Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Setup
2.3. Data Collection
2.4. Data Analysis
2.5. Economic Analysis
3. Results and Discussion
3.1. Clonal Productivity Responses to Stand Density and Fertilizer Application
3.2. Early Growth/Productivity in Relation to the Incidence of Pests and Disease
3.3. Productivity in relation to LAI and Foliar N
3.4. Profitability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Energy. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy. In Economic Availability of Feedstocks; Langholtz, M.H., Stokes, B.J., Eaton, L.M., Eds.; ORNL/TM-2016/160; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; Volume 1, 448p. Available online: http://energy.gov/eere/bioenergy/2016-billion-ton-report (accessed on 23 February 2021). [CrossRef] [Green Version]
- Alternative Fuels Data Center. Energy Independence and Security Act of 2007. US Department of Energy, Energy Efficiency and Renewable Energy Office. Available online: https://afdc.energy.gov/laws/eisa.html (accessed on 29 March 2021).
- Hay, J.F. Corn for Biofuel Production. Farm Energy. 3 April 2019. Available online: https://farm-energy.extension.org/corn-for-biofuel-production/ (accessed on 18 March 2021).
- Larson, J.A.; English, B.C.; De La Torre Ugarte, D.G.; Menard, R.J.; Hellwinckel, C.M.; West, T.O. Economic and environmental impacts of the corn grain ethanol industry on the United States agricultural sector. J. Soil Water Conserv. 2010, 65, 267–279. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Debnath, D.; Zhong, J.; Ferin, K.; Van Loock, A.; Khanna, M. The economic, and environmental costs and benefits of the renewable fuel standard. Environ. Res. Lett. 2021, 16, 034021. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Nichols, E.G.; Hazel, D.W. Early Clonal survival and growth of poplars grown on North Carolina Piedmont and Mountain marginal lands. BioEnergy Res. 2016, 9, 548–558. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Implementing Regulation (EU) 2020/1294 of 15 September 2020 on the Union Renewable Energy Financing Mechanism. Official Journal of the European Union, L303/1-L303/16. 17 September 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R1294&from=EN (accessed on 27 February 2021).
- Matzenberger, J.; Kranzl, L.; Tromborg, E.; Junginger, M.; Daioglou, V.; Sheng Goh, C.; Keramidas, K. Future perspectives of international bioenergy trade. Renew. Sustain. Energy Rev. 2015, 43, 926–941. [Google Scholar] [CrossRef]
- FORISK Consulting. Wood bioenergy US. Forisk Res. Q. 2021. Quarter 1. Available online: https://forisk.com/wordpress//wp-content/assets/2021_Q1_WBUS_Free-Summary_20210203.pdf (accessed on 28 April 2021).
- Townsend, P.A.; Haider, N.; Boby, L.; Heavey, J.; Miller, T.A.; Volk, T.A. A Roadmap for Poplar and Willow to Provide Environmental Services and to Build the Bioeconomy; WSU Extension-EM115E; Washington State University (WSU): Washington, WA, USA, 2019; Peer-Reviewed. [Google Scholar]
- Cser, H.; Boby, L. Biomass feedstocks characteristics. South Reg. Ext. For. 2015. SREF-BE-005. Available online: https://sref.info/resources/publications/biomass-feedstocks-characteristics-1 (accessed on 18 April 2021).
- Balatinecz, J.J.; Kretschmann, D.E. Properties and utilization of poplar wood. In Poplar Culture in North America; Dickmann, D.I., Isebrands, J.G., Eckenwalder, J.E., Richardson, J., Eds.; National Research Council of Canada: Ottawa, ON, Canada, 2001; pp. 277–291. [Google Scholar]
- Tyndall, J.; Schulte, L.; Hall, R. Expanding the US Cornbelt Biomass Portfolio: Forester perceptions of the potential for woody biomass. Small Scale For. 2011, 10, 287–303. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Cunningham, M.W.; Hall, R.B.; Mirck, J.; Rockwood, D.L.; Stanturf, J.A.; Volk, T.A. Woody biomass from short rotation energy crops. In Sustainable Production of Fuels, Chemicals and Fibers from Forest Biomass; Zhu, J.Y., Zhang, X., Pan, X., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 27–63. [Google Scholar] [CrossRef]
- Wright, J.; Gallagher, T. Sustainable Production Practices of Short Rotation Woody Crops in the Southeastern United States: A Guidebook for Cottonwood and Hybrid Poplar. IBSS Southeastern Partnership for Integrated Biomass Supply Systems. Available online: http://www.se-ibss.org/publications-and-patents/extension-and-outreach-publications/sustainable-production-practices-of-short-rotation-woody-crops-in-the-southeastern-united-states-a-guidebook-for-cottonwood-and-hybrid-poplar (accessed on 15 February 2021).
- Miller, R.O.; Bender, B.A. Planting Density Effects on Biomass Growth of Hybrid Poplar Varieties in Michigan; Forest Biomass Innovation Center Research Report; Michigan State University: East Lansing, MI, USA, 2016; Available online: https://www.canr.msu.edu/uploads/396/36452/2016c.pdf (accessed on 3 March 2021).
- Shifflett, S.; Hazel, D.; Frederick, D.; Nichols, E.G. Species Trials of short rotation woody crops on two wastewater application sites in North Carolina, USA. BioEnergy Res. 2014, 7, 157–173. [Google Scholar] [CrossRef]
- Coyle, D.R.; Coleman, M.D.; Durant, J.A.; Newman, L.A. Survival and growth of 31 Populus clones in South Carolina. Biomass Bioenergy 2006, 30, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; LeBauer, D.; Dietze, M. Predicting yields of short-rotation hybrid poplar (Populous spp.) for the United States through model-data synthesis. Ecol. Soc. Am. 2013. [Google Scholar] [CrossRef] [Green Version]
- Hinchee, M.; Rottmann, W.; Mullinax, L.; Zhang, C.; Shujun, C.; Cunningham, M.; Pearson, L.; Nehra, N. Short-rotation woody crops for bioenergy and biofuels applications. In Biofuels; Tomes, D., Lakshmanan, P., Songstad, D., Eds.; Springer: New York, NY, USA, 2011; pp. 139–156. [Google Scholar] [CrossRef]
- Fang, S.; Xu, X.; Lu, S.; Tang, L. Growth dynamics and biomass production in short-rotation poplar plantations: 6-year results for three clones at four spacings. Biomass Bioenergy 2000, 41, 415–425. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Johnston, M.; Van Rees, K.C.J. Hybrid poplar growth in bioenergy production systems: Biomass prediction with a simple process-based model (3PG). Biomass Bioenergy 2010, 34, 687–702. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, A.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Campbell, J.E.; Lobell, D.B.; Genova, R.C.; Field, C.B. The global potential of bioenergy on abandoned agriculture lands. J. Environ. Sci. Technol. 2008, 42, 5791–5794. [Google Scholar] [CrossRef]
- Finzi, A.C.; Norby, R.J.; Calfapietra, C.; Gallet-Budynek, A.; Gielen, B.; Holmes, W.E.; Hoosbeek, M.R.; Iversen, C.M.; Jackson, R.B.; Kubiske, M.E.; et al. Increases in Nitrogen Uptake Rather than Nitrogen-Use Efficiency Support Higher Rates of Temperate Forest Productivity under Elevated CO2. Proc. Natl. Acad. Sci. USA 2007, 104, 14014–14019. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Dickmann, D.I. Responses of two hybrid Populus clones to flooding, drought, and nitrogen availability. I. Morphology and growth. Can. J. Bot. 1992, 70, 2265–2270. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Yield in 8 year-old hybrid poplar plantations on abandoned farmland along climatic and soil fertility gradients. For. Ecol. Manag. 2012, 267, 228–239. [Google Scholar] [CrossRef]
- Dipesh, K.; Blazier, M.; Pelkki, M.; Liechty, H. Genotype influences survival and growth of eastern cottonwood (Populus deltoides L.) managed as a bioenergy feedstock on retired agricultural sites of the Lower Mississippi Alluvial Valley. New For. 2017, 48, 95–114. [Google Scholar] [CrossRef]
- Crouse, D.A. Soils and Plant Nutrients. North Carolina State Extension Publications. Available online: https://content.ces.ncsu.edu/extension-gardener-handbook/1-soils-and-plant-nutrients (accessed on 21 March 2021).
- Flynn, R. Calculating Fertilizer Costs; Cooperative Extension Service; College of Agricultural, Consumer and Environmental Sciences, New Mexico State University: Las Cruces, NM, USA, 2014. [Google Scholar]
- Brown, K.; Van den Driessche, R. Effects of nitrogen and phosphorus fertilization on the growth and nutrition of hybrid poplars on Vancouver Island. New For. 2005, 29, 89–104. [Google Scholar] [CrossRef]
- Coyle, D.R.; Aubrey, D.P.; Siry, J.P.; Volfovicz-Leon, R.R.; Coleman, M.D. Optimal nitrogen application rates for three intensively-managed hardwood tree species in the southeastern USA. For. Ecol. Manag. 2013, 303, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Lasa, B.; Jauregui, I.; Aranjuelo, I.; Sakalauskiene, S.; Aparicio-Tejo, P.M. Influence of stage of development in the efficiency of nitrogen fertilization on poplar. J. Plant Nutr. 2016, 39, 87–98. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Nichols, E.G.; Hazel, D.W. Productivity and cost-effectiveness of short-rotation hardwoods on various land types in the Southeastern USA. Int. J. Phytorem. 2019. [Google Scholar] [CrossRef]
- Long, H.; Li, X.; Wang, H.; Jia, J. Biomass resources and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- El Kasmioui, O.; Ceulemans, R. Financial analysis of the cultivation of short rotation woody crops for bioenergy in Belgium: Barriers and opportunities. BioEnergy Res. 2013, 6, 336–350. [Google Scholar] [CrossRef]
- Shifflett, S.D.; Hazel, D.W.; Nichols, E.G. Sub-soiling and genotype selection improves Populus productivity grown on a North Carolina sandy soil. Forests 2016, 794, 74. [Google Scholar] [CrossRef] [Green Version]
- Ghezehei, S.B.; Nichols, E.G.; Maier, C.A.; Hazel, D.W. Adaptability of Populus to physiography and growing conditions in the Southeastern USA. Forests 2019, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture, Agricultural Statistics Service. 2012 Census of Agriculture, Volume 1, Chapter 1: National Level Data, Land: 2012 and 2007 (Table 8). Available online: https://www.nass.usda.gov/Publications/AgCensus/2012/Full_Report/Volume_1,_Chapter_1_US/st99_1_008_008.pdf (accessed on 16 April 2021).
- Web Soil Survey. 2016. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 1 March 2021).
- Ghezehei, S.B.; Wright, J.; Zalesny, R.S., Jr.; Nichols, E.G.; Hazel, D.W. Matching site-suitable poplars to rotation length for optimized productivity. For. Ecol. Manag. 2020, 45, 117670. [Google Scholar] [CrossRef]
- Schreiner, E.J. Rating Poplars for Melampsora Leaf Rust Infection; Forest Research Note NE-90; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1959; pp. 1–3.
- North Carolina Tree Seedling Catalog 2020–2021. Available online: https://www.ncforestservice.gov/nursery/pdf/NCFS_Tree_Seedling_Catalog.pdf (accessed on 15 February 2021).
- Historic North Carolina Delivered Timber Prices, 1988–2020. NC State Extension. Available online: https://content.ces.ncsu.edu/historic-north-carolina-delivered-timber-prices-1988-2014 (accessed on 21 April 2021).
- Miller, R.O.; Bender, B.A. Sources of variation in hybrid poplar biomass production throughout Michigan, USA. Michigan State University, Forest Biomass Innovation Center Research Report, 2016 (d). In Proceedings of the A Presentation in the 25th International Poplar Symposium, Berlin, Germany, 13–16 September 2016. [Google Scholar]
- Zalesny, R.S., Jr.; Stanturf, J.A.; Gardiner, E.S.; Perdue, J.H.; Young, T.M.; Coyle, D.R.; Headlee, W.L.; Bañuelos, G.S.; Hass, A. Ecosystem services of woody crop production systems. BioEnergy Res. 2016, 9, 465–491. [Google Scholar] [CrossRef] [Green Version]
- Dillen, S.Y.; Djomo, S.N.; Al Afas, N.; Vanbeveren, S.; Ceulemans, R. Biomass yield and energy balance of a short-rotation poplar coppice with multiple clones on degraded land during 16 years. Biomass Bioenergy 2013, 56, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Christersson, L. Biomass production of intensively grown poplars in the southernmost part of Sweden: Observations of characters, traits and growth potential. Biomass Bioenergy 2006, 30, 497–508. [Google Scholar] [CrossRef]
- Vande Walle, I.; Van Camp, N.; Van de Casteele, L.; Verheyen, K.; Lemeur, R. Short-rotation forestry of birch, maple, poplar and willow in Flanders (Belgium) I-Biomass production after 4 years of tree growth. Biomass Bioenergy 2007, 31, 267–275. [Google Scholar] [CrossRef]
- Sonon, L.S.; Kissel, D.E.; Saha, U. Cation Exchange Capacity and Base Saturation. University of Georgia Extension. 2017. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/C%201040_2.PDF (accessed on 27 March 2021).
- Ghezehei, S.B.; Shifflett, S.D.; Hazel, D.W.; Nichols, E.G. SRWC bioenergy productivity and economic feasibility on marginal lands. J. Environ. Manage. 2015, 160, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Stellacci, A.; Campi, P.; Vitti, C.; Modugno, F.; Mastrorilli, M. Feasibility of SRC species for growing in Mediterranean conditions. BioEnergy Res. 2016, 9, 208–223. [Google Scholar] [CrossRef]
- Verlinden, M.S.; Broeckx, L.S.; Van den Bulcke, J.; Van Acker, J.; Ceulemans, R. Comparative study of biomass determinants of 12 poplar (Populus) genotypes in a high-density short-rotation culture. For. Ecol. Manag. 2013, 307, 101–111. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Tosi, L.; Fabre, B.; Frey, P. Three-year investigations on leaf rust of poplar cultivated for biomass production in Umbria, Central Italy. Biomass Bioenergy 2013, 49, 315–322. [Google Scholar] [CrossRef]
- Coyle, D.R.; Hart, E.R.; McMillin, J.D.; Rule, L.C.; Hall, R.B. Effects of repeated cottonwood leaf beetle defoliation on Populus growth and economic value over an 8-year harvest rotation. For. Ecol. Manag. 2008, 255, 3365–3373. [Google Scholar] [CrossRef]
- Ceulemans, R.; Pontailler, J.; Mau, F.; Guittet, J. Leaf allometry in young poplar stands: Reliability of leaf area index estimation, site and clone effects. Biomass Bioenergy 1993, 4, 315–321. [Google Scholar] [CrossRef]
- Ridge, C.R.; Hinckley, T.M.; Stettler, R.F.; Van Volkenburgh, E. Leaf growth characteristics of fast-growing poplar hybrids Populus trichocarpa × P. deltoides. Tree Physiol. 1986, 1, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.; Landsberg, J.; Linder, S. Tamm Review: Insights gained from light use and leaf growth efficiency indices. For. Ecol. Manag. 2016, 379, 232–342. [Google Scholar] [CrossRef]
- dLteif, A.; Whalen, J.K.; Bradley, R.L.; Camire, C. Diagnostic tools to evaluate the foliar nutrition and growth of hybrid poplars. Can. J. For. Res. 2008, 38, 2138–2147. [Google Scholar]
- Wilson, A.; Nzokou, P.; Güney, D.; Kulaç, Ş. Growth response and nitrogen use physiology of Fraser fir (Abies fraseri), red pine (Pinus resinosa), and hybrid poplar under amino acid nutrition. New For. 2013, 44, 281–295. [Google Scholar] [CrossRef]
- Pope, P.E.; McLaughlin, R.A.; Hansen, E.A. Biomass and nitrogen dynamics of hybrid poplar on two different soils: Implications for fertilization strategy. Can. J. For. Res. 1988, 18, 223–230. [Google Scholar]
- Schweier, J.; Becker, G. Economics of poplar short rotation coppice plantations on marginal land in Germany. Biomass Bioenergy 2013, 59, 494. [Google Scholar]

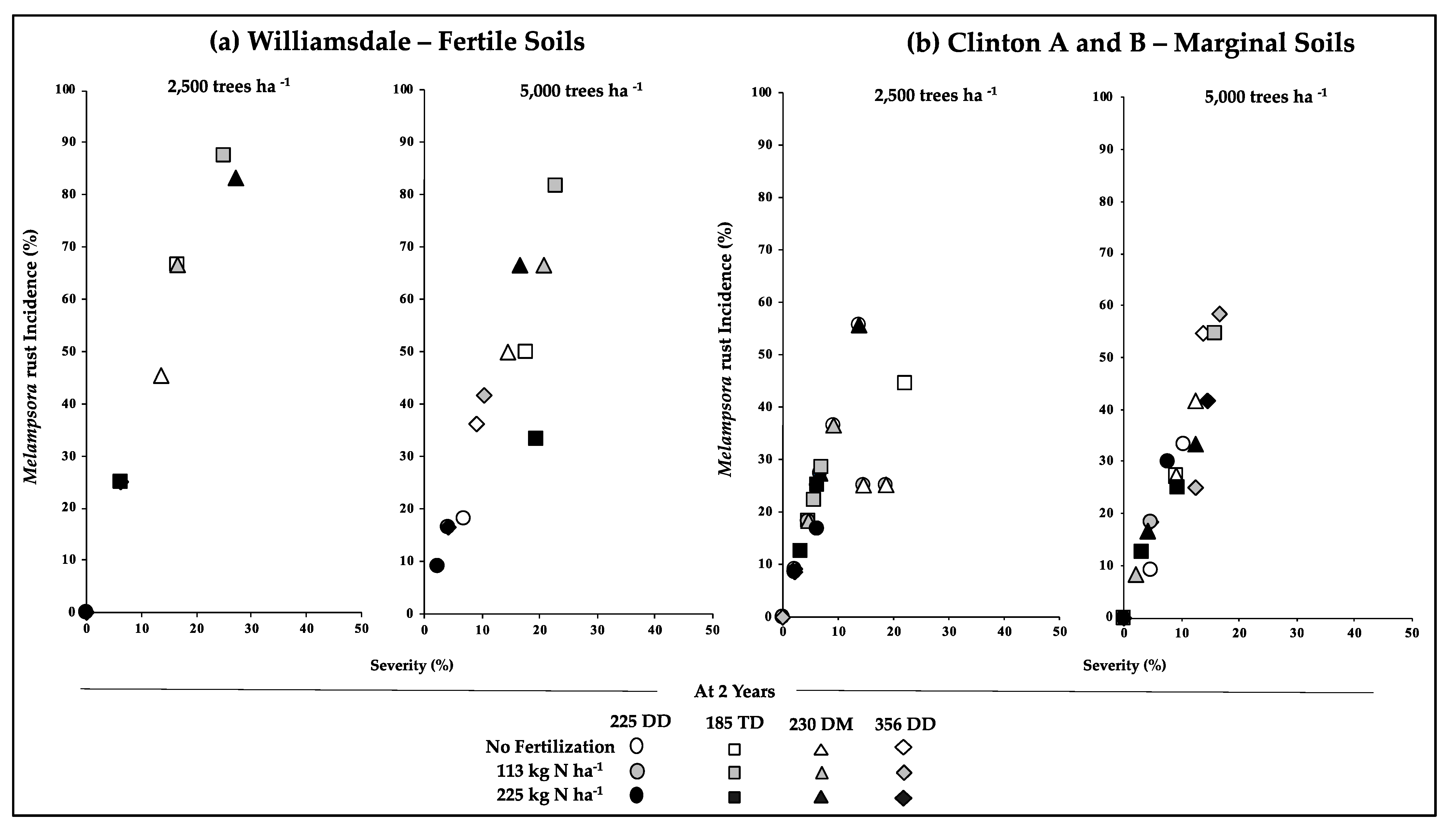

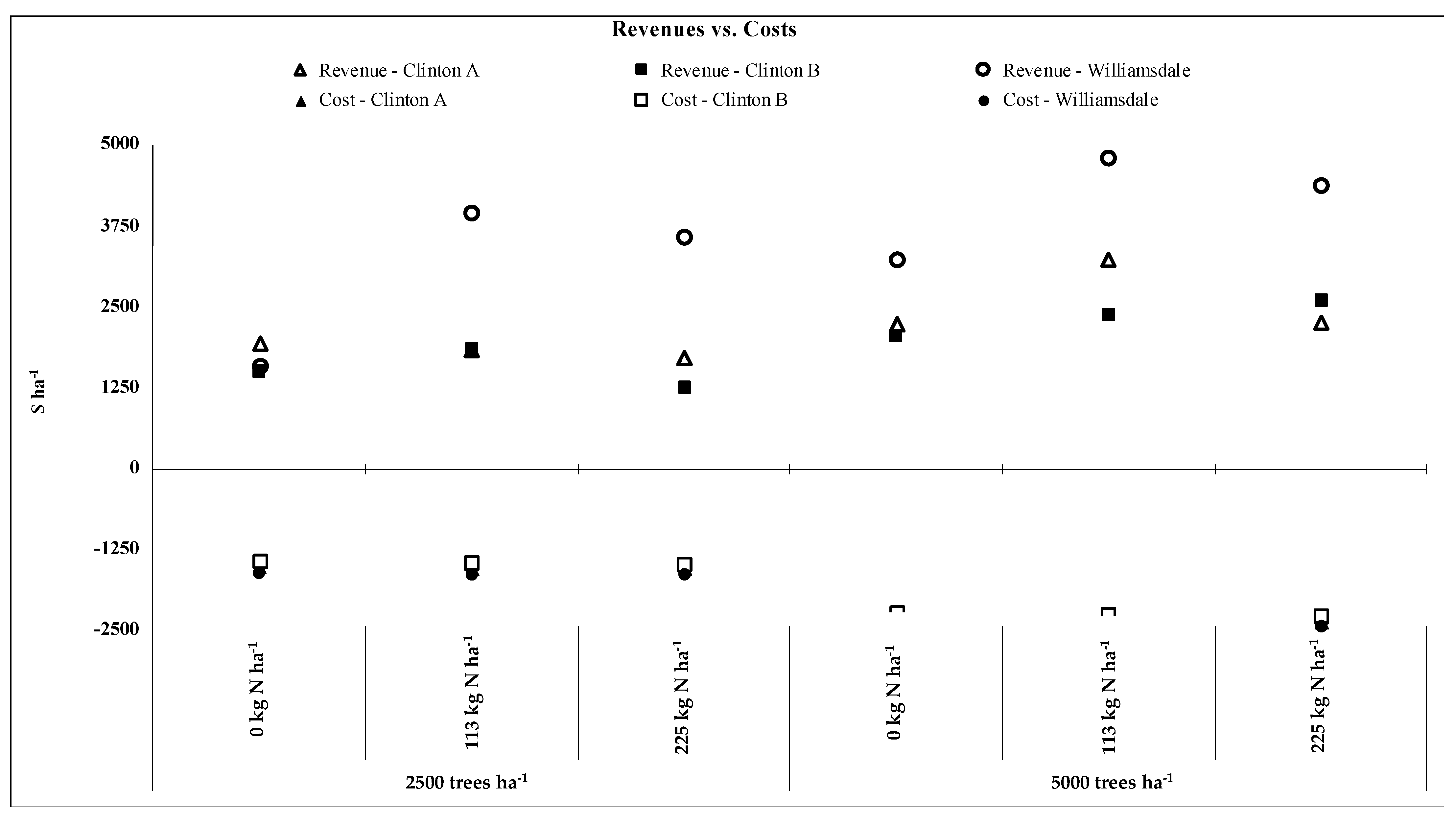
| Site Characteristics | Clinton A | Clinton B | Williamsdale |
|---|---|---|---|
| Soil Quality | Marginal | Marginal | Fertile |
| Location | 35° 1′ 20.47″ N; 78° 16′ 24.62″ W | 35° 1′ 20″ N; 78° 16′ 23.90″ W | 34° 45′ 51.06″ N; 78°5′ 59.04″ W |
| Soil Series c | Wagram loamy sand | Orangeburg loamy fine sand | Noboco loamy fine sand |
| Mean Annual Rainfall (mm) a,b | 1371 | 1371 | 1480 |
| Elevation (msl) a | 50.6 | 50.6 | 17 |
| Mean Annual T (C) a,b | 16.7 | 16.7 | 16.7 |
| Mean Daily Humidity (%) a,b | 71.9 | 71.9 | 73.4 |
| Mean Plant Available Water (cm3 cm−3) a,b | 0.18 | 0.18 | 0.2 |
| Soil pH d | 6.2 | 6.1 | 6 |
| Cation Exchange Capacity (Meq/100g) d | 2.3 | 2.1 | 6.9 |
| Soil P (kg ha−1) d | 504 | 605 | 1011 |
| Soil K (kg ha−1) d | 131 | 112 | 410 |
| Soil Mg (kg ha−1) d | 102 | 92 | 308 |
| Soil Ca (kg ha−1) d | 559 | 517 | 1630 |
| Soil NO-3 (kg ha−1) d | 3.7 | 2.5 | 4.1 |
| Prior Crop | Sorghum | Sorghum | Corn |
| Treatments | Mean Stem Biomass (Mg ha−1) | |||
|---|---|---|---|---|
| Clinton A | Clinton B | Williamsdale | ||
| Stand Density | 5000 trees ha−1 2500 trees ha−1 MSD (⍺= 0.05) | 93.9 A 65.3 B 18.02 | 85.6 A 55.9 B 16.69 | 148.6 A 124.9 A 27.98 |
| Fertilizer Rate | 225kg N ha−1 113 kg N ha−1 0 kg N ha−1 MSD (⍺= 0.05) | 73.5 A 93.6 A 71.8 A 26.6 | 69.6 A 78.4 A 63.7 A 24.6 | 164.8 A 154.8 A 93.1 B 36.06 |
| Clone | ‘140’ ‘356’ ‘230’ ‘185’ MSD (⍺ = 0.05) | 114.8 A 103.2 A 54.0 B 48.9 B 33.80 | 95.1 A 89.8 A 56.3 B 38.5 B 31.30 | 214.3 A 223.8 A 60.2 B 16.5 B 52.81 |
| Overall | 79.4 | 69.8 | 129.7 | |
| Stand Density | Fertilizer Rates | Clone (Genomic Group) | Stands Age: Six Years | Stand Density: Two Years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Green Stem Biomass ± 1SD (Mg ha−1) | LAI (m2 m−2) (SD) | Foliar N% (SD) | |||||||||
| Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | |||
| 5000 trees ha−1 |
225 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 107.3 ± 48.8 62.1 ± 78.4 52.3 ± 14.6 103.2 ± 52.8 | 114.4 ± 52.3 55.0 ± 31.5 68.0 ± 28.5 136.3 ± 39.4 | 219.1 ± 44.3 18.5 ± 11.0 66.8 ± 16.1 329 ±193.4 | 1.2 (0.5) 1.8 (0.9) 1.9 (0.8) 1.3 (0.6) | 0.9 (0.3) 1.2 (0.3) 1 (0.2) 0.9 (0.3) | 1.2 (0.2) 1.4 (0.2) 1.2 (0.3) 1.3 (0.2) | 2 (0.2) 2.1 (0.1) 1.9 (0.2) 1.8 (0.4) | 1.7 (0.2) 1.7 (0.3) 1.8 (0.2) 1.8 (0.2) | 2.1 (0.1) 2 (0.2) 2 (0.2) 2 (0.1) |
|
113 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 146.2 ± 41.6 61.5 ± 42.0 90.2 ± 16.8 170.2 ± 60.3 | 140.5 ± 51.5 48.0 ± 12.2 74.5 ± 19.7 80.6 ± 57.0 | 378.1 ± 42.5 22.8 ± 6.8 70.2 ± 23.5 225.2 ± 15.9 | 1 (0.3) 1.6 (0.4) 1.4 (0.5) 1.5 (0.5) | 1.2 (0.4) 1.8 (0.5) 1.5 (0.3) 1 (0.1) | 2.2 (0.1) 2.5 (0.2) 2.7 (0.1) 2.5 (0.1) | 1.7 (0.3) 1.8 (0.2) 1.7 (0.2) 1.3 (0.3) | 1.6 (0.1) 1.6 (0.3) 1.6 (0.1) 1.9 (0.1) | 2.2 (0.1) 2.1 (0.2) 2 (0.3) 2.1 (0.1) | |
|
0 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 141.7 ± 61.4 44.6 ± 27.2 43.5 ± 17.8 93.2 ± 55.6 | 112.2 ± 27.9 44.9 ± 14.4 62.7 ± 19.8 76.4 ± 71.8 | 215.7±135.2 14.3 ± 7.8 51.4 ± 20.7 184.7 ± 58.3 | 1.5 (0.4) 2.1 (0.4) 2.2 (0.2) 1.4 (0.3) | 1 (0.1) 2.1 (0.1) 1.1 (0.4) 1.1 (0.1) | 1.9 (0.4) 2.2 (0.6) 1.8 (0.3) 2.2 (0.6) | 2.1 (0.3) 1.8 (0.1) 1.9 (0) 2 (0.2) | 1.9 (0.1) 1.8 (0.1) 1.8 (0.2) 1.8 (0.1) | 2.2 (0.2) 2.1 (0.6) 2.1 (0.2) 2.1 (0.1) | |
| 2500 trees ha−1 |
225 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 102.1 ± 14.8 30.0 ± 13.5 44.0 ± 10.9 70.1 ± 43.2 | 57.4 ± 25.7 38.4 ± 7.1 38.8 ± 5.7 43.2 ± 12.8 | 240.6 ± 57.3 15.2 57.8 ± 23.5 203.2 ± 45.1 | 1.8 (0.3) 1.5 (0.5) 1.3 (0.4) 1.1 (0.4) | 1.3 (0.5) 1.1 (0.4) 1.1 (0.5) 1.6 (0.6) | 1.2 (0.5) 1.3 (0.6) 1.7 (0.9) 1.5 (0.7) | 1.8 (0.1) 1.6 (0.4) 1.8 (0.1) 1.7 (0.3) | 1.7 (0.4) 2.2 (0.3) 2.1 (0.3) 1.9 (0.2) | 2 (0.2) 1.9 (0) 1.9 (0.2) 1.9 (0.1) |
|
113 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 83.9 ± 39.7 46.3 ± 4.0 45.3 ± 5.4 89.7 ± 33.4 | 80.4 ± 54.6 24.3 ± 15.4 53.1 ± 11.2 107.9 ± 58.8 | 180.1 ± 28.8 13.1 ± 2.4 80.1 ± 24.9 298.0 ± 65.9 | 1.8 (0.3) 1.5 (0.5) 1.3 (0.4) 1.1 (0.4) | 1.8 (0.4) 1.9 (0.3) 1.5 (0.2) 1.4 (0.3) | 1.8 (0.8) 1.5 (0.6) 1.9 (1) 1.4 (0.6) | 1.8 (0.1) 1.6 (0.4) 1.8 (0.1) 1.7 (0.3) | 1.7 (0.1) 1.6 (0) 2 (0.3) 1.9 (0.2) | 2.1 (0.5) 1.8 (0.2) 2.1 (0.2) 2.1 (0.1) | |
|
0 (kg N ha−1) | ‘140’ (DD) ‘185’ (TD) ‘230’ (DM) ‘356’ (DD) | 107.7 ± 12.1 51.0 ± 37.2 48.6 ± 21.1 71.4 | 65.6 ± 46.4 21.3 ± 16.0 40.4 ± 12.7 90.0 ± 22.5 | 54.1 ± 36.0 12.2 ± 4.4 25.5 ± 9.2 137.5 ± 32.1 | 1.5 (0.6) 1.8 (0.8) 2.1 (0.2) 2.1 (0.6) | 1.3 (0.5) 1.4 (0.3) 1.9 (0.3) 2.1 (0.1) | 2.3 (0.5) 2.3 (0.8) 2.4 (0.4) 2.1 (0.6) | 1.9 (0.2) 2 (0.3) 1.8 (0.2) 1.6 (0.2) | 1.7 (0.2) 1.9 (0.1) 1.6 (0.3) 1.4 (0.2) | 2.1 (0.2) 2.1 (0.1) 1.9 (0.1) 2.1 (0.1) | |
| Stand Density | Fertilizer Rates | Clone | Stand Age: 6 Years | Stand Age: 12 Years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPV ($USD ha−1) at (Price = $27 Mg−1) | Break-Even Price ($USD Mg−1 Green Biomass) | NPV ($USD ha−1) (Price = $27 USD Mg−1) | Break-Even Price ($USD Mg−1 Green Biomass) | Stem MAI, (Mg ha−1 year−1) | |||||||||||||
| Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | Clinton A | Clinton B | Williamsdale | |||
| 2500 Trees ha−1 | 0 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($1300) ($1686) ($1702) ($1547) | ($1509) ($1810) ($1681) ($1343) | ($1838) ($2123) ($2032) ($1270) | 38.0 57.8 59.7 47.1 | 48.3 106.7 65.9 40.7 | 58.9 191.8 102.3 35.4 | $33 ($1630) ($1677) ($1240) | ($1195) ($2045) ($1679) ($728) | ($1939) ($2651) ($2425) ($520) | - 37.6 38.4 32.6 | 32.9 59.2 40.8 29.4 | 40.5 110.2 63.3 28.2 | 25.3 12.0 11.4 16.8 | 15.4 5.0 9.5 21.2 | 11.3 2.5 5.3 28.7 |
| 113 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($1485) ($1741) ($1748) ($1446) | ($1431) ($1813) ($1617) ($1244) | ($1004) ($2139) ($1684) ($202) | 43.4 62.2 63.1 41.9 | 43.4 97.0 55.3 37.5 | 32.0 181.7 46.6 27.3 | ($1023) ($1744) ($1763) ($912) | ($935) ($2010) ($1458) ($408) | $181 ($2659) ($1520) $2185 | 30.9 39.5 39.9 30.2 | 30.6 54.7 36.0 28.0 | - 104.7 34.0 23.9 | 19.7 10.9 10.6 21.1 | 18.9 5.7 12.5 25.3 | 37.5 2.7 16.7 62.1 | |
| 225 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($1372) ($1862) ($1767) ($1589) | ($1598) ($1727) ($1724) ($1694) | ($606) ($2139) ($1849) ($860) | 39.3 85.3 64.6 48.1 | 52.9 69.1 68.5 63.6 | 29.1 160.3 57.1 30.7 | ($684) ($2067) ($1799) ($1298) | ($1386) ($1750) ($1742) ($1658) | $1,195 ($2,636) ($1,913) $559 | 29.0 50.1 40.6 33.0 | 34.9 42.1 41.9 39.7 | - 93.4 39.5 25.7 | 24.0 7.1 10.3 16.5 | 13.5 9.0 9.1 10.2 | 50.1 3.2 12.0 42.3 | |
| 5000 Trees ha−1 | 0 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($1963) ($2623) ($2630) ($2292) | ($2085) ($2543) ($2422) ($2329) | ($1629 ($2998) ($2746) ($1839) | 39.3 80.8 82.3 49.2 | 43.6 78.6 62.1 54.6 | 33.5 220.9 76.0 35.8 | ($772) ($2634) ($2655) ($1702) | ($1182) ($2473) ($2132) ($1869) | ($69) ($3492) ($2861) ($595) | 28.4 46.2 46.8 32.6 | 30.0 44.8 37.8 34.6 | - 118.4 47.5 27.8 | 33.3 10.5 10.2 21.9 | 26.4 10.6 14.7 18.0 | 44.9 3.0 10.7 38.5 |
| 113 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($1969) ($2545) ($2349) ($1806) | ($1930) ($2559) ($2379) ($2337) | ($561) ($2977) ($2655) ($1601) | 38.9 64.8 50.6 36.3 | 39.1 75.7 55.9 53.2 | 27.9 147.9 61.6 33.1 | ($723) ($2348) ($1796) ($263) | ($676) ($2451) ($1942) ($1825) | $2656 ($3385) ($2579) $57 | 28.2 39.3 33.2 27.1 | 28.1 43.5 35.2 34.0 | - 82.5 40.4 - | 34.3 14.4 21.2 40.0 | 33.0 11.3 17.5 18.9 | 78.8 4.7 14.6 46.9 | |
| 225 (kg N ha−1) | ‘140’ ‘185’ ‘230’ ‘356’ | ($2257) ($2565) ($2631) ($2285) | ($2131) ($2535) ($2447) ($1982) | ($1670) ($3033) ($2705) ($922) | 46.0 64.7 73.0 47.0 | 43.7 69.1 59.7 39.9 | 33.6 178.8 64.2 29.1 | ($1493) ($2360) ($2547) ($1571) | ($1201) ($2341) ($2090) ($781) | ($75) ($3484) ($2664) $1795 | 31.2 39.2 42.8 31.6 | 30.0 40.7 36.7 28.5 | - 97.5 41.6 - | 25.2 14.6 12.3 24.3 | 26.9 12.9 16.0 32.0 | 45.6 3.9 13.9 68.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezehei, S.B.; Ewald, A.L.; Hazel, D.W.; Zalesny, R.S., Jr.; Nichols, E.G. Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments. Forests 2021, 12, 869. https://doi.org/10.3390/f12070869
Ghezehei SB, Ewald AL, Hazel DW, Zalesny RS Jr., Nichols EG. Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments. Forests. 2021; 12(7):869. https://doi.org/10.3390/f12070869
Chicago/Turabian StyleGhezehei, Solomon B., Alexander L. Ewald, Dennis W. Hazel, Ronald S. Zalesny, Jr., and Elizabeth Guthrie Nichols. 2021. "Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments" Forests 12, no. 7: 869. https://doi.org/10.3390/f12070869
APA StyleGhezehei, S. B., Ewald, A. L., Hazel, D. W., Zalesny, R. S., Jr., & Nichols, E. G. (2021). Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments. Forests, 12(7), 869. https://doi.org/10.3390/f12070869







