Abstract
The effect and responses to drought stress were analyzed in Quercus ilex L. seedlings using a nontargeted metabolomic approach, implementing the approaches of previous studies in which other -omics platforms, transcriptomics, and proteomics were employed. This work aimed to characterize the Q. ilex leaf metabolome, determining possible mechanisms and molecular markers of drought tolerance and identifying putative bioactive compounds. Six-month-old seedling leaves subjected to drought stress imposed by water withholding under high-temperature and irradiance conditions were collected when leaf fluorescence decreased by 20% (day 17) and 45% (day 24) relative to irrigated seedlings. A total of 3934 compounds were resolved, with 616 being variable and 342 identified, which belonged to five chemical families. Out of the identified compounds, 33 were variable, mostly corresponding to amino acids, carboxylic acids, benzenoids, flavonoids and isoprenoids. Epigallocatechin, ellagic acid, pulegone, indole-3-acrylic acid and dihydrozeatin-O-glucoside were up-accumulated under drought conditions at both sampling times. An integrated multi-omics analysis of phenolic compounds and related enzymes was performed, revealing that some enzymes involved in the flavonoid pathways (chalcone synthase, anthocyanidin synthase and anthocyanidin reductase) were up-accumulated at day 24 in non-irrigated seedlings. Some putative markers of tolerance to drought in Q. ilex are proposed for assisting breeding programs based on the selection of elite genotypes.
1. Introduction
According to climate data and derived predictive models, longer and more intense episodes of drought, accompanied by high temperatures and irradiance, are expected to occur throughout the 21st century [1]. These will be more drastic in specific areas such as the Mediterranean Basin [2,3], the natural habitat of the forest tree species under study, Holm oak (Quercus ilex L.), with defined zones of low survival probability [4]. It has been argued that tree mortality and dieback are caused by drought and high-temperature conditions, accompanied by biotic stress (insects, fungi), which for some species is becoming a serious threat [5,6]. Even though it is considered the most drought-tolerant species within the European Quercus genus [7], episodes of tree damage and mortality have been observed in the last 50 years in natural stands and reforested areas [8]. Known as the decline syndrome, the causal agents are not well defined, with a combination of biotic (e.g., Phytophthora cinnamomii) and abiotic (drought) agents proposed as possible drivers [9,10].
Quercus ilex is a nondomesticated species, with biological characteristics (e.g., allogamy, wind pollen and animal seed dispersal) that make the exploitation of natural variability and the selection of elite genotypes the only plausible approach in plant breeding programs, which serve as the basis of sustainable management, conservation and reforestation programs [11,12,13]. For such a purpose, the characterization of the variability and the mechanisms of tolerance to stresses at the molecular level and the search for gene, gene products and metabolites to be used as markers should be a priority for selecting resilient genotypes for ulterior propagation and introduction in the dehesa and natural forests [14,15]. This requires the use of different techniques of morphometry, cell biology, physiology and molecular biology because tree mortality is not easy to predict [16].
We have previously investigated the responses to drought in Q. ilex individuals from different Andalusian populations to confirm differences in response and characterize the mechanisms of tolerance and of the genes implicated [17,18]. Thus, studies have been carried out on morphometry, physiology, classic biochemistry, transcriptomics and proteomics approaches [7,19,20,21,22,23,24]. In order to provide a more complete and realistic view of the processes and mechanisms, we should implement our previous work with the third gene expression level, that of metabolites, by using a nontargeted metabolomics approach. For that, we used a holistic, nontargeted, MS-based metabolomics approach. Such an approach has been recently used in the phytochemical analysis and variability between Q. ilex acorn morphotypes [25]. It has been reported that specific metabolites such as osmolytes, phenolics, and other secondary compounds play a key role in the response and tolerance to drought in particular and to abiotic stresses in general [26,27,28,29,30].
It was in the early 2000s that MS-based metabolomics started to be used in plant biology research [31], with pioneer papers dealing with stress response and water deficits appearing by the end of the decade [32,33]. Most of the work published focused on model systems and crops [34], with much less research carried out on trees [35,36,37]. Some of the published work used the genus Quercus and Q. ilex species as the experimental system [3,38,39,40].
Here, we characterized the leaf metabolome of Q. ilex in six-month-old seedlings and the changes that take place in response to drought stress by water withholding under high-temperature and irradiance conditions. Drought was imposed by water withholding in seedlings grown in perlite for 28 days, as previously described in San-Eufrasio et al. [7]. The analysis included two time points corresponding to a leaf fluorescence decrease of 20% and 45% relative to irrigated seedlings. The final goal was the characterization of the Q. ilex leaf metabolome, the identification of novel metabolites with an emphasis on those with bioactivity previously reported, the characterization of tolerance to drought from a metabolomics point of view, the identification of possible markers of tolerance and the integratation of the metabolite data with those obtained by us and other groups using other -omics or classic approaches. The knowledge generated can be translated to ecological studies and to breeding programs based on the molecular-assisted selection of elite resilient genotypes to be used in restoration and reforestation projects.
2. Materials and Methods
2.1. Plant Material, Treatment and Experimental Design
Mature and healthy acorns were collected from trees located in Almaden de la Plata (Seville, Andalusia, Spain; 37°52′ N, 6°28′ W) and germinated as previously described in San-Eufrasio et al. [7]. Six-month-old seedlings grown in 3 L black plastic pots containing perlite were subjected to drought conditions by water withholding for 28 days. The irrigated, control, and seedling samples were maintained at 100% moisture. The experiment was performed by July 2018 in Cordoba, Andalusia, Southern Spain (37°54′ N, 4°43′ O), where extreme dry conditions prevail throughout the whole month (under a mean 37 °C temperature, 28 W m−2 solar irradiance, and 41% humidity).
An experiment based on a completely randomized design was developed by using ten biological replicates per treatment [7]. Out of the biological replicates, three asymptomatic (nondamaged) non-irrigated seedlings were randomly selected for metabolomics. All leaves were collected when the leaf chlorophyll fluorescence dropped by 20% and 45% in the non-irrigated seedlings compared to the irrigated ones (at days 17 and 24, respectively) [7]. After collection, leaves from the three biological replicates per treatment and sampling time were washed with tap water, blot dried with filter paper, shock-frozen in liquid nitrogen, and stored at −80 °C until metabolite extraction.
2.2. Extraction of Metabolites
Metabolites were extracted from leaves as described by Valledor et al. [41], with minor modifications. An extraction solution containing 600 µL of ice-cold methanol–chloroform–water (5:2:2) was added to 50 mg dry weight leaf tissue and vortexed for 10 s. The mixture was sonicated (ultrasonic bath, 40 kHZ for 10 min) and after centrifugation at 20,000× g at 4 °C for 4 min, the supernatant was transferred to a new tube. Then, 200 µL of cold methanol–chloroform–water (5:2:2) was added to the pellets and the process was repeated once. After combining both supernatants, they were vacuum dried at 30 °C (Speedvac, Eppendorf Vacuum Concentrator Plus/5301, Eppendorf, Leicestershire, UK). Dried extracts were reconstituted in methanol, centrifuged at 20,000× g for 10 min, and filtered through 0.22 μm PTPE membranes (Thermo Fisher Scientific, Courtaboeuf, France) and the filtrate was collected in 1.5 mL LC/MS certified sample vials.
2.3. Metabolite Identification and Quantification Using LC–Orbitrap MS Analysis
Dried extracts were re-dissolved in 1 mL of 50% methanol and 5 µL of sample were subjected to chromatographic separation with a Dionex Ultimate 3000 RS UHPLC system (Thermo Fisher Scientific, Bremen, Germany) equipped with an Acquity UPLC BEH (bridged ethyl hybrid) C18 column (1.7 μm, 100 × 2.1 mm, Waters Corporation, Manchester, UK) at 40 °C. A fifteen-minute mobile phase gradient was employed. A gradient elution chromatography was performed with solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol), as follows: (i) 95% A for 0.5 min; (ii) a linear increase from 5% to 100% in solvent B for 10 min; and (iii) return to 95% A for 2.9 min. A flow rate of 0.5 mL/min was used.
Column eluent was analyzed using a quadrupole Orbitrap Q Exactive hybrid mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated electrospray ionization source (HESI) operating in positive and negative polarities. The operating parameters, in positive ion mode, were a sheath gas flow rate at 60 kV, an auxiliary gas flow rate at 25 kV, a sweep gas flow rate at 2 kV, a spray voltage at 3.50 kV, a capillary temperature at 320 °C, an S-lens RF level at 50 kV, and an auxiliary gas heater temperature at 400 °C. For negative ion mode, all parameters remained the same except that the spray voltage was set to 3.00 kV. The Xcalibur v3.1 software was used for instrument control and data acquisition. Spectra data were acquired in full scan (FS) mode at a resolution of 70,000 (full-width half-maximum, FWHM at m/z 200) for MS1, and in a data-dependent (dd-MS2/dd-SIM) manner for MS2, fragmenting the five most abundant precursor ions per MS1 scan (TopN, 5), acquiring MS/MS data between 200 and 2000 m/z at a resolution of 17.500.
Three biological replicates of each treatment and quality control mix (QC) were analyzed. The QC samples were prepared using equal volumes of all samples and were injected after every six samples for continuous quality assurance and to promote confidence in the data. Moreover, the QC samples were analyzed in a data-dependent (dd-MS2/dd-SIM) manner for feature annotation. All acquired data were exported by Xcalibur software to be analyzed by the Compound Discoverer v3.1 software (Thermo Fisher Scientific, Bremen, Germany). Raw data were deposited in the NIH Common Fund’s National Metabolomics Data Repository (NMDR) website, the Metabolomics Workbench, https://www.metabolomicsworkbench.org, accessed on 13 September 2020. The data can be accessed directly via DataTrack ID:3106.
2.4. Data Processing
UPLC-MS/MS data treatment, alignment, peak selection, deconvolution, normalization, and annotation were performed using the Compound Discoverer v3.1 software (Thermo Fisher Scientific, Bremen, Germany). The alignment was performed with a retention time with a maximum shift of 0.1 min and a mass tolerance of 5 ppm. Then, peak selection and deconvolution allowed the detection of feature groups across all samples. Moreover, elemental compositions (chemical formula hypothesis) for all compounds were predicted and the chemical background was hidden using blank samples. The metabolites were annotated using a ddMS2 similarity search (sustained by the agreement between theoretical and experimental isotopic patterns) in mzCloud (https://www.mzcloud.org/, accessed on 13 September 2020), and the formula or exact mass (mass error ≤ 5 ppm) were searched in ChemSpider (https://www.chem-spider.com/, accessed on 13 September 2020), which was also used for the literature references. Finally, metabolites were classified and mapped to biological pathways using the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/, accessed on 13 September 2020).
Considering the areas of the corresponding chromatographic peaks of the MS1 precursor, a relative comparison among the different samples was performed. Neutral masses obtained in the positive and negative modes were evaluated to avoid duplicities (neutral mass in different modes and similar retention time) while retaining the most intense peaks. A table (Table S1) was generated that included the following information: mass-to-charge ratio (m/z), retention time, and the abundance of the metabolites in the samples.
2.5. Statistical Analysis
All the statistical analyses were performed in the environment RStudio v1.2.158 running under R v3.6.1. Three biological replicates were used for all statistical procedures. The metabolomic data were pre-processed using missing value imputation (Random Forest algorithm with a threshold of 0.35), abundance balancing (values were sample-centric and then each value was multiplied by the average intensity (sum of the peak area of all variables within a sample) of all samples) and filtering (consistency-based criteria with a threshold of 0.5) using pRocessomics (available on web direction: http://github.com/Valledor/pRocessomics, accessed on 27 August 2021).
A multivariate analysis (principal component analysis, PCA) with all samples, including QC samples, was performed. With this analysis, the robustness of the analytical procedure was demonstrated by the tight clustering of the QC samples. In addition, QCs located in the center of the PCA plot ensure that the separation between the groups is not random but is due to a real variability. In addition, to determine which metabolites were different between conditions, a one-way Kruskal–Wallis test per single metabolite was carried out on area values. A PCA was carried out with these metabolites (cut-off of the p-value < 0.05).
2.6. Muti-Omics Integrated Analysis
Datasets of transcriptomics and proteomics, previously reported by Guerrero-Sánchez et al. [24], and the dataset of metabolomics generated in this work were used to perform a multi-omics integration by using the KEGG (Kyoto Encyclopedia of Genes and Genomes) metabolic pathway database. The presence of transcripts, proteins and metabolites related to the following pathways were manually selected: phenylpropanoids; lignins; coumarins; stilbenoids; flavonoids; flavones and flavonols; anthocyanins; and isoflavonoids. The resulting molecular profile obtained for the different pathways was followed by a manual integration of Q. ilex phenolic metabolism. Once the integration was carried out, the levels of transcripts, proteins and metabolites were statistically analyzed (p-value < 0.05 in the Student t-test) under drought conditions.
3. Results and Discussion
3.1. Drought Treatment in Q. ilex Seedlings
Studies on drought stress in Q. ilex seedlings are justified considering that this is the main cause of plant mortality upon transplanting from the nursery [42,43], and hence, the main cause of the failure of restoration and reforestation programs. Because there is great variability in the response to drought stress between individuals and populations [7,21], the final goal of the investigation is to select highly tolerant genotypes, characterize the mechanisms of tolerance and identify key molecular markers associated with resilience to be used in breeding programs. The latter should be sustained by in vitro holistic -omics strategies, covering the different levels of the central dogma of molecular biology and the omics cascade, which connect genotype and phenotype from genomics, transcriptomics, proteomics and metabolomics.
The current research was intended to implement previous studies on the effect and responses to drought in Q. ilex seedlings, in which morphometric (growth, damage symptoms, mortality), physiological (water content, photosynthesis), classic biochemistry (pigments, sugar, amino acids, phenolics), and omics (transcriptomics and proteomics) approaches were used [7,24]. Six-month-old seedlings from highly drought-tolerant, asymptomatic individuals located in the Almaden de la Plata (Sevilla, Andalusia, Spain), grown on perlite, were subjected to drought stress imposed by water withholding under high-temperature and irradiance conditions [7]. Leaf samples were collected at day 17 and 24, when leaf fluorescence dropped to values of 20 and 45% with respect to the irrigated seedlings. The soil matric potential was −15 (day 17) and −26 (day 24) kPa in the non-irrigated seedlings, the relative leaf water content at day 24 was 85% (irrigated seedlings) and 54% (non-irrigated ones), and the quantum-yield (Qy) was 0.73 (irrigated seedlings) and 0.60 (day 17) and 0.40 (day 24) (non-irrigated ones), indicating severe stress conditions. There were no differences in photosynthetic rate and stomatal conductance between treatments at day 9; on the contrary, they were very much impaired under drought conditions at day 24, with values of 11 (irrigated seedlings) and 2 (non-irrigated ones) µmol CO2 m−2 s−1 (photosynthetic efficiency), and 0.18 (irrigated seedlings) and 0.05 (non-irrigated ones) mol H2O m−2 s−1 (stomatal conductance) [7]. At day 24, there were no significant differences between irrigated and non-irrigated seedlings in terms of the content of photosynthetic pigments, and there was a small reduction in the content of anthocyanins [7], proving the integrity of the photosynthetic machinery. Leaves from non-irrigated seedlings had higher levels in total sugars, amino acids and phenolics [7], which is quite a common response to drought, as these compounds enhance osmoprotection and prevent water loss [26,38].
3.2. Untargeted Metabolome Profiling in Q. ilex Leaves
The leaf metabolome from irrigated and non-irrigated Q. ilex seedling leaves at the two sampling times (days 17 and 24) was analyzed by LC-Q-Orbitrap-MS of water-methanolic extracts, operating in the positive and negative ion modes (Table 1).

Table 1.
LC-MS metabolic profile of leaf tissue extracts from irrigated and non-irrigated seedlings. The number of resolved positive and negative ion features are shown, corresponding to the mean of three biological replicates.
In total, 1152–1312 (negative) and 1318–1523 (positive) ion features were resolved at both treatments and sampling times (Table 1). Qualitative differences (absence of a variable ion in the three biological replicates of a treatment) allowed 127 up- and 117 down-accumulated and 87 up- and 128 down-accumulated ions to be identified at days 17 and 24, respectively (Table 1). Quantitative differences (p-value < 0.05 in the Kruskal–Wallis test) identified 123 up- and 102 down-accumulated, and 218 up- and 105 down-accumulated ions at days 17 and 24, respectively (Table 1). These values are lower than those previously reported for Q. ilex acorns by using Q-TOF [25], Quercus suber leaves by using a double extraction system [44], and Nicotiana tabacum leaves by using IT-TOF [45]. The original dataset was filtered for consistency (present in the three replicates) and differences between samples (p-value < 0.05 in the Kruskal–Wallis test) (Table 1). Around 20% of the whole metabolome visualized changed in abundance, with a similar number of compounds being more/less abundant, and showing quantitative/qualitative changes in non-irrigated leaves, except for those more abundant at day 24. Venn diagrams were performed with the total (3934), variable (616) and annotated (342) features identified in irrigated and non-irrigated Q. ilex leaf seedlings at days 17 and 24 (Figure S1). Out of 616 variable metabolites, 54 were present at both treatments and both sampling times, representing a small percentage of the total (8.77%). Regarding variable compounds, 137 variable metabolites were commonly identified at both treatments and both sampling times. On the other hand, 15 metabolites were commonly observed at both sampling times in the non-irrigated seedlings, whereas 10 and 41 were detected at days 17 and 24, respectively, which indicates the existence of permanent and transitory changes.
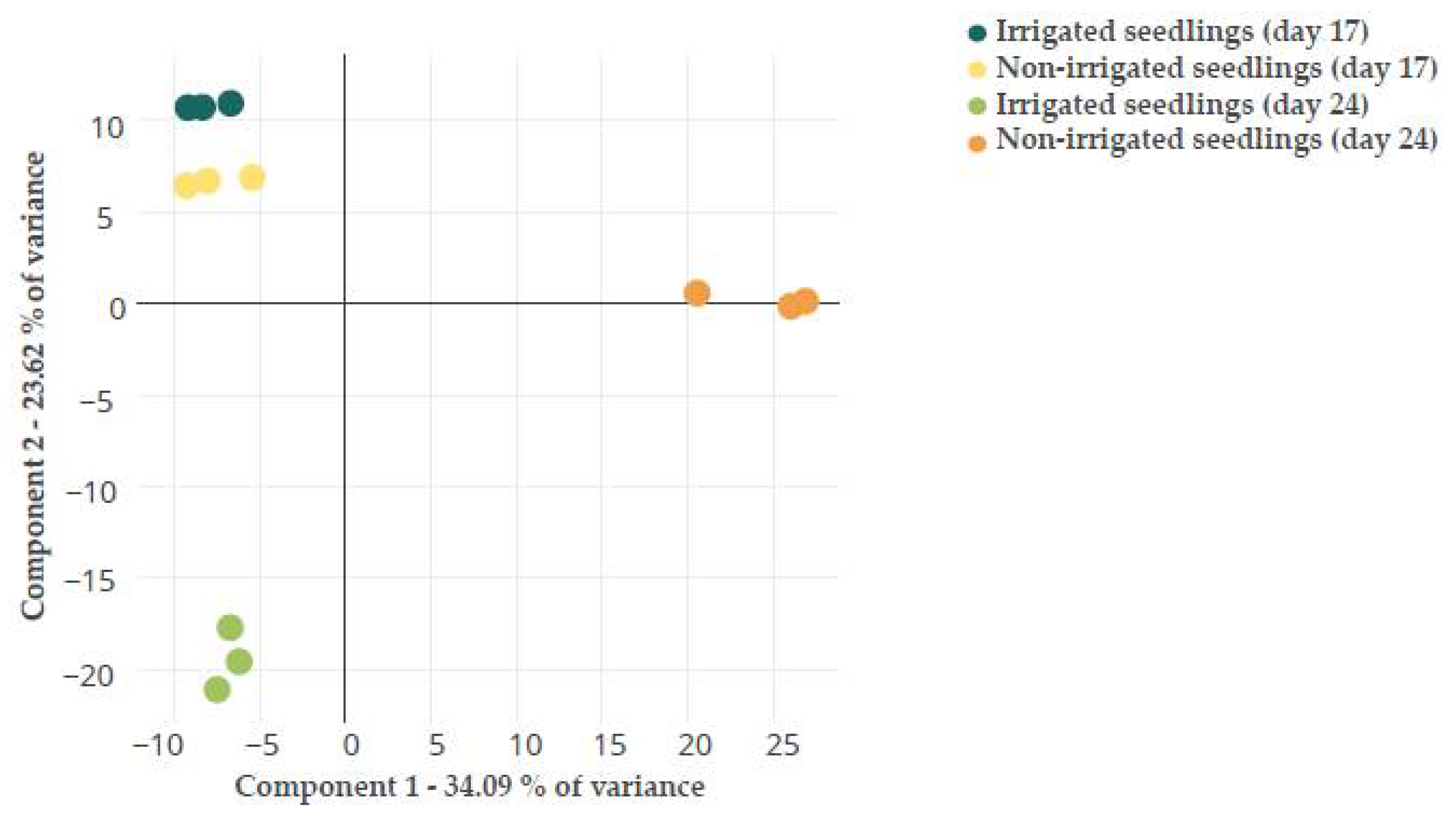
A PCA analysis was performed to reduce the dimensionality of the data and visualize the relationship among samples. The PCA of the total dataset (Figure S2) showed that the different replicates within samples, including the QC ones, were grouped, demonstrating the high quality of the data. PC1 and PC2 accounted for a low percentage of the variance, at 15.10% and 12.84%, respectively. This is quite common for Q. ilex -omics analysis [24]. The PCA test of the variable features dataset (616) discriminated treatments and sampling times (Figure 1); thus, PC1 (34.09% of the variance) separated non-irrigated seedlings at day 24 for the rest of the treatments, whereas PC2 (23.62% of the variance) separated both sampling times. A similar PCA analysis has been previously performed with the variable transcripts and proteins at the two treatments and sampling times [24]. Higher variability was found among replicates in transcriptomics than proteomics or metabolomics data. A different distribution of the samples was obtained with the different -omics datasets. From the protein and transcript PCA, PC1 clearly separated treatments and PC2 sampling times [24]. This indicates that the transcript and protein profiles were more variable at the assayed times than the metabolite one, and that changes in transcripts and proteins occurred earlier than in metabolites.
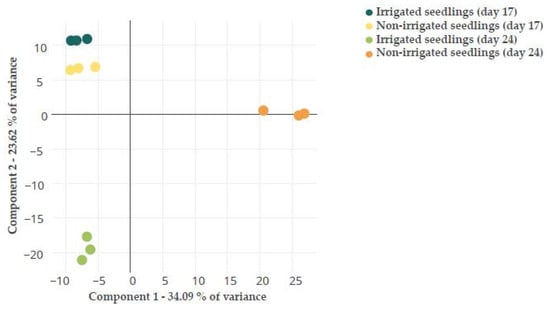
Figure 1.
PCA analysis of the variable features dataset identified in Q. ilex leaf seedlings during both treatments (irrigated and non-irrigated seedlings) and both sampling times (days 17 and 24).
From the m/z of the ion precursor and derived fragments, 342 compounds were annotated (Table 2 and Table S1), with redundancy for some of them. They belonged to different chemical families (Table 2), with 22 having three or more identified compounds. The secondary metabolism group, and concretely, that of phenolic compounds, at the number of 131, was the most represented. Out of 342 annotated compounds, 28 were included in very heterogeneous classes (Table 2 and Table S1). These data show, as previously observed in Q. ilex acorns [25], the complexity and richness of the Q. ilex secondary metabolites.

Table 2.
Total number of annotated compounds classified by chemical families.
3.3. Differential Metabolite Abundance Analysis
Next, data on annotated variable compounds are presented and discussed. Out of the 342 annotated compounds, 33 were variable (p-value < 0.05 in the Kruskal–Wallis test) (Table 3), belonging to the following groups: amino acids (9), carbohydrates and carbohydrate conjugates (3), phenolics (9), terpenoids (6), phytohormones (2), and others (4). The number of identified variable compounds is lower than that reported for Q. suber [44], but it is in the range of that reported in other Quercus spp., or even plant species [46,47]. From our previous proteomics and transcriptomics analysis [24], the number of expected variable metabolites, according to the enzyme protein and transcript profiles, should be much higher, which clearly indicates the limitation of the metabolomics strategy.

Table 3.
List of variable annotated metabolites identified in Q. ilex seedlings subjected to drought conditions.
As previously shown in previous studies on metabolome changes under drought conditions, and in agreement with data presented here, amino acids, carbohydrates, organic acids, and the group of secondary metabolites (phenolics, and terpenoids) are the most affected [44,61,62]. Some preliminary studies on changes in the metabolome profile have been caried out in Quercus spp., with agreements on changes in chemical groups, but differences in individual components can be explained by the different species or genotype, experimental design or metabolomics platform. As an example, Aranda et al. [47] reported an experiment with older seedlings using GC-MS. Some annotated variable compounds have not been previously identified in Quercus spp. (e.g., aesculin, dihydrophaseic acid), others are of very limited distribution or not reported in the plant kingdom (e.g., 7-oxoheptanoate and 10-hydroxy-2decenoic-acid), whereas others are ubiquitous (e.g., aspartate, L-tyrosine) (Table 3). In addition, for some of them, bioactivities and health benefits have been claimed (e.g., emodin, miglitol, 7-deoxyloganin). As some of the compounds have been described in animal systems or microorganisms, its identification should be considered questionable, and it should be validated by NMR and other approaches. Those that were not previously described in nonplant systems are kept out of the discussion. The pattern observed depended on the compound, with qualitative and quantitative differences among treatments, and up- or down-accumulation tendencies at both or individual sampling times.
Regarding the phenolics compounds, they are the major secondary metabolites in the genus Quercus, with some of them showing bioactivity and having interesting pharmacological properties (epigallocatechin, ellagic acid) [53]. The clearest metabolome response of Q. ilex to drought stress was the changes in the phenolic profile with variable phenolic compounds of the different chemical groups, from simple benzenoids to more complex flavonoids and tannins through coumarins and monolignols (Table 3), which agrees with Almeida et al. [44]. Benzenoids (C6–C1, C6–C2, C6–C3 and C6–C7), either increased (3,4-Dihydroxymandelaldehyde, L-dopa) or decreased (Isovanillic acid) under drought conditions. The implication of L-dopa and other catecholamines in the responses to biotic and abiotic stresses has been suggested [63] and associated with its allelochemical potential [64]. Coumarins have been previously reported in the genus Quercus, being present in bark, contributing to the taste of wines produced and stored in wood barrels [65]. Three compounds belonging to this group were identified as variable ones: the simplest coumarin, aesculin, and scoparone. They were down-accumulated in the response to drought in Q. ilex, except for aesculin at day 24. Both epigallocatechin and ellagic acid were up-accumulated under drought conditions at both sampling times [66,67].
Quercus spp., and concretely Q. ilex, are rich in terpenoids, mostly mono- and sesquiterpenes [44]. They may contribute to the drought-tolerant phenotype as, for example, they are components of the cuticular waxes [68]. In Q. ilex under drought conditions, the amount of terpenoids in root exudates and terpene emissions increased [69,70]; additionally, in Q. suber, an increase in the terpenoid leonuridine was a characteristic late response to drought [44]. Among the annotated variable terpenoids in this work, the following compounds are included: the triterpenoid saponin arjunic acid (down-accumulated in the non-irrigated seedlings at day 24), the sesquiterpenoid dihydrophaseic acid (detected at days 17, in the irrigated seedlings, and 24 in the non-irrigated ones), and the monoterpenes pulegone and (+)-exo-5-hydroxycamphor (detected in the irrigated seedlings at day 17). A clear tendency, up- or down-accumulated, for this group has not been found, which is not surprising, as it has been previously discussed in [71].
As for the amino acids annotated in the Q. ilex leaf metabolome, L-tyrosine was observed in the non-irrigated seedlings, being ten times more accumulated at day 24 than at day 17, which agrees with Fabregas and Fernie [72]. Tyrosine has proven to be accumulated in drought-stressed chickpea plants and is also more abundant in drought-tolerant varieties [73]. Other annotated variable amino acids were also up-accumulated at day 24 (homoarginine, GABA, L-citrulline, L-methionine sulfoxide, L-tyrosine and L-dopa), even though they were not detected, or even at lower abundance in the non-irrigated seedlings at day 17. Amino acid accumulation under stress conditions has been reported in several plants species, including the genus Quercus [44,47,72], with GABA and proline being two clear examples linked to a stress-tolerant character [74,75]. In Quercus spp., the up-accumulation of GABA and glutamic acid was one of the clearest responses to drought in Quercus spp. [47].
Among the changes in the group of carbohydrates in the metabolic profile of seedlings subjected to drought, the increase in the levels of glucose, fructose, oligosaccharides or derivatives (polyols and other osmotic active compounds) seems to be a general tendency. In Q. ilex, a slight increase in glucose, fructose, and galactose, but not sucrose or raffinose, has been previously reported [47]. These sugars were not identified in the present work, which can be due to the employed extraction and MS analysis. Out of the 28 annotated carbohydrates, only three were variable in response to drought, namely N-acetyl-beta-D-galactosamine (decreased under drought conditions at both sampling times), (−)-quebrachitol (less abundant in the non-irrigated seedlings at both sampling times) and miglitol (increased under drought conditions at both sampling times). To our knowledge, not much information has been reported about the presence of N-acetyl-beta-D-galactosamine and miglitol in plant tissues [76]. Quebrachitol is one of the major methylated cyclitols in some plant species but has not been previously reported in the genus Quercus. With no clear biological roles assigned, its chemical nature suggests its participation in the response to osmotic stress [77].
Indole-3-acrylic acid, generated from tryptophan and reported as an auxin growth regulator, was identified in this study (Table 3). As most of the plant hormones, auxins seem to play a role in the responses to abiotic stresses, either directly or through hormone signaling pathway crosstalk [46,78,79]. Another variable growth regulator annotated was the cytokinin dihydrozeatin-O-glucoside that increased in abundance at both sampling times. In this direction, a few published works have reported an increase in cytokinin levels in plants under drought conditions [80,81].
Abscisic acid (ABA) has been reported as the key player in plant responses to drought. ABA has been identified in this metabolomic study, but statistically significant differences between samples have not been found. The most typical effect of ABA is stomata closing, which reduces water loss and gas exchange. In our system, the photosynthetic rate and stomatal conductance were only significantly reduced at late times of the experiment, even though no changes in ABA were observed. For some of the up-accumulated compounds, some previous evidence may support a possible role in drought tolerance. This is the case of the riboflavin precursor 6,7-dimethyl-8-(1-D-ribityl)lumazine. The riboflavin treatment of tobacco plants enhanced drought tolerance [82]. Other metabolites have been shown to be changed in response to drought in Q. ilex and other Quercus spp., and these changes are either general or species-specific. Such changes have not been observed with the employed individual or under our experimental conditions. It is the case of some organic acids such as succinic and malic acids.
Because the search of possible molecular markers is related to Q. ilex drought tolerance response, we propose those metabolites that were up-accumulated in non-irrigated seedlings (Table 3). This list includes L-citrulline, L-tyrosine, epigallocatechin, ellagic acid, pulegone, indole-3-acrylic acid, dihydrozeatin-O-glucoside, 2-furoic acid and pantothenic acid.
3.4. Integrated Multi-Omics Data
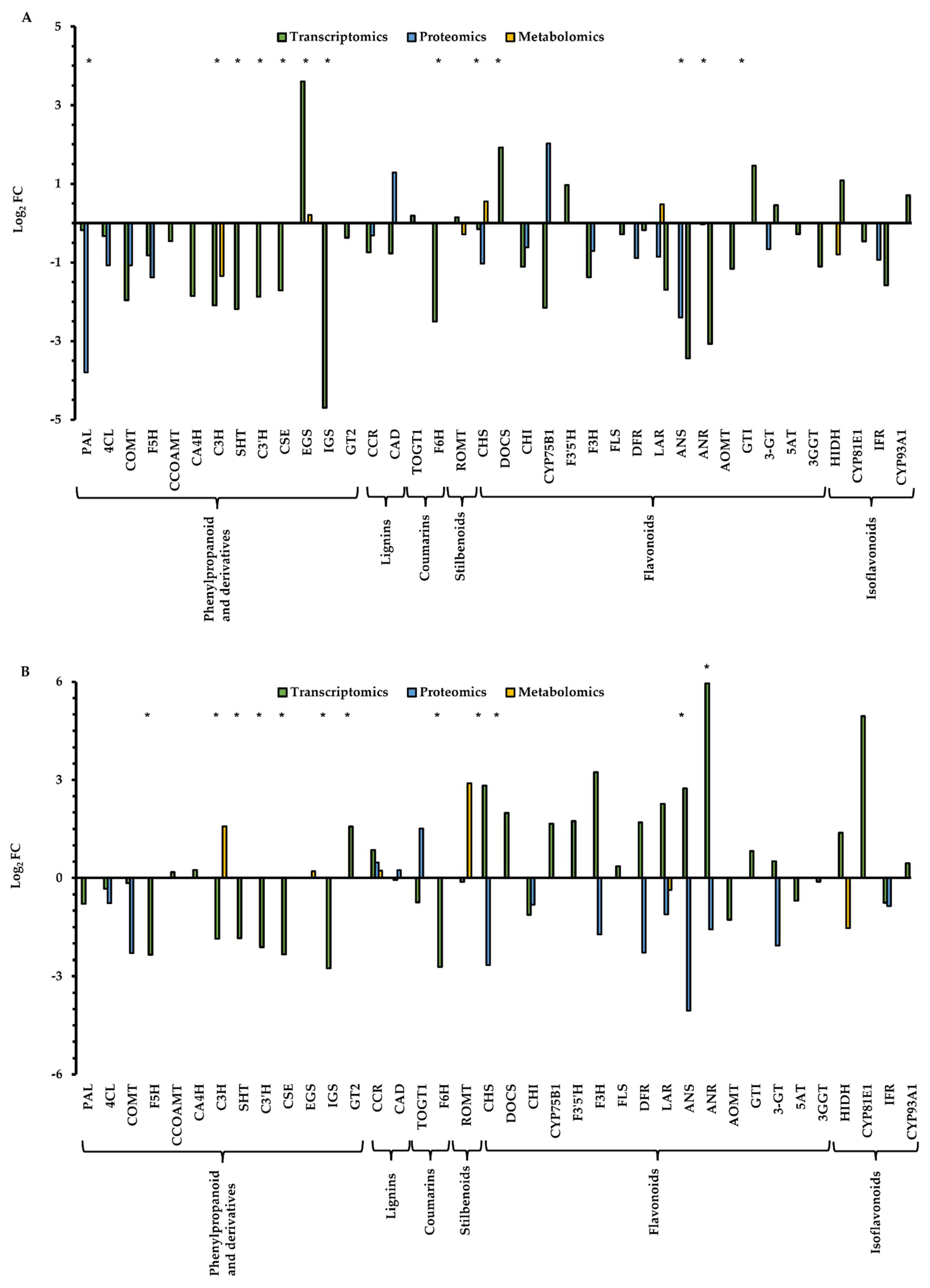
A total number of 25,169 transcripts, 3312 proteins that were previously reported in Guerrero-Sánchez et al. [24], and 342 metabolites reported in this work, were annotated in Q. ilex seedlings. As expected, the transcriptomic dataset is the most comprehensive and informative and includes more than 10,000 transcripts, followed by the proteomics dataset with about 3000 proteins, and, finally, the metabolomics dataset containing a few hundred meabolites [83]. An integrated multi-omics analysis of phenolic compounds’ andrelated enzymes was performed, considering that the phenolic metabolism pathway is the most represented in the metabolome of Q. ilex. All the transcripts, proteins and metabolites related to the biosynthesis of phenylpropanoids and derivates, lignins, coumarins, stilbenoids, diarylheptanoids and gingerols, flavonoids, flavones and flavonols, anthocyanins, and isoflavonoids were selected (Table S2). A total of 38 enzymes involved in phenolics metabolism were identified in the pathways analyzed, with the biosynthesis of phenylpropanoid and flavonoids being the most represented (13 and 16, respectively). The number of enzymes found on the three omics levels was quite low (leucoanthocyanidin reductase, cinnamoyl-CoA reductase and chalcone synthase), which indicates that the data integration is challenging when a combined analysis of metabolomics data is integrated with other omics data [84]. However, when the presence of enzymes is identified at two omics levels, the number of enzymes is higher, at 18 out of 38. The rest of the enzymes were detected only at the transcript level (Table S2).
The enzymes involved in the pathways of lignins, stilbenoids and isoflavonoids were not affected by drought conditions (Figure 2). In contrast, some enzymes involved in the pathways of phenylpropanoids and derivates, coumarins and flavonoids were altered in the Q. ilex seedlings subjected to drought. Most of the enzymes involved in the pathways of phenylpropanoids and derivates and coumarins were down-accumulated in response to drought (Figure 2). It is remarkable the significant late response to drought observed in the flavonoid pathways in some enzymes (chalcone synthase, anthocyanidin synthase and anthocyanidin reductase) that were up-accumulated at day 24 (Figure 2). Flavonoids have a high antioxidant activity against reactive oxygen species (ROS), which are enhanced under drought stress [72,85]. Chalcone synthase is a key enzyme in the flavonoid biosynthesis pathway that monitors the changes in response to drought in many plants. In Nicotiana bentamiana and Populus, the expression of the chalcone synthase gene increased under drought conditions [86,87]. Recently, chalcone synthase has been proposed as a putative marker of drought tolerance, which was up-accumulated in Q. ilex seedlings from several populations subjected to drought [88]. Li et al. [89] reported that anthocyanidin synthase from Morus alba contributes to the protection of plants against abiotic stress such as drought by improving the ROS-scavenging ability. Kubra et al. [90] also showed that the expression of the anthocyanidin synthase gene in Arachis hypogaea was significantly increased in drought. In anthocyanidin reductase, although it was described as a down-accumulated gene in response to drought in Camellia sinensis [91], other studies reported that its expression is increased (e.g., Vitis vinifera) under this abiotic stress [92]. Wang et al. [93] described that, in C. sinensis, this enzyme was first decreased and then increased in response to drought stress. Considering the role of these enzymes in the response to drought as well as their identification in at least two omics approaches, they could be proposed as markers of resilience to drought stress to be used in breeding and reforestation programs. Therefore, they deserve further attention and in-depth functional study and validation as potential molecular markers.
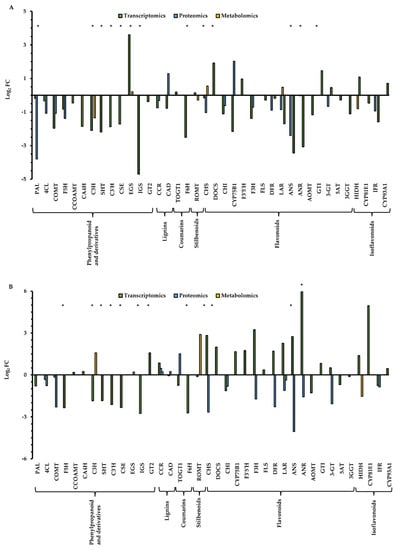
Figure 2.
Integrated multi-omics analysis of phenolic compound-related enzymes in Q. ilex. The asterisk indicates significant differences in the differential expression of the enzymes related to phenolic compounds in response to drought at days 17 (A) and 24 (B) at the transcriptomic, proteomic and metabolomic levels. Expression levels are represented as log2 of fold change (FC). PAL: phenylalanine ammonia-lyase; 4CL: 4-coumarate CoA ligase; COMT: caffeic acid 3-O-methyltransferase; F5H: ferulate 5-hydroxylase; CCOAMT: caffeoyl-CoA O-methyltransferase; CA4H: cinnamate 4-hydroxylase; C3H: coumarate 3-hydroxylase; SHT: shikimate O-hydroxycinnamoyltransferase; C3′H: p-coumaroyl ester 3′-hydroxylase; CSE: caffeoylshikimate esterase; EGS: eugenol synthase; IGS: isoeugenol synthase; GT2: cinnamate beta-D-glucosyltransferase; CCR: cinnamoyl-CoA reductase; CAD: cinnamyl alcohol dehydrogenase; TOGT1: scopoletin glucosyltransferase; F6H: feruloyl CoA ortho-hydroxylase; ROMT: trans-resveratrol di-O-methyltransferase; CHS: chalcone synthase; DOCS: NAD(P)H-dependent 6′-deoxychalcone synthase; CHI: chalcone isomerase; CYP75B1: flavonoid 3′-hydroxylase; F3′5′H: flavonoid 3′,5′-hydroxylase; F3H: flavanone-3-hydroxylase; FLS: flavonol synthase; DFR: dihydroflavonol reductase; LAR: leucoanthocyanidin reductase; ANS: anthocyanidin synthase; ANR: anthocyanidin reductase; AOMT: flavonoid 3′,5′-methyltransferase; GTI: flavonol 3-O-glucosyltransferase; HIDH: 2-hydroxyisoflavanone dehydratase; CYP81E1: isoflavone 2′-hydroxylase; IFR: isoflavone reductase; CYP93A1: 3,9-dihydroxypterocarpan 6A-monooxygenase; 3-GT: anthocyanidin 3-O-glucosyltransferase; 5AT: anthocyanin 5-aromatic acyltransferase; 3GGT: anthocyanidin 3-O-glucoside 2″-O-glucosyltransferase. Asterisk indicates significant differences between irrigated and non-irrigated seedlings (* p < 0.05).
4. Conclusions
The molecular study of the effect and responses to drought stress in Q. ilex is a key feature in assisting breeding programs based on the selection of elite genotypes and hence, maintaining the sustainability of Mediterranean ecosystems and the agrosilvopastoral system “dehesa” under stress and climate change conditions. For this, two omics approaches, transcriptomics and proteomics, were employed with this species to decipher molecular mechanisms involved in the response to drought as well as to identify gene, gene products and molecular markers associated with drought tolerance [24,93]. As a complement analysis, in this work, changes in the metabolome profile of Q. ilex seedlings subjected to drought conditions by water withholding was performed. The metabolomic analysis, at two sampling times (days 17 and 24) under drought conditions, revealed important changes in the metabolome (3934 features resolved, with 616 variable and 342 annotated compounds). The chemical groups more affected by the stress were amino acids, carbohydrates, organic acids, and the group of secondary metabolites, including phenolics and terpenoids. The list of annotated variable compounds included some that were not previously identified in Quercus spp. (e.g., aesculin, dihydrophaseic acid), that were not previously reported in the plant kingdom (e.g., 7-oxoheptanoate and 10-hydroxy-2decenoic-acid), or that were ubiquitous (e.g., aspartate, L-tyrosine). It is remarkable that some of them have been previously described in other plant species as being bioactive or having health benefits (e.g., epigallocatechin, ellagic acid, emodin, 7-deoxyloganin). Among the variable compounds, such as L-citrulline, L-tyrosine, epigallocatechin, ellagic acid, pulegone, indole-3-acrylic acid, dihydrozeatin-O-glucoside, 2-furoic acid and pantothenic acid, some could be considered putative markers of tolerance as they are accumulated in non-irrigated seedlings at the two sampling times. The integrated multi-omics analysis was performed with transcripts and proteins of phenolic pathway enzymes that were previously reported [24] and the metabolome analysis presented here. The flavonoid biosynthesis pathway was the most represented in Q. ilex, with a total of 16 transcripts, 10 proteins and 2 metabolites identified. Three enzymes (chalcone synthase, anthocyanidin synthase and anthocyanidin reductase) were up-accumulated at day 24 in response to drought and identified in at least two omics approaches. These enzymes, together with the up-accumulated metabolites at both sampling times, could be proposed as markers of drought tolerance to be used in breeding programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13040551/s1, Table S1. (A) List of the total metabolites obtained in three biological replicates of Q. ilex leaf seedlings under drought conditions at two sampling times (days 17 and 24), showing the mass-to-charge ratio (m/z), retention time, and abundance. (B) List of annotated variable metabolites (p-value < 0.05 in the Kruskal–Wallis test) showing the ID, neutral mass (Da), retention time (min) and description. Table S2. List of enzymes involved in the phenolics metabolism identified at the transcriptomic, proteomic and metabolomic levels in Q. ilex. The presence of the enzyme is shown with an “x”. Figure S1. Venn diagrams of the total 3934 (A), and 616 variable (B) features, and 342 annotated compounds (C) in irrigated and non-irrigated Q. ilex leaf seedlings at both sampling times (days 17 and 24). Figure S2. PCA analysis of the total dataset of metabolites including the quality control mix (QC).
Author Contributions
Conceptualization, J.V.J.-N.; methodology, M.T.-P., C.L.-H., R.V.-F., V.M.G.-S., Á.I.-G., J.V.J.-N. and M.-D.R.; software, M.T.-P., C.L.-H. and V.M.G.-S.; writing—original draft preparation, M.-D.R. and J.V.J.-N.; writing—review and editing, M.-Á.C., J.V.J.-N. and M.-D.R.; supervision, J.V.J.-N.; funding acquisition, J.V.J.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant ENCINOMICS-2 PID2019-109038RB-I00 from the Spanish Ministry of Economy and Competitiveness.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
C.L.-H. is grateful for the contract “Severo Ochoa Program (BP17-112, Government of Principado de Asturias, Spain)”. M.-Á.C. and M.-D.R. are grateful for the awards of Ramón y Cajal (RYC-2017-23706), and the Juan de la Cierva-Incorporación (IJC2018-035272-I) contracts by the Spanish Ministry of Science, Innovation and Universities, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Touma, D.; Ashfaq, M.; Nayak, M.A.; Kao, S.C.; Diffenbaugh, N.S. A multi-model and multi-index evaluation of drought characteristics in the 21st century. J. Hydrol. 2015, 526, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.J.; Meireles, C.I.; Gomes, C.J.P.; Ribeiro, N.; Almeida, M.C. The Impact of Climate Change on Forest Development: A Sustainable Approach to Management Models Applied to Mediterranean-Type Climate Regions. Plants 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Assessment of the impacts of climate change on Mediterranean terrestrial ecosystems based on data from field experiments and long-term monitored field gradients in Catalonia. Environ. Exp. Bot. 2018, 152, 49–59. [Google Scholar] [CrossRef]
- Quinto, L.; Navarro-Cerrillo, R.M.; Palacios-Rodriguez, G.; Ruiz-Gómez, F.; Duque-Lazo, J. The current situation and future perspectives of Quercus ilex and Pinus halepensis afforestation on agricultural land in Spain under climate change scenarios. New For. 2020, 52, 145–166. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Bussotti, F.; Pollastrini, M. Opportunities and Threats of Mediterranean Evergreen Sclerophyllous Woody Species Subjected to Extreme Drought Events. Appl. Sci. 2020, 10, 8458. [Google Scholar] [CrossRef]
- San-Eufrasio, B.; Sánchez-Lucas, R.; López-Hidalgo, C.; Guerrero-Sánchez, V.M.; Castillejo, M.Á.; Maldonado-Alconada, A.M.; Jorrín-Novo, J.V.; Rey, M.-D. Responses and differences in tolerance to water shortage under climatic dryness conditions in seedlings from Quercus spp. and Andalusian Q. ilex populations. Forests 2020, 11, 707. [Google Scholar] [CrossRef]
- Ogaya, R.; Liu, D.; Barbeta, A.; Peñuelas, J. Stem mortality and forest dieback in a 20-years experimental drought in a Mediterranean holm oak forest. Front. For. Glob. Change 2020, 2, 89. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Moreno, G.; Solla, A. Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric. For. Meteorol. 2013, 169, 92–99. [Google Scholar] [CrossRef]
- Ruiz-Gómez, F.J.; Pérez-de-Luque, A.; Navarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Curr. For. Rep. 2019, 5, 251–266. [Google Scholar] [CrossRef]
- Soto, A.; Lorenzo, Z.; Gil, L. Differences in fine-scale genetic structure and dispersal in Quercus ilex L. and Q. suber L.: Consequences for regeneration of Mediterranean open woods. Heredity 2007, 99, 601–607. [Google Scholar] [CrossRef]
- Guzmán, B.; Rodríguez López, C.M.; Forrest, A.; Cano, E.; Vargas, P. Protected areas of Spain preserve the neutral genetic diversity of Quercus ilex L. irrespective of glacial refugia. Tree Genet. Genomes 2015, 11, 124. [Google Scholar] [CrossRef]
- Fernández i Marti, A.; Romero-Rodríguez, C.; Navarro-Cerrillo, R.M.; Abril, N.; Jorrín-Novo, J.V.; Dodd, R.S. Population genetic diversity of Quercus ilex subsp. ballota (Desf.) Samp. reveals divergence in recent and evolutionary migration rates in the Spanish dehesas. Forests 2018, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Bechtold, U. Plant life in extreme environments: How do you improve drought tolerance? Front. Plant Sci. 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, M.T.; San-José, M.D.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm oak somatic embryogenesis: Current status and future perspectives. Front. Plant Sci. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trugman, A.T.; Anderegg, L.D.; Anderegg, W.R.; Das, A.J.; Stephenson, N.L. Why is tree drought mortality so hard to predict? Trends Ecol. Evol. 2021, 36, 520–532. [Google Scholar] [CrossRef]
- Rey, M.D.; Castillejo, M.Á.; Sánchez-Lucas, R.; Guerrero-Sanchez, V.M.; López-Hidalgo, C.; Romero-Rodríguez, C.; Valero-Galván, J.; Sghaier-Hammami, B.; Simova-Stoilova, L.; Echevarría-Zomeño, S.; et al. Proteomics, holm oak (Quercus ilex L.) and other recalcitrant and orphan forest tree species: How do they see each other? Int. J. Mol. Sci. 2019, 20, 692. [Google Scholar] [CrossRef] [Green Version]
- Escandón, M.; Castillejo, M.Á.; Jorrín-Novo, J.V.; Rey, M.D. Molecular Research on Stress Responses in Quercus spp.: From Classical Biochemistry to Systems Biology through Omics Analysis. Forests 2021, 12, 364. [Google Scholar] [CrossRef]
- Jorge, I.; Navarro, R.M.; Lenz, C.; Ariza, D.; Jorrín, J. Variation in the holm oak leaf proteome at different plant developmental stages, between provenances and in response to drought stress. Proteomics 2006, 6, 207–214. [Google Scholar] [CrossRef]
- Echevarría-Zomeño, S.; Ariza, D.; Jorge, I.; Lenz, C.; Del Campo, A.; Jorrín, J.V.; Navarro, R.M. Changes in the protein profile of Quercus ilex leaves in response to drought stress and recovery. J. Plant Physiol. 2009, 166, 233–245. [Google Scholar] [CrossRef]
- Valero-Galvan, J.; Gonzalez-Fernandez, R.; Navarro-Cerrillo, R.M.; Gil-Pelegrin, E.; Jorrin-Novo, J.V. Physiological and proteomic analyses of drought stress response in Holm oak provenances. J. Proteome Res. 2013, 12, 5110–5123. [Google Scholar] [CrossRef] [PubMed]
- Simova-Stoilova, L.P.; Romero-Rodriguez, M.C.; Sánchez-Lucas, R.; Navarro-Cerrillo, R.M.; Medina-Auñón, A.; Jorrin Novo, J.V. 2-DE proteomics analysis of drought treated seedlings of Quercus ilex supports a root active strategy for metabolic adaptation in response to water shortage. Front. Plant Sci. 2015, 6, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simova-Stoilova, L.P.; López-Hidalgo, C.; Sanchez-Lucas, R.; Valero-Galvan, J.; Romero-Rodríguez, C.; Jorrin-Novo, J.V. Holm oak proteomic response to water limitation at seedling establishment stage reveals specific changes in different plant parts as well as interaction between roots and cotyledons. Plant Sci. 2018, 276, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Sánchez, V.M.; Castillejo, M.Á.; López-Hidalgo, C.; Alconada, A.M.M.; Jorrín-Novo, J.V.; Rey, M.D. Changes in the transcript and protein profiles of Quercus ilex seedlings in response to drought stress. J. Proteom. 2021, 243, 104263. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Menéndez, M.; Jorrin-Novo, J.V. Phytochemical composition and variability in Quercus ilex acorn morphotypes as determined by NIRS and MS-based approaches. Food Chem. 2021, 338, 127803. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Meijón, M.; Feito, I.; Oravec, M.; Delatorre, C.; Weckwerth, W.; Majada, J.; Valledor, L. Exploring natural variation of Pinus pinaster Aiton using metabolomics: Is it possible to identify the region of origin of a pine from its metabolites? Mol. Ecol. 2016, 25, 959–976. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [Green Version]
- Siriwach, R.; Matsuda, F.; Yano, K.; Hirai, M.Y. Drought stress responses in context-specific genome-scale metabolic models of arabidopsis thaliana. Metabolites 2020, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants–biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef]
- Warren, C.R.; Aranda, I.; Cano, F.J. Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics 2012, 8, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; Ribeiro-Barros, A.I.; António, C. Experimental design and sample preparation in forest tree metabolomics. Metabolites 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Zhang, J.; Li, J.; Sun, P.; Zhang, Y.; Xin, X. Genome-wide transcriptomic analysis of a desert willow, Salix psammophila, reveals the function of hub genes SpMDP1 and SpWRKY33 in drought tolerance. BMC Plant Biol. 2019, 19, 365. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Ubach, A.; Barbeta, A.; Sardans, J.; Guenther, A.; Ogaya, R.; Oravec, M.; Urban, O.; Peñuelas, J. Topsoil depth substantially influences the responses to drought of the foliar metabolomes of Mediterranean forests. Perspect. Plant Ecol. Evol. Syst. 2016, 21, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Conrad, A.O.; McPherson, B.A.; Wood, D.L.; Madden, L.V.; Bonello, P. Constitutive phenolic biomarkers identify naïve Quercus agrifolia resistant to Phytophthora ramorum, the causal agent of sudden oak death. Tree Physiol. 2017, 37, 1686–1696. [Google Scholar] [CrossRef]
- Li, Q.; Yan, L.; Ye, L.; Zhou, J.; Zhang, B.; Peng, W.; Zhang, X.; Li, X. Chinese black truffle (Tuber indicum) alters the ectomycorrhizosphere and endoectomycosphere microbiome and metabolic profiles of the host tree Quercus aliena. Front. Microbiol. 2018, 9, 2202. [Google Scholar] [CrossRef] [Green Version]
- Valledor, L.; Escandón, M.; Meijón, M.; Nukarinen, E.; Cañal, M.J.; Weckwerth, W. A universal protocol for the combined isolation of metabolites, DNA, long RNA s, small RNA s, and proteins from plants and microorganisms. Plant J. 2014, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Leiva, M.J.; Fernández-Alés, R. Variability in seedling water status during drought within a Quercus ilex subsp. ballota population, and its relation to seedling morphology. For. Ecol. Manag. 1998, 111, 147–156. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, T.; Pinto, G.; Correia, B.; Gonçalves, S.; Meijón, M.; Escandón, M. In-depth analysis of the Quercus suber metabolome under drought stress and recovery reveals potential key metabolic players. Plant Sci. 2020, 299, 110606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, M.; Zhao, G.; Lu, H.; Zhang, Z.; Zou, C. Chromatographic Profiling with Machine Learning Discriminates the Maturity Grades of Nicotiana tabacum L. leaves. Separations 2021, 8, 9. [Google Scholar] [CrossRef]
- Ullah, N.; Yüce, M.; Neslihan Öztürk Gökçe, Z.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genet. 2017, 18, 969. [Google Scholar] [CrossRef]
- Aranda, I.; Cadahía, E.; Fernández de Simón, B. Specific leaf metabolic changes that underlie adjustment of osmotic potential in response to drought by four Quercus species. Tree Physiol. 2020, 41, 728–743. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Rodrigues, A.M.; Perdiguero, P.; António, C.; Atkin, O.K.; Li, M.; Collada, C.; Gil, L. A molecular approach to drought-induced reduction in leaf CO2 exchange in drought-resistant Quercus ilex. Physiol. Plant. 2018, 162, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Simon, J.; Rennenberg, H. Drought and air warming affect the species-specific levels of stress-related foliar metabolites of three oak species on acidic and calcareous soil. Tree Physiol. 2013, 33, 489–504. [Google Scholar] [CrossRef]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercus rubra. Glob. Change Biol. 2015, 21, 4177–4195. [Google Scholar] [CrossRef]
- Kim, J.J.; Ghimire, B.K.; Shin, H.C.; Lee, K.J.; Song, K.S.; Chung, Y.S. Comparison of phenolic compounds content in indeciduous Quercus species. J. Med. Plant Res. 2012, 6, 5228–5239. [Google Scholar] [CrossRef] [Green Version]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A.; Rocha-Guzmán, N.E. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food. Compost. Anal. 2017, 63, 38–46. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Başyiğit, B.; Sağlam, H.; Köroğlu, K.; Karaaslan, M. Compositional analysis, biological activity, and food protecting ability of ethanolic extract of Quercus infectoria gall. J. Food Process. Preserv. 2020, 44, e14692. [Google Scholar] [CrossRef]
- Mezni, F.; Stiti, B.; Fkiri, S.; Ayari, F.; Slimane, L.B.; Ksouri, R.; Khaldi, A. Phenolic profile and in vitro anti-diabetic activity of acorn from four African Quercus species (Q. suber, Q. canariensis, Q. coccifera and Q. ilex). South Afr. J. Bot. 2022, 146, 771–775. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.E.; Ricardo-da-Silva, J.M.; Pérez-Coello, M.S.; Laureano, O. Evaluation of Portuguese and Spanish Quercus pyrenaica and Castanea sativa species used in cooperage as natural source of phenolic compounds. Eur. Food Res. Technol. 2013, 237, 367–375. [Google Scholar] [CrossRef]
- Zahri, S.; Belloncle, C.; Charrier, F.; Pardon, P.; Quideau, S.; Charrier, B. UV light impact on ellagitannins and wood surface colour of European oak (Quercus petraea and Quercus robur). Appl. Surf. Sci. 2007, 253, 4985–4989. [Google Scholar] [CrossRef]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and biological properties of bioactive phenolic compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Miranda, I.; Sousa, V.; Ferreira, J.; Pereira, H. Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 2017, 12, e0179268. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Gas chromatographic-mass spectrometric analysis of volatile compounds in oak wood used for ageing of wines and spirits. Chromatographia 1998, 47, 427–432. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Świędrych, A.; Lorenc-Kukuła, K.; Skirycz, A.; Szopa, J. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol. Biochem. 2004, 42, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.D.C.; Barbosa de Lima, R.; Dantas dos Santos, W.; Ferrarese-Filho, O. The role of L-DOPA in plants. Plant Signal. Behav. 2014, 9, 28275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winstel, D.; Gautier, E.; Marchal, A. Role of oak coumarins in the taste of wines and spirits: Identification, quantitation, and sensory contribution through perceptive interactions. J. Agric. Food Chem. 2020, 68, 7434–7443. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.N.; de Toledo Picoli, E.A.; de Souza, G.A.; Farag, M.A.; Scotti, M.T.; Filho, J.M.B.; Da Silva, M.S.; Tavares, J.F. Phenolics metabolism provides a tool for screening drought tolerant Eucalyptus grandis hybrids. Aust. J. Crop Sci. 2017, 11, 1016–1024. [Google Scholar] [CrossRef]
- Simões, R.; Rodrigues, A.; Ferreira-Dias, S.; Miranda, I.; Pereira, H. Chemical composition of cuticular waxes and pigments and morphology of leaves of Quercus suber trees of different Provenance. Plants 2020, 9, 1165. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2007, 131, 211–225. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [Green Version]
- Haberstroh, S.; Kreuzwieser, J.; Lobo-do-Vale, R.; Caldeira, M.C.; Dubbert, M.; Werner, C. Terpenoid emissions of two Mediterranean woody species in response to drought stress. Front. Plant Sci. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2017, 42, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2021, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Nazeam, J.A.; Al-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef]
- Wu, Z.C.; Zhang, J.Q.; Zhao, J.T.; Li, J.G.; Huang, X.M.; Wang, H.C. Biosynthesis of quebrachitol, a transportable photosynthate, in Litchi chinensis. J. Exp. Bot. 2018, 69, 1649–1661. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Moncaleán, P.; Pereira, C.; Pěnčík, A.; Petřík, I.; Pavlović, I.; Novák, O.; Strnad, M.; Goicoa, T.; Ugarte, M.D.; et al. Cytokinins are involved in drought tolerance of Pinus radiata plants originating from embryonal masses induced at high temperatures. Tree Physiol. 2021, 41, 912–926. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Fiorani, F.; Brzobohatý, B.; Černý, M.; Spichal, L.; et al. Cytokinins: Their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Jin, X.; Yang, Y.; Lin, Z.; Zhang, Y. The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul. 2014, 72, 269–277. [Google Scholar] [CrossRef]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, B.; Mendes, P. Bioinformatics approaches to integrate metabolomics and other systems biology data. In Plant Metabolomics; Saito, K., Dixon, R.A., Willmitzer, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 57, pp. 105–115. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Yao, H.; Peng, X.; Wang, R.; Li, F.; Wang, Z.; Zhao, M.; Jin, L. Overexpression of Chalcone Synthase Improves Flavonoid Accumulation and Drought Tolerance in Tobacco. Preprints 2019, 2019060103. [Google Scholar] [CrossRef]
- Ahmed, U.; Rao, M.J.; Qi, C.; Xie, Q.; Noushahi, H.A.; Yaseen, M.; Shi, X.; Zheng, B. Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules 2021, 26, 5546. [Google Scholar] [CrossRef]
- San-Eufrasio, B.; Bigatton, E.D.; Guerrero-Sánchez, V.M.; Chaturvedi, P.; Jorrín-Novo, J.V.; Rey, M.D.; Castillejo, M.Á. Proteomics data analysis for the identification of proteins and derived proteotypic peptides of potential use as putative drought tolerance markers for Quercus ilex. Int. J. Mol. Sci. 2021, 22, 3191. [Google Scholar] [CrossRef]
- Li, J.; Zhao, A.; Yu, M.; Li, Y.; Liu, X.; Chen, X. Function analysis of anthocyanidin synthase from Morus alba L. by expression in bacteria and tobacco. Electron. J. Biotechnol. 2018, 36, 9–14. [Google Scholar] [CrossRef]
- Kubra, G.; Khan, M.; Munir, F.; Gul, A.; Shah, T.; Hussain, A.; Caparrós-Ruiz, D.; Amir, R. Expression characterization of flavonoid biosynthetic pathway genes and transcription factors in peanut under water deficit conditions. Front. Plant Sci. 2021, 12, 1140. [Google Scholar] [CrossRef]
- Singh, K.; Rani, A.; Paul, A.; Dutt, S.; Joshi, R.; Gulati, A.; Ahuja, P.S.; Kumar, S. Differential display mediated cloning of anthocyanidin reductase gene from tea (Camellia sinensis) and its relationship with the concentration of epicatechins. Tree Physiol. 2009, 29, 837–846. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.S.; Zhang, C.; Kurjogi, M.M.; Pervaiz, T.; Zheng, T.; Zhang, C.; Lide, C.; Shangguan, L.; Fang, J. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci. Rep. 2017, 7, 13134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Xin, H.; Wang, M.; Ma, Q.; Wang, L.; Kaleri, N.A.; Wang, Y.; Li, X. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).