Abstract
Forest stand density has been shown to have different, albeit small, effects on soil carbon. We hypothesized that the absence of a density effect on soil carbon (C) storage could be explained by a loss of old soil C. This replacement of old by fresh C could result in zero net C sequestration by soils but could also alter the quality of the soil organic matter. We used one afforestation experiment in Siberia, in which three tree species (spruce, larch and Scots pine) have been grown for the last 30 years at 18 levels of stand density, ranging originally from 500 to 125,000 stems per ha. We selected five density levels and studied the C and nitrogen (N) contents in mineral soils at 0–5 cm depth. The age of the soil C was measured under larch and spruce for three levels of density by radiocarbon (14C) dating. In all soil samples, we determined the stability of the soil organic matter (SOM) by assessing two indices: C decomposability (mineralization of C per unit of soil C) and primability (susceptibility of the SOM to microbial priming). The stand density affected the soil C and N contents differently depending on the tree species. Only under spruce did both the C and N contents increase with density; under larch and pine, the covariation was insignificant and N even tended to decline with a density increase. With the 14C data, we were able to show the strong dilution of old SOM by fresh C derived from the trees; the effect was stronger with a higher density. This provides the first evidence that a density increase increases the fractions of new C versus old C and this can happen without altering the total C contents such as under larch. Although the stand density altered the soil C and N contents only under spruce, it altered C decomposability under all tree species; with a density increase, the C decomposability declined under spruce but increased under larch and pine. This is relevant to predicting C losses from forest soils with different tree species and densities. Higher C losses would occur under larch and pine with higher densities but under spruce, a density increase would reduce the losses of C from the soil. Furthermore, although no significant covariation of stand density with C primability was detected, we first observed strong tree species effects on C primability. Twice as much C was lost from the soil under larch than under spruce or pine by an equal addition of C-glucose. This indicated that elevated C deposition from roots and exudates to the soil as predicted due to an elevated CO2 concentration would most strongly accelerate the soil C turnover and C losses under larch than under spruce and Scots pine. Overall, the tree species altered the susceptibility of the soil C to an elevated C input and the stand density had a strong effect on the decomposability of the SOM, which is an important parameter of C stability. The effect of stand density is, therefore, important to consider even if the stand density does not affect the total soil C.
1. Introduction
Forest soils store large amounts of soil organic matter, which plays a crucial role in forest ecosystems including nutrient provision, carbon sequestration, water regulation, soil structuring and biodiversity promotion. Trees are the main source of SOM in soils through above- and below-ground litter inputs. It is surprising that the effects of stand density and thinning on soil C stocks are not consistent among the published studies and are often small [1]. Forest stands planted with a high density of stems generally have a larger basal area than low-density stands. High-density stands should, therefore, have higher C inputs into the soil than low-density stands, which should also increase their soil C stocks compared with low-density stands [2]. A meta-analysis showed, however, that afforested plantations with a high density (>1600 stems ha−1; 53 observations) did not have greater soil C stocks (mean depth: 0–26 cm) compared with plantations with a low planting density (<1600 stems ha−1; 55 observations) over 21 years [2]. Recent case studies, on the other hand, show contrasting results regarding the stand density effect on the soil C stocks. For instance, case studies in China (Fraxinus mandshurica plantations [3] and Cunninghamia lanceolata plantations [4]) and in Canada (Populus plantations [5]) showed increased soil C stocks (or contents for [4]) in high-density afforested stands compared with low-density afforested stands 10 to 17 years after planting. Conversely, no clear effects of the stand density on the soil C stocks were detected eight years after a plantation establishment in a case study in Uruguay (Eucalyptus and Pinus plantations [6]). Another study of 103 Quercus stands in Spain suggested a positive tree density effect on the soil C stocks [7]. Conversely, lower soil C stocks in high-density stands (842 stems ha−1) than in low-density stands (450 trees ha−1) were observed in a mature (65- to 75-year-old) Pinus densiflora forest in South Korea [8]. Finally, no stand density effect on soil C stocks was observed in mature Douglas fir (Pseudotsuga menziesii) plantations in France [9].
The thinning of forests, in which a portion of the stems is removed, would be expected to reduce the soil C stocks due to a reduced litter input and/or increased rates of decomposition because of higher temperatures and moisture [10]. However, most studies on the effects of stand thinning on soil C stocks have reported no significant effects on the soil C stocks of mineral soil [11,12,13,14,15,16,17,18,19,20,21,22] although a few have documented soil C losses [23,24,25,26] even in deeper soil horizons (down to 1 m depth) [27]. A recent meta-analysis including 53 studies did not find a remarkable thinning effect on the soil C stocks although the soil CO2 efflux increased by almost 30% [28]. Similarly, an earlier meta-analysis [18] based on 28 observations (11 studies) found no significant effects of partial cutting (compared with uncut controls) on the soil C stocks.
Although many studies have addressed tree species effects on the forest floor C dynamics or mineral soil and forest floor C pools [29,30,31,32], they provide limited information on the “stability” of these pools, particularly in the mineral soil where C can persist for centuries through the association of soil organic matter (SOM) with minerals or the occlusion of SOM within aggregates [33]. Tree species effects on the stability of SOM have mostly been deduced from ex situ estimates of CO2 efflux from the soil by heterotrophic respiration [34]. This measure mainly provides information on the amount and stability of C in the SOM that is not protected by the soil mineral matrix via aggregation or other physicochemical interactions with the soil minerals [35].
Planting trees could dilute old soil C by adding new C. Even if net changes in the soil C are small due to the mineralization of the old soil C, the addition of newly derived C from trees to the soil could cause the total soil C to be qualitatively different. This is important to SOM stability and future soil carbon losses because the response of the soil heterotrophic respiration to the altered temperature is largely determined by the quality and availability of the SOM; thus, the tree species may determine the soil CO2 flux response to the temperature increase without affecting the net C content in the soil. The susceptibility of the SOM to microbial priming (primability) might also depend on the tree species and stand density. This is potentially important because priming influences the oxidation of the old soil carbon reservoirs in deep soil. In many cases, priming has been sufficiently large to induce soil carbon losses in response to long-term CO2 enrichment, influencing the total carbon balance response of the system [36,37]. In these cases, the priming effect is a major modulator of the net carbon balance response. Thus, the priming effect is now acknowledged to be sufficiently large to warrant its inclusion in global models of carbon cycling [38,39]. Unfortunately, the controls over priming are extremely difficult to discern. In particular, the effects of plant species on soil priming are very poorly understood. The goal of this work was to discern the effects of three individual Siberian tree species and stand densities on the contents and stability of the SOM. We show here that the stand density affects the decomposability of the soil C to a greater effect than the soil C contents. This implies that the effect of stand density is important to consider even if the stand density does not affect the total soil C. Moreover, we demonstrate for the first time strong tree species effects on the primability of the soil C; this is important for predicting the soil C response to global climate change under different tree species.
2. Materials and Methods
2.1. Research Sites and Experimental Setup
The afforestation experiment with varied stand densities was established by the idea and under the supervision of Dr. Aleksey Buzykin in the southern taiga zone, 135 km north of Krasnoyarsk. An 18 ha area was selected for the uniformity of the gray forest soil, which was at the time managed as pasture. In 1982, 2-year-old seedlings of Scots pine (Pinus sylvestries), larch (Larix gmelini) and spruce (Picea abies) were planted, each at 18 different densities ranging from 500 to 128,000 trees per ha [40]. Due to density-dependent self-mortality, the current stocking density as determined in 2016 had declined and ranged from 400 to 19,000 trees per ha. An intensive forest survey was performed in 2013–2016 and included the stem diameter, stem height, base cone height, crown architecture and tree mortality. Overall, all morphological characteristics except the tree height were strongly affected by the stocking density. We selected five density levels for each tree species, capturing the full range of densities but not sampling all of them (Table 1). We collected mineral soil samples (0–10 cm depth) with composite samples generated from 5–10 collections along a transect between 2 neighboring trees at fixed intervals. Three transects were sampled at each plot and this resulted in three mixed soil samples that served as replicates. The experimental design included pseudo-replications in the definition of [41]; therefore, all conclusions regarding the significant differences were produced under the assumption that there were no other factors at the area of the experiment that affected the soil properties greater than the tree species and densities.

Table 1.
The initial and current density levels of five selected plots with three tree species.
2.2. Soil Sample Preparation and Analysis
Each soil sample was divided into two subsamples; one was air-dried at room temperature for further incubation studies and the other was used for the C and N determination. For this, the samples were dried at 60 °C over 1–2 weeks and then ground with a mechanical mill (40 mesh). Samples of approximately 15–20 mg of soil were weighed for the analysis. The total C and N contents were measured with an online C/N analyzer (Carlo Erba). Subsamples from each of the three replicates and from three plots with spruce and larch were mixed and sent to the Arizona AMS laboratory for 14C dating.
2.3. Determining the Priming of Soil Organic Matter and C Decomposability
For each sample, we added 40 g (dry weight) of soil to specimen cups (120 mL) and adjusted the soil moisture content to 70% of the water holding capacity. The specimen cups were placed in mason jars (473 mL) for one week of preincubation (22 °C) to allow for a recovery from any physical disturbance of the soils prior to the incubation. After preincubation, the weekly substrate addition treatments began. One set of soils received only deionized water (control) and the treatment samples received 13C-labeled glucose. We determined priming using an isotope mass balance, introducing an artificially high 13C tracer with the substrate additions. To achieve the 13C signal, universally labeled 13C-glucose (D-Glucose-13C, 97 atom%; Cambridge Isotope Laboratories, Tewksbury, MA, USA) was added to natural abundance glucose such that the added substrate had a δ13C signature of 1357‰. Most priming studies apply substrates in a single addition; however, repeated substrate additions have been proposed to better represent the substrate inputs through root exudation in the field [42,43,44]. A single substrate addition has also been reported to induce stronger priming than multiple additions, possibly overestimating the priming effect [44,45]. For these reasons, we elected to use multiple substrate additions (one per week) over the 5-week period. Each treatment had three replicates and all soils were incubated under the same conditions as the preincubation. Each week, we sealed the jars to measure the CO2 fluxes during three measurement periods: 0–2 days, 2–5 days and 5–7 days after the weekly substrate addition [43]. These periods were short enough that jars did not become anoxic. After each sampling, the jars were opened to room air before resealing for the next measurement interval. A 20 mL gas sample was taken for the CO2 concentration with a gas-tight syringe. For the 13C-CO2 values, a 20 mL gas sample was injected into a Picarro 2131 isotope spectrometer interfaced with a Picarro Small Sample Introduction Module (SSIM) and 16-port Multiplexer, allowing the automatic processing of up to 8 syringes in 1 cycle. CO2-free air was used to dilute the gas samples when the CO2 concentrations were greater than 2000 ppm. Labeled glucose was added at 1000 μg C g−1 every week in a 1 mL deionized water solution; only 1 mL deionized water was added to the control samples.
2.4. Priming Calculation
The mass balance was used to separate the SOM-derived CO2 from the glucose-derived CO2 (Equation (1)) and priming was the difference of the SOM-derived CO2 between the glucose-amended and control samples (Equation (2)):
where CSOM-control is CO2-C (mg g−1) from the control, CSOM-glucose and Ctotal are CO2-C (mg g−1) derived from the SOM and glucose in the glucose-amended samples, δtotal and δglucose are the δ13C of CO2 from the glucose-amended samples and glucose solution (1357‰) and δSOM-control is the δ13C of CO2 from the control samples. We presented the relative priming (%) estimated as:
CSOM-glucose = Ctotal (ẟtotal − ẟglucose)/(ẟSOM-control − ẟglucose)
Priming = CSOM-glucose − CSOM-control
Relative priming = Priming (mg g−1)/CSOM-control (mg g−1) × 100%.
Finally, the soil C decomposability was estimated by dividing the amount of CO2 emitted over the incubation time from the control sample by the soil C contents in the given sample:
Soil C decomposability = CSOM-control (mg g−1)/soil C (mg g−1).
2.5. Statistical Data Analysis
All measurements were performed in duplicate for each soil sample and the mean values of the duplicates from each soil sample were used for the statistics. All parameters were tested for a normality of distribution and a homogeneity of variance with Kolmogorov–Smirnov and Levene’s tests, respectively. The main effects of the tree species and stand density were determined by a two-way analysis of variance (ANOVA) with three replicates (three mixed soil samples). The independent factors were the tree species (three levels) and stand density (five levels); the dependent variables were the C and N contents. We considered the effect significant at p < 0.050. When the interaction of the species versus the density was significant, a one-way ANOVA was performed for each tree species separately to determine if the main effect of the stand density was significant. In the two-way ANOVA, and only when the main effects were significant, post-hoc comparisons with the Tukey honest significant difference (HSD) test were performed to discern under which species and at what density level the studied soil parameters were different. A power function was computed to determine the links between the C and N contents and soil age, C decomposability and stand density. The effects of the stand density and tree species on the amount of CO2 produced in an incubation experiment and on the relative priming were also studied with a two-way ANOVA. All statistics were carried out with the statistical package STATISTICA (7.0 for Windows [46]).
3. Results
Both the C and N contents were strongly affected by the tree species and stand density and the effects were highly interactive (Table 2). The highest soil C content was under spruce; it differed from larch (p = 0.013) and Scots pine (p = 0.040). Spruce also increased the soil N contents compared with larch (p = 0.046) and Scots pine (p = 0.031). Scots pine and larch were not different in the soil C or N contents.

Table 2.
The results (p-values) of the two-way ANOVA with tree species and stand densities as main factors and their interactions (n = 3). ND: not determined because of the lack of interactions between the tree species versus density. Significant values (p < 0.050) are highlighted in bold.
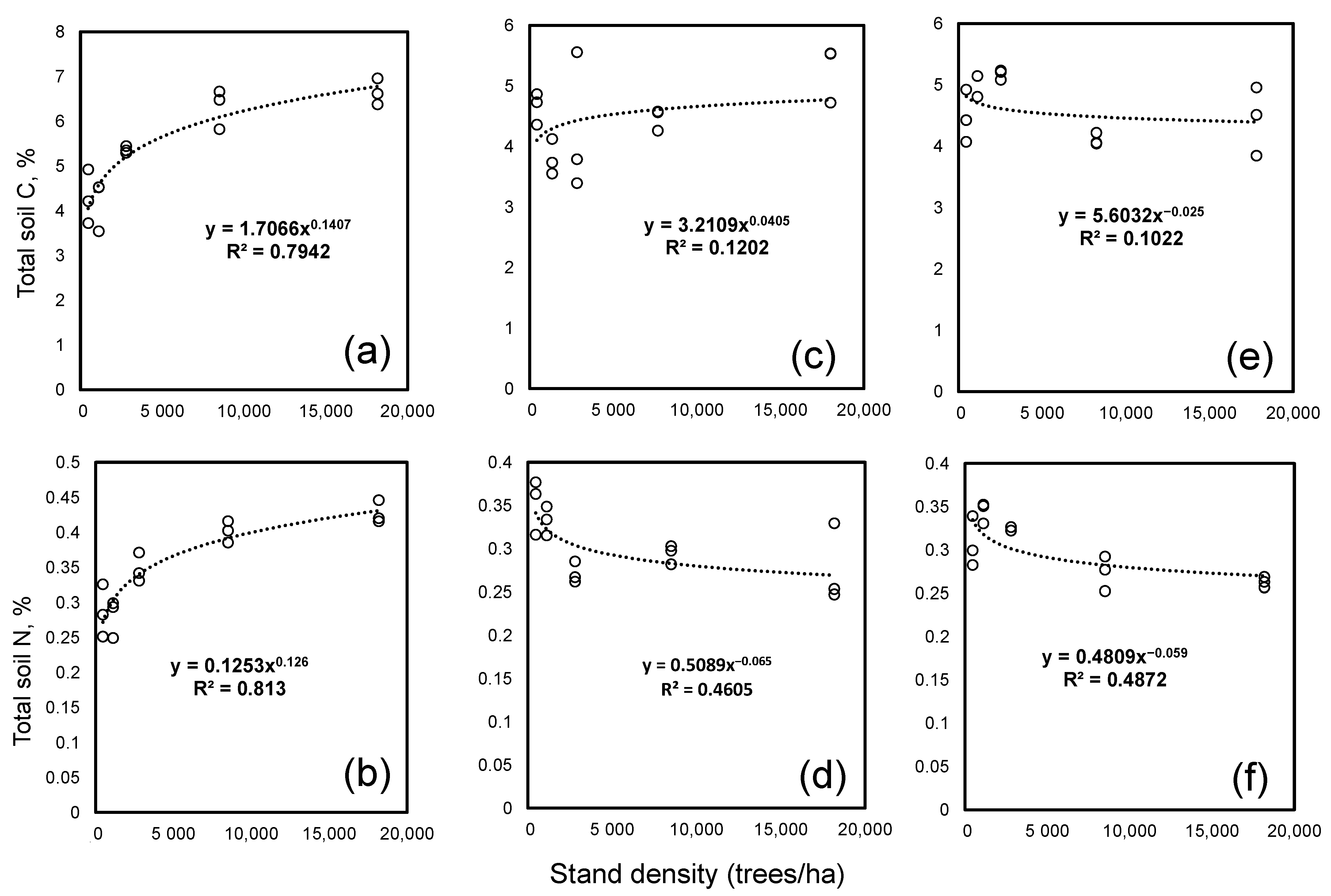
The dependency of the stand density effects on the C and N contents from the tree species is illustrated on Figure 1. It could be seen that a clear positive effect of the density on the soil C and N contents was detectable only under spruce. Here, the soil C and N contents increased with the stand densities. This supported the hypothesis that an increasing stand density increases the soil C sequestration. In contrast, no effect of the stand density was significant for the C content in soils under larch but it was significant for the N content (Table 2). Under pine, the main effect of the density was significant for both C and N (Table 2) but the concentrations of both elements increased up to 2523 stems per ha and declined when the density was higher (Figure 2).
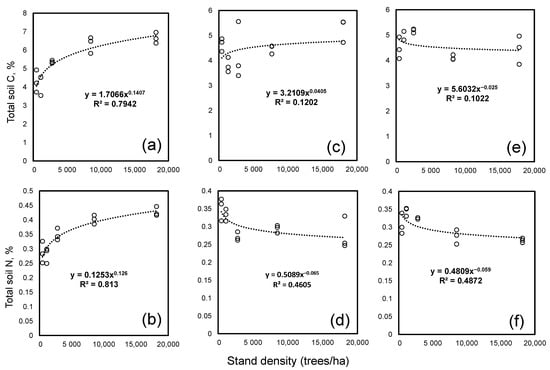
Figure 1.
Soil C and N contents under spruce (a,b), larch (c,d) and Scots pine (e,f) at five levels of density. For each species and density level we had three replicates, which are shown.
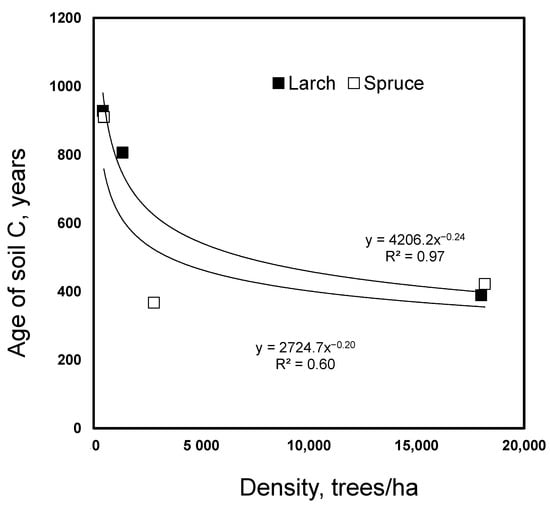
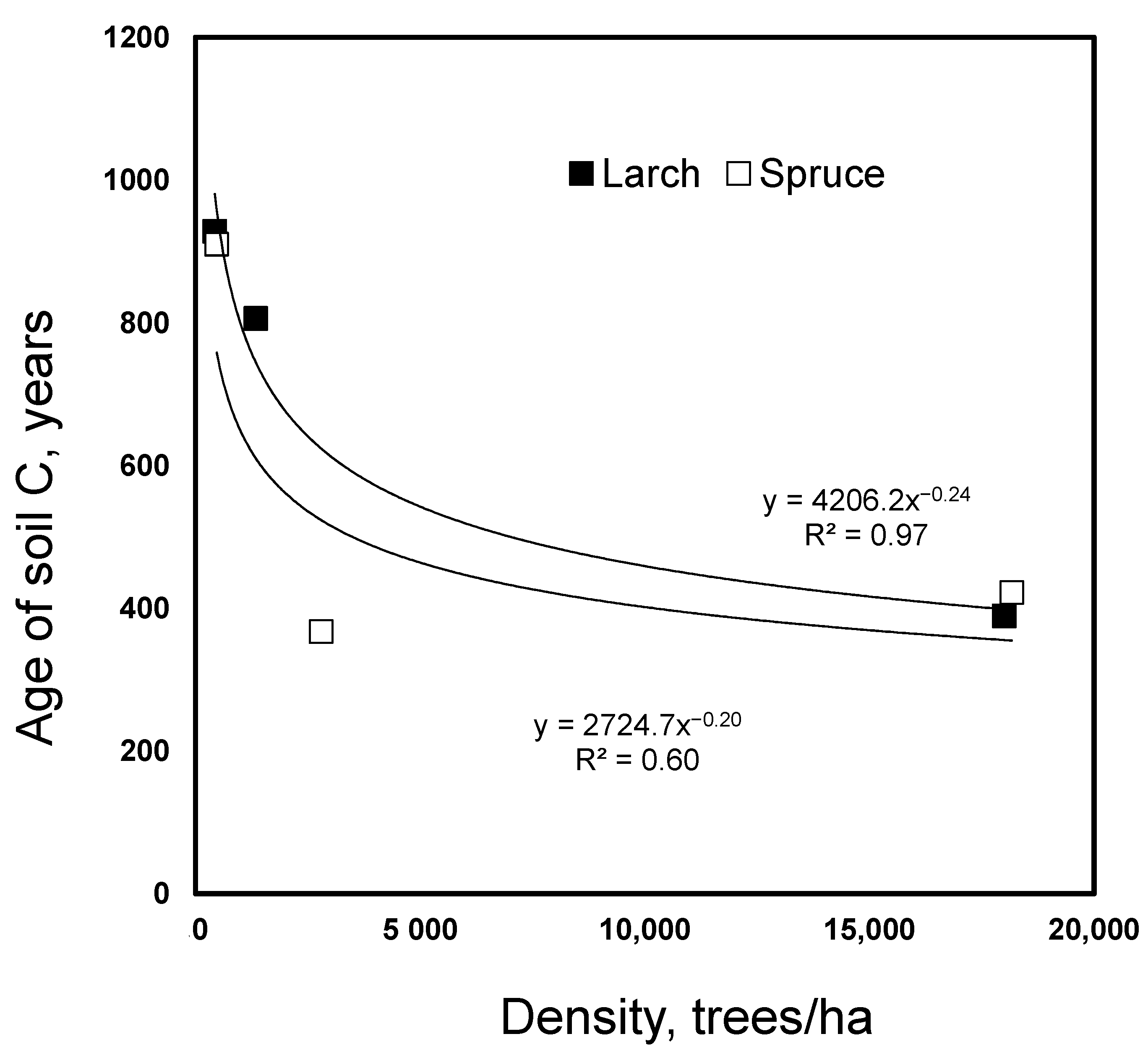
Figure 2.
Dependency of age of soil carbon on stand density for spruce and larch.
A covariation analysis revealed a significant (p < 0.050) relationship between the stand density and the soil C and N contents only under spruce; the stand density there explained 83 and 84% of the variation in the soil C and N (Figure 2), respectively. Under the other two tree species, the density explained less than 20% of the variation for either element. Both the C and N contents under spruce were related to the stand density through the power function with the exponents near to 1/8. The exponents for C were 0.13 with the upper and lowest values within 95% confidence of 0.17 and 0.10. The exponent for N was 0.125, an exact 1/8, with a 95% confidence interval between 0.09–0.15. Thus, the significant accumulation of C and N with an increasing forest stand density was observed only for spruce.
The age of the soil C, determined with 14C, declined with an increasing stand density under both spruce and larch from 900–980 to 350–390 years (Figure 2). As the density increase caused a soil C increase only under spruce, a similar decline in the proportion of old soil organic C under larch indicated that fresh C entered the soil without a net C accumulation.
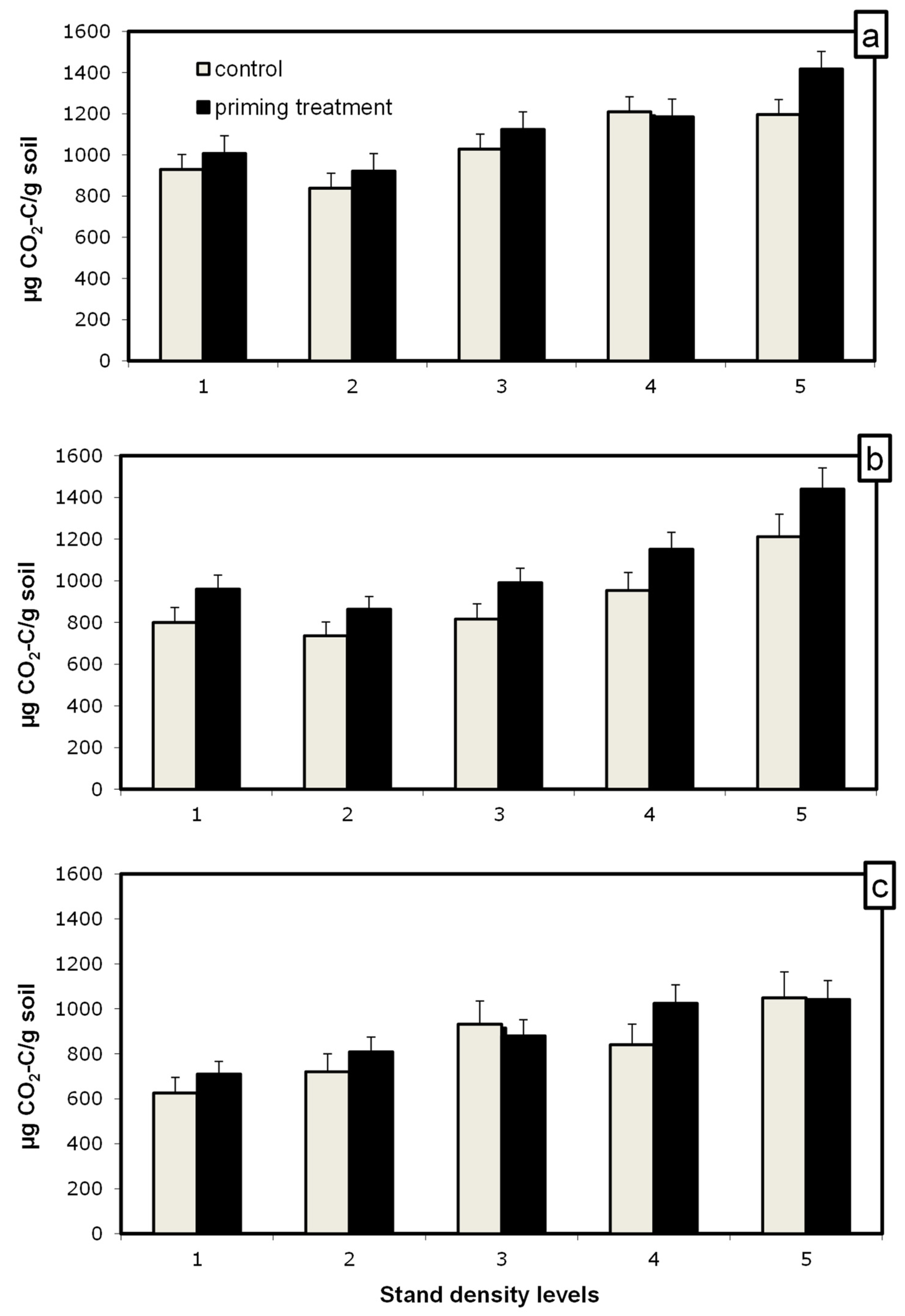
The tree species respired a similar amount of C-CO2 (Figure 3); the main effect of the tree species was not significant (p > 0.050). The stand density affected the amount of CO2 produced; more CO2 was emitted with a higher density of all tree species. This could be attributed to higher C contents in high-density stands by spruce. Under larch and Scots pine, a higher density mineralization rate could be due to other factors.
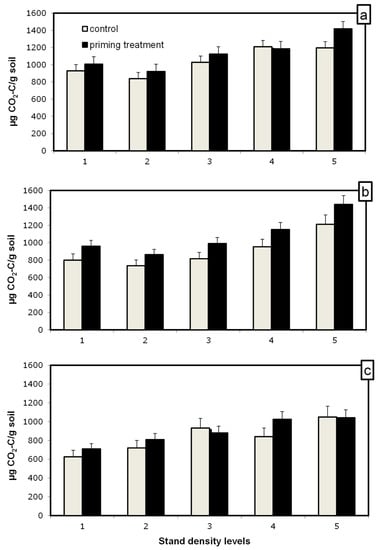
Figure 3.
Amount of C-CO2 produced from mineralization of SOM during 5 weeks of incubation of soil samples with glucose addition for priming estimation (black bars) and without (grey bars) for spruce (a), larch (b) and Scots pine (c). The difference between the black and grey bars indicates the priming effect. Bars represent mean values ± SE, n = 3.
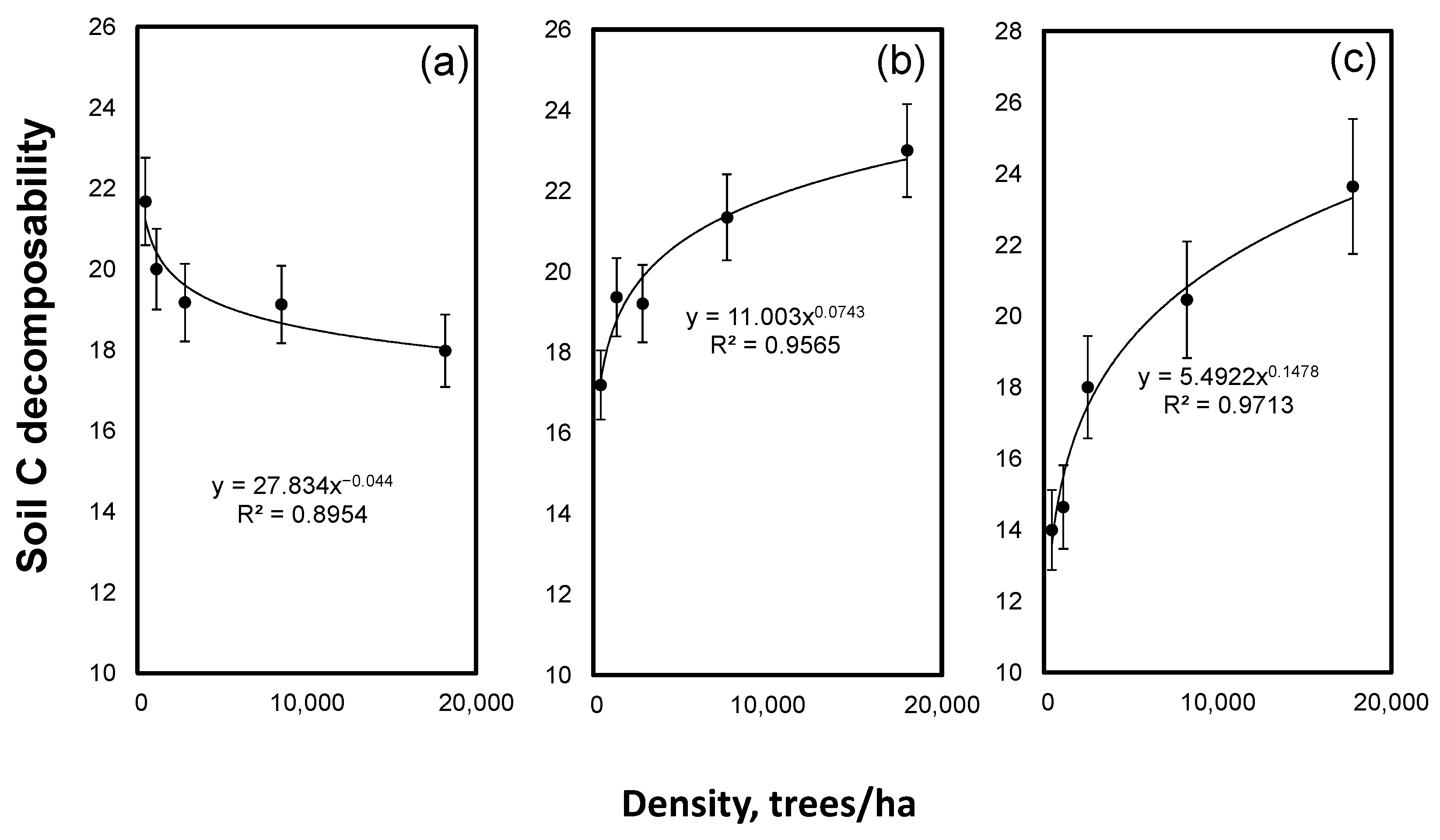
The estimated C decomposability showed a strong interaction between the density and tree species. The density declined the soil C decomposability under spruce whereas an increase in the density increased the decomposability of the soil C under larch and Scots pine (Figure 4).
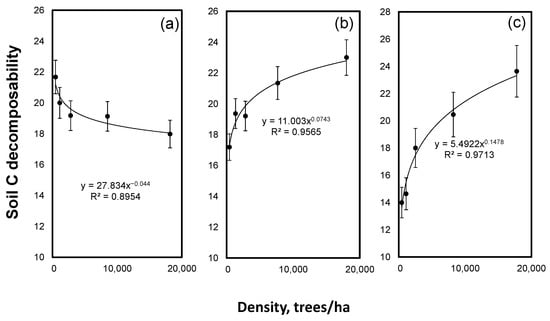
Figure 4.
Soil C decomposability versus forest stand density for spruce (a), larch (b) and Scots pine (c). Mean values ± SE, n = 3.
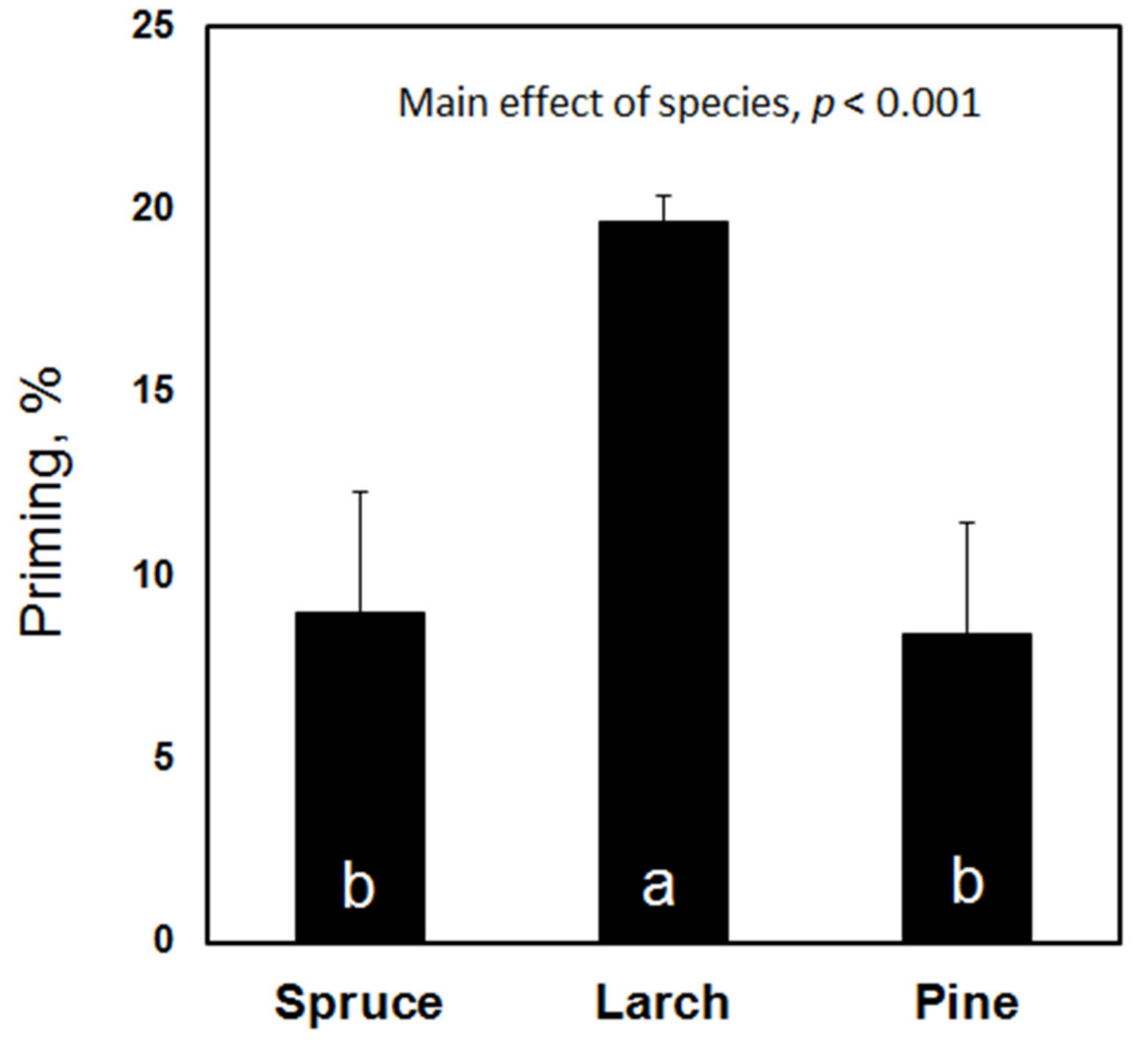
In contrast to the decomposability, the priming effect was not altered by the stand density but it was strongly affected by the tree species (Figure 5). The highest priming was measured in the soil samples under larch; the addition of easily degradable C increased the mineralization of the native SOM by almost 20%. The priming effects under spruce and Scots pine were significantly lower (for both, p < 0.001) than under larch with the mean values reaching only 8–9%.
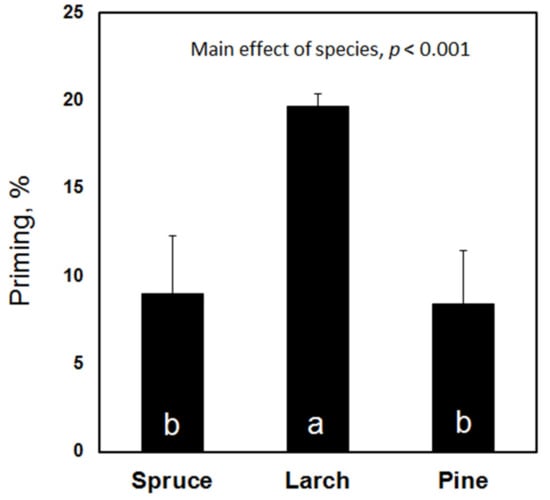
Figure 5.
Priming effect and relative increase in C mineralization rate in the presence of easily degradable C. Similar letters within the bars indicate no significant difference.
4. Discussion
Our work aimed to elucidate the interactions between the tree species and stand density effects on the soil C sequestration and stability of the SOM. We used two indices of C stability: (a) soil C decomposability estimated as the C mineralization rate per unit of soil C; and (b) the susceptibility of soil C to microbial priming. The major findings of our work were: (a) the stand density strongly altered the decomposability of the soil C but the direction of change depended on the tree species; (b) the stand density had no impact on the primability of the SOM but the tree species affected the susceptibility of the SOM to microbial priming; and finally (c) radiocarbon dating revealed a strong impact of the stand density on the soil C even when no changes in the C contents were observed.
A literature review suggested that the effect of the tree species has been studied to a greater extent than that of the stand density [29,30,31,32,47,48]. Although the thinning of forest stands is a major operation in forest management, the discussion of forest management effects on the soil C sequestration has been predominantly centered on the changes of the C stocks in soils [12]. Only recently have researchers started to consider the tree species effects on the stability of the SOM in soils [49]. For instance, using a 40-year-old common garden experiment with replicated plots of eleven temperate tree species, researchers measured five SOM stability indices including heterotrophic respiration, C in aggregate occluded particulate organic matter (POM) and mineral-associated SOM and bulk SOM δ15N and Δ 14C [49]. The stability of the SOM varied substantially among the tree species and this variability was independent of the amount of organic C in the soils. Thus, when considering forest soils as C sinks, the stability of the C stocks must be considered in addition to their size. The effect of the stand density on the stability of the soil C has previously not been considered. We concentrated on the SOM changes in the mineral soil because: (a) the tree species and density effect on the litter layer accumulation was relatively better established [50]; and (b) the litter C can easily be lost through fires and other disturbances so mineral soil C is more likely to be a key reservoir for long-term C storage [12].
We found a strong and positive effect of the stand density on the soil C and N contents only under spruce (Figure 1). The stand density explained the high (83–84%) variability in the soil C and N contents. An accumulation of C in the soil is a balance between a fresh C deposition and C losses. The C deposition to mineral soil is mostly determined by the below-ground NPP, which is positively related to the density of the stand [51]. The effect of the stand density on the C mineralization is less known but we have shown that an increase in the stand density under spruce declined the decomposability of the soil C (Figure 4). A higher C and N accumulation in the soils under a high-density treatment in spruce covariated with a lower degradability of the SOM. This is new but the mechanistic explanation of the cause and effects needs further investigation. Nevertheless, the observed correlation of the stand density and C contents suggested that the C accumulation under spruce was constrained by the C deposition rather than by C losses. This was in line with the observations that, among boreal and temperate forest tree species, spruce is the champion of soil C sequestration [52,53,54]. By contrast, in the soils under Scots pine and larch, the density had no or very small effects if not all density levels were considered on the C and N contents (Figure 1). In the Scots pine soil, the C and N contents were even slightly lower at a higher density, indicating that the C mineralization exceeded the C deposition. Thus, the stand density effects on the soil C and N depended on the tree species and the interpretation of the results from the afforestation experiments must take into account the differences in the stand densities.
Despite the lack of a stand density effect on the soil C and N under larch, a strong effect of the density was observed on the age of the soil C, as evidenced by radiocarbon dating (Figure 2). The effect was as strong as for spruce where a significant accumulation of C was found in the high-density treatments. The observed decline in the age of the soil C from 900 to 450 years with a density increase under larch indicated a strong dilution of the old soil C by fresh C derived from the trees. As the stand density increase in larch did not increase the soil C content, large losses of the old soil C seem to be a plausible explanation for the observed density effect on the soil C age.
If half of the old soil C, present in the soil before afforestation, was substituted by new tree-derived C, a profound change in the stability of the SOM could be expected. This was tested by the incubation study that demonstrated that the C decomposability increased with a density increase under Scots pine and larch and decreased under spruce. Although the stand density did not affect priming, the tree species altered the susceptibility of the SOM to microbial priming with an acceleration of SOM mineralization under an elevated C input. The strongest priming was observed under larch, the most dominant tree species in Eurasian boreal forests. The soil under larch lost two times more C than the soils under Scots pine and spruce under an elevated C input. Global climate change alters the tree species composition worldwide [55,56]. Several scenarios predict a shift in the species composition in Russian boreal forests [57]. If larch forests, as predicted, are partially replaced by spruce and Scots pine [58], less carbon will be lost from the soils due to priming, providing negative feedback to global climate change.
Overall, we demonstrated that the stand densities affected the soil C accumulation differently depending on the tree species. Species where no extra accumulation of either C or N due to an increased stand density was found still altered the stability of the soil C, a very important parameter for predicting the future changes in soil C in response to global changes. To our knowledge, this work is the first to demonstrate a species-dependent effect of stand density on the decomposability of soil C and the first to demonstrate tree species effects on the susceptibility of SOM to microbial priming.
5. Conclusions
We have shown that the soil C content is not always affected by the stand density but the soil stability almost certainly is. The decomposability of the soil C increased with a density increase under spruce and declined under larch and Scots pine. This warrants a further intensification of the research on the forest stand density effects on soil C, especially when changes in C contents are minor. The high priming effect under larch is also highly relevant for explaining the current and future roles of the Russian boreal forests dominated by larch in the global C cycle. Overall, our results provide a strong reason for including the forest stand density together with the tree species into global biogeochemical models predicting soil C responses to altered environmental conditions.
Author Contributions
Conceptualization, O.V.M. and C.-H.C.; methodology, M.I.M. and R.S.S.; formal analysis, O.V.M.; investigation, O.V.M. and M.I.M.; data curation, R.S.S. and C.-H.C.; writing—original draft preparation, O.V.M.; writing—review and editing, M.I.M. and C.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (RFBR, grant numbers 18-54-52005 and 19-29-05122) and by the Ministry of Science and Technology (Taiwan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118–127. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Sun, X.; Sun, H.; Wang, W.; Razaq, M. Effects of stand density on soil organic carbon storage in the top and deep soil layers of Fraxinus mandshurica plantations. Austrian J. For. Sci. 2019, 136, 27–44. [Google Scholar]
- Farooq, T.; Ma, X.; Rashid, M.; Wu, W.; Xu, J.; Tarin, M.; He, Z.; Wu, P. Impact of stand density on soil quality in Chinese fir (Cunninghamia lanceolate) monoculture. Appl. Ecol. Environ. Res. 2019, 17, 3553–3566. [Google Scholar] [CrossRef]
- Truax, B.; Fortier, J.; Gagnon, D.; Lambert, F. Planting density and site effects on stem dimensions, stand productivity, biomass partitioning, carbon stocks and soil nutrient supply in hybrid poplar plantations. Forests 2018, 9, 293. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Vance, E.D.; Califra, Á.; Del Giorgio, F.; Martínez, L.; González-Barrios, P. Eucalyptus and Pinus stand density effects on soil carbon sequestration. For. Ecol. Manag. 2016, 368, 28–38. [Google Scholar] [CrossRef]
- González, I.G.; Corbí, J.G.; Cancio, A.F.; Ballesta, R.J.; Cascón, M.G. Soil carbon stocks and soil solution chemistry in Quercus ilex stands in Mainland Spain. Eur. J. For. Res. 2012, 131, 1653–1667. [Google Scholar] [CrossRef]
- Noh, N.J.; Kim, C.; Bae, S.W.; Lee, W.K.; Yoon, T.K.; Muraoka, H.; Son, Y. Carbon and nitrogen dynamics in a Pinus densiflora forest with low and high stand densities. J. Plant Ecol. 2013, 6, 368–379. [Google Scholar] [CrossRef]
- Cécillon, L.; Soucémarianadin, L.N.; Berthelot, A.; Duverger, M.; De Boisseson, J.M.; Gosselin, F.; Guenet, B.; Barthès, B.; De Danieli, S.; Barrier, R.; et al. piCaSo: Pilotage sylvicole et contrôle pédologique des stocks de carbone des sols forestiers. Rep. ADEME 2017, 103. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85082819757&origin=inward&txGid=f5b2dbd15c3d3d529b5291c5fe536486 (accessed on 30 January 2022).
- Vesterdal, L.; Dalsgaard, M.; Felby, C.; Raulund-Rasmussen, K.; Jørgensen, B.B. Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For. Ecol. Manag. 1995, 77, 1–10. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Stupak, I.; Vesterdal, L. Distribution of biomass and carbon in even-aged stands of Norway spruce (Picea abies (L.) Karst.): A case study on spacing and thinning effects in northern Denmark. Scand. J. For. Res. 2006, 21, 470–488. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Hoover, C.M. Management impacts on forest floor and soil organic carbon in northern temperate forests of the US. Carbon Balance Manag. 2011, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.; Kolka, R.; Palik, B.; McDonald, R.; Jurgensen, M. Long-term management impacts on carbon storage in Lake States forests. For. Ecol. Manag. 2011, 262, 424–431. [Google Scholar] [CrossRef]
- Jurgensen, M.; Tarpey, R.; Pickens, J.; Kolka, R.; Palik, B. Long-term Effect of Silvicultural Thinnings on Soil Carbon and Nitrogen Pools. Soil Sci. Soc. Am. J. 2012, 76, 1418–1425. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Wu, T. Effect of forest structural change on carbon storage in a coastal Metasequoia glyptostroboides stand. Sci. World J. 2013, 2013, 830509. [Google Scholar] [CrossRef]
- Ruiz-Peinado, R.; Bravo-Oviedo, A.; López-Senespleda, E.; Montero, G.; Río, M. Do thinnings influence biomass and soil carbon stocks in Mediterranean maritime pinewoods? Eur. J. For. Res. 2013, 132, 253–262. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, S.; Liu, S.; Oeding, J. A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 2013, 10, 3691–3703. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef]
- Noormets, A.; Epron, D.; Domec, J.C.; McNulty, S.G.; Fox, T.; Sun, G.; King, J.S. Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For. Ecol. Manag. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- Strukelj, M.; Brais, S.; Paré, D. Nine-year changes in carbon dynamics following different intensities of harvesting in boreal aspen stands. Eur. J. For. Res. 2015, 134, 737–754. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.H.; Li, G.; Yoon, T.K.; Lee, S.-T.; Kim, C.; Son, Y. Effects of thinning intensity on nutrient concentration and enzyme activity in Larix kaempferi forest soils. J. Ecol. Environ. 2016, 40, 2. [Google Scholar] [CrossRef]
- Mattson, K.G.; Smith, H.C. Detrital organic matter and soil CO2 efflux in forests regenerating from cutting in West Virginia. Soil Biol. Biochem. 1993, 25, 1241–1248. [Google Scholar] [CrossRef]
- Chiti, T.; Perugini, L.; Vespertino, D.; Valentini, R. Effect of selective logging on soil organic carbon dynamics in tropical forests in central and western Africa. Plant Soil 2015, 399, 283–294. [Google Scholar] [CrossRef]
- Moreno-Fernández, D.; Díaz-Pinés, E.; Barbeito, I.; Sánchez-González, M.; Montes, F.; Rubio, A.; Cañellas, I. Temporal carbon dynamics over the rotation period of two alternative management systems in Mediterranean mountain Scots pine forests. For. Ecol. Manag. 2015, 348, 186–195. [Google Scholar] [CrossRef]
- Mushinski, R.M.; Gentry, T.J.; Boutton, T.W. Forest organic matter removal leads to long-term reductions in bacterial and fungal abundance. Appl. Soil Ecol. 2019, 137, 106–110. [Google Scholar] [CrossRef]
- Gross, C.D.; James, J.N.; Turnblom, E.C.; Harrison, R.B. Thinning Treatments Reduce Deep Soil Carbon and Nitrogen Stocks in a Coastal Pacific Northwest Forest. Forests 2018, 9, 238. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, D.; Li, W.; Sun, D.; Jin, C.; Yuan, F.; Wang, A.; Wu, J. The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Cepáková, Š.; Tošner, Z.; Frouz, J. The effect of tree species on seasonal fluctuations in water-soluble and hot water-extractable organic matter at post-mining sites. Geoderma 2016, 275, 19–27. [Google Scholar] [CrossRef]
- Mueller, K.E.; Hobbie, S.; Chorover, J.; Reich, P.; Eisenhauer, N.; Castellano, M.; Chadwick, O.A.; Dobies, T.; Hale, C.M.; Jagodzinski, A.; et al. Effects of litter traits, soil biota, and soil chemistry on soil carbon stocks at a common garden with 14 tree species. Biogeochemistry 2015, 123, 313–327. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A.; Zech, W. Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 2002, 242, 171–182. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A.; Zech, W. The effect of single tree species on soil microbial activities related to C and N cycling in the Siberian artificial afforestation experiment. Plant Soil 2002, 242, 183–196. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Plante, A.F.; Conant, R.T.; Carlson, J.; Greenwood, R.; Shulman, J.M.; Haddix, M.L.; Paul, E.A. Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol. Biochem. 2010, 42, 1991–1996. [Google Scholar] [CrossRef]
- Paterson, E.; Thornton, B.; Midwood, A.J.; Osborne, S.M.; Sim, A.; Millard, P. Atmospheric CO2 enrichment and nutrient additions to planted soil increase mineralisation of soil organic matter. Soil Biol. Biochem. 2008, 40, 2434–2440. [Google Scholar] [CrossRef]
- Taneva, L.; Gonzalez-Meler, M.A. Decomposition kinetics of soil C of different age from a forest exposed to 8 years of elevated atmospheric CO2 concentration. Soil. Biol. Biochem. 2008, 40, 2670–2677. [Google Scholar] [CrossRef]
- Wutzler, T.; Reichstein, M. Colimitation of decomposition by substrates and decomposers—A comparison of model formulations. Biogeosciences 2008, 5, 749–759. [Google Scholar] [CrossRef]
- Chapin, F.S.; McFarland, J.; McGuire, A.D.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global C cycle: Linking plant-soil C dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar]
- Sobachkin, R.S.; Sobachkin, D.S.; Buzykin, A.I. The influence of stand density on growth of tree conifer species. In Trees and Soil Interactions: Implications to Global Change; Binkley, D., Menyailo, O.V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2005; pp. 247–255. [Google Scholar]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 2005, 37, 445–454. [Google Scholar] [CrossRef]
- Liu, X.-J.A.; Sun, J.; Mau, R.L.; Finley, B.K.; Compson, Z.G.; van Gestel, N.; Brown, J.R.; Schwartz, E.; Dijkstra, P.; Hungate, B.A. Labile carbon input determines the direction and magnitude of the priming effect. Appl. Soil Ecol. 2017, 109, 7–13. [Google Scholar] [CrossRef]
- Qiao, N.; Schaefer, D.; Blagodatskaya, E.; Zou, X.; Xu, X.; Kuzyakov, Y. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Chang. Biol. 2014, 20, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Hu, G.; Dai, W.; Jiang, P.; Bai, E. The priming effect of soluble carbon inputs in organic and mineral soils from a temperate forest. Oecologia 2015, 178, 1239–1250. [Google Scholar] [CrossRef]
- StatSoft. Statistica for Windows (Computer Program Manual); StatSoft: Tulsa, OK, USA, 1997. [Google Scholar]
- Menyailo, O.V.; Abraham, W.R.; Conrad, R. Tree species affect atmospheric CH4 oxidation without altering community composition of soil methanotrophs. Soil Biol. Biochem. 2010, 42, 101–107. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A. Interactive effects of tree species and soil moisture on methane consumption. Soil Biol. Biochem 2003, 35, 625–628. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Eissenstat, D.M.; Trumbore, S.; Freeman, K.H.; Hobbie, S.E.; Chorover, J.; Oleksyn, J.; Reich, P.B.; Mueller, C.W. Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Glob. Chang. Biol 2019, 25, 1529–1546. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Litton, C.M.; Ryan, M.G.; Knight, D.H. Effects of tree density and stand age on carbon allocation patterns in postfire lodgepole pine. Ecol. Appl. 2004, 14, 460–475. [Google Scholar] [CrossRef]
- Berger, T.W.; Neubauer, C.; Glatzel, G. Factors controlling soil carbon and nitrogen stores in pure stands of Norway spruce (Picea abies) and mixed species stands in Austria. For. Ecol. Manag. 2002, 159, 3–14. [Google Scholar] [CrossRef]
- Hagen-Thorn, A.; Callesen, I.; Armolaitis, K.; Nihlgård, B. The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land. For. Ecol. Manag. 2004, 195, 373–384. [Google Scholar] [CrossRef]
- Hansson, K.; Olsson, B.A.; Olsson, M.; Johansson, U.; Berggren Kleja, D. Differences in soil properties in adjacent stands. For. Ecol. Manag. 2011, 262, 522–530. [Google Scholar] [CrossRef]
- Pastor, J.; Post, W.M. Response of northern forests to CO2-induced climate change. Nature 1988, 334, 55–58. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczó, Z.; Várbíró, G.; Varga, C.; Tóth, J.A.; Demeter, I.; Veperdi, G.; Berki, I. Long-term effects of climate change on carbon storage and tree species composition in a dry deciduous forest. Glob. Chang. Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef]
- Krankina, O.N.; Dixon, R.K.; Kirilenko, A.P.; Kobak, K.I. Global Climate Change Adaptation: Examples from Russian Boreal Forests. Clim. Chang. 1997, 36, 197–215. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Dvinskaya, M.L.; Ranson, K.J.; Im, S.T. Expansion of Evergreen Conifers to the Larch-Dominated Zone and Climatic Trends. Russ. J. Ecol. 2005, 36, 164–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).