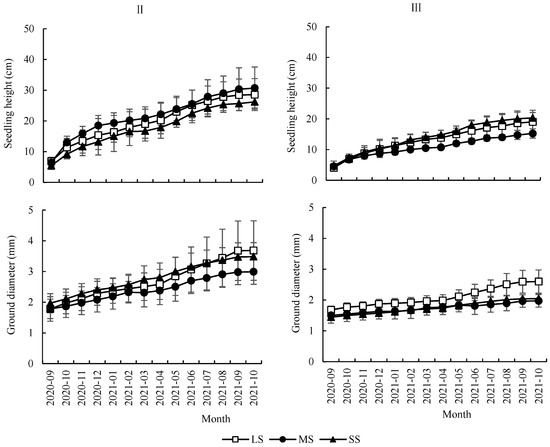
Figure 4.
Monthly dynamic changes of seedling height and ground diameter of Quercus acutissima seedlings from different sizes of seeds under different forest gaps. LS: large seed; MS: medium seed; SS: small seed.
3.3.2. Relative Growth Rate
RGRH was significantly affected by month but not affected by seed size or forest gap (Table 1). RGRH decreased significantly with the increasing month in a waved pattern, with a slight increase from May to July 2021 (Figure 5). The month, seed size, and forest gap showed significantly effects on RGRD (Table 1). RGRD first increased and then decreased with the increasing month, with higher values between December 2020 and May 2020 to September 2020 (Figure 5). Among seed sizes, RGRD from LS was significantly greater than that of MS and SS (p < 0.01), while the difference between MS and SS was not significant (p > 0.05). RGRD in gap II was significantly greater than that of understory III (Table 1). The interaction of the month and forest gap showed a significant effect on RGRD (Table 1). As the time increased, the difference in RGRD among forest gaps became smaller (Figure 5).
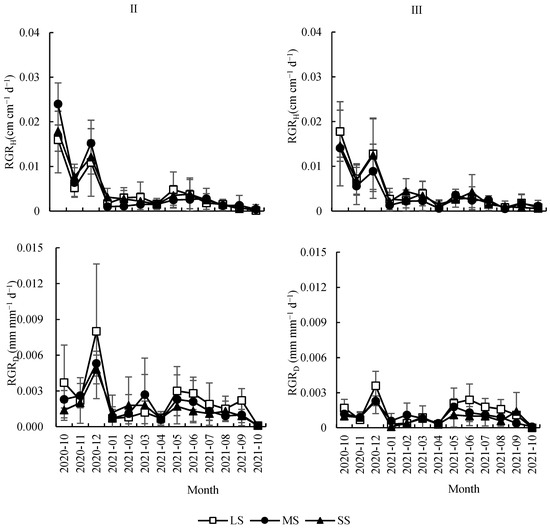
Figure 5.
Monthly dynamic changes of the relative growth of height and ground diameter of Quercus acutissima seedlings from different sizes of seeds under different forest gaps. LS: large seed; MS: medium seed; SS: small seed.
3.4. Effects of Forest Gap and Seed Size on Seedling Biomass and Biomass Allocation Dynamics
The age, seed size, and forest gap showed significant effects on SB, and there was a significant interaction between seed size and forest gap (Table 2). The SB of biennial seedlings was lower than that of three-year-old seedlings (p < 0.01), with an average of 1.65 ± 0.88 g and 2.82 ± 1.77 g, respectively. SB was greater in gap II than in gap III (Figure 6). With different seed sizes, the difference in SB between LS and MS was not significant, and both were greater than the SB from SS. The larger the seeds, the stronger the light intensity, and then the greater the SB (Figure 6).

Table 2.
Three-factor analysis of variance of age, seed size, and gap size on seed germination and seedling indexes of Quercus acutissima. SB: seedling biomass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; R/S: root/shoot ratio; P/NP: photosynthetic tissues/non-photosynthetic tissues; SLA: specific leaf area; LAR: leaf area ratio; RL: root length; RSA: root surface area; RV: root volume; SRL: specific root length; SRA: specific root surface area.
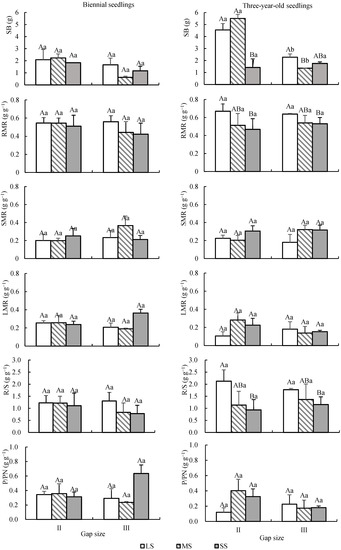
Figure 6.
Dynamic changes of biomass and biomass allocation of Quercus acutissima seedlings from different sizes of seeds under different forest gaps. LS: large seed; MS: medium seed; SS: small seed. Different capital letters indicate that there is a significant difference between different seed size groups under the same gap (p < 0.05); different lower letters indicate that there is a significant difference between gaps under the same seed size (p < 0.05).
The age and gap size had no significant effects on RMR, SMR, LMR, R/S, or P/PN. The seed size showed significant effects on RMR and R/S, but no significant effects on SMR, LMR, and P/PN (Table 2). Among different seed sizes, LS was significantly greater than SS in RMR and R/S, but MS was not significantly different from LS and SS, respectively (Figure 6).
3.5. Effects of Forest Gap and Seed Size on Leaf Characteristics Dynamic
Results showed that age had a significant effect on SLA and no significant effect on LAR. Seed size and forest gap had no significant effect on SLA and LAR (Table 2). The SLA of three-year-old seedlings was significantly larger than that of biennial seedlings (p < 0.01), and the average values were 360.79 ± 214.22 cm2 g−1 and 169.08 ± 29.04 cm2 g−1, respectively (Figure 7).
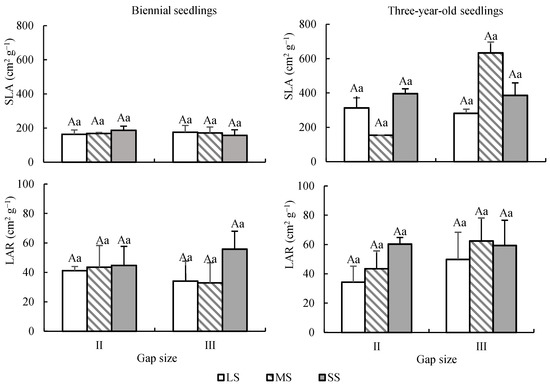
Figure 7.
Dynamic changes of leaf characteristics of Quercus acutissima seedlings from different sizes of seeds under different forest gaps. LS: large seed; MS: medium seed; SS: small seed. Different capital letters indicate that there is a significant difference between different seed size groups under the same gap (p < 0.05), and different lower letters indicate that there is a significant difference between gaps under the same seed size (p < 0.05).
3.6. Effects of Forest Gap and Seed Size on Root Characteristics Dynamics
Among the root indicators, age had a significant effect on RV and SRA, seed size had a significant effect on RSA, forest gap had a significant effect on RSA and RV, and the effects of all other factors were not significant for root system indicators (Table 2). RV of biennial seedlings was lower than that of three-year-old seedlings (p < 0.05), with an average of 0.71 ± 0.35 cm3 and 1.02 ± 0.68 cm3, respectively. However, the SRA of biennial seedlings was larger than that of three-year-old seedlings (p < 0.05), and the average values were 30.13 ± 8.97 cm2 g−1 and 21.87 ± 13.34 cm2 g−1. Among different seed sizes, LS had higher RSA than SS, while MS was not significantly different from LS and SS. RSA and RV were higher in II than in III (Figure 8).
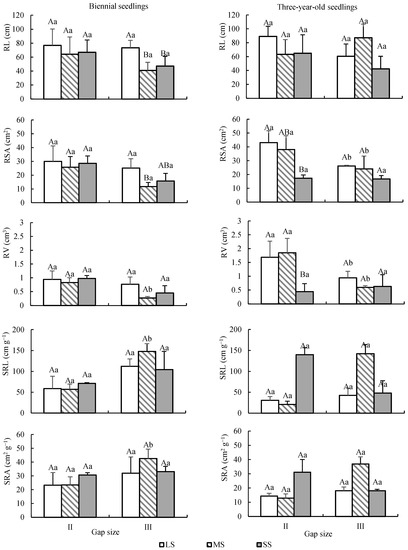
Figure 8.
Dynamic changes of root characteristics of Quercus acutissima seedlings from different sizes of seeds under different forest gaps. LS: large seed; MS: medium seed; SS: small seed. Different capital letters indicate that there is a significant difference between different seed size groups under the same gap (p < 0.05), and different lower letters indicate that there is a significant difference between gaps under the same seed size (p < 0.05).
3.7. Correlation Analysis of Seedling Characteristics
SB was significantly and positively correlated with RSA and RV, and significantly and negatively correlated with SRL and SRA, which indicated that root characteristics were the main factors determining seedling growth. RMR was significantly negatively correlated with LMR, P/PN, SRL, and SRA, and was significantly positively correlated with R/S, RSA, and RV. RSA and RV were significantly negatively correlated with SRL and SRA (Table 3), indicating that there were differences in root resource utilization.

Table 3.
Correlation analysis among seedling characteristics of Quercus acutissima. SB: seedling biomass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; R/S: root/shoot ratio; P/NP: photosynthetic tissues/non-photosynthetic tissues; SLA: specific leaf area; LAR: leaf area ratio; RL: root length; RSA: root surface area; RV: root volume; SRL: specific root length; SRA: specific root surface area. *, p < 0.05; **, p < 0.01.
The results of the PCA showed that the first and second principal components represented 65.73% of the total variables (Figure 9). The coefficient of each index, eigenvalue, variance contribution rate, and accumulated contribution rate are shown in Table A1. The first principal component (41.57%) reflected the root system characteristics related to biomass, including R/S, RMR, RV, RSA, SRA, SB, and SRL. The second principal component (24.16%) was related to the biomass distribution characteristics of ground parts, including LMR, P/NP, SMR, and SLA.
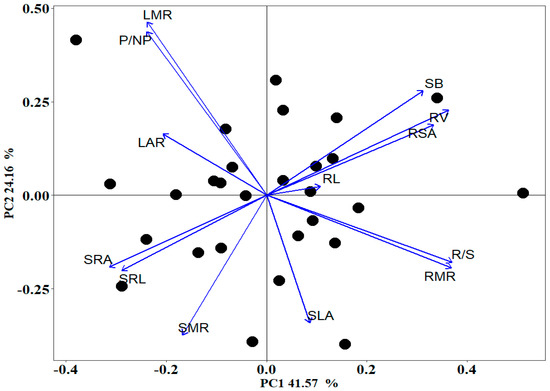
Figure 9.
Principal component analysis of Quercus acutissima seedling traits. SB: seedling biomass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; R/S: root/shoot ratio; P/NP: photosynthetic tissues/non-photosynthetic tissues; SLA: specific leaf area; LAR: leaf area ratio; RL: root length; RSA: root surface area; RV: root volume; SRL: specific root length; SRA: specific root surface area.
4. Discussion
Precipitation was observed to be the major factor affecting soil water content in nature [11,40]. In this study, there was minimal precipitation from January to June and a significantly increased rainfall in July 2019. The seeds of Q. acutissima were found to germinate in July. Therefore, soil water content was a dominant factor for the seed germination of Q. acutissima, which is in agreement with those obtained for Pinus sylvestris L. [11] and Pinus pinaster Ait. [13]. In addition, we found that forest gaps had a significantly modulatory influence on soil water content. From November 2020 to April 2021, the soil water content of forest gap I was the lowest, which was related to less precipitation in the same period. The higher light radiation in large forest gaps than in understory contributed to the higher temperature and thus higher evaporation, leading to variable soil humidity [41]. The seeds of Q. acutissima are mild recalcitrance and have poor dehydration tolerance. Research on Q. robur L. [42] found that the lower the soil water content was, the poorer the germination capacity was. Thus, the low soil water content and long duration of drought were the major factors for the almost zero seedling emergence rate of gap I. The soil water content in gap III was the highest, and was the most important reason for the higher seedling emergence rate in the understory. However, the highest germination rate was in II, in addition to its relation to the higher soil water content, which was also associated with higher temperature. These findings clearly indicated that the smaller forest gaps were more favorable for seed germination. Moreover, we found that the seedling emergence rate of large and medium seeds was higher than that of small seeds. This finding is consistent with prior research [28,29,30,31]. The germination advantage of large seeds has been related to their high nutrient contents [5]. Large seeds had the highest germination rate and had a high-stress tolerance ability, which was in line with the finding of Paz and Martínez-Ramos [33]. Together, the above findings suggest that seed germination of Q. acutissima is determined mainly by precipitation and is simultaneously obviously regulated by the size of the forest gap and by seeds.
Growth is an important indicator of plant adaptation to external environments [43]. In this study, results showed that the seedlings of Q. acutissima in gap II had higher seedling height, ground diameter, and biomass than those in gap III. This indicated that the forest gap contributed to the growth of seedlings, which was in line with the results found by Collet et al. [17] and Diaci et al. [18]. The seedling size of Q. acutissima in forest gaps was higher than in understory, indicating a high demand for light, consistent with results from other research [16,25,26]. There were no significant effects of forest gaps on the relative growth rate of seedling height. Grogan et al. [23] suggested that seedling height determines growth and survival after forest gap formation. For the relative ground diameter growth rate, gap II was significantly greater than that of understory III. The high relative ground diameter growth rate reflects the increased storage capacity of the seedlings and their ability to adapt to the understorey habitat [43,44]. Therefore, the adaption ability of seedlings in forest gaps meant that they performed better than those in the understory. Additionally, we found that large seeds of Q. acutissima had higher biomass, ground diameter, and relative growth rate of ground diameter than small seeds, which led to the higher adaptation ability of large seeds compared to small seeds. furthermore, the reserved nutrients of large seeds affects the growth of seedlings over a longer period [35,36]. Previous studies [33,34], however, considered that the relative growth rate of small seeds was higher than that of large seeds. Quero et al. [34] found only two species supported this conclusion. These indicated that the effect of seed size on the growth of seedlings was complicated. Forest gaps and large seeds contributed to the rapid increase in seedling ground diameter, which was an important approach to improving adaptation ability to the environment within the forest.
Morphological adaptation is one of the primary ways for plants to adapt to the external environment [45]. It suggests there was no significant difference in biomass allocation of seedlings of Q. acutissima among forest gaps. The investigation of the natural regeneration of seedlings under Q. acutissima forest also showed that no significant difference existed in biomass allocation between 2–4-year-old seedlings. This was believed to be related to severe soil drought during its growth period [38]. The annual rainfall was 519.72 mm in 2020, close to the rainfall from 2014 to 2016, and thus seedlings were under severe drought stress. Therefore, the biomass allocation of seedlings was limited by severe drought stress. Seedlings of Q. acutissima in gap II had higher total root surface area and root volume compared to III, indicating a higher ability for the absorption of soil water and nutrients. The root is the major organ for storing non-structural carbohydrates [46,47]. The larger root volume was beneficial for Q. acutissima to store more non-structural carbohydrates and to improve the adaptability of seedlings in the understory [38]. Herrear [48] also argued that root development of Quercus suber L. was crucial for understory survival. Among seeds of different sizes, large seeds generally have a higher root mass ratio and root/shoot ratio than small seeds. In the understory, a high root/shoot ratio was beneficial to its adaptation to drought stress [47,49]. Seedlings from large seeds of Q. acutissima had a higher total root surface area than seedlings from small seeds, which indicated that the root development of large seeds was better than that of small seeds. Correlation analysis showed that the seedling biomass of Q. acutissima was significantly positively correlated with total root surface area and root volume. Principal component analysis showed that root characteristics were the key factors affecting seedling biomass. Therefore, the regulation of roots was an important way for seedlings of Q. acutissima to adapt to the environment within the forest, which was consistent with the results of previous studies [38].
Through research on seed germination and the early seedling growth of Q. acutissima, it was found that the two stages showed different requirements for forest gaps, which was consistent with the conclusions of other scholars regarding some tree species [3,10]. The low precipitation in 2019 resulted in an extremely low rate of seed germination under a large forest gap, and seedlings could not be established. Changes in precipitation patterns would have an important impact on natural forest regeneration [9]. The small gap and understory had higher soil water content, and seed germination was less affected. Rozas [16] also believed that Quercus seeds are large and nutrient-rich, and seed germination is not a key process that limits natural regeneration. In terms of growth, the growth of seedlings in gap II was better than that in III, which indicated that the smaller gap was suitable for the growth of early seedlings. It is easier to establish a seedling bank under the forest or under the small forest gap, which provides the foundation for rapid growth after the appearance of the larger forest gap in the future. Therefore, this paper agrees that the advanced seedling bank in the forest understory is the main source of natural regeneration [22,23]. Seed size significantly affected the germination and early seedling growth of Q. acutissima. Large seeds had obvious advantages in germination, seedling diameter growth, and root development, especially in the understory. Therefore, large seeds improved the adaptability of Q. acutissima in the understory, which was consistent with the conclusion of Quero et al. [34]. Overall, seed germination is not the key process that restricts the natural regeneration of Q. acutissima, but the growth and survival of seedlings are the key limiting stages of natural regeneration.
5. Conclusions
This study showed that the precipitation in this area was the key factor limiting the germination of Q. acutissima seeds under its plantations. The gap affected the germination of Q. acutissima seeds by regulating soil water content. In this paper, a suitable gap size promotes the germination of Q. acutissima seeds, but excessively large gaps are prone to severe drought and can cause seed germination failure. In the early growth of seedlings, the small forest gap was significantly higher than that under the forest. Therefore, seed germination and seedling growth depend on gaps differently. Large seeds have the highest germination capacity, seedling ground diameter growth rate, and root development, especially under the forest canopy, which shows that they are most conducive to the early establishment of seedlings. Therefore, forest gap and seed size are the key factors for the establishment of the understory seedling bank of the Q. acutissima plantation. In future research, it is necessary to strengthen the monitoring of precipitation and environmental factors in the forest. The results give insight into the relationship between seedling growth and gap size, and provide a basis for the regeneration and management of Q. acutissima plantation.
Author Contributions
Conceptualization, B.C. and P.M.; formal analysis, X.K. and Y.P.; investigation, J.Z., C.T., Y.G., X.C., and S.W.; resources, P.G. and J.D.; data curation, R.N.; writing—original draft preparation, X.K. and K.W.; writing—and review and editing, P.M. and B.C.; supervision, B.C.; funding acquisition, B.C. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Central Finance Forestry Reform and Development Fund, grant number [2020]TG08, Germplasm resources nursery project of saline alkali tolerant tree species in the Yellow River Delta, grant number 2019-370505-05-03-035206, and Key R & D projects in Shandong Province, grant number 2017GNC10121.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Coefficient, eigenvalue, variance contribution rate, and the accumulated contribution rate of principal components. SB: seedling biomass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; R/S: root/shoot ratio; P/NP: photosynthetic tissues/non-photosynthetic tissues; SLA: specific leaf area; LAR: leaf area ratio; RL: root length; RSA: root surface area; RV: root volume; SRL: specific root length; SRA: specific root surface area.
Table A1.
Coefficient, eigenvalue, variance contribution rate, and the accumulated contribution rate of principal components. SB: seedling biomass; RMR: root mass ratio; SMR: stem mass ratio; LMR: leaf mass ratio; R/S: root/shoot ratio; P/NP: photosynthetic tissues/non-photosynthetic tissues; SLA: specific leaf area; LAR: leaf area ratio; RL: root length; RSA: root surface area; RV: root volume; SRL: specific root length; SRA: specific root surface area.
| Index | The First Principal Component | The Second Principal Component |
|---|---|---|
| R/S | 0.86 | −0.32 |
| RMR | 0.86 | −0.34 |
| RV | 0.84 | 0.41 |
| RSA | 0.77 | 0.34 |
| SRA | −0.73 | −0.34 |
| SB | 0.73 | 0.50 |
| SRL | −0.67 | −0.35 |
| LMR | −0.55 | 0.82 |
| P/NP | −0.56 | 0.78 |
| SMR | −0.39 | −0.66 |
| SLA | 0.20 | −0.60 |
| RL | 0.25 | 0.06 |
| LAR | −0.48 | 0.30 |
| Eigenvalue | 5.40 | 3.15 |
| Variance contribution rate (%) | 41.57 | 24.16 |
| Accumulated contribution rate (%) | 41.57 | 65.73 |
References
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, Y.; Wang, H. Development strategy and management countermeasures of planted forests in China Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar] [CrossRef]
- Pröll, G.; Darabant, A.; Gratzer, G.; Katzensteiner, K. Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur. J. For. Res. 2015, 134, 293–308. [Google Scholar] [CrossRef]
- Rey, P.J.; Alcántara, J.M. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): Connecting patterns of seed dispersal to seedling establishment. J. Ecol. 2000, 88, 622–633. [Google Scholar] [CrossRef]
- Mao, P.; Guo, L.; Gao, Y.; Qi, L.; Cao, B. Effects of seed size and sand burial on germination and early growth of seedlings for coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 2019, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, H.E.; Hutchins, S.A.; Liu, B.W. The role of birds and mammals in Korean pine (Pinus koraiensis) regeneration dynamics. Oecologia 1996, 107, 120–130. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, H.; Yang, W. Climate change-induced water stress suppresses the regeneration of the critically endangered forest tree Nyssa yunnanensis. PLoS ONE 2017, 12, e0182012. [Google Scholar] [CrossRef]
- Dodson, E.K.; Root, H.T. Conifer regeneration following stand-replacing wildfire varies along an elevation gradient in a ponderosa pine forest, Oregon, USA. For. Ecol. Manag. 2013, 302, 163–170. [Google Scholar] [CrossRef]
- Khaine, I.; Woo, S.Y.; Kwak, M.; Lee, S.H.; Je, S.M.; You, H.; Lee, T.; Jang, J.; Lee, H.K.; Cheng, H.C.; et al. Factors affecting natural regeneration of tropical forests across a precipitation gradient in Myanmar. Forests 2018, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Szwagrzyk, J.; Szewczyk, J.; Bodziarczyk, J. Dynamics of seedling banks in beech forest: Results of a 10-year study on germination, growth and survival. For. Ecol. Manag. 2001, 141, 237–250. [Google Scholar] [CrossRef]
- Pardos, M.; Montes, F.; Aranda, I.; Cañellas, I. Influence of environmental conditions on germinant survival and diversity of Scots pine (Pinus sylvestris L.) in central Spain. Eur. J. For. Res. 2007, 126, 37–47. [Google Scholar] [CrossRef]
- Gasser, D.; Messier, C.; Beaudet, M.; Lechowicz, M.J. Sugar maple and yellow birch regeneration in response to canopy opening, liming and vegetation control in a temperate deciduous forest of Quebec. For. Ecol. Manag. 2010, 259, 2006–2014. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, E.; Juez, L.; Bravo, F. Environmental influences on post-harvest natural regeneration of Pinus pinaster Ait. in Mediterranean forest stands submitted to the seed-tree selection method. Eur. J. For. Res. 2010, 129, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.J.; Tan, H.; Li, F.Q.; Chen, M.; Zhang, J.X. Microclimate regimes following gap formation in a montane secondary forest of eastern Liaoning Province, China. J. For. Res. 2007, 18, 167–173. [Google Scholar] [CrossRef]
- Bílek, L.; Remeš, J.; Zahradník, D. Natural regeneration of senescent even-aged beech (Fagus sylvatica L.) stands under the conditions of central Bohemia. J. For. Sci. 2009, 55, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Rozas, V. Regeneration patterns, dendroecology, and forest-use history in an old-growth beech-oak lowland forest in Northern Spain. For. Ecol. Manag. 2003, 182, 175–194. [Google Scholar] [CrossRef]
- Collet, C.; Lanter, O.; Pardos, M. Effects of canopy opening on the morphology and anatomy of naturally regenerated beech seedlings. Trees—Struct. Funct. 2002, 16, 291–298. [Google Scholar] [CrossRef]
- Diaci, J.; Adamic, T.; Rozman, A. Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: Influences of light regime, herb competition and browsing. For. Ecol. Manag. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Čater, M.; Diaci, J. Divergent response of European beech, silver fir and Norway spruce advance regeneration to increased light levels following natural disturbance. For. Ecol. Manag. 2017, 399, 206–212. [Google Scholar] [CrossRef]
- Soto, D.P.; Jacobs, D.F.; Salas, C.; Donoso, P.J.; Fuentes, C.; Puettmann, K.J. Light and nitrogen interact to influence regeneration in old-growth Nothofagus-dominated forests in south-central Chile. For. Ecol. Manag. 2017, 384, 303–313. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Madsen, P.; Hahn, K. Natural regeneration in a beech-dominated forest managed by close-to-nature principles—A gap cutting based experiment. Can. J. For. Res. 2008, 38, 1716–1729. [Google Scholar] [CrossRef]
- Grogan, J.; Landis, R.M.; Ashton, M.S.; Galvão, J. Growth response by big-leaf mahogany (Swietenia macrophylla) advance seedling regeneration to overhead canopy release in southeast Pará, Brazil. For. Ecol. Manag. 2005, 204, 399–412. [Google Scholar] [CrossRef]
- d’Oliveira, M.V.N.; Ribas, L.A. Forest regeneration in artificial gaps twelve years after canopy opening in Acre State Western Amazon. For. Ecol. Manag. 2011, 261, 1722–1731. [Google Scholar] [CrossRef]
- Petritan, A.M.; Nuske, R.S.; Petritan, I.C.; Tudose, N.C. Gap disturbance patterns in an old-growth sessile oak (Quercus petraea L.)-European beech (Fagus sylvatica L.) forest remnant in the Carpathian Mountains, Romania. For. Ecol. Manag. 2013, 308, 67–75. [Google Scholar] [CrossRef]
- Kunstler, G.; Curt, T.; Bouchaud, M.; Lepart, J. Growth, mortality, and morphological response of European beech and downy oak along a light gradient in sub-Mediterranean forest. Can. J. For. Res. 2005, 35, 1657–1668. [Google Scholar] [CrossRef]
- Holladay, C.A.; Kwit, C.; Collins, B. Woody regeneration in and around aging southern bottomland hardwood forest gaps: Effects of herbivory and gap size. For. Ecol. Manag. 2006, 223, 218–225. [Google Scholar] [CrossRef]
- Baskin, J.M.; Lu, J.J.; Baskin, C.C.; Tan, D.Y.; Wang, L. Diaspore dispersal ability and degree of dormancy in heteromorphic species of cold deserts of northwest China: A review. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 93–99. [Google Scholar] [CrossRef]
- Wang, T.T.; Chu, G.M.; Jiang, P.; Niu, P.X.; Wang, M. Effects of sand burial and seed size on seed germination, seedling emergence and seedling biomass of Anabasis Aphylla. Pak. J. Bot. 2017, 49, 391–396. [Google Scholar]
- Larios, E.; Búrquez, A.; Becerra, J.X.; Venable, D.L. Natural selection on seed size through the life cycle of a desert annual plant. Ecology 2014, 95, 3213–3220. [Google Scholar] [CrossRef] [Green Version]
- Baraloto, C.; Forget, P.M.; Goldberg, D.E. Seed mass, seedling size and neotropical tree seedling establishment. J. Ecol. 2005, 93, 1156–1166. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Twiddle, J.C.; Dickie, J.B.; Westoby, M. A brief history of seed size. Science 2005, 307, 576–580. [Google Scholar] [CrossRef] [Green Version]
- Paz, H.; Martínez-Ramos, M. Seed mass and seedling performance within eight species of Psychotria (Rubiaceae). Ecology 2003, 84, 439–450. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R.; Poorter, L. Seed-mass effects in four Mediterranean quercus species (Fagaceae) growing in contrasting light environments. Am. J. Bot. 2007, 94, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Vidal, E.; Sampedro, L.; Zas, R. Is the benefit of larger seed provisioning on seedling performance greater under abiotic stress? Environ. Exp. Bot. 2017, 134, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Bladé, C.; Vallejo, V.R. Seed mass effects on performance of Pinus halepensis Mill. seedlings sown after fire. For. Ecol. Manag. 2008, 255, 2362–2372. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Wang, H. Managing planted forests for multiple uses under a changing environment in China. N. Z. J. For. Sci. 2014, 44, S3. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ni, R.; Kan, X.; Lin, Q.; Mao, P.; Cao, B.; Gao, P.; Dong, J.; Mi, W.; Zhao, B. Effects of Precipitation and Soil Moisture on the Characteristics of the Seedling Bank under Quercus acutissima Forest Plantation in Mount Tai, China. Forests 2022, 13, 545. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Liu, Y. The effect of low irradiance on growth, photosynthetic characteristics, and biomass allocation in two deciduous broad-leaved tree seedlings in southeast of Hubei Province. Acta Ecol. Sin. 2010, 30, 6082–6090. [Google Scholar]
- Mao, P.; Mu, H.; Cao, B.; Qin, Y.; Shao, H.; Wang, S.; Tai, X. Dynamic characteristics of soil properties in a Robinia pseudoacacia vegetation and coastal eco-restoration. Ecol. Eng. 2016, 92, 132–137. [Google Scholar] [CrossRef]
- Diaci, J.; Pisek, R.; Boncina, A. Regeneration in experimental gaps of subalpine Picea abies forest in the Slovenian Alps. Eur. J. For. Res. 2005, 124, 29–36. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Blake, P.S. Indeterminate development in desiccation-sensitive seeds of Quercus robur L. Seed Sci. Res. 1994, 4, 127–133. [Google Scholar] [CrossRef]
- Brüllhardt, M.; Rotach, P.; Bigler, C.; Nötzli, M.; Bugmann, H. Growth and resource allocation of juvenile European beech and sycamore maple along light availability gradients in uneven-aged forests. For. Ecol. Manag. 2020, 474, 118314. [Google Scholar] [CrossRef]
- Delagrange, S.; Messier, C.; Lechowicz, M.J.; Dizengremel, P. Physiological, morphological and allocational plasticity in understory deciduous trees: Importance of plant size and light availability. Tree Physiol. 2004, 24, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Puglielli, G.; Laanisto, L.; Poorter, H.; Niinemets, Ü. Global patterns of biomass allocation in woody species with different tolerances of shade and drought: Evidence for multiple strategies. New Phytol. 2021, 229, 308–322. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Pedroso, F.K.J.V.; Prudente, D.A.; Bueno, A.C.R.; Machado, E.C.; Ribeiro, R.V. Drought tolerance in citrus trees is enhanced by rootstock-dependent changes in root growth and carbohydrate availability. Environ. Exp. Bot. 2014, 101, 26–35. [Google Scholar] [CrossRef]
- Herrera, J. Acorn predation and seedling production in a low-density population of cork oak (Quercus suber L.). For. Ecol. Manag. 1995, 76, 197–201. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).