Abstract
Phellodon is a genus of ectomycorrhizal fungi belonging to the group known as the stipitate hydnoids. It is associated with coniferous trees in forest ecosystems and is widely distributed in the northern hemisphere. Phellodon, together with Hydnellum, and Sarcodon, is classified in the Bankeraceae, members of which are generally considered as symbiotic fungi. Ectomycorrhizal fungi can help plant roots fix nitrogen and improve the absorption capacity of soil nutrients by trees, so they play an important role in ecosystem protection. Taxonomic and phylogenetic studies of Chinese Phellodon collections were carried out. Four new Phellodon species were discovered from southwestern China based on a combination of morphological characters and molecular data. Phellodon atroardesiacus is characterized by the blackish blue to dark grey pileus, dark grey to ash grey spines, and presence of clamp connections in spines. Phellodon cinereofuscus is distinguished by a cottony tomentose pileal margin, long spines which become clay-buff when dry, and echinulate basidiospores. Phellodon stramineus is characterized by a depressed and tomentose pileus, straw buff-colored pileal surface, and dark grey to ash grey spines. Phellodon yunnanensis is distinguished by a clay-pink to brown pileus, pale brown to white spines, and the presence of clamp connections in the outer layer of stipe. Detailed descriptions, illustrations, and ecological traits for the new taxa are provided. Phylogenetic analyses inferred from the internal transcribed spacer (ITS) regions confirmed that the four new species are distinct within Phellodon.
1. Introduction
Phellodon P. Karst. is a genus of ectomycorrhizal fungi associated with conifer trees in forest ecosystems. The genus Phellodon, together with Hydnellum P. Karst., and Sarcodon Quél. ex P. Karst., belongs to stipitate hydnoids, all of which are classified in the family Bankeraceae.
Ectomycorrhizal fungi are an important bridge between plant roots and soil and have ecological functions such as improving the absorption capacity of trees to soil nutrients [1]. Ectomycorrhizal fungal agents can also be widely used in seedling breeding of trees, exsitu protection of tree species, restoration and reconstruction of damaged ecosystems, and other processes [2]. Stipitate hydnoids are the emphasis of conservation in Europe because of their declining numbers [3]. Many British stipitate hydnoids species (14 species) were included in the UK Biodiversity Action Plan as priority species [4].
Phellodon was established by Petter Adolf Karsten and its type species is P. niger (Fr.) P. Karst [5]. According to the modern definition, species in Phellodon are characterized by the basidiomata consisting of a stipe and pileus with hydnoid hymenophores, uniform to duplex context, hyaline and echinulate basidiospores [3]. Due to indeterminate growth, basidiomata of Phellodon are confluent and acquire irregular shape [6].
Around 18 species have been described in the genus according to He, et al. [7]. Most of these species were recorded from North America [6]. In the 20th century, species of Phellodon were described based only on morphological characteristics [8,9,10,11,12,13,14,15]. In recent years, molecular studies have been used to infer species limits in Phellodon. Parfitt et al. [3] combined morphological methods with DNA sequencing of the ITS1 region to clear the classification status of the known Phellodon species from the UK, which revealed more terminal clusters than conventionally recognized taxa. Baird et al. [7] conducted a study to reevaluate the species of stipitate hydnoid fungi from temperate southeastern United States; species of Phellodon were recorded and Bankera fuligineoalba (J.C. Schmidt) Pouzar was recombined in Phellodon. Then, they discovered a new species, P. mississippiensis R.E. Baird, L.E. Wallace & G. Baker, from the southern United States, which was observed to have rare clamp connections in the subhymenial hyphae [16]. The taxonomy and phylogeny of Phellodon are not well studied from China, and only one species, P. subconfluens H.S. Yuan & F. Wu in Liaoning Province, was recently described by Mu, et al. based on morphological characters and molecular data [17].
During investigations on macrofungi from Yunnan Province, southwestern China, some specimens with stipitate hydnoid basidiomata were collected. Morphological characters and phylogenetic analyses based on the internal transcribed spacer (ITS) regions indicated that these specimens represented four undescribed species of Phellodon. The aims of this study are to confirm the taxonomic affinities of the new species, explore the species diversity of Phellodon in southwestern China, and infer the evolutionary relationships among representative species of Phellodon.
2. Materials and Methods
2.1. Morphological Studies
The studied specimens were deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macromorphological descriptions were based on field notes and herbarium specimens. Three specimens were examined of each of the 4 suspected new species, and 30 spores were counted per specimen. Microscopic characters, measurements, and drawings were made from slide preparations stained with Cotton Blue and Melzer’s reagent and observed at magnifications up to ×1000 under a light microscope (Nikon Eclipse E 80i microscope, Nikon, Tokyo, Japan) following Sun, et al. [18] and Han, et al. [19]. In the text, the following abbreviations were used: IKI = Melzer’s reagent, IKI– = negative in Melzer’s reagent, KOH = 5% potassium hydroxide, CB = Cotton Blue, CB+ = cyanophilous, CB− = acyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = variation in the L/W rationes between the specimens studied, and n = number of spores measured from given number of specimens. A field Emission Scanning Electron Microscope (FESEM) Hitachi SU-8010 (Hitachi, Ltd., Tokyo, Japan) was used to photograph the morphology of the basidiospores. Sections were studied at magnifications up to 1500× following Sun et al. [18].
2.2. Molecular Study and Phylogenetic Analysis
A CTAB rapid plant genome ex traction kit DN14 (Aidlab Biotechnologies, Beijing, China) was used to acquire total genomic DNA from dried specimens according to the manufacturer’s instructions with some modifications [20,21]. The primer pairs ITS5/4 and MS1/MS2 were used to amplify ITS and the small subunit of mitochondrial rRNA gene (mtSSU) for one-way. The primer pairs LR0R/LR7, NS1/NS4, AF/Cr and 5F/7Cr were used to amplify the large subunit of nuclear ribosomal RNA gene (nLSU), the small subunit of nuclear ribosomal RNA gene (nSSU), DNA-directed RNA polymerase II subunit 1 (RPB1) and DNA-directed RNA polymerase II subunit 2 (RPB2) respectively [22] for two-way.
The PCR procedure for ITS and mtSSU was as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 40 s, 56 °C for 45 s and 72 °C for 1 min and a final extension of 72 °C for 10 min. The PCR procedure for nrLSU and nrSSU was as follows: initial denaturation at 94 °C for 1 min, followed by 35 cycles at 94 °C for 30 s, 50 °C for 1 min and 72 °C for 1.5 min and a final extension of 72 °C for 10 min. The PCR procedure for RPB1 and RPB2 was as follows: initial denaturation at 94 °C for 2 min, 9 cycles at 94 °C for 45 s, 60 °C for 45 s, followed by 36 cycles at 94 °C for 45 s, 53 °C for 1 min, 72 °C for 1.5 min and a final extension of 72 °C for 10 min. The PCR products were purified and sequenced in Beijing Genomics Institute, China, with the same primers. All newly generated sequences were submitted to GenBank (Table 1).

Table 1.
A list of species, specimens and GenBank accession numbers of sequences used in this study.
The sequences were aligned in MAFFT 6 [23] with the G-INS-i option provided by the CBRC (http://mafft.cbrc.jp/alignment/server/, accessed on 15 November 2020) and manually adjusted in BioEdit [24]. The sequences of Sarcodon imbricatus (L.) P. Karst. and S. leucopus (Pers.) Maas Geest. & Nannf. were used as outgroups. Phylogenetic analyses were performed as reported in Zhu, et al. [25] and Liu et al. [26]. The best-fit model of nucleotide evolution for the datasets was selected with AIC (Akaike Information Criterion) using MrModeltest 2.3 [27,28]. Maximum Parsimony (MP), Maximum Likelihood (ML), and Bayesian Inference (BI) analyses were performed based on ITS sequences.
The MP analysis was performed in PAUP* version 4.0b10 [29] with the heuristic search. All characters were equally weighted, and gaps were treated as missing data. Max-trees was set to 5000, branches of zero length were collapsed and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BS) analysis with 1000 replicates [30]. Descriptive tree statistics, tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each Maximum Parsimonious Tree (MPT) generated. Only the Maximum Parsimony best tree from all searches was kept.
The ITS region was divided into three partitions, ITS1, 5.8s, and ITS2, for the ML and Bayesian analyses [18]. ML searches were conducted with RA×ML-HPC2 under the GTRGAMMA model, with all model parameters estimated by the program. To assess branch support, 1000 rapid bootstrap replicates were run with the GTRCAT model.
BI was performed using MrBayes 3.2.6 on Abe through the Cipres Science Gateway (www.phylo.org, accessed on 18 November 2020) with 2 independent runs, each one beginning from random trees with 4 simultaneous independent Chains, performing 2 million replicates, sampling one tree every 100 generations. The first 25% of the sampled trees were discarded as burn-in. The remaining ones were used to construct a majority rule consensus and to calculate Bayesian posterior probabilities (BPP) of the clades.
Phylogenetic trees were constructed using FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 19 November 2020). Branches that received bootstrap support for Maximum Parsimony (MP), Maximum Likelihood (ML) and Bayesian Posterior Probabilities (BPP) greater than or equal to 75% (MP and ML) and 0.95 (BPP) were considered as significantly supported, respectively.
3. Results
3.1. Phylogenetic Analyses
The ITS dataset included sequences from 59 fungal specimens representing 25 taxa. The dataset had an aligned length of 721 characters, of which 322 characters were constant, 43 were variable and parsimony-uninformative, and 356 were parsimony-informative. Maximum parsimony analysis yielded four equally parsimonious trees (TL = 1110, CI = 0.577, RI = 0.840, RC = 0.485, HI = 0.423). The best-fit model selected for these three partitions of ITS sequences was GTR+G for ITS1, JC for 5.8s, and HKY + G for ITS2. Bayesian and MP analyses resulted in similar topologies as the ML analysis, with an average standard deviation of split frequencies of 0.006202. The ML topology is shown with MP (≥50%), ML (≥50%), and BPP (≥0.95) supported values at the nodes (Figure 1).
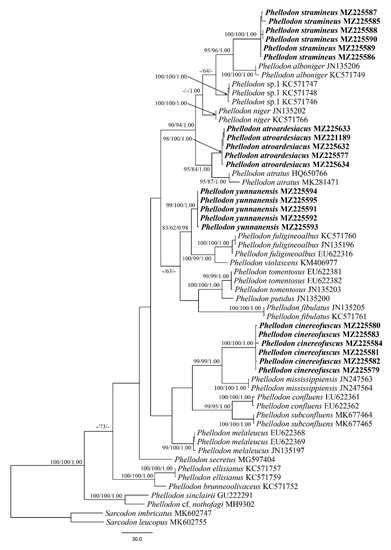
Figure 1.
Maximum parsimony (MP) phylogram of the Phellodon species based on ITS sequences data. The supported branches are labeled with parsimony bootstrap proportions higher than 50%, maximum likelihood bootstraps higher than 50% and Bayesian posterior probabilities more than 0.95. Bold names = New species.
The phylogeny inferred from ITS sequences demonstrated that sampled specimens of the four new species: Phellodon atroardesiacus, P. cinereofuscus P. stramineus, and Phellodon yunnanensis formed distinct well-supported lineages (Figure 1).
3.2. Taxonomy

Figure 2.
Basidiomata of Phellodon species. (a,b). P. atroardesiacus, (c,d). P. cinereofucus, (e,f). P. stramineus, (g,h). P. yunnanensis. Scale bars: 2 cm.
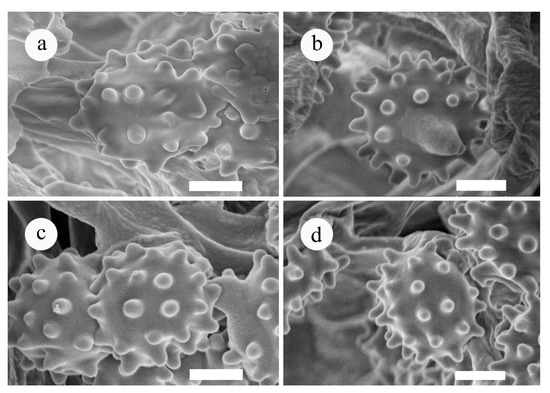
Figure 3.
SEM of basidiospores of Phellodon species. (a). P. atroardesiacus, (b). P. cinereofucus, (c). P. stramineus (d). P. yunnanensis. Scale bars: 1.5 µm.
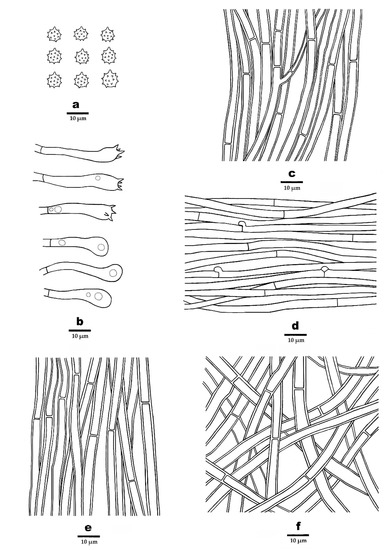
Figure 4.
Microscopic structures of P. atroardesiacus (drawn from the holotype). (a). Basidiospores. (b). Basidia and basidioles. (c). Hyphae from spines. (d). Hyphae from context. (e). Hyphae from inner layer of stipe. (f). Hyphae from outer layer of stipe.
MycoBank: 840306
Diagnosis—Differs from other Phellodon species by the blackish blue to dark grey pileus, scrobiculate at center, a mass of tomentum on pileus, short spines, and the presence of clamp connections in spines.
Etymology—atroardesiacus (Lat.): refers to the blackish blue basidiomata.
Holotype—CHINA. Xizang Autonomous Region, Chayu County, Cibagou Nature Reserve, on the ground of Pinus densata forest, approx. 97°04′ E 28°35′ N, elev. approx. 2900 m, 10 September 2020, Cui 18449 (BJFC 035310).
Fruitbody—Annual, centrally stipitate, single to concrescent, without odor or taste when fresh. Pileus orbicular to suborbicular, up to 5.5 cm in diam, 1.5 cm thick at the center. Pileal surface blackish blue to dark grey when fresh and becoming vinaceous brown upon drying, zonate, tomentose, scrobiculate at the center of the pileus; margin ash grey when fresh, becoming black after drying, up to 2 mm wide. Spines soft, dark greyish blue to ash grey when fresh, becoming fragile, pale mouse grey upon drying, up to 5 mm long. Context greyish blue, tough, azonate, up to 1 cm thick. Stipe cylindrical, glabrous, black, up to 5 cm long, 1 cm in diam.
Hyphal structure—Hyphal system monomitic; generative hyphae mostly with simple septa; all the hyphae IKI–, CB–; all the hyphae turned to olive green to black in KOH. Generative hyphae of pileal surface clay-buff, thick-walled, rarely branched, with simple septa, regularly arranged to parallel, 2.5–5 µm in diam. Generative hyphae in context clay-buff, slightly thick-walled, occasionally branched, with simple septa, parallel arranged, 3–6 µm in diam. Generative hyphae in spines vinaceous brown, thin-walled, occasionally branched, mostly with simple septa, occasionally with clamp connections, more or less parallel along the spines, 2–4 µm in diam. Generative hyphae in stipe clay-buff, thick-walled in the outer layer, rarely branched, mostly bearing simple septa, interwoven, 3–6 µm in diam; thick-walled in the inner layer, rarely branched, with simple septa, parallel along the stipe, 2.5–5 µm in diam.
Cystidia—Cystidia and other sterile hyphal elements absent.
Basidia—Clavate, bearing four sterigmata, 20–35 × 5–6 µm; sterigmata 2–4 µm long; basidioles similar to basidia in shape, but slightly smaller.
Spores—Basidiospores subglobose to globose, hyaline, thin-walled, echinulate, IKI–, CB–, 4–5 × (3–)3.5–4.5 µm, L = 4.45 µm, W = 3.78 µm, Q = 1–1.43 (n = 90/3, without the ornamentation).
Additional specimens (paratypes) examined—CHINA. Xizang Autonomous Region, Chayu County, Cibagou Nature Reserve, on ground of Pinus densata forest, alt. 2900 m, 10 September 2020, Cui 18457 (BJFC 035318) & Cui 18458 (BJFC 035319) & Cui 18459 (BJFC 035320).
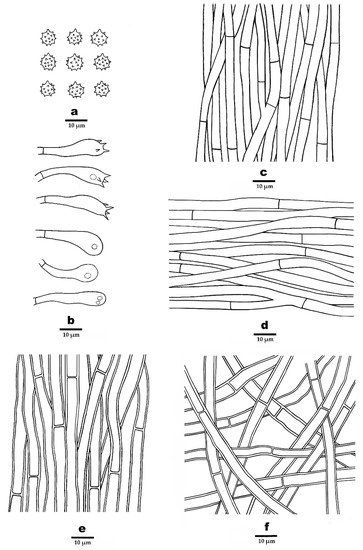
Figure 5.
Microscopic structures of P. cinereofucus (drawn from the holotype). (a). Basidiospores. (b). Basidia and basidioles. (c). Hyphae from spines. (d). Hyphae from context. (e). Hyphae from inner layer of stipe. (f). Hyphae from outer layer of stipe.
MycoBank: 840307
Diagnosis—Differs from other Phellodon species by the cottony tomentose pileal margin, long spines which become clay-buff when dry, and echinulate basidiospores.
Etymology—cinereofuscus (Lat.): refers to the grey to pale brown spines.
Holotype—CHINA. Yunnan Province, Mouding County, Huafoshan Nature Reserve, on the ground of Pinus and Fagaceae forest, approx. 101°26′ E 25°19′ N, elev. approx. 2250 m, 13 September 2018, Cui 16962 (BJFC 030261).
Fruitbody—Basidiomata annual, centrally or eccentrically stipitate, single to concrescent, with a fenugreek odor when fresh. Pileus irregularly shaped, infundibuliform, up to 11 cm in diam, 0.5 cm thick at the center. Pileal surface reddish brown to cinnamon brown when fresh and becoming greyish brown upon drying, color deeper at the center, zonate, glabrous, with radially aligned stripes at maturity; mature margin white when fresh and becoming cream to buff-yellow after drying, up to 1 cm wide. Spines soft, greyish brown to white when fresh, becoming fragile, buff to cinnamon-buff upon drying, up to 6 mm long. Context vinaceous buff, tough, azonate, up to 0.5 cm thick. Stipe cylindrical, glabrous, clay-buff to reddish buff, up to 4 cm long, 1.5 cm in diam.
Hyphal structure—Hyphal system monomitic; generative hyphae mostly with simple septa; all the hyphae IKI–, CB–; all tissues turning olive green to black in KOH. Generative hyphae in pileal surface hyaline to clay-buff, slightly thin-walled on the surface, rarely branched, with simple septa, regularly arranged to parallel, 3–6 µm in diam. Generative hyphae in context hyaline to clay-buff, thin-walled, occasionally branched, regularly arranged, with simple septa, 4–6.5 µm in diam. Generative hyphae in spines hyaline to clay-buff, thin-walled, mostly branched, with simple septa, more or less parallel along the spines, 2–4 µm in diam. Generative hyphae in stipe hyaline to clay-buff, thick-walled in the outer layer, rarely branched, bearing simple septa, interwoven, 3–7 µm in diam; thick-walled in the inner layer, with simple septa, parallel along the stipe, 3–6 µm in diam.
Cystidia—Cystidia and other sterile hyphal elements absent.
Basidia—Clavate, bearing four sterigmata and a basal simple septum, 17–34 × 5–7 µm; sterigmata 1–4 µm long; basidioles similar to basidia in shape, but slightly smaller.
Spores—Basidiospores subglobose to globose, hyaline, thin-walled, echinulate, IKI–, CB–, 4–5 × (3.5–)4–4.5 µm, L = 4.6 µm, W = 4.05 µm, Q = 1–1.25 (n = 90/3, without the ornamentation).
Additional specimens (paratypes) examined—CHINA. Yunnan Province, Nanhua County, Yulu Town, Sapiwu Village, on the ground of mixed forest dominated by trees of Pinus and Quercus, approx. 101°16′ E 25°11′ N elev. approx. 1800 m, 10 August 2016, Cui 14231 (BJFC 029099); Chuxiong, Zixishan Forest Park, on the ground of Fagaceae forest, approx. 101°24′ E 25°1′ N elev. approx. 2100 m, 13 September 2018, Cui 16944 (BJFC 030243) & Cui 16945 (BJFC 030244); Mouding County, Huafoshan Nature Reserve, on the ground of Pinus and Fagaceae forest, approx. 101°26′ E 25°19′ N, elev. approx. 2250 m, 13 September 2018, Cui 16963 (BJFC 030262).
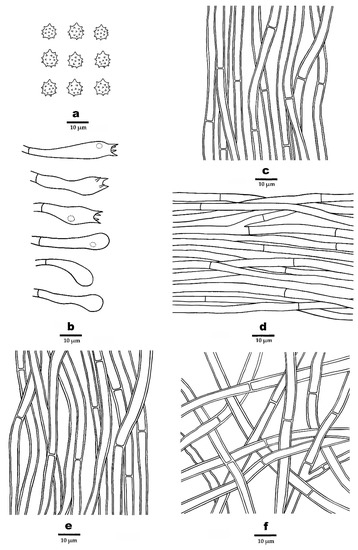
Figure 6.
Microscopic structures of P. stramineus (drawn from the holotype). (a). Basidiospores. (b). Basidia and basidioles. (c). Hyphae from spines. (d). Hyphae from context. (e). Hyphae from inner layer of stipe. (f). Hyphae from outer layer of stipe.
MycoBank: 840308
Diagnosis—Differs from other Phellodon species by the greyish brown to olivaceous buff depressed and tomentose pileus, and long basidia with moderately long sterigmata.
Etymology—stramineus (Lat.), refers to the straw buff-colored pileal surface.
Holotype—CHINA. Yunnan Province, Mouding County, Huafoshan Nature Reserve, on the ground of forest dominated by Pinus yunnanensis and Fagaceae, approx. 101°26′ E 25°19′ N, elev. approx. 2250 m, 13 September 2018, Cui 16959 (BJFC 030258).
Fruitbody—Basidiomata annual, centrally or eccentrically stipitate, single to concrescent, with a fenugreek odor when dry. Pileus depressed or infundibuliform, up to 8 cm in diam, 5 cm thick at the center. Pileal surface straw buff when fresh and becoming buff upon drying, zonate, tomentose, with radially aligned stripes; margin dark grey to pale mouse grey when fresh, up to 3 mm wide. Spines soft, dark grey to ash grey when fresh, becoming fragile, pale mouse-grey to clay-buff upon drying, up to 3 mm long. Context tough, azonate, up to 3 mm thick. Stipe cylindrical, glabrous, olivaceous buff, up to 5.5 cm long, 0.8 cm in diam.
Hyphal structure—Hyphal system monomitic; generative hyphae mostly with simple septa; all the hyphae IKI–, CB–; tissues of pileus and stipe hyaline, while tissues of mature spines turned olive green in KOH. Generative hyphae hyaline and slightly thick-walled in pileus surface, rarely branched, with simple septa, regularly arranged to parallel, 4–6 µm in diam. Generative hyphae in context hyaline, thick-walled, occasionally branched, with simple septa, regularly arranged, 3–5 µm in diam. Generative hyphae in spines hyaline to clay-buff, thin-walled, occasionally branched, with simple septa, more or less parallel along the spines, 2–4 µm in diam. Generative hyphae in stipe hyaline to clay-buff, thick-walled, without branches, mostly bearing simple septa, subparallel along the stipe, 2–5 µm in diam.
Cystidia—Cystidia and other sterile hyphal elements absent.
Basidia—Clavate, bearing four sterigmata and a basal simple septum, 18–55 × 5–7 µm; sterigmata 1.5–5 µm long; basidioles similar to basidia in shape, but slightly smaller.
Spores—Basidiospores subglobose to globose, hyaline, thin-walled, echinulate, IKI–, CB–, 4–5.5(–6) × 4–5(–5.5) µm, L = 5.06 µm, W = 4.38 µm, Q = 1–1.5 (n = 90/3, without the ornamentation).
Additional specimens (paratypes) examined—CHINA. Yunnan Province, Chuxiong, Zixishan Forest Park, on the ground of Fagaceae forest, approx. 101°24′ E 25°1′ N elev. approx. 2250 m, 13 September 2018, Cui 16942 (BJFC 030241) & Cui 16943 (BJFC 030242); Mouding County, Huafoshan Nature Reserve, on the ground of forest dominated by Pinus yunnanensis and Fagaceae, approx. 101°26′ E 25°19′ N, elev. approx. 2250 m, 13 September 2018, Cui16956 (BJFC 030255) & Cui 16961 (BJFC 030260) & Cui 16964 (BJFC 030263).
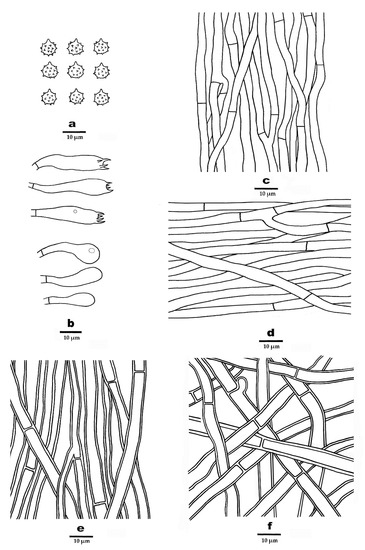
Figure 7.
Microscopic structures of P. yunnanensis (drawn from the holotype). (a). Basidiospores. (b). Basidia and basidioles. (c). Hyphae from spines. (d). Hyphae from context. (e). Hyphae from inner layer of stipe. (f). Hyphae with clamps from outer layer of stipe.
MycoBank: 840309
Diagnosis—Differs from other Phellodon species by a combination of glabrous pileus and stipe, moderately long spines, the presence of clamp connections in the outer layer of stipe, and tissues turning brown in KOH.
Etymology—yunnanensis (Lat.): referring to the holotype locality of the species in Yunnan Province.
Holotype—CHINA. Yunnan Province, Lanping County, Tongdian Town, Jianganchang, on the ground of Pinus armandii and Rhododendron forest, approx. 99°32′ E 26°41′ N, elev. approx. 2600 m, 18 September 2018, Cui 17129 (BJFC 030429).
Fruitbody—Basidiomata annual, centrally or eccentrically stipitate, solitary or gregarious, with a fenugreek odor when fresh. Pileus irregularly shaped, depressed or infundibuliform, up to 8 cm in diam., 3 mm thick at the center. Pileal surface clay pink to brown when fresh and becoming greyish brown upon drying, zonate, glabrous, with radially aligned stripes; margin blunt or irregular, fawn to white when fresh, becoming greyish brown with age, up to 3 mm wide. Spines soft, pale brown to white when fresh, becoming fragile, buff to cinereous upon drying, up to 5 mm long. Context tough, azonate, up to 3 mm thick. Stipe cylindrical, glabrous, basal tomentum absent, fawn, up to 3.5 cm long, 1.5 cm in diam.
Hyphal structure—Hyphal system monomitic; generative hyphae mostly with simple septa, occasionally with clamp connections; all the hyphae IKI–, CB–; tissues of pileus and stipe turning olive green to black, while tissues of mature spines turning brown in KOH. Generative hyphae in pileal surface hyaline, thin-walled to slightly thick-walled on the surface, rarely branched, with simple septa, regularly arranged to interwoven, 3–6.5 µm in diam. Generative hyphae in context hyaline, thin-walled, occasionally branched, with simple septa, regularly arranged, 2–6 µm in diam. Generative hyphae in spines hyaline, thin-walled, occasionally branched, with simple septa, more or less parallel along the spines, 2–4 µm in diam. Generative hyphae in stipe hyaline, slightly thick-walled in the outer layer, rarely branched, mostly bearing simple septa, occasionally with clamp connections, interwoven, 3–10 µm in diam., slightly thick-walled in the inner layer, rarely branched, with simple septa, subparallel along the stipe, 2–6 µm in diam.
Cystidia—Cystidia and other sterile hyphal elements absent.
Basidia—Clavate, bearing four sterigmata and a basal simple septum, 24–27 × 6–7 µm; sterigmata 1.5–5 µm long; basidioles similar to basidia in shape, but slightly smaller.
Spores—Basidiospores subglobose to globose, hyaline, thin-walled, echinulate, IKI–, CB–, 3.5–4.5(–5) × 3–4 (–4.5) µm, L = 3.99 µm, W = 3.64 µm, Q = 1.08–1.12 (n = 90/3, without the ornamentation).
Additional specimens (paratypes) examined—CHINA. Yunnan Province, Chuxiong, Zixishan Forest Park, on the ground of Pinus and Fagaceae forest, approx. 101°24′ E 25°1′ N elev. approx. 2300 m, 12 August 2016, Cui 14292 (BJFC 029160) & Cui 14294 (BJFC 029162); Xiangri-La, on the ground of Pinus forest, alt. 3200 m, 17 September 2018, Cui 17097 (BJFC 030397); Lanping County, Tongdian Town, Jianganchang, on the ground of Pinus armandii and Rhododendron forest, approx. 99°32′ E 26°41′ N, elev. approx. alt 2600 m, 18 September 2018, Cui 17131 (BJFC 030431).
4. Discussion
In the present study, four new species of Phellodon are described from southwestern China based on morphological characters and phylogenetic analyses of the ITS sequences. Due to the lack of multilocus sequences of other species in the genus, we are unable to construct a multilocus phylogenetic tree for the time being. We have sequenced the existing specimens in our laboratory and provided the available sequences of nLSU, nSSU, mtSSU, RPB1 and RPB2 genes of the genus which might be useful for future studies.
Phellodon atroardesiacus is closely related to P. atratus K. Harrison in our phylogenetic analyses (Figure 1). Morphologically, P. atratus is similar to P. atroardesiacus in having a slate gray to black pileus and similar basidiospores (3.5–4.5 × 3.3–4.1 µm, [31]). However, P. atratus differs from P. atroardesiacus by having longer and narrower basidia (26–35 × 4.5–5.2 µm), and a lack of clamp connections [31]. These two species are then grouped together with P. niger. Macromorphologically, P. niger resembles P. atroardesiacus in having single to concrescent basidiomata, spongy tomentose pileus, and grey or bluish grey spines. Furthermore, P. niger can be distinguished from P. atroardesiacus by lacking clamp connections [5]. In addition, these three species are distinct from each other in the phylogenetic tree (Figure 1). Phellodon fibulatus K.A. Harrison resembles P. atroardesiacus in having single to gregarious basidiomata and abundant clamp connections in the spines. However, P. fibulatus is different from P. atroardesiacus by its greyish orange pileus and tissues becoming greyish in KOH [6].
Phellodon cinereofuscus is closely related to P. mississippiensis in the phylogenetic tree (Figure 1). Morphologically, P. mississippiensis is similar to P. cinereofuscus in having single to concrescent basidiomata, and white to pale orange, or light orange, spines. However, there are clamp connections in the subhymenial hyphae in P. mississippiensis while they are absent in P. cinereofuscus. Moreover, P. mississippiensis can be distinguished from P. cinereofuscus in having shorter basidia (16–22 × 5–6 µm, [6]). Phellodon cinereofuscus may be confused with P. fibulatus by having similar-colored pileal margin and similar-sized basidiospores and basidia. However, clamp connections are absent in P. cinereofuscus while present in P. fibulatus. Furthermore, P. fibulatus differs from P. cinereofuscus in having a longer stipe (3–7 cm, [12]). Phellodon tomentosus (L.) Banker is similar to P. cinereofuscus in having a brownish yellow to yellowish brown pileus which is cottony tomentose and often fibrillose at the margin. However, P. tomentosus differs from P. cinereofuscus in having thicker basidiomata (up to 2 mm, [17]) and clamp connections in stipe tissue. Moreover, our phylogenetic analyses showed that P. tomentosus is distinctly different from P. cinereofuscus (Figure 1).
Phellodon stramineus is closely related to P. alboniger (Fr.) Karsten in the phylogenetic tree (Figure 1). Morphologically, P. alboniger might be confused with P. stramineus by having single to concrescent basidiomata. However, P. alboniger is distinguished by its narrower basidiospores (3.8–5.2 × 3.6–4.4 µm) and shorter basidia (24–36 × 5–6.4 µm, [10]).
Phellodon yunnanensis is closely related to P. violascens (Alb. & Schwein.) A.M. Ainsw. and P. fuligineoalbus in our phylogenetic analyses (Figure 1). Phellodon violascens differs from P. yunnanensis by its white to flesh brown basidiomata, lack of clamp connections, and larger basidiospores (4.5–5.4 × 4.3–4.5 µm, [32]). Phellodon fuligineoalbus differs from P. yunnanensis in having yellow-white or light brown basidiomata, longer spines (up to 6 mm), lack of clamp connections, and larger basidiospores (4–6 × 4–5 µm, [16]). Phellodon fibulatus may be confused with P. yunnanensis by having clamp connections. However, clamp connections are abundant and occur in all parts in P. fibulatus, whereas in P. yunnanensis clamps are found only in the outer layer of stipe. Furthermore, it differs from P. yunnanensis in having tawny rhizomorphs and basal tomentum [6]. In addition, P. fibulatus is distinct from P. yunnanensis in the ITS-based phylogenetic analyses (Figure 1).
Stipitate hydnoid fungi, as critical functional components of forest ecosystems, are sensitive to nitrogen deposition. The diversity of stipitate hydnoid fungi can reflect the conservation state of forest ecosystems. Although approximately 16 stipitate hydnoid fungi have been recorded in China [7,33,34], species concepts for many of those fungi are still obscure. Therefore, comprehensive studies on the species diversity, taxonomy, and phylogeny of the hydnoid fungi are needed in the future.
5. Conclusions
In this study, four new species of ectomycorrhizal fungi belonging to Phellodon are described from southwestern China based on morphological characters, ecological distributions and ITS-based phylogeny.
Author Contributions
B.-K.C. designed the research; B.-K.C., X.-L.H., X.J., S.L. and C.-G.S. prepared the samples; C.-G.S., X.J. and S.L. conducted the molecular experiments and analyzed the data; C.-G.S., X.-L.H. and B.-K.C. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by the National Natural Science Foundation of China (No. 31870008), Beijing Forestry University Outstanding Young Talent Cultivation Project (No. 2019JQ03016), and the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (No. 2019HJ2096001006).
Data Availability Statement
The data and results of this study are available upon reasonable request. Please contact the main author of this publication.
Acknowledgments
We express our gratitude to Jia-Hui Xing (China), Yuan-Yuan Chen (China), Min Wang (China), Yi-Fei Sun (China), Yan Wang (China), Yu-Li Han (China) for help during field collections and molecular studies. Genevieve Gates (Australia) is thanked for improving the English of the text.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Erland, S.; Taylor, A.F.S. Resupinate ectomycorrhizal fungal genera. In Ectomycorrhizal Fungi Key Genera in Profile; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 347–363. [Google Scholar] [CrossRef]
- Yu, L.; Guo, L.D.; Ma, K.P. The role of mycorrhizal fungi in ecosystems. Acta Phytoecol. Sin. 2002, 26, 739–745. [Google Scholar]
- Parfitt, D.; Ainsworth, A.M.; Simpson, D.; Rogers, H.J.; Boddy, L. Molecular and morphological discrimination of stipitate hydnoids in the genera Hydnellum and Phellodon. Mycol. Res. 2007, 3, 761–777. [Google Scholar] [CrossRef]
- UK Biodiversity Group. Anon UK biodiversity group tranche 2 action plans. In Plants and Fungi; English Nature: Peterborough, ON, Canada, 1998. [Google Scholar]
- Karsten, P.A. Enumeratio hydnearum Fr. fennicarum, systemate novo dispositarum. Rev. Mycol. Toulouse 1881, 3, 19–21. [Google Scholar]
- Baird, R.; Wallace, L.E.; Baker, G.; Scruggs, M. Stipitate hydnoid fungi of the temperate southeastern United States. Fungal Divers. 2013, 62, 41–114. [Google Scholar] [CrossRef]
- He, M.Q. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Fries, E.M. Observationes Mycologicae; Gerh. Bonnier: Copenhagen, Denmark, 1815; p. 230. [Google Scholar] [CrossRef]
- Banker, H.J. A contribution to a revision of the North American Hydnaceae. Mem. Torrey Bot. Club 1906, 12, 99–194. [Google Scholar] [CrossRef][Green Version]
- Coker, W.C.; Beers, A.H. The Stipitate Hydnums of the Eastern United States; University of North Carolina Press: Chapel Hill, NC, USA, 1951; p. 211. [Google Scholar]
- Pouzar, Z. Sbírejte losákovité houby. Ceská Mykol. 1955, 9, 95–96. [Google Scholar]
- Harrison, K.A. A new species of Phellodon possessing clamp connections. Can. J. Bot. 1972, 50, 1219–1221. [Google Scholar] [CrossRef]
- Stalpers, J.A. The aphyllophoraceous fungi I. keys to the species of the Thelephorales. Stud. Mycol. 1993, 35, 1–168. [Google Scholar]
- Pegler, D.N.; Roberst, P.J.; Spooner, B.M. British Chanterelles and Tooth Fungi; Kew: Royal Botanic Gardens, UK, 1997. [Google Scholar]
- Karsten, P.A. Enumeratio boletinearum et polyporearum fennicarum, systemate novo dispositarum. Rev. Mycol. Toulouse 1881, 3, 16–19. [Google Scholar]
- Baird, R.E.; Wallace, L.E.; Baker, G. Stipitate hydnums of the southern United States 1: Phellodon mississippiensis sp. nov. Mycotaxon 2013, 123, 183–191. [Google Scholar] [CrossRef]
- Mu, Y.H.; Wu, F.; Yuan, H.S. Hydnaceous fungi of China 7. Morphological and molecular characterization of Phellodon sub confluens sp. nov. from temperate, deciduous forests. Phytotaxa 2019, 414, 280–288. [Google Scholar] [CrossRef]
- Sun, Y.F.; Costa-Rezende, D.H.; Xing, J.H.; Zhou, J.L.; Zhang, B.; Gibertoni, T.B.; Gates, G.; Glen, M.; Dai, Y.C.; Cui, B.K. Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia 2020, 44, 206–239. [Google Scholar] [CrossRef]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Cui, B.K.; Li, H.J.; Ji, X.; Zhou, J.L.; Song, J.; Si, J.; Yang, Z.L.; Dai, Y.C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 2019, 97, 137–392. [Google Scholar] [CrossRef]
- Chen, J.J.; Cui, B.K.; Dai, Y.C. Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 2016, 37, 21–36. [Google Scholar] [CrossRef]
- Liu, S.; Shen, L.L.; Wang, Y.; Xu, T.M.; Gates, G.; Cui, B.K. Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front. Microbiol. 2021, 12, 631166. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhu, L.; Song, J.; Zhou, J.L.; Si, J.; Cui, B.K. Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Front. Microbiol. 2019, 10, 812. [Google Scholar] [CrossRef]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from east Asia. Front. Microbiol 2021, 12, 644979. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinform. 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program. Distributed by the Author; Evolutionary Biology Center: Uppsala University, Uppsala, Swden, 2004. [Google Scholar]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Harrison, K.A. New or little known north American stipitate Hydnums. Can. J. Bot. 1964, 42, 1205–1233. [Google Scholar] [CrossRef]
- Hrouda, P. Hydnaceous fungi of the Czech Republic and Slovakia. Czech Mycol. 1999, 51, 99–155. [Google Scholar] [CrossRef]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Mu, Y.H.; Hu, Y.P.; Wei, Y.L.; Yuan, H.S. Hydnaceous fungi of China 8. Morphological and molecular identification of three new species of Sarcodon and a new record from southwest China. MycoKeys 2020, 66, 83–103. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).