Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings
Abstract
1. Introduction
2. Materials and Methods
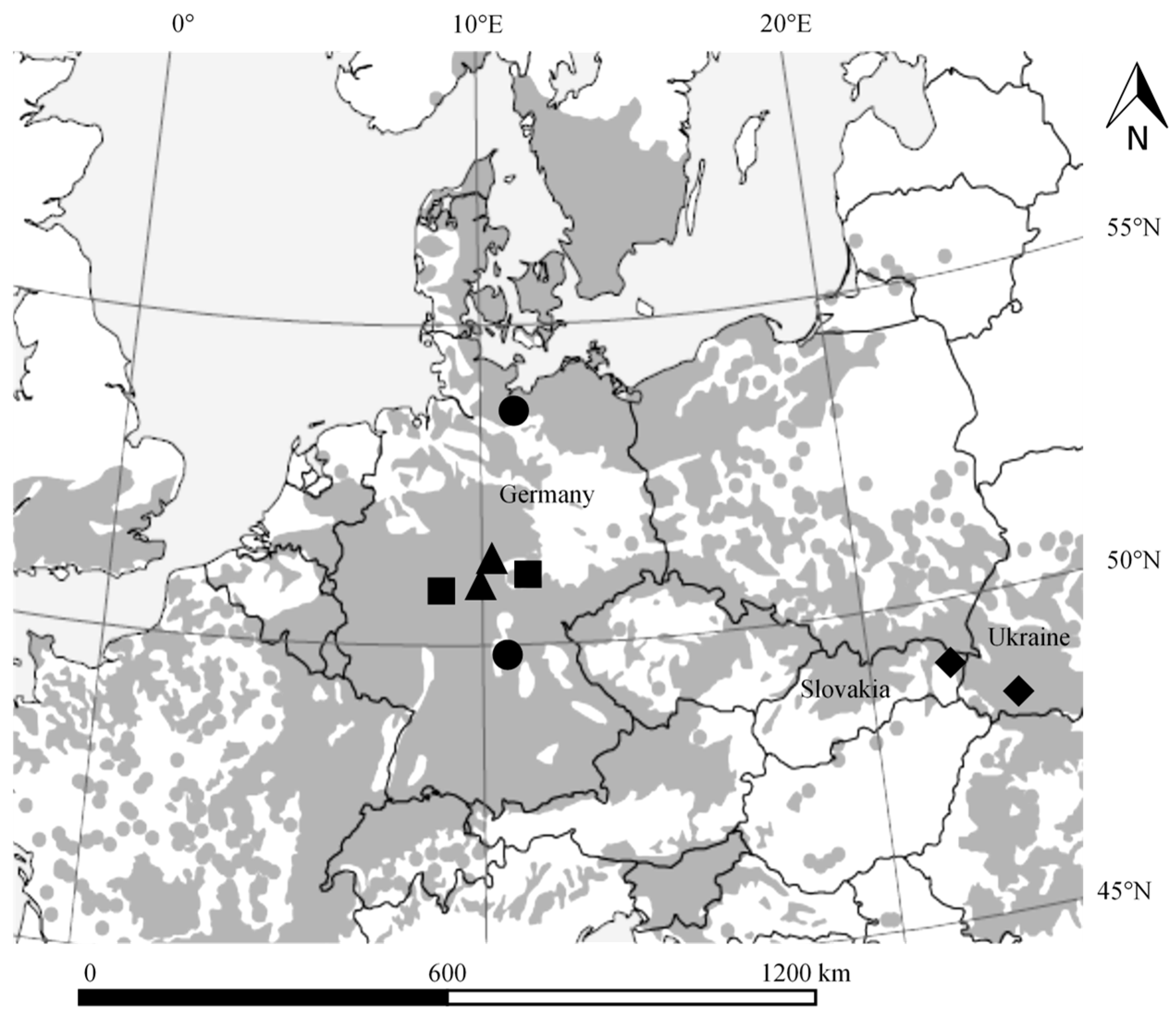
2.1. Study Sites
2.2. Sampling Design and Data Collection (Terrestrial Laser Scanning)
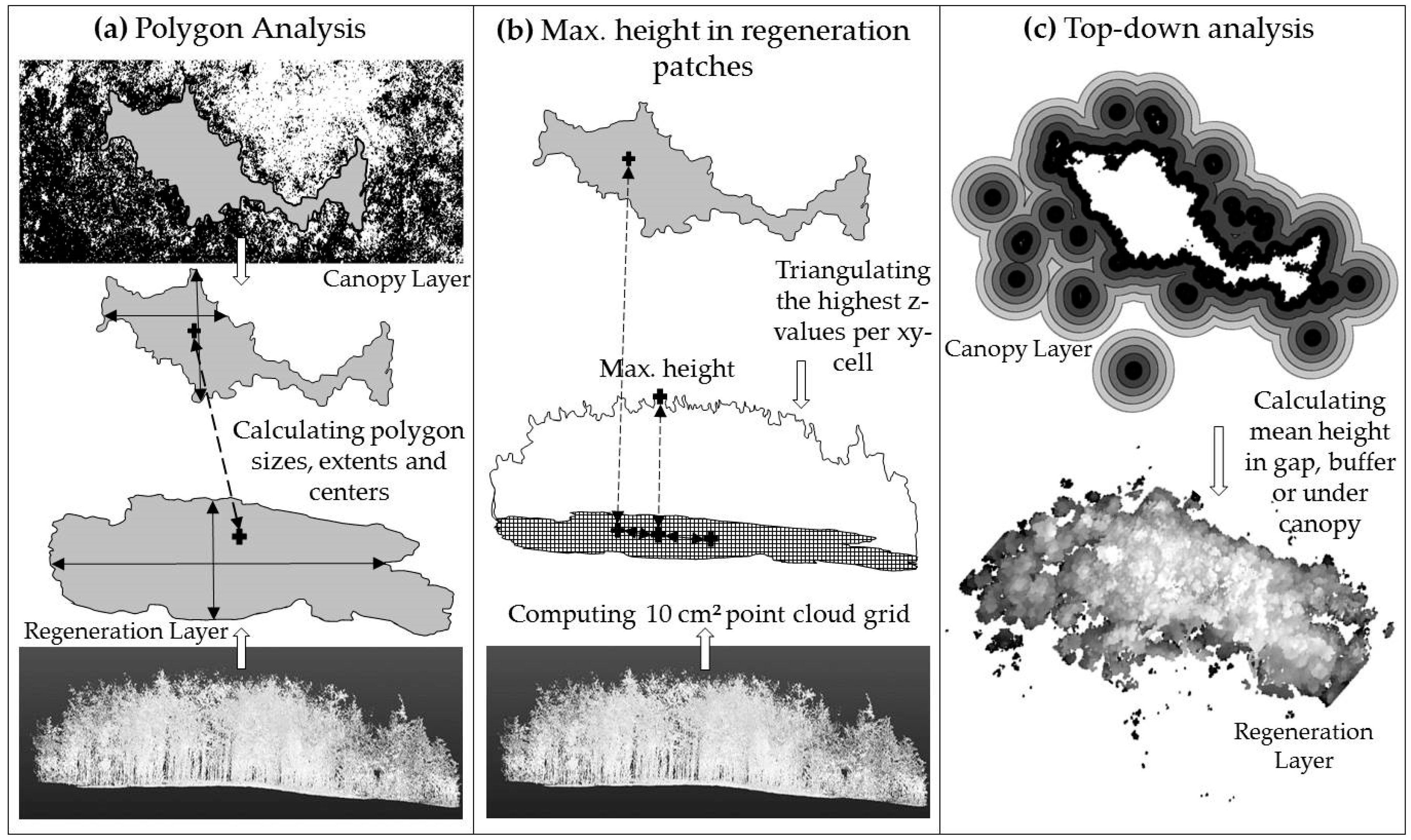
2.3. Data Analysis of Gap and Understory Characteristics—Size, Shape and Center
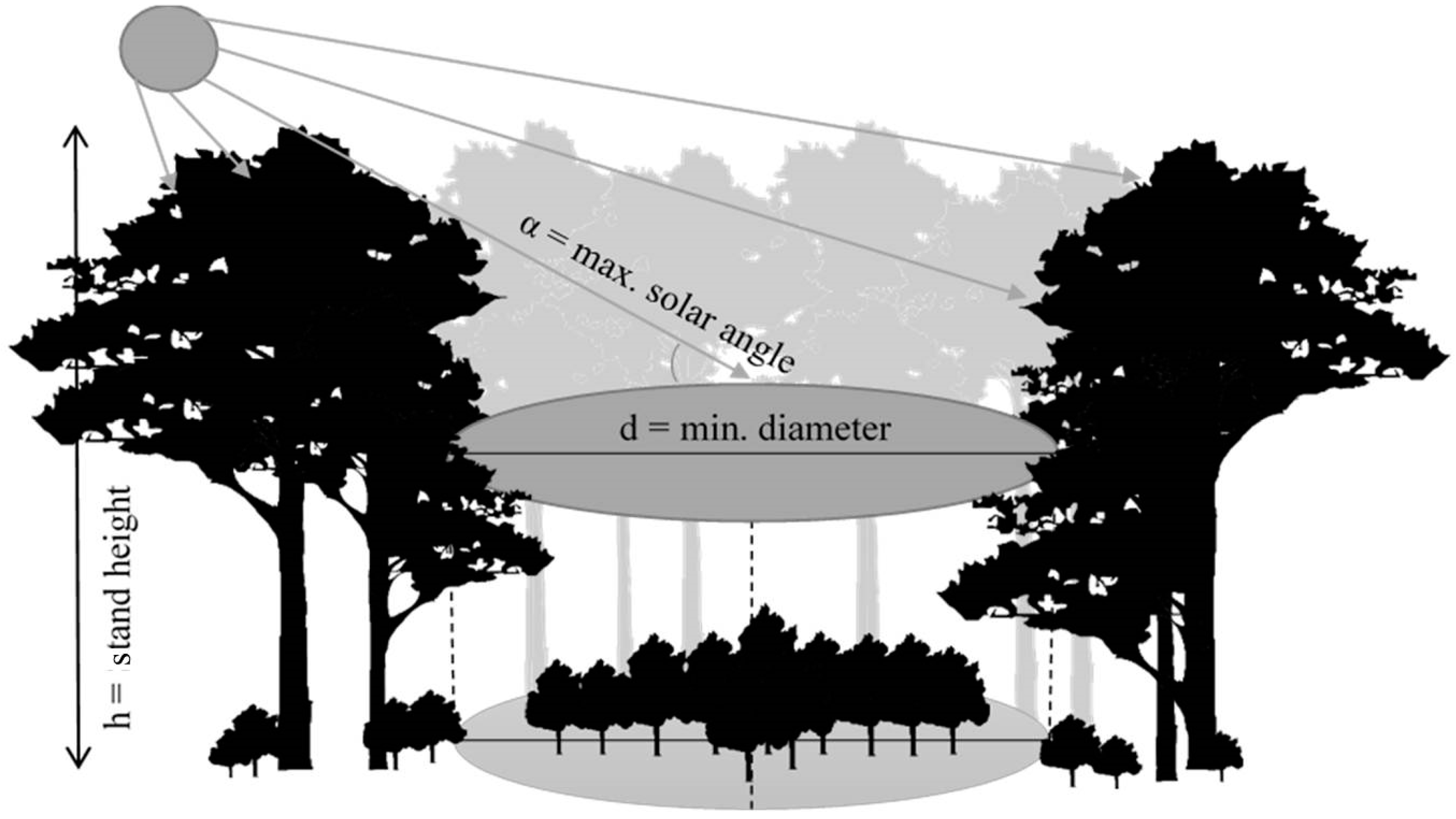
2.3.1. Direct Radiation on the Forest Floor
2.3.2. Top-Down Analysis
2.4. Statistical Analysis
3. Results
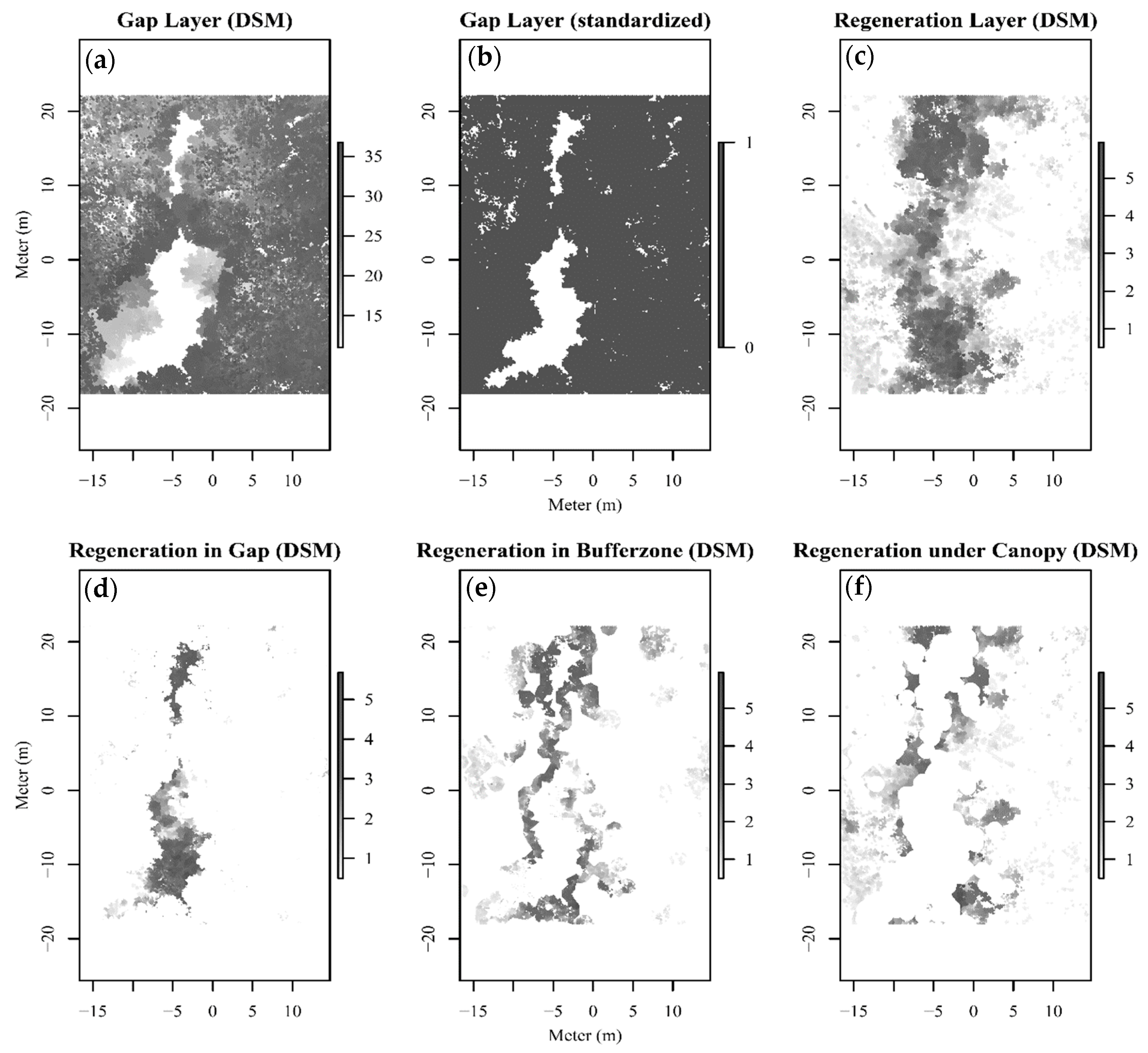
3.1. Gap, Understory and Light Regulating Characteristics
3.2. Regeneration Height in Dependency of Canopy Closure
3.3. Spatial Distribution Pattern of Regeneration Areas
4. Discussion
4.1. Light Availability
4.2. Spatial Distribution Patterns of Regeneration Height
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Löhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention forestry to maintain multifunctional forests: A world perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Nagel, T.A.; Zenner, E.; Brang, P. Research in Old-Growth Forests and Forest Reserves: Implications for Integrated Forest Management. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Freiburg, Germany, 2013; pp. 44–50. [Google Scholar]
- Coates, K.D. Tree recruitment in gaps of various size, clearcuts and undisturbed mixed forest of interior British Columbia, Canada. For. Ecol. Manag. 2002, 155, 387–398. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Meyer, P.; Ammer, C. 6 Anthropogene Störungen 6.1 Waldnutzungen. In Störungsökologie; Wohlgemuth, T., Jentsch, A., Seidl, R., Eds.; UTB GmbH: Stuttgart, Germany, 2019; pp. 273–303. [Google Scholar]
- Diaci, J.; Adamic, T.; Rozman, A. Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: Influences of light regime, herb competition and browsing. For. Ecol. Manag. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Meyer, P.; Tabaku, T.; Lüpke, B.V. Die Struktur albanischer Rotbuchen-Urwälder—Ableitungen für eine naturnahe Buchenwirtschaft. Forstwiss. Cent. 2003, 122, 47–58. [Google Scholar] [CrossRef]
- Drößler, L.; von Lüpke, B. Canopy gaps in two virgin beech forest reserves in Slovakia. J. For. Sci. 2005, 51, 446–457. [Google Scholar] [CrossRef]
- Canham, C.D.; Denslow, J.S.; Platt, W.J.; Runkle, J.R.; Spies, T.A.; White, P.S. Light regimes beneath closed canopies and treefall-gaps in temperate and tropical forests. Can. J. For. Res. 1990, 20, 620–631. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J.G. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. The gap theory in forest dynamics. Bot. Mag. Tokyo 1992, 105, 375–383. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of Eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Brokaw, N.V.L. The definition of treefall gap and its effect on measures of forest dynamics. Biotropica 1982, 11, 158–160. [Google Scholar] [CrossRef]
- Runkle, J.R. Guidelines and Sample Protocol for Sampling Forest Gaps; General Technical Report PNW-GTR-283; Forest Service: Washington, DC, USA, 1992. [Google Scholar]
- Canham, C.D. Growth and canopy architecture of shade-tolerant trees: Response to canopy gaps. Ecology 1988, 69, 786–795. [Google Scholar] [CrossRef]
- Canham, C.D. Different responses to gaps among shade-tolerant tree species. Ecology 1989, 70, 548–550. [Google Scholar] [CrossRef]
- Brown, N. A gradient of seedling growth from the center of a tropical rain forest canopy gap. For. Ecol. Manag. 1996, 82, 239–244. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Coates, K.D. Conifer seedling response to northern temperate forest gaps. For. Ecol. Manag. 2000, 127, 249–269. [Google Scholar] [CrossRef]
- Malcolm, D.C.; Mason, W.L.; Clarke, G.C. The transformation of conifer forests in Britain—Regeneration, gap size and silvicultural systems. For. Ecol. Manag. 2001, 151, 7–23. [Google Scholar] [CrossRef]
- Collet, C.; Lanter, O.; Pardos, M. Effects of canopy opening on the morphology and anatomy of naturally regenerated beech seedlings. Trees 2002, 16, 291–298. [Google Scholar] [CrossRef]
- Mitamura, M.; Yamamura, Y.; Nakano, T. Large-scale canopy opening causes decreased photosynthesis in the saplings of shade-tolerant conifer, Abies veitchii. Tree Physiol. 2008, 29, 137–145. [Google Scholar] [CrossRef]
- Wagner, S.; Fischer, H.; Huth, F. Canopy effects on vegetation caused by harvesting and regeneration treatments. Eur. J. For. Res. 2011, 130, 17–40. [Google Scholar] [CrossRef]
- Emborg, J. Understorey light conditions and regeneration with respect to the structural dynamics of a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 1998, 106, 83–95. [Google Scholar] [CrossRef]
- Petriţan, A.M.; von Lüpke, B.; Petriţan, I.C. Effects of shade on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry 2007, 80, 397–412. [Google Scholar] [CrossRef]
- Petriţan, A.M.; von Lüpke, B.; Petriţan, I.C. Influence of light availability on growth, leaf morphology and plant architecture of beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.) and ash (Fraxinus excelsior L.) saplings. Eur. J. For. Res. 2009, 128, 61–74. [Google Scholar] [CrossRef]
- De Lima, R.A.F. Gap size measurement: The proposal of a new field method. For. Ecol. Manag. 2005, 214, 413–419. [Google Scholar] [CrossRef]
- Green, P.T. Canopy gaps in rain forest on Christmas Island, Indian Ocean: Size distribution and methods of measurement. J. Trop. Ecol. 1996, 12, 427–434. [Google Scholar] [CrossRef]
- Seidel, D.; Ammer, C.; Puettmann, K. Describing forest canopy gaps efficiently, accurately, and objectively: New prospects through the use of terrestrial laser scanning. Agric. For. Meteorol. 2015, 213, 23–32. [Google Scholar] [CrossRef]
- Hobi, M.L.; Ginzler, C.; Commarmot, B.; Bugmann, H. Gap pattern of the largest primeval beech forest of Europe revealed by remote sensing. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Koukoulas, S.; Blackburn, G.A. Quantifying the spatial properties of forest canopy gaps using LiDAR imagery and GIS. Int. J. Remote Sens. 2004, 25, 3049–3072. [Google Scholar] [CrossRef]
- NLF (Niedersächsische Landesforsten): Entscheidungshilfen zur Behandlung und Entwicklung von Buchenbeständen. Available online: https://www.nw-fva.de/fileadmin/user_upload/Verwaltung/Publikationen/Merkblaetter/Bu_Nds_Entscheidungshilfen_zur_Behandlung_und_Entwicklung_von_Buchenbestaenden.pdf (accessed on 30. July 2018).
- Stiers, M.; Willim, K.; Seidel, D.; Ehbrecht, M.; Kabal, M.; Ammer, C.; Annighöfer, P. A quantitative comparison of the structural complexity of managed, lately unmanaged and primary European beech (Fagus sylvatica L.) forests. For. Ecol. Manag. 2018, 430, 357–365. [Google Scholar] [CrossRef]
- Willim, K.; Stiers, M.; Annighöfer, P.; Ammer, C.; Ehbrecht, M.; Kabal, M.; Stillhard, J.; Seidel, D. Assessing understory complexity in beech-dominated Forests (Fagus sylvatica L.)-from managed to primary forests. Sensors 2019, 19, 1684. [Google Scholar] [CrossRef]
- EUFORGEN. Distribution Map of Beech (Fagus Sylvatica). 2009. Available online: http://www.euforgen.de (accessed on 28 May2018).
- Ehbrecht, M.; Schall, P.; Juchheim, J.; Ammer, C.; Seidel, D. Effective number of layers: A new measure for quantifying three-dimensional stand structure based on sampling with terrestrial LiDAR. For. Ecol. Manag. 2016, 380, 212–223. [Google Scholar] [CrossRef]
- Seidel, D.; Beyer, F.; Hertel, D.; Fleck, S.; Leuschner, C. 3D-laser scanning: A non-destructive method for studying above-ground biomass and growth of juvenile trees. Agric. For. Meteorol. 2011, 151, 1305–1311. [Google Scholar] [CrossRef]
- Messier, C. Managing light and understory vegetation in boreal and temperate broadleaf-conifer forests. In Silviculture of Temperate and Boreal Broadleaf-Conifer Mixtures; Land Management, Handbook; Comeau, P.G., Thomas, K.D., Eds.; BC Ministry of Forests: Victoria, BC, Canada, 1996; pp. 59–81. [Google Scholar]
- Kolari, P.; Pumpanen, J.; Kulmala, L.; Ilvesniemi, H.; Nikinmaa, E.; Grönholm, T.; Hari, P. Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For. Ecol. Manag. 2006, 221, 241–248. [Google Scholar] [CrossRef]
- Roussel, J.R.; Auty, D. lidR: Airborne LiDAR Data Manipulation and Visualization for Forestry Applications. R Package Version 2.0.0. 2019. Available online: https://CRAN.R-project.org/package=lidR (accessed on 20 February 2019).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.6–7. 2017. Available online: https://CRAN.R-project.org/package=raster (accessed on 20 February 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 20 February 2019).
- Hobi, M.L.; Commarmot, B.; Bugmann, H. Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J. Veg. Sci. 2015, 26, 323–336. [Google Scholar] [CrossRef]
- Zhu, J.; Matsuzaki, T.; Lee, F.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Beaudet, M.; Messier, C. Growth and morphological responses of yellow birch, sugar maple and beech seedlings growing under a natural light gradient. Can. J. For. Res. 1998, 28, 1007–1015. [Google Scholar] [CrossRef]
- Brokaw, N.V.L.; Busing, R.T. Niche versus chance and tree diversity in forest gaps. Tree 2000, 15, 183–188. [Google Scholar] [CrossRef]
- Runkle, J.R. Gap regeneration in some old-growth forests of the eastern United States. Ecology 1981, 62, 1041–1051. [Google Scholar] [CrossRef]
- Schröter, M.; Härdtle, W.; von Oheimb, G. Crown plasticity and neighborhood interactions of European beech (Fagus sylvatica L.) in an old-growth forest. Eur. J. For. Res. 2012, 131, 787–798. [Google Scholar] [CrossRef]
- Seidel, D.; Ruzicka, K.J.; Puettmann, K. Canopy gaps affect the shape of Douglas-fir crowns in the western Cascades, Oregon. For. Ecol. Manag. 2016, 363, 31–38. [Google Scholar] [CrossRef]
- Wagner, S.; Madsen, P.; Ammer, C. Evaluation of different approaches for modelling individual tree seedling height growth. Trees 2009, 23, 701–715. [Google Scholar] [CrossRef]
- Riegel, G.M.; Miller, R.F.; Krueger, W.C. Competition for resources between understory vegetation and overstory Pinus ponderosa in northeastern Oregon. Ecol. Appl. 1992, 2, 71–85. [Google Scholar] [CrossRef]
- Ammer, C. Response of Fagus sylvatica seedlings to root trenching overstory Picea abies. Scand. J. For. Res. 2002, 17, 408–416. [Google Scholar] [CrossRef]
- Petriţan, I.C.; von Lüpke, B.; Petriţan, A.M. Effects of root trenching of overstorey Norway spruce (Picea abies) on growth and biomass of underplanted beech (Fagus sylvatica) and Douglas fir (Pseudotsuga menziesii) saplings. Eur. J. For. Res. 2011, 130, 813–828. [Google Scholar] [CrossRef]
- Modrý, M.; Hubený, D.; Rejšek, K. Differential response of naturally regenerated European shade tolerant tree species to soil type and light availability. For. Ecol. Manag. 2004, 188, 185–195. [Google Scholar] [CrossRef]
- Rozenbergar, D.; Mikac, S.; Anic, I.; Diaci, J. Gap regeneration patterns in relationship to light heterogeneity in two old-growth beech-fir forest reserves in South East Europe. Forestry 2007, 80, 431–443. [Google Scholar]

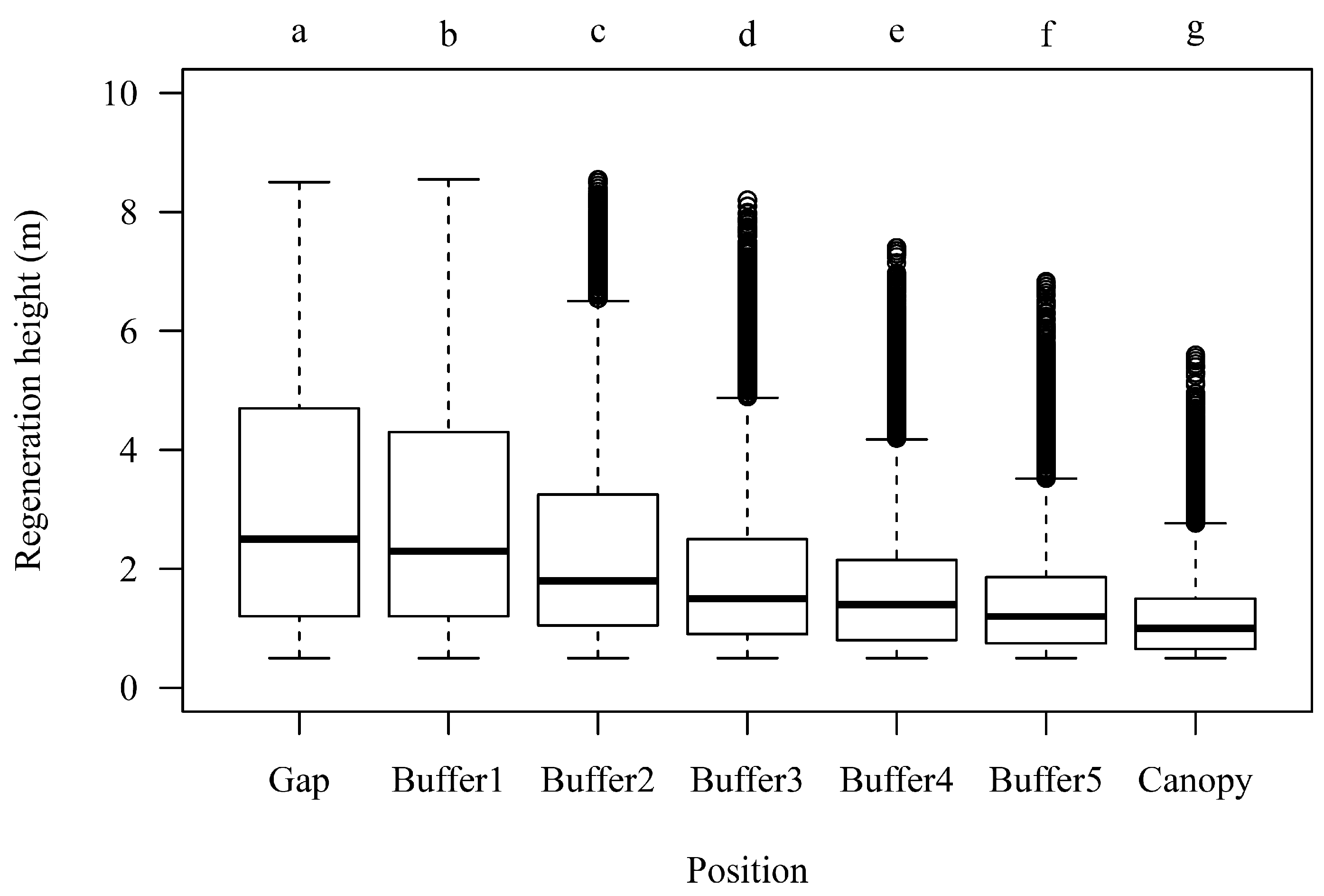
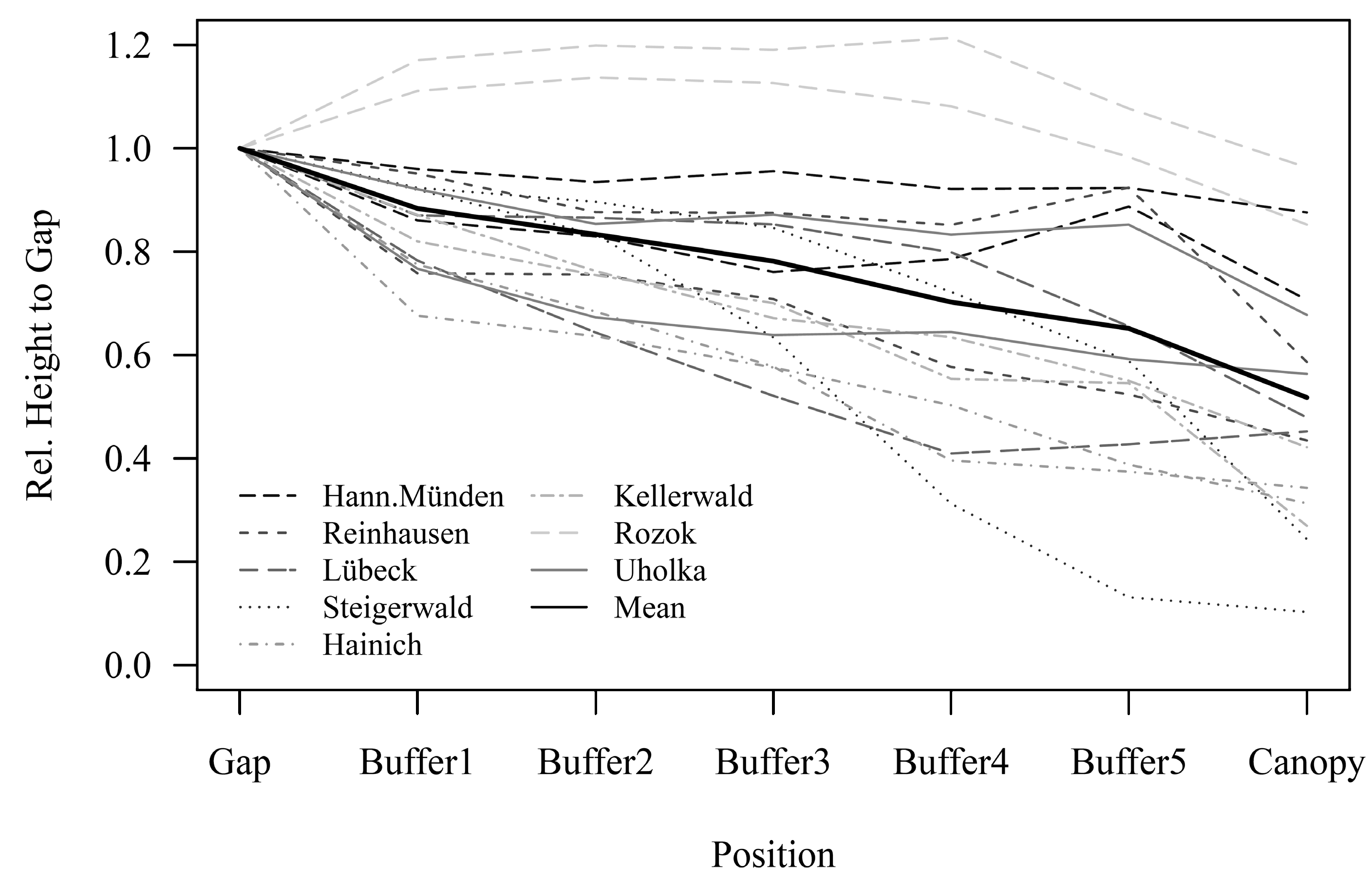
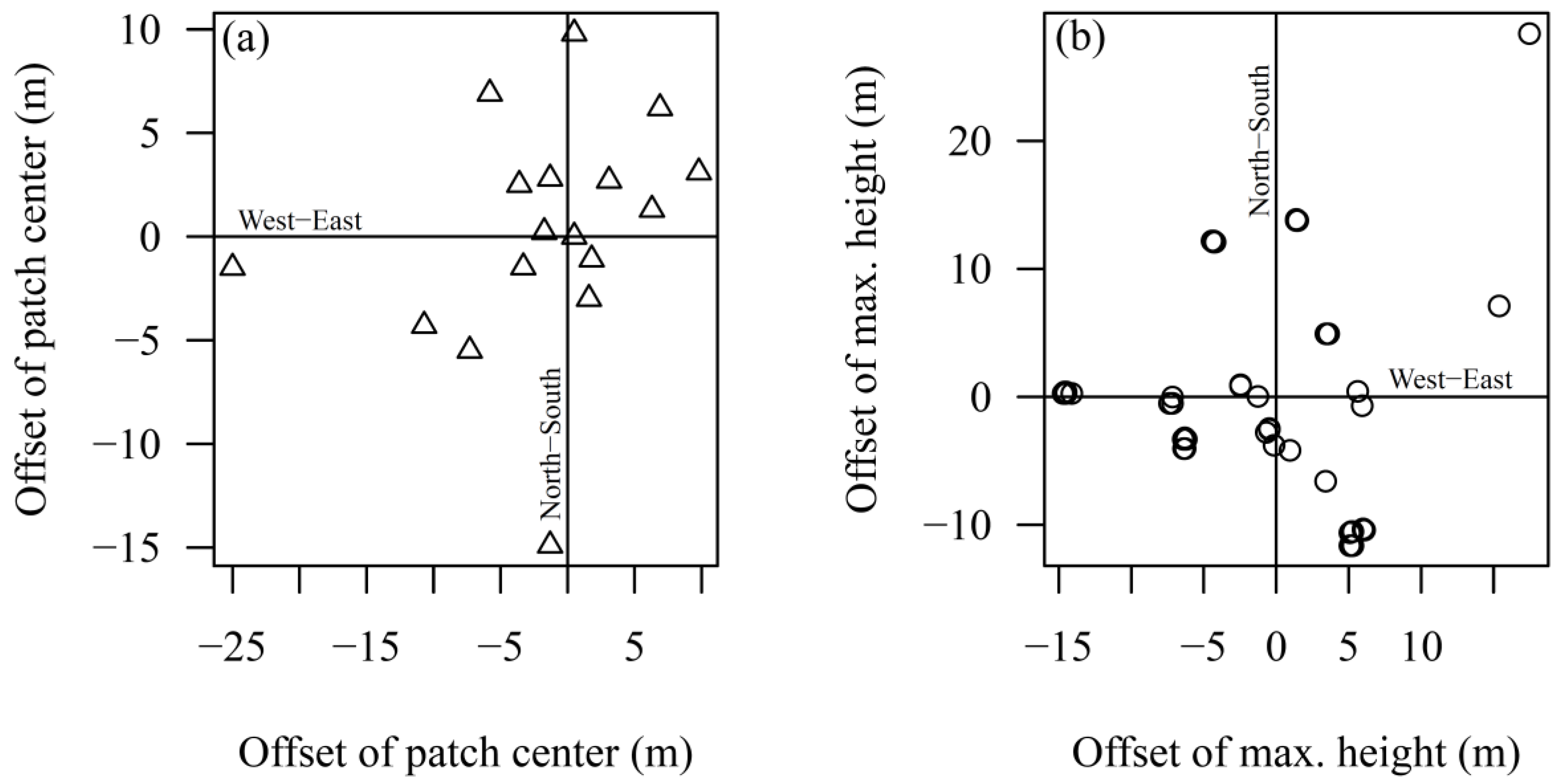
| Country | Management Type | Study Sites | MAT (°C) | MAP (mm) | Elevation (m a.s.l.) | Stand Age (years) |
|---|---|---|---|---|---|---|
| Germany | Traditionally managed | Hann. Münden | 6.5–7.5 | 750–1050 | 270–410 | 81 |
| Reinhausen | 8 | 740 | 190–310 | 98 | ||
| Alternatively managed | Ebrach | 7–8 | 850 | 320–480 | 111 | |
| Lübeck | 8–8.5 | 625–725 | 40–90 | 131 | ||
| National Park (lately unmanaged) | Kellerwald | 6–8 | 600–800 | 540–635 | 184 | |
| Hainich | 7–8 | 600–800 | 330–380 | 183 | ||
| Slovakia | Primary forest (unmanaged) | Rožok | 6–7 | 780 | 580–745 | Uneven-aged |
| Ukraine | Uholka | 7 | 1407 | 700–840 | Uneven-aged |
| Study Area | Management Type | Latitude | Max. Solar Angle | Min. Diameter (m) | Plot | Stand Height (m) | Canopy Gap (m2) | Gap Ratio (d/h) | Max. Extension (m) | Direction-ratio (NS/WO) | Regeneration Area (m2) | Max. Height (m) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | WE | ||||||||||||
| Hann. Münden | Traditional | 51° | 62.43° | 17.20 | 1 | 34.50 | 169.21 | 1.11 | 38.3 | 13.1 | 2.92 | 775.76 | 3.6 |
| 2 | 31.40 | 68.29 | 0.22 | 7.0 | 23.4 | 0.30 | 748.77 | 4.5 | |||||
| 78.22 | 0.51 | 15.9 | 12.4 | 1.28 | |||||||||
| Reinhausen | Traditional | 51° | 62.43° | 17.13 | 1 | 32.40 | 17.10 | 0.15 | 5.0 | 6.0 | 0.83 | 266.71 | 3.9 |
| 36.32 | 0.21 | 6.7 | 9.6 | 0.70 | |||||||||
| 11.76 | 0.18 | 5.9 | 4.0 | 1.48 | |||||||||
| 17.39 | 0.17 | 5.5 | 4.4 | 1.25 | |||||||||
| 56.26 | 0.43 | 13.9 | 11.6 | 1.20 | |||||||||
| 31.06 | 0.25 | 8.0 | 8.8 | 0.91 | |||||||||
| 26.49 | 0.13 | 4.1 | 10.1 | 0.41 | |||||||||
| 2 | 33.20 | 107.75 | 0.58 | 19.3 | 13.0 | 1.48 | 332.87 | 3.6 | |||||
| Ebrach | Alternative | 49° | 64.43° | 18.11 | 1 | 37.85 | 175.27 | 0.49 | 18.4 | 20.6 | 0.89 | 335.69 | 7.5 |
| 125.62 | 0.41 | 15.7 | 13.8 | 1.14 | |||||||||
| 193.13 | 0.82 | 31.0 | 28.4 | 1.10 | |||||||||
| 2 | 37.84 | 41.84 | 0.17 | 6.5 | 15.6 | 0.42 | 429.08 | 8.6 | |||||
| 26.24 | 0.12 | 4.7 | 8.0 | 0.59 | |||||||||
| 54.37 | 0.19 | 7.1 | 12.0 | 0.59 | |||||||||
| 87.74 | 0.25 | 9.6 | 20.8 | 0.46 | |||||||||
| 55.98 | 0.18 | 7.0 | 14.7 | 0.48 | |||||||||
| Lübeck | Alternative | 53° | 60.43° | 19.33 | 1 | 33.85 | 79.65 | 0.35 | 12.0 | 12.5 | 0.96 | 242.04 | 5.5 |
| 2 | 34.29 | 174.34 | 0.59 | 20.2 | 15.4 | 1.31 | 168.33 | 4.8 | |||||
| Kellerwald | National Park | 51° | 62.43° | 16.45 | 1 | 31.75 | 246.03 | 1.19 | 37.8 | 14.1 | 2.68 | 587.77 | 5.9 |
| 2 | 31.25 | 94.23 | 0.40 | 12.4 | 22.5 | 0.55 | 931.79 | 7.6 | |||||
| 96.55 | 0.38 | 12.2 | 12.7 | 0.96 | |||||||||
| 64.78 | 0.37 | 11.6 | 8.3 | 1.40 | |||||||||
| Hainich | National Park | 51° | 62.43° | 19.63 | 1 | 38.45 | 40.31 | 0.24 | 9.1 | 9.8 | 0.93 | 642.79 | 5.1 |
| 2 | 36.75 | 131.21 | 0.58 | 21.4 | 12.0 | 1.78 | 568.66 | 5.7 | |||||
| Rožok | Primary Forest | 48° | 65.43° | 20.25 | 1 | 43.75 | 112.00 | 0.48 | 20.9 | 11.9 | 1.76 | 975.87 | 4.2 |
| 66.60 | 0.37 | 16.1 | 10.6 | 1.52 | |||||||||
| 229.04 | 0.51 | 22.2 | 14.3 | 1.55 | |||||||||
| 2 | 44.85 | 91.22 | 0.35 | 15.6 | 17.6 | 0.89 | 1331.81 | 6.5 | |||||
| 33.77 | 0.17 | 7.7 | 11.0 | 0.70 | |||||||||
| 309.25 | 0.42 | 18.9 | 25.3 | 0.75 | |||||||||
| 38.09 | 0.23 | 10.3 | 8.3 | 1.24 | |||||||||
| Uholka | Primary Forest | 48° | 65.43° | 20.64 | 1 | 45.50 | 86.12 | 0.39 | 17.8 | 10.7 | 1.66 | 596.19 | 5.6 |
| 2 | 44.80 | 475.68 | 0.61 | 27.3 | 27.3 | 1.37 | 736.21 | 7.0 | |||||
| Position | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Plot | Gap | Buffer1 | Buffer2 | Buffer3 | Buffer4 | Buffer5 | Canopy | p | F |
| Hann. Münden | 1 | 1.17 a | 1.12 b | 1.09 c | 1.12 d | 1.08 e | 1.08 e | 1.03 e | 0.000 | 28.02 |
| 2 | 1.63 a | 1.39 b | 1.35 c | 1.24 d | 1.28 e | 1.44 e | 1.15 f | 0.000 | 358.1 | |
| Reinhausen | 1 | 1.89 a | 1.44 b | 1.43 b | 1.34 c | 1.09 d | 0.99 e | 0.82 f | 0.000 | 906.4 |
| 2 | 1.34 a | 1.28 b | 1.18 c | 1.18 c | 1.14 c | 1.24 a | 0.79 d | 0.000 | 214.4 | |
| Lübeck | 1 | 2.38 a | 1.87 b | 1.53 c | 1.24 d | 0.98 e | 1.20 ef | 1.08 f | 0.000 | 1730 |
| 2 | 1.67 a | 1.45 b | 1.45 b | 1.43 b | 1.34 c | 1.09 d | 0.80 e | 0.000 | 450.9 | |
| Ebrach | 1 | 5.13 a | 4.74 b | 4.59 c | 4.34 d | 3.70 e | 3.20 f | 1.25 g | 0.000 | 1510 |
| 2 | 5.54 a | 5.09 b | 4.61 c | 3.51 d | 1.73 e | 0.73 f | 0.57 f | 0.000 | 1035 | |
| Hainich | 1 | 2.63 a | 1.78 b | 1.68 c | 1.57 d | 1.32 e | 1.02 f | 0.82 g | 0.000 | 1237 |
| 2 | 2.90 a | 2.25 b | 1.99 c | 1.68 d | 1.15 e | 1.09 e | 0.99 e | 0.000 | 1370 | |
| Kellerwald | 1 | 4.17 a | 3.63 b | 3.18 c | 2.79 d | 2.64 e | 2.29 f | 1.76 g | 0.000 | 3280 |
| 2 | 5.41 a | 4.44 b | 4.09 c | 3.79 d | 2.99 e | 2.95 e | 1.46 f | 0.000 | 3439 | |
| Rožok | 1 | 1.20 a | 1.33 b | 1.37 c | 1.35 bc | 1.29 d | 1.18 a | 1.02 e | 0.000 | 169.9 |
| 2 | 1.21 a | 1.41 b | 1.45 c | 1.44 cd | 1.46 c | 1.29 e | 1.16 f | 0.000 | 493 | |
| Uholka | 1 | 2.21 a | 1.69 b | 1.49 c | 1.41 d | 1.42 d | 1.31 e | 1.24 f | 0.000 | 1119 |
| 2 | 2.99 a | 2.76 b | 2.56 c | 2.61 c | 2.49 c | 2.55 c | 2.03 d | 0.000 | 167.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiers, M.; Willim, K.; Seidel, D.; Ammer, C.; Kabal, M.; Stillhard, J.; Annighöfer, P. Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings. Forests 2019, 10, 637. https://doi.org/10.3390/f10080637
Stiers M, Willim K, Seidel D, Ammer C, Kabal M, Stillhard J, Annighöfer P. Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings. Forests. 2019; 10(8):637. https://doi.org/10.3390/f10080637
Chicago/Turabian StyleStiers, Melissa, Katharina Willim, Dominik Seidel, Christian Ammer, Myroslav Kabal, Jonas Stillhard, and Peter Annighöfer. 2019. "Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings" Forests 10, no. 8: 637. https://doi.org/10.3390/f10080637
APA StyleStiers, M., Willim, K., Seidel, D., Ammer, C., Kabal, M., Stillhard, J., & Annighöfer, P. (2019). Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings. Forests, 10(8), 637. https://doi.org/10.3390/f10080637






