Abstract
Mangroves play an important role in carbon sequestration. However, mangroves can be sources of greenhouse gas (GHG) emissions. In this study, methane (CH4) emissions and related soil properties were determined in multiple mangroves in Taiwan, including Kandelia obovata and Avicennia marina mangroves. K. obovata possess prop roots, whereas pneumatophores are found in A. marina. Our results showed that mangrove soils were significant sources of CH4 emissions, which should be accounted for in mangrove carbon budgets. In particular, CH4 emissions in the A. marina mangroves were approximately 50- to 100-fold those of the K. obovata mangroves and the adjoining mudflats. Multiple regression analyses indicated that the soil salinity and pH in K. obovata mangroves and the soil redox potential and organic content in the mudflats were the key factors affecting CH4 emissions. However, the pneumatophore density alone explained approximately 48% of the variation in CH4 emissions in the A. marina mangroves. More pneumatophores resulted in higher CH4 emissions in the A. marina mangroves. Thus, compared with the assessed soil properties, the contribution of pneumatophores to the transportation of CH4 from soil was more significant. In addition to soil properties, our results demonstrated that the root structure may also affect GHG emissions from mangroves.
Keywords:
Avicennia marina; greenhouse gas; Kandelia obovata; mangroves; methane; pneumatophore; soil 1. Introduction
Anthropogenic activities, such as deforestation and fossil fuel combustion, have generated a great amount of carbon dioxide (CO2) emissions, with accompanying greenhouse effects and global warming [1,2]. To alleviate climate change, it is essential to develop mitigation strategies to increase the carbon storage capacity and capture ability in natural ecosystems [3,4]. Vegetated coastal ecosystems (VCEs), including mangroves, seagrass meadows, and tidal marshes, are known as the major “blue carbon” sinks, and hold the potential for higher organic carbon (OC) sequestration than terrestrial forests [4,5].
Mangroves provide multiple ecosystem functions and services, such as coastal protection, water quality improvement, nutrient cycling, carbon sequestration, and fisheries [6,7,8]. Expressed as economic value, the mangrove payment of ecosystem services (PES, ~91,000 $US dollars ha−1) is much greater than that of seagrass meadows (~13,000 $US dollars ha−1) and salt marshes (~1500 $US dollars ha−1) [9]. Serrano et al. [10] reported that the soil carbon stock in 1-m-thick mangroves in Australia was approximately 251 Mg C ha−1, which was 1.5~2.5 times greater than the levels in seagrass meadows (112 Mg C ha−1) and salt marshes (168 Mg C ha−1). In addition, Donato et al. [11] demonstrated carbon storage in mangrove soils to be approximately three to five times higher than that in upland forests in tropical zones. Thus, mangroves play an essential role in sequestering carbon and alleviating climate change.
Recently, mangroves were found to be not only carbon sinks, but also greenhouse gas (GHG) sources [4,12,13]. Frequent tidal inundation has been found to restrict the transportation of oxygen across the soil–air surface, creating anaerobic environments in mangrove soils [14]. Subsequently, the redox potential (ORP) of soils was reduced, and other oxidants were substituted as electron acceptors for microbial metabolism. Thus, anaerobic respiration generally occurs after aerobic respiration as follows: (1) denitrification; (2) manganese, iron, and sulfate reduction reactions; and (3) methanogenesis [15,16]. GHGs, including CO2, N2O, and CH4, may be produced and emitted from soils through a series of microbial respiration processes.
To precisely quantify the carbon budgets and storage in mangrove ecosystems, GHG emissions should not be neglected [3,17,18,19]. Rosentreter et al. [12] demonstrated that carbon storage in carbon dioxide equivalents was reduced by 20% by CH4 emissions, as the global warming potential of CH4 is 28 times as great as that of CO2 over a 100-year time scale. In addition, based on the global methane budget in 2017 [2], wetlands were the major sources of CH4 in natural ecosystems, especially in subtropical and tropical zones. Thus, to establish carbon budgets accurately, CH4 emissions from mangrove ecosystems should be considered for evaluation.
Both biotic (e.g., mangrove tree species) and abiotic (e.g., soil properties) factors play important roles in affecting GHG emissions. Soil properties such as water content, organic matter content, pH, salinity, ORP, and temperature can influence CH4 emissions, as each parameter may regulate microbial processes [18,20,21,22,23]. He et al. [24] reported that the root structure of mangrove trees contributed in various ways to CH4 emissions from soils. The effect of pneumatophores on CH4 emissions remains uncertain. How biotic and abiotic factors influence CH4 emissions must be determined and evaluated.
Mangroves are widely distributed along the western coast of Taiwan. Two dominant mangrove species, Kandelia obovata and Avicennia marina, are distributed on the north and south coasts, respectively. Each of these mangrove species has a unique root structure—prop roots are observed in the K. obovata mangroves and pneumatophores occur in the A. marina mangroves. The pneumatophores of A. marina are known to contribute to CH4 emissions [25,26]. However, compared with soil properties, the relationship between pneumatophores and CH4 emissions remains unclear. Our previous study [13] showed that there was a seasonal variation of CH4 emissions in mangroves, where CH4 emissions were higher in warm seasons (spring and summer). The study by [13] also found that soil properties affected CH4 emissions greatly in K. obovata mangroves, but none of soil properties significantly influenced CH4 emissions in A. marina mangroves. In this study, we hypothesize that CH4 emissions would increase with increasing the pneumatophore density. The objectives of this study were (1) to quantify CH4 emissions from the soils of K. obovata mangroves (prop roots), A. marina mangroves (pneumatophores), and adjoining mudflats; (2) to characterize the effects of soil properties on CH4 emissions in these three types of habitats; and (3) to determine the effects of pneumatophore density on CH4 emissions in A. marina mangroves.
2. Materials and Methods
2.1. Site Description
Nine research sites (Figure 1) were established along the western coast of Taiwan, from north to south: A—Wazihwei (WZ); B—Zhuwei (ZW); C—Xinfeng (XF); D—Zhunan (ZN); E1—Fangyuan Kandelia obovata (FY-K); E2—Fangyuan Avicennia marina (FY-A); F—Budai (BD); G—Beimen (BM); H—Chiku (CK). The climate is dry and mild in winter and humid and hot in summer (Table 1). K. obovata mangroves dominated at Site A, B, C, and D. A. marina mangroves dominated at Site F, G, and H. At Site E, both species were codominant in the mangroves (E1 and E2). Compared with the A. marina mangroves, a higher density and tree height were observed in the K. obovata mangroves (Table 1). The tides at all of the mangroves were semidiurnal tides, and the tidal range was highest at Site E and decreased gradually to the north and south. The mean immersion times at the sites during flood periods ranged from 1.2–18.2 h/day. The soil texture for all of the mangroves was silt. The median grain size was the greatest at Site C (0.058 ± 0.017 mm) and the smallest at Site E1 (0.015 ± 0.001 mm).
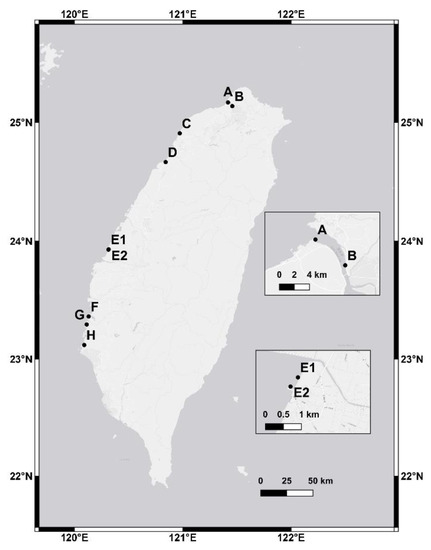
Figure 1.
Location of the research sites: A—Wazihwei (WZ); B—Zhuwei (ZW); C—Xinfeng (XF); D—Zhunan (ZN); E1—Fangyuan Kandelia obovata (FY-K); E2—Fangyuan Avicennia marina (FY-A); F—Budai (BD); G—Beimen (BM); H—Chiku (CK) in Taiwan (map sources: QGIS 2.18.14).

Table 1.
Climatic conditions, mangrove characteristics and soil texture at the mangrove sites: A—Wazihwei (WZ); B—Zhuwei (ZW); C—Xinfeng (XF); D—Zhunan (ZN); E1—Fangyuan Kandelia obovata (FY-K); E2—Fangyuan Avicennia marina (FY-A); F—Budai (BD); G—Beimen (BM); H—Chiku (CK).
2.2. Methane Emissions Measurements
The CH4 emissions and soil properties were determined for a complete seasonal cycle during 2019–2020 (Table 1) within the two species of mangroves and adjoining mudflats (located in the open space outside of mangroves). Mudflats were available for the CH4 measurements at Site C, D, E2, F, and G. The CH4 emission measurements were adapted from Lin et al. [13]. Briefly, we used an ultraportable greenhouse gas analyzer (LGR915-0001, Los Gatos Research, San Jose, CA, USA) connected to an in situ closed-path chamber through a polyvinyl chloride (PVC) tube to determine the CH4 emissions from the soils of the mangroves and mudflats. A semicircular transparent acrylic chamber attached to a stainless-steel ring (with a diameter of 30 cm and height of 15 cm) was placed to avoid crab holes and was inserted into a 10-cm depth of soil to create the air volume (10.6 L) over a 0.071 m2 surface area. The CH4 fluxes within the chamber were monitored by the gas analyzer and were documented by a data logger using a 20-s recording interval in 10-min sessions. The CH4 emissions were determined by Equation (1).
where F is the CH4 emission in terms of µmol CH4 m−2 h−1, S is the slope of linear regression line between CH4 concentration (ppm) and logging time point (20 s), V is chamber volume (L), R is ideal gas constant = 0.082 (L atm K−1 mol−1), T is the absolute temperature (K), A is the bottom area of chamber (i.e., 0.071 m2), and 180 is time conversion constant = (1 h × (60 min/hour) × ((60 s/min)/20 s)). The CH4 detection limit was 0.01 to 100 ppm.
The CH4 emissions were measured from the soils of the mangroves and mudflats with three to five replications (five in most cases) during emersion periods (2 h before and after the low tide) at each site in each season. The distance between each randomly selected soil sample was at least 5 m in order to eliminate any potential interactions between the soil samples resulting from the site disturbance.
Based on our previous research [13], significant CH4 emissions were observed in spring and summer. To determine the effects of pneumatophore density on CH4 emissions, we quantified CH4 emissions under different densities of pneumatophores in A. marina mangroves at Sites E2 and G in spring or summer. The density of pneumatophores was classified into the following four levels: (1) 0–20, (2) 20–50, (3) 50–80, and (4) at least 80 pneumatophores within the chamber (0.071 m2; Figure 2). The measurements of the CH4 emissions at each level were carried out in three replicates. To represent the density of pneumatophores, the number of pneumatophores was divided by the surface area (0.071 m2) of the chamber.

Figure 2.
Photographs of the pneumatophores of A. marina within the chambers (diameter = 30 cm): (a) 0–20, (b) 20–50, (c) 50–80, and (d) >80 pneumatophores (unit: number of pneumatophores in 0.071 m2).
2.3. Soil Sample Analyses
The soil properties in the top 10-cm layer were evaluated after CH4 measurements with five and two replications in the mangroves and mudflats, respectively, at each site in each season. In the field, a portable pH meter (WD-35634-40, OAKTON Instruments, Vernon Hills, IL, USA) was applied to measure the soil pH, and a redox potential meter (ORP30, CLEAN L’eau, Taoyuan City, Taiwan) was used to determine the soil ORP and temperature. As the mangrove soils were wet and free of particles, the instrument manual [28] suggested that the soil pH could be measured in situ. The soil pH and ORP were measured by inserting the meters into the soil at a depth of 5 cm. The soil samples were then collected using stainless-steel cores (with a diameter of 7 cm and length of 80 cm), and syringes (with a diameter of 2.9 cm and length of 5 cm) were used to retrieve the top 10 cm of the soil core samples for two subsamples. One subsample was used for the soil bulk density, water, and organic matter content analysis [13,29], while the other was used for the salinity measurement. These subsamples were transferred into 50-mL centrifuge tubes, stored in a portable cooler, and then transported to the laboratory for further analysis. The subsamples were stored in a −20 °C freezer and were analyzed within two weeks at the laboratory. At the laboratory, the pore water was derived from the soil subsample with a syringe, and the salinity was then measured using a portable refractometer (Refractometer FG-201, Hangzhou Chincan Trading Co., Ltd., Hangzhou, Zhejiang Province, China).
2.4. Statistical Analyses
As the Shapiro–Wilk test results demonstrated that the CH4 emissions and soil property datasets were nonnormally distributed (p-value < 0.05), the nonparametric Kruskal–Wallis test was utilized to determine the differences in CH4 emissions and soil properties among the studied sites and habitat types. When the results showed a significant difference (p-value <0.05), Dunn’s test was implemented as a post hoc analysis to determine which sites, habitat types, or levels differed. Stepwise multiple regression analysis was used to evaluate the effects of the soil properties on the CH4 emissions across the studied sites in the mangrove ecosystems for both species. R software (Version 3.6.1, https://www.r-project.org/) [30] and SigmaPlot 14.0 (Systat Software, San Jose, CA, USA) were applied for the statistical analyses, and p < 0.05 was considered statistically significant in this study.
3. Results
3.1. CH4 Emissions and Soil Properties
In the mangroves and adjoining mudflats, CH4 emissions were significantly different among the studied sites (Figure 3, and Table 2 and Table 3). The mean CH4 fluxes from the mangrove soils ranged from 0.8 to 94.1 µmol-CH4 m−2 h−1. Higher CH4 emissions were observed in the soils of the Avicennia marina mangroves (Figure 3 and Table 2). Compared with the Kandelia obovata mangroves (−1.7 to 16.6 µmol-CH4 m−2 h−1) and mudflats (−0.7 to 7.6 µmol-CH4 m−2 h−1), CH4 emissions were significantly greater in the A. marina mangroves (2.1 to 765.9 µmol-CH4 m−2 h−1; Figure 4). Soil properties other than the soil temperature varied distinctly among the mangroves (Table 2). However, only the soil bulk density, organic matter, and water content differed distinctly among the mudflats.
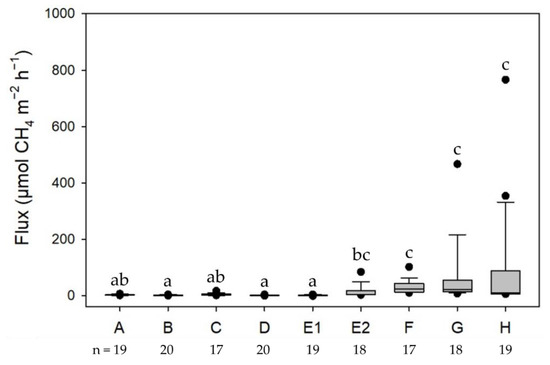
Figure 3.
CH4 emissions from the mangrove soils: A—Wazihwei (WZ); B—Zhuwei (ZW); C—Xinfeng (XF); D—Zhunan (ZN); E1—Fangyuan Kandelia obovata (FY-K); E2—Fangyuan Avicennia marina (FY-A); F—Budai (BD); G—Beimen (BM); H—Chiku (CK). The same letters over different bars suggest no significant differences among sites according to the Kruskal–Wallis test and Dunn’s test.

Table 2.
CH4 emissions and soil characteristics (mean ± standard error) of the mangroves at A—Wazihwei (WZ); B—Zhuwei (ZW); C—Xinfeng (XF); D—Zhunan (ZN); E1—Fangyuan Kandelia obovata (FY-K); E2—Fangyuan Avicennia marina (FY-A); F—Budai (BD); G—Beimen (BM); H—Chiku (CK). Different letters represent significant site differences according to the Kruskal–Wallis test and Dunn’s test at the significance level. ORP—redox potential.

Table 3.
CH4 emissions and soil characteristics (mean ± standard error) of the mudflats at C—Xinfeng (XF); D—Zhunan (ZN); E—Fangyuan (FY); F—Budai (BD); G—Beimen (BM). Different letters represent significant site differences according to the Kruskal–Wallis test and Dunn’s test at the significance level. ORP—redox potential.
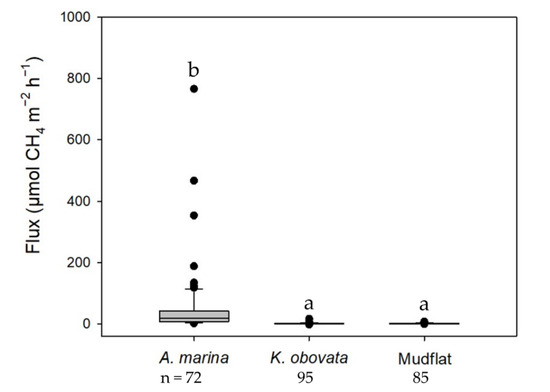
Figure 4.
CH4 emissions from the A. marina and K. obovata mangroves and the adjoining mudflats soils. Different letters represent significant differences among habitats with the Kruskal–Wallis test and Dunn’s test.
3.2. Effects of Soil Properties and Pneumatophores on Methane Emissions in Mangrove Ecosystems
The results of the multiple regressions demonstrated that the soil pH and salinity exerted significant negative effects on CH4 emissions (CH4 flux = −3.19 × pH − 0.50 × salinity + 24.69; p-value < 0.01; R2 = 0.43) in the K. obovata mangroves. There was an exponential relation between salinity and CH4 flux, but a linear relationship between pH and CH4 flux (Figure 5). However, nonsignificant correlations were found between the soil properties and CH4 emissions in the A. marina mangroves. In the mudflats, the CH4 emissions were negatively related to the soil ORP, but positively related to the organic matter content (CH4 flux = −0.01 × ORP + 0.65 × organic matter content − 0.45, p-value < 0.01, R2 = 0.57) (Figure 6). The results demonstrate that the CH4 flux ranged from 4.8 to 161.3 µmol-CH4 m−2 h−1 with 57 to 1814 pneumatophores m−2, and greater numbers of pneumatophores produced higher CH4 emissions in the A. marina mangroves (Figure 7).
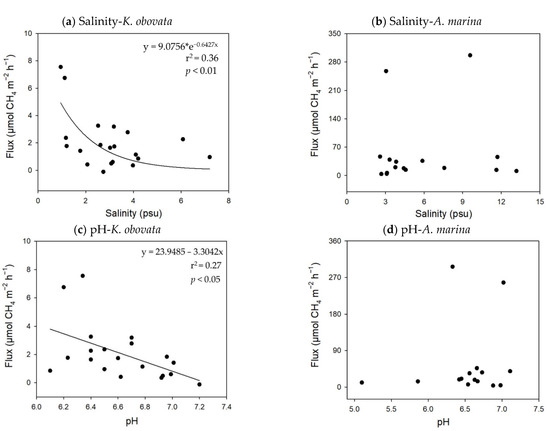
Figure 5.
The relationship between soil salinity, pH, and CH4 flux in the two mangrove species: (a,c) K. obovata (n = 20) and (b,d) A. marina (n = 16).
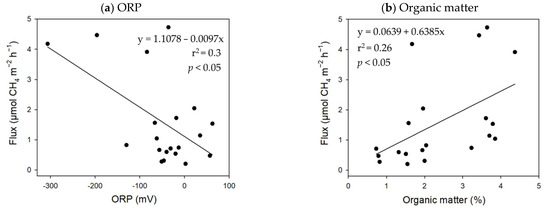
Figure 6.
The relationships between the soil (a) ORP and (b) organic matter with CH4 flux in the mudflats (n = 20).
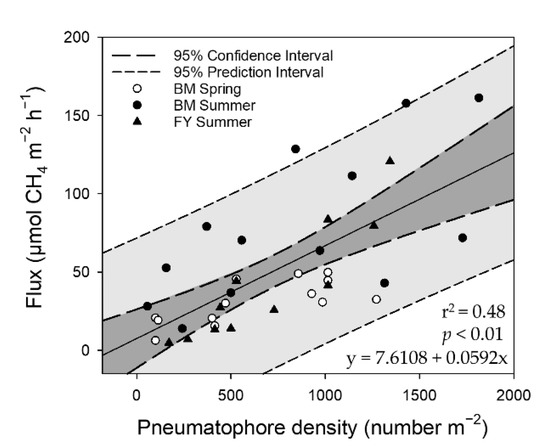
Figure 7.
The relationships between the pneumatophore density and CH4 flux for the A. marina mangroves: E2—Fangyuan (FY); G—Beimen (BM).
4. Discussion
Large variations in CH4 emissions were observed at the nine mangroves (−1.7 to 765.9 µmol-CH4 m−2 h−1; Figure 4) as a result of spatial and habitat differences. However, compared with other studies, CH4 emissions in the studied mangrove ecosystems were still within the range of previous estimates (Kandelia obovata, 0.8 to 72.5 µmol-CH4 m−2 h−1, Chen et al. [31]; Avicennia marina, 0.2 to 1087.5 µmol-CH4 m−2 h−1, Allen et al. [21]; mudflat, 5.6 to 87.5 µmol-CH4 m−2 h−1, Xiang et al. [32]). As a result, the studied mangrove soils were significant sources of CH4 emissions, which should be accounted for in the carbon budgets of mangrove ecosystems.
The current study found that CH4 emissions in the A. marina mangroves were approximately 50- to 100-fold those of the K. obovata mangroves and adjoining mudflats (Figure 4). The CH4 emissions in vegetated soils were generally higher than those in unvegetated soils (mudflats), as more labile organic compounds originating from plants were provided for methanogenic communities [32,33,34]. However, this observation was not necessarily recapitulated in the K. obovata mangroves in this study. This suggests that differences in mangrove species and root structure may lead to various patterns of GHG emissions.
As GHGs were generated mainly as a result of microbial activities that were greatly affected by soil characteristics, GHG emissions were expected to be regulated by the soil properties [31]. Indeed, the multiple regression analyses indicated that the soil salinity and pH in the K. obovata mangroves, and the soil redox potential and organic matter content in the mudflats were the main soil properties influencing CH4 emissions. Previous findings have reported salinity to be inversely related to CH4 emissions in mangroves, with relationships illustrated by either linear [35] or logarithmic regressions [18]. Sulfate is among the most abundant ions in seawater. In high salinity environments, the higher availability of sulfate ions enhanced the activities of sulfate-reducing bacteria, which might inhibit methanogenic bacteria obtaining organic substrates; thus, less CH4 emissions were produced [19,36,37,38,39]. Methanogens have been found to be sensitive to soil pH, and demonstrated better growth in the pH range of 6 to 8 [40,41]. It appears that the soil pH in the K. obovata mangroves was favorable for methanogenesis. The soil organic matter content exhibited a significantly positive correlation with CH4 emissions, as high levels of organic matter provide additional carbon sources for methanogens, generating a greater quantity of CH4 [14,23,42]. In addition, methanogenic bacteria are highly active in lower redox potential conditions [26,30,43]. As a result, higher CH4 emissions were observed from anaerobic soils with a high organic matter in the mudflats.
Compared with the K. obovata mangroves and mudflats, however, the multiple regression results demonstrated that none of the soil properties explained the large proportion of variation in CH4 emissions from the A. marina mangroves. Similar results were also observed by Lin et al. [13]. As the pneumatophores provided gas exchange conduits between soils and the atmosphere, CH4 was likely emitted through the pneumatophores from the deep soil [19,25,26]. Our results showed that the density of pneumatophores in the A. marina mangroves explained approximately 48% of the variation in CH4 emissions (Figure 7). Livesley et al. [26] reported that 24% of the variation in CH4 flux resulted from pneumatophores. Thus, the significance of pneumatophores in transporting CH4 from the soil to the atmosphere in mangroves cannot be neglected. In summary, the soil properties and pneumatophores were the primary factors affecting CH4 emissions in K. obovata and A. marina mangroves, respectively.
To further evaluate the effects of pneumatophore density on CH4 emissions in different climatic regions in A. marina mangroves, our results were compared with other data derived from previous research [25,26,36,44]. The linear regression lines of CH4 emissions on the pneumatophore density presented in previous studies were transformed into the same units (µmol-CH4 m−2 h−1) and were redrawn in this study (Figure 8). Even though the sampling times were different between the studies (Figure 8), the results indicate that the slopes of the regression lines were always steeper in tropical regions than in subtropical or temperate regions, despite the high variation in slopes within tropical regions. As higher temperatures facilitate methanogen activity in soils [18,21], these comparisons suggest that with the same density of pneumatophores, more CH4 is emitted from A. marina mangroves in tropical regions. In addition, CH4 emissions were positively correlated with pneumatophore density in A. marina mangroves across all climate regions.
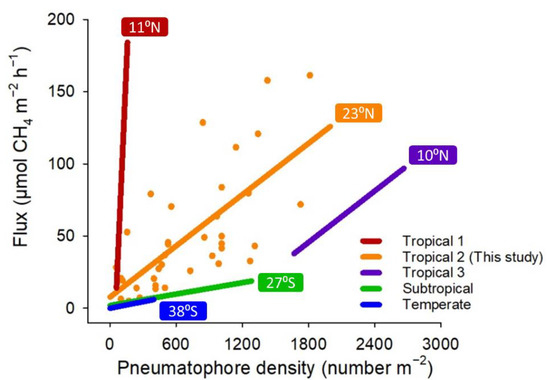
Figure 8.
Comparisons of linear regression lines of CH4 emissions on pneumatophore density in A. marina mangroves in different climatic regions. The numbers in color frames represent the latitudes of the studied sites. Tropical 1: Purvaja et al. [36]; tropical 3: Krithika et al. [25]; subtropical: Kreuzwieser et al. [44]; temperate: Livesley et al. [26]. Sampling time: tropical 1: pre-monsoon, monsoon, post-monsoon, and summer; tropical 3: dry and wet; subtropical: spring and winter; temperate: spring, summer, fall, and winter.
5. Conclusions
In this study, CH4 emissions and soil characteristics were determined in multiple mangroves of Taiwan, including in the Kandelia obovata (subtropical zone) and Avicennia marina (tropical zone) mangroves. Our results showed that CH4 emissions in the A. marina mangroves were significantly greater than those in the K. obovata mangroves and the adjoining mudflats. The multiple regression analyses indicated that the soil salinity and pH in the K. obovata mangroves, and the soil redox potential and organic matter content in the mudflats, were the main soil properties affecting CH4 emissions across the studied mangroves. However, this study also showed that pneumatophore density alone in A. marina mangroves explained approximately 48% of the variation in CH4 emissions. Thus, compared with the assessed soil properties, the contribution of pneumatophores to the transportation of CH4 from the soils was more significant. In addition to the soil properties, our results demonstrated that the root structure may also affect GHG emissions in mangroves.
Author Contributions
Conceptualization, H.-J.L.; data curation, C.-W.L., Y.-C.K., W.-J.L., and C.-W.H.; formal analysis, C.-W.L. and C.-W.H.; funding acquisition, H.-J.L.; investigation, C.-W.L., Y.-C.K., W.-J.L., and C.-W.H.; methodology, H.-J.L.; project administration, H.-J.L.; supervision, H.-J.L.; validation, C.-W.H. and H.-J.L.; writing, C.-W.L. and H.-J.L. All of the authors have read and agreed to the published version of the manuscript.
Funding
The study was granted by the Ministry of Science and Technology (MOST) of Taiwan (106-2621-M-005-005-MY3; 109-2811-M-005-519) and the “Innovation and Development Center of Sustainable Agriculture” from The Featured Areas Research Center Program within the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Acknowledgments
We are grateful for the support of the Ministry of Science and Technology (MOST) of Taiwan under grant no. 106-2621-M-005-005-MY3 and no. 109-2811-M-005-519. This work was also financially supported in part by the “Innovation and Development Center of Sustainable Agriculture” from The Featured Areas Research Center Program within the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Jackson, R.B.; Saunois, M.; Bousquet, P.; Canadell, J.G.; Poulter, B.; Stavert, A.R.; Bergamaschi, P.; Niwa, Y.; Segers, A.; Tsuruta, A. Increasing anthropogenic methane emissions arise equally from agricultural and fossil fuel sources. Environ. Res. Lett. 2020, 15, 071002. [Google Scholar] [CrossRef]
- O’Connor, J.J.; Fest, B.J.; Sievers, M.; Swearer, S.E. Impacts of land management practices on blue carbon stocks and greenhouse gas fluxes in coastal ecosystems—A meta-analysis. Glob. Chang. Biol. 2020, 26, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76. [Google Scholar] [CrossRef]
- Yulianto, G.; Soewardi, K.; Adrianto, L. The role of mangrove in support of coastal fisheries in Indramayu Regency, West Java, Indonesia. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2016, 9, 1020–1029. [Google Scholar]
- Herrera-Silveira, J.A.; Pech-Cardenas, M.A.; Morales-Ojeda, S.M.; Cinco-Castro, S.; Camacho-Rico, A.; Sosa, J.P.C.; Mendoza-Martinez, J.E.; Pech-Poot, E.Y.; Montero, J.; Teutli-Hernandez, C. Blue carbon of Mexico, carbon stocks and fluxes: A systematic review. PeerJ 2020, 8, e8790. [Google Scholar] [CrossRef] [PubMed]
- Nellemann, C.; MacDevette, M.; Manders, T.; Eickhout, B.; Svihus, B.; Prins, A.G.; Kaltenborn, B.P. The Environmental Food Crisis: The Environment’s Role in Averting Future Food Crises; A UNEP Rapid Response Assessment Programme: GRID-Arendal: Arendal, Norway, 2009. [Google Scholar]
- Serrano, O.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I.; Canto, R.; Phinn, S.; Arias-Ortiz, A.; Bai, L.; Baldock, J.; Bedulli, C.; et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 2019, 10, 4313. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.H.; Eyre, B.D. Methane emissions partially offset “blue carbon” burial in mangroves. Sci. Adv. 2018, 4, 4985. [Google Scholar] [CrossRef]
- Lin, C.W.; Kao, Y.C.; Chou, M.C.; Wu, H.H.; Ho, C.W.; Lin, H.J. Methane Emissions from Subtropical and Tropical Mangrove Ecosystems in Taiwan. Forests 2020, 11, 470. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.D.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Pfitzner, J.; Tirendi, F.; Zagorskis, I.; Brunskill, G.J.; Clough, B.F. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar. Geol. 2001, 179, 85–103. [Google Scholar] [CrossRef]
- Li, C.; Mosier, A.; Wassmann, R.; Cai, Z.; Zheng, X.; Huang, Y.; Tsuruta, H.; Boonjawat, J.; Lantin, R. Modeling greenhouse gas emissions from rice-based production systems: Sensitivity and upscaling. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A. Estimating the full greenhouse gas emissions offset potential and profile between rehabilitating and established mangroves. Sci. Total Environ. 2019, 665, 419–431. [Google Scholar] [CrossRef]
- Al-Haj, A.N.; Fulweiler, R.W. A synthesis of methane emissions from shallow vegetated coastal ecosystems. Glob. Chang. Biol. 2020, 26, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuation in three mode of greenhouse gases emission in relation to soil labile carbon pools in degraded mangrove, Sundarban, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef]
- Allen, D.E.; Dalal, R.C.; Rennenberg, H.; Meyer, R.L.; Reeves, S.; Schmidt, S. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Yu, D.; Tam, N.F.; Ye, Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Hernández, M.E.; Junca-Gómez, D. Carbon stocks and greenhouse gas emissions (CH4 and N2O) in mangroves with different vegetation assemblies in the central coastal plain of Veracruz Mexico. Sci. Total Environ. 2020, 741, 140276. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guan, W.; Xue, D.; Liu, L.; Peng, C.; Liao, B.; Hu, J.; Yang, Y.; Wang, X.; Zhou, G. Comparison of methane emissions among invasive and native mangrove species in Dongzhaigang, Hainan Island. Sci. Total Environ. 2019, 697, 133945. [Google Scholar] [CrossRef] [PubMed]
- Krithika, K.; Purvaja, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224. [Google Scholar]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Central Weather Bureau of Taiwan. Available online: https://www.cwb.gov.tw/V8/C/ (accessed on 23 January 2021).
- OAKTON Instruments, IL, USA. Instruction Manual: pH Spear, Oakton 35634-40. Available online: http://www.4oakton.com/Assets/manual_pdfs/35634series.pdf (accessed on 25 February 2021).
- Li, S.B.; Chen, P.H.; Huang, J.S.; Hsueh, M.L.; Hsieh, L.Y.; Lee, C.L.; Lin, H.J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Boil. 2018, 24, 4195–4210. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 11 February 2020).
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, D.; Ding, W.; Yuan, J.; Lin, Y. Invasion chronosequence of Spartina alterniflora on methane emission and organic carbon sequestration in a coastal salt marsh. Atmos. Environ. 2015, 112, 72–80. [Google Scholar] [CrossRef]
- Segarra, K.E.A.; Samarkin, V.; King, E.; Meile, C.; Joye, S.B. Seasonal variations of methane fluxes from an unvegetated tidal freshwater mudflat (Hammersmith Creek, GA). Biogeochemistry 2013, 115, 349–361. [Google Scholar] [CrossRef]
- Wang, H.; Liao, G.; D’Souza, M.; Yu, X.; Yang, J.; Yang, X.; Zheng, T. Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1873–1885. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Valach, A.; Shortt, R.; Kasak, K.; Rey-Sanchez, C.; Hemes, K.S.; Baldocchi, D.; Lai, D.Y. Methane emissions reduce the radiative cooling effect of a subtropical estuarine mangrove wetland by half. Glob. Chang. Biol. 2020, 26, 4998–5016. [Google Scholar] [CrossRef] [PubMed]
- Purvaja, R.; Ramesh, R.; Frenzel, P. Plant-mediated methane emission from an Indian mangrove. Glob. Chang. Biol. 2004, 10, 1825–1834. [Google Scholar] [CrossRef]
- Chauhan, R.; Datta, A.; Ramanathan, A.L.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Li, X.; Mitsch, W.J. Methane emissions from created and restored freshwater and brackish marshes in southwest Florida, USA. Ecol. Eng. 2016, 91, 529–536. [Google Scholar] [CrossRef]
- Cotovicz, L.C., Jr.; Knoppers, B.A.; Brandini, N.; Poirier, D.; Santos, S.J.C.; Abril, G. Spatio-temporal variability of methane (CH4) concentrations and diffusive fluxes from a tropical coastal embayment surrounded by a large urban area (Guanabara Bay, Rio de Janeiro, Brazil). Limnol. Oceanogr. 2016, 61, S238–S252. [Google Scholar] [CrossRef]
- Chang, T.C.; Yang, S.S. Methane emission from wetlands in Taiwan. Atmos. Environ. 2003, 37, 4551–4558. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Wong, Y.S.; Ye, Y. Effect of wastewater discharge on greenhouse gas fluxes from mangrove soils. Atmos. Environ. 2011, 45, 1110–1115. [Google Scholar] [CrossRef]
- Gonsalves, M.J.; Fernandes, C.E.; Fernandes, S.O.; Kirchman, D.L.; Bharathi, P.L. Effects of composition of labile organic matter on biogenic production of methane in the coastal sediments of the Arabian Sea. Environ. Monit. Assess. 2011, 182, 385–395. [Google Scholar] [CrossRef]
- Konnerup, D.; Betancourt-Portela, J.M.; Villamil, C.; Parra, J.P. Nitrous oxide and methane emissions from the restored mangrove ecosystem of the Ciénaga Grande de Santa Marta, Colombia. Estuar. Coast. Shelf Sci. 2014, 140, 43–51. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol. 2003, 5, 423–431. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).