Early Growth Response of Nine Timber Species to Release in a Tropical Mountain Forest of Southern Ecuador
Abstract
1. Introduction
2. Materials and Methods
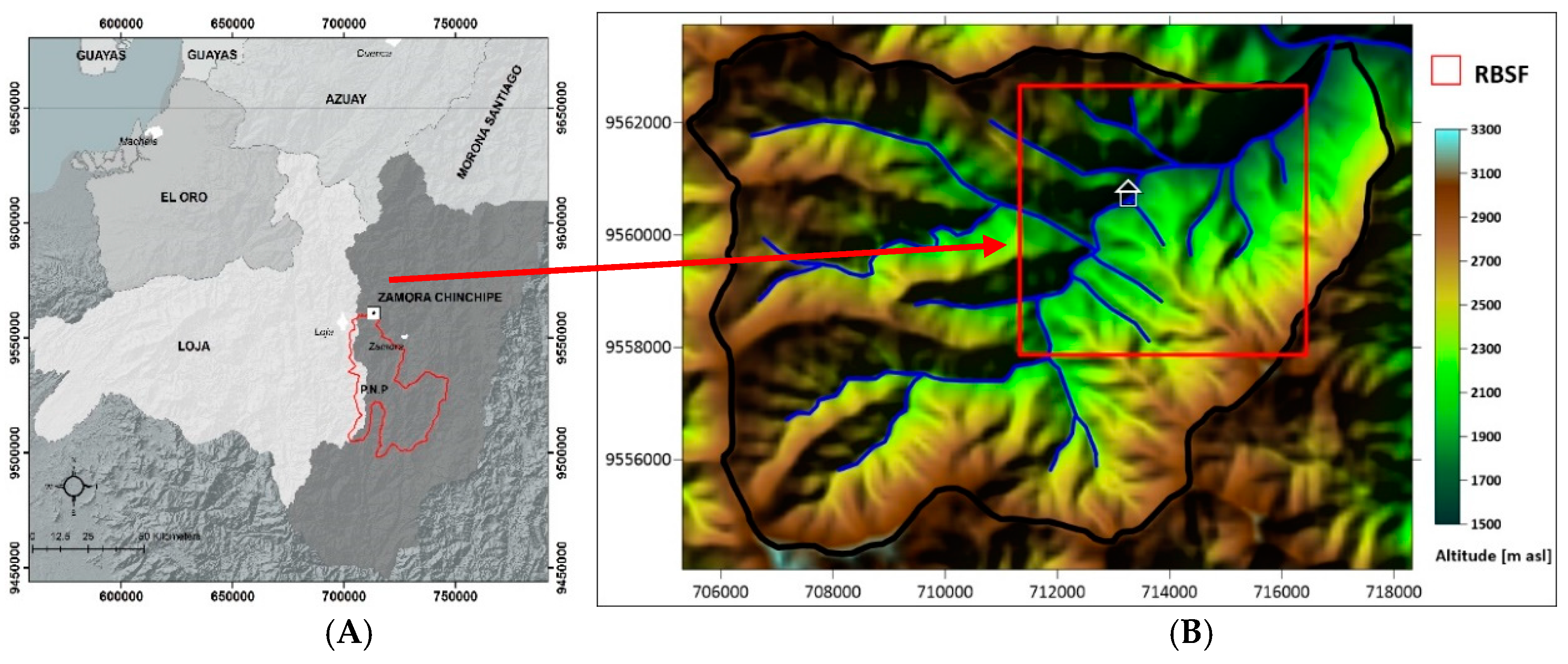
2.1. Study Area
2.2. Experimental Design
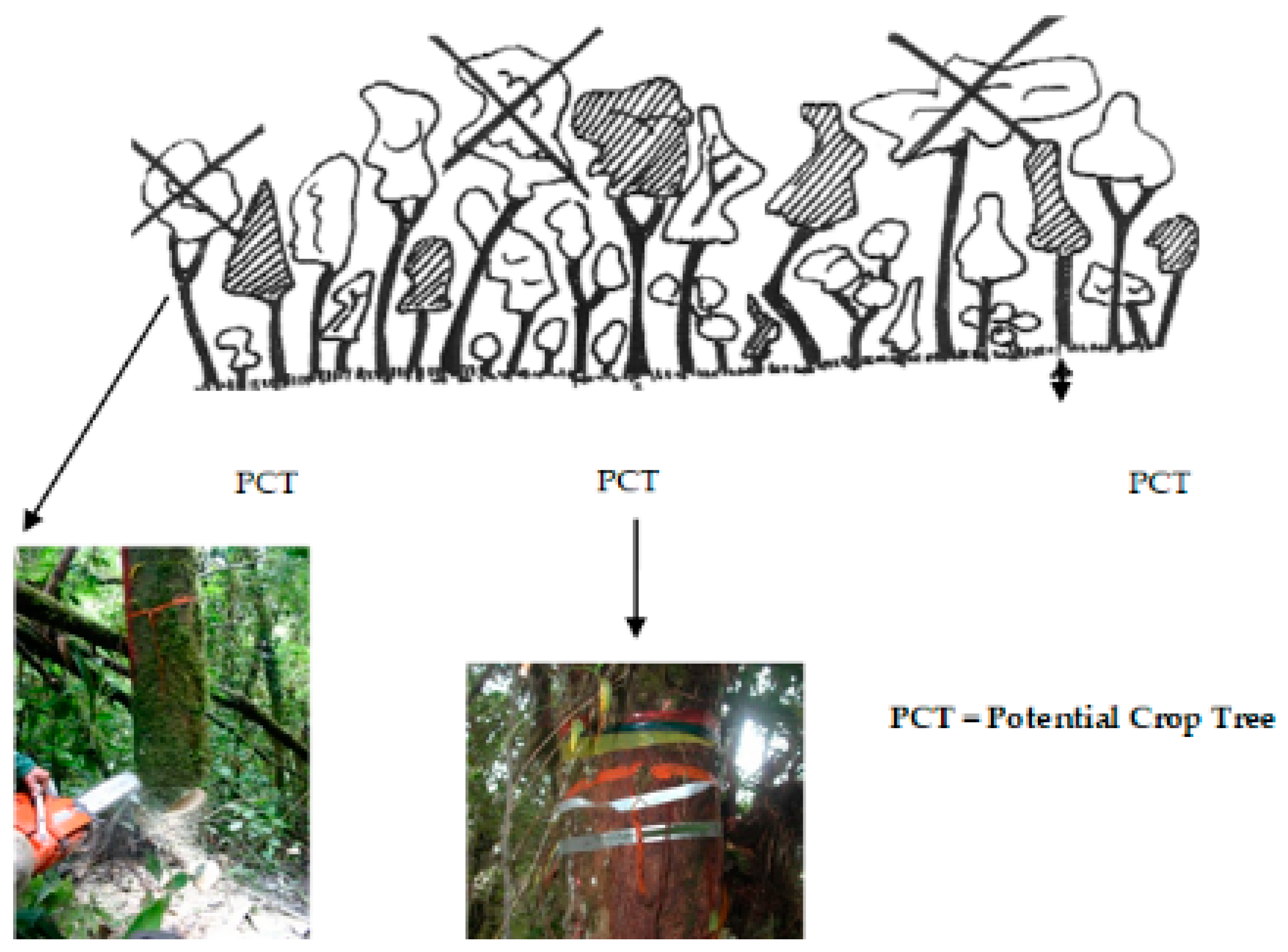
2.3. Silvicultural Treatment
2.4. Data Analysis
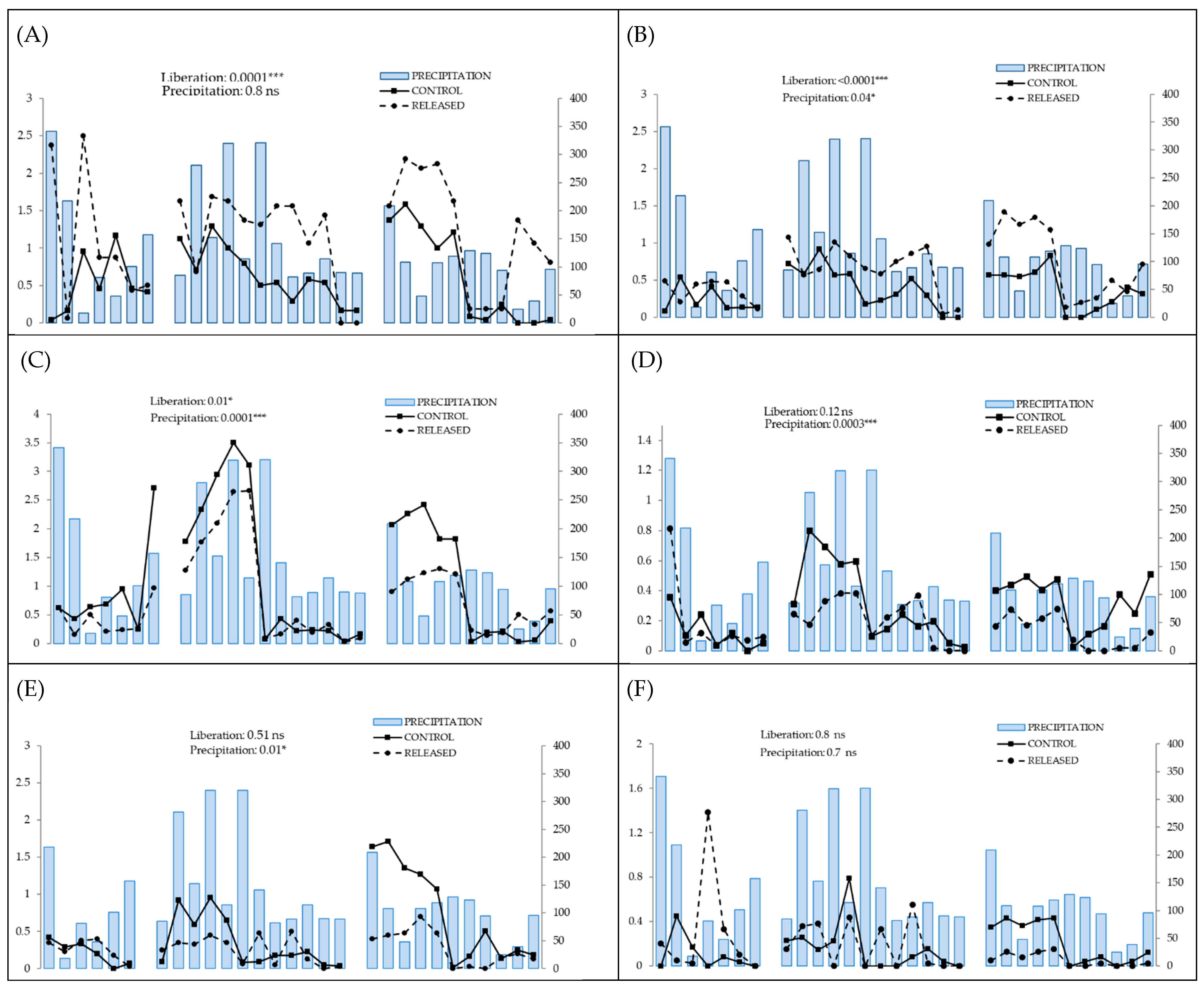
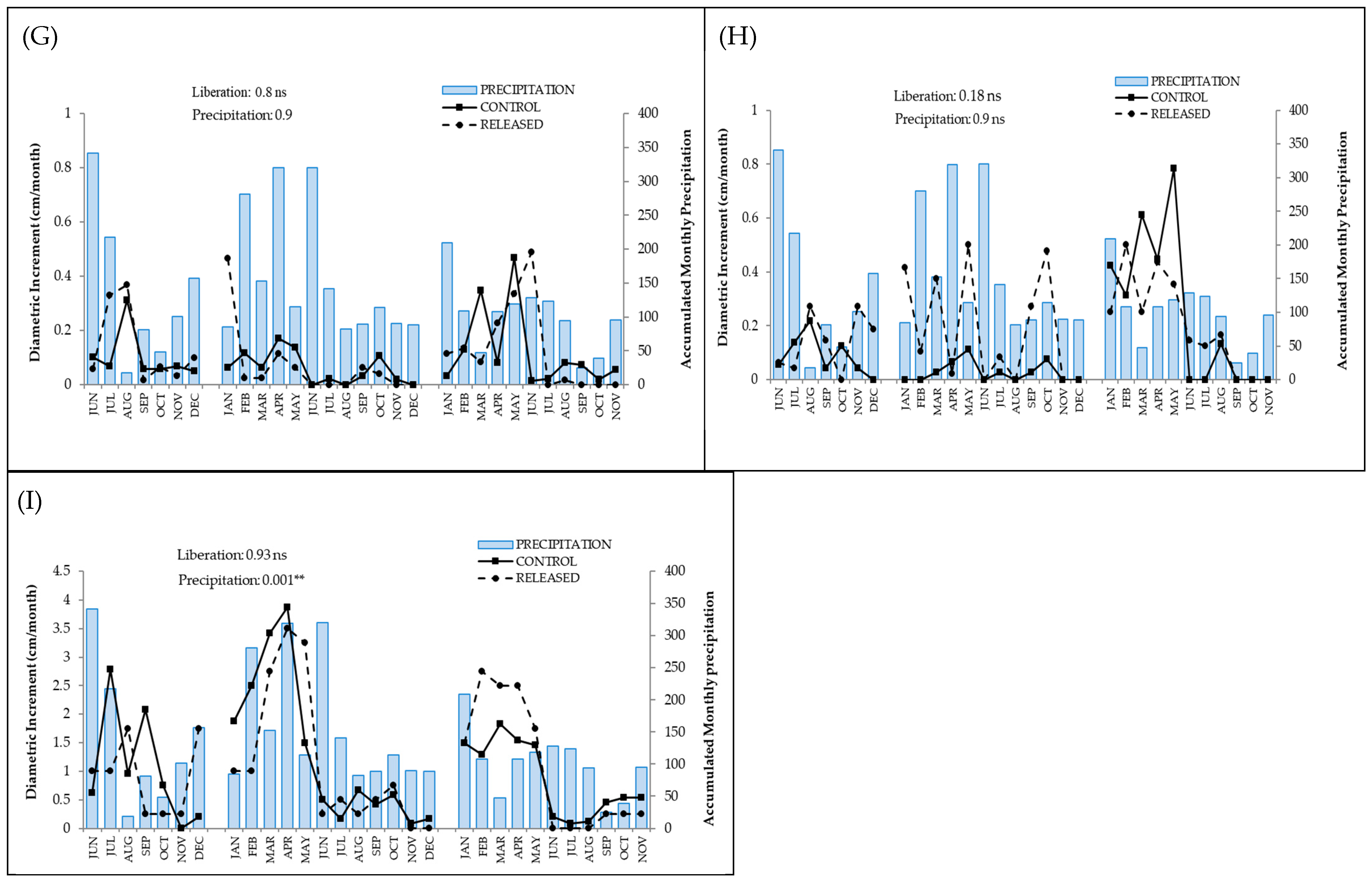
3. Results
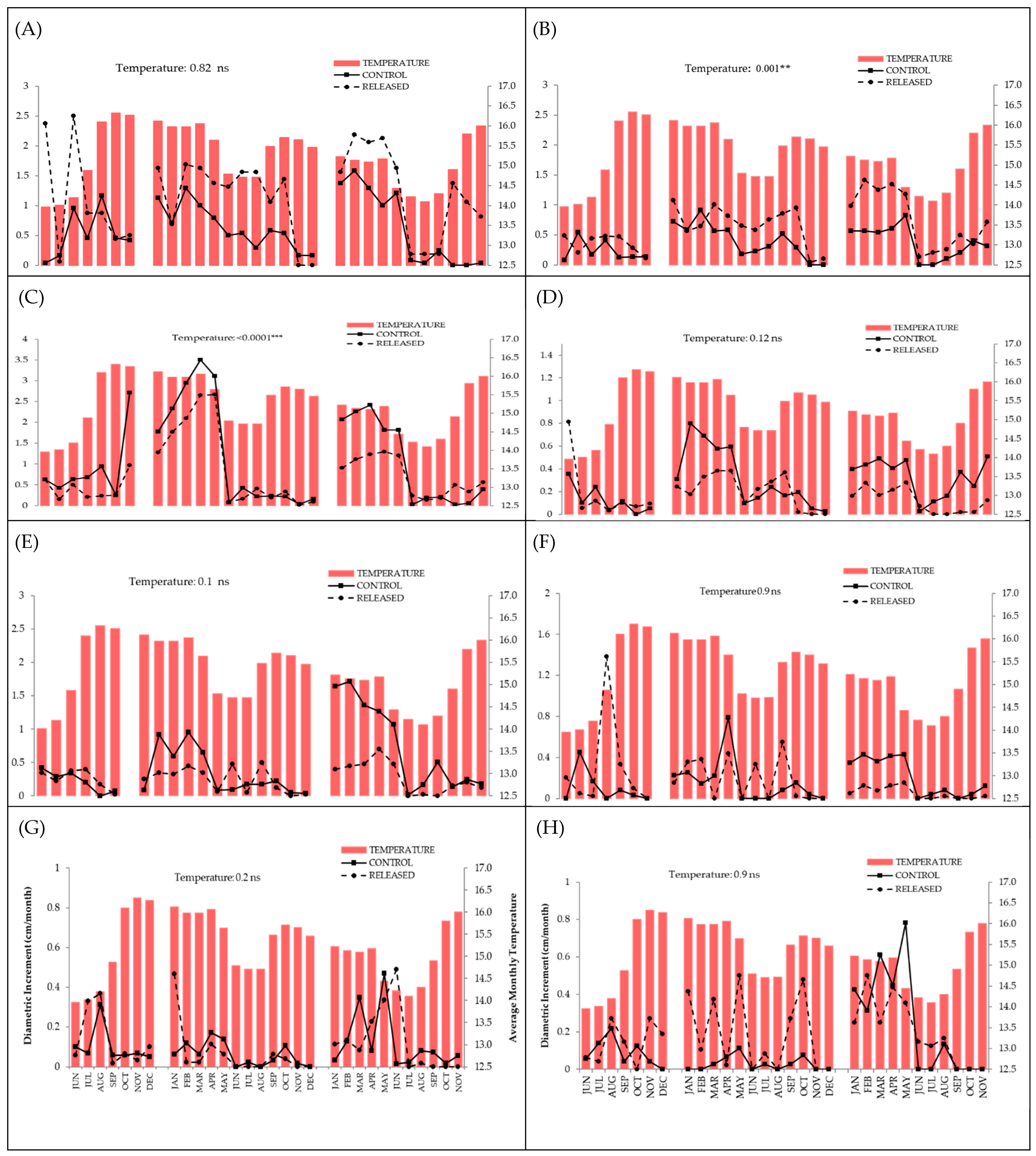
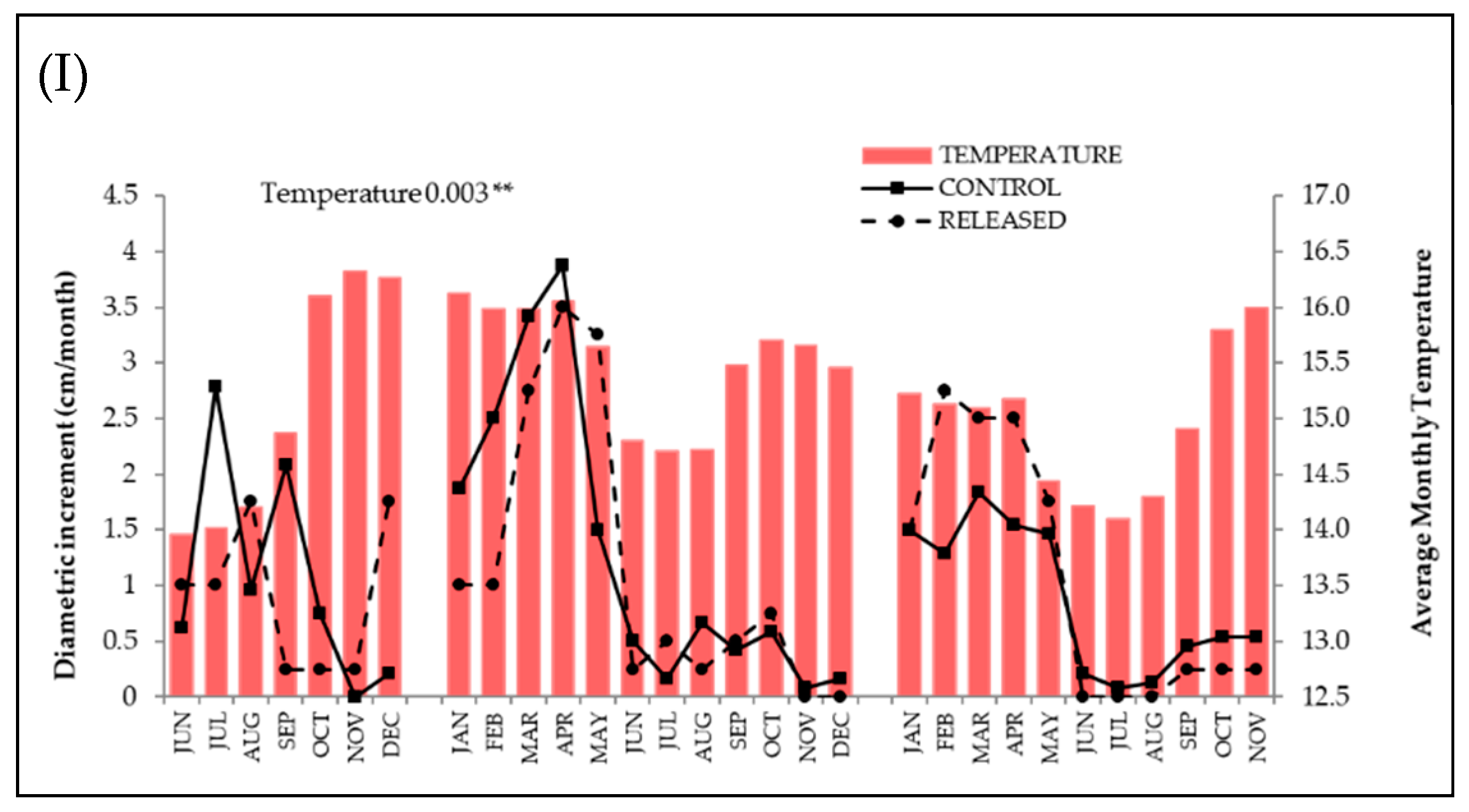
3.1. Positive Response to Release
3.2. Negative Response to Release
3.3. Null Response to Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olander, L.P.; Gibbs, H.K.; Steininger, M.; Swenson, J.J.; Murray, B.C. Reference scenarios for deforestation and forest degradation in support of REDD: A review of data and methods. Environ. Res. Lett. 2008, 3, 025011. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.; Bendix, J.; Kottke, I.; Makeschin, F.; Mosandl, R. Gradients in a Tropical Mountain Ecosystem of Ecuador; Springer Science & Business Media: Berlin, Germany, 2008; ISBN 978-3-540-73526-7. [Google Scholar]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Sprugel, D.G. When branch autonomy fails: Milton’s Law of resource availability and allocation. Tree Physiol. 2002, 22, 1119–1124. [Google Scholar] [CrossRef]
- Malhi, Y.; Phillips, O.L. Tropical forests and global atmospheric change: A synthesis. Philos. Trans. R. Soc. B 2004, 359, 549–555. [Google Scholar] [CrossRef]
- Clark, D.A.; Clark, D.B. Assessing the growth of tropical rain forest trees: Issues for forest modeling and management. Ecol. Appl. 1999, 9, 981–997. [Google Scholar] [CrossRef]
- Canadell, J.G.; Schulze, E.D. Global potential of biospheric carbon management for climate mitigation. Nat. Commun. 2014, 5, 52–82. [Google Scholar] [CrossRef]
- Wadsworth, F.H. Forest Production for Tropical America; USDA Forest Service: Washington, DC, USA, 2000.
- Peña-Claros, M.; Fredericksen, T.S.; Alarcón, A.; Blate, G.M.; Choque, U.; Leaño, C.; Licona, J.C.; Mostacedo, B.; Pariona, W.; Villegas, Z.; et al. Beyond reduced-impact logging: Silvicultural treatments to increase growth rates of tropical trees. For. Ecol. Manag. 2008, 256, 1458–1467. [Google Scholar] [CrossRef]
- Fredericksen, T.S.; Putz, F.E. Silvicultural intensification for tropical forest conservation. Biodivers. Conserv. 2003, 12, 1445–1453. [Google Scholar] [CrossRef]
- Andresen, E.; Pedroza-Espino, L.; Allen, E.B.; Pérez-Salicrup, D.R. Effects of selective vegetation thinning on seed removal in secondary forest succession. Biotropica 2005, 37, 145–148. [Google Scholar] [CrossRef]
- Lampretch, H. Silvicultura en los Trópicos: Dinámica de los Bosques Tropicales Húmedos; Deutshe Gesellschaft für Techinische Zusammenarbeit: Eschborn, Germany; Instituto de Silvicultura de la Universidad de Gottingen: Gottingen, Germany, 1990. [Google Scholar]
- Kammesheidt, L.; Dagang, A.A.; Schwarzwäller, W.; Weidelt, H.J. Growth patterns of dipterocarps in treated and untreated plots. For. Ecol. Manag. 2003, 174, 437–445. [Google Scholar] [CrossRef]
- Wadsworth, F.H.; Zweede, J.C. Liberation: Acceptable production of tropical forest timber. For. Ecol. Manag. 2006, 233, 45–51. [Google Scholar] [CrossRef]
- Vincent, M.; Krause, C.; Zhang, S.Y. Radial growth response of black spruce roots and stems to commercial thinning in the boreal forest. Forestry 2009, 82, 557–571. [Google Scholar] [CrossRef]
- Vitali, V.; Brang, P.; Cherubini, P.; Zingg, A.; Nikolova, P.S. Radial growth changes in Norway spruce montane and subalpine forests after strip cutting in the Swiss Alps. For. Ecol. Manag. 2016, 364, 145–153. [Google Scholar] [CrossRef]
- Adame, P.; Brandeis, T.J.; Uriarte, M. Diameter growth performance of tree functional groups in Puerto Rican secondary tropical forests. For. Syst. 2014, 23, 52–63. [Google Scholar] [CrossRef]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Mohren, G.M.J.; Zuidema, P.A. Understanding recruitment failure in tropical tree species: Insights from a tree-ring study. For. Ecol. Manag. 2014, 312, 108–116. [Google Scholar] [CrossRef]
- Pélissier, R.; Pascal, J.P. Two-year tree growth patterns investigated from monthly girth records using dendrometer bands in a wet evergreen forest in India. J. Trop. Ecol. 2000, 16, 429–446. [Google Scholar] [CrossRef]
- Da Silva, R.P.; dos Santos, J.; Tribuzy, E.S.; Chambers, J.Q.; Nakamura, S.; Higuchi, N. Diameter increment and growth patterns for individual tree growing in Central Amazon, Brazil. For. Ecol. Manag. 2002, 166, 295–301. [Google Scholar] [CrossRef]
- Silva, J.N.M.; de Carvalho, J.D.; do Ca Lopes, J.; De Almeida, B.F.; Costa, D.H.M.; de Oliveira, L.D.; Skovsgaard, J.P. Growth and yield of a tropical rain forest in the Brazilian Amazon 13 years after logging. For. Ecol. Manag. 1995, 71, 267–274. [Google Scholar] [CrossRef]
- Günter, S.; Cabrera, O.; Weber, M.; Stimm, B.; Zimmermann, M.; Fiedler, K.; Knuth, J.; Boy, J.; Wilcke, W.; Lost, S.; et al. Natural forest management in neotropical mountain rain forests—An ecological experiment. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin, Germany, 2008; pp. 347–359. ISBN 978-3-540-73526-7. [Google Scholar]
- Leoni, J.M.; da Fonseca, S.F.; Schöngart, J. Growth and population structure of the tree species Malouetia tamaquarina (Aubl.) (Apocynaceae) in the central Amazonian floodplain forests and their implication for management. For. Ecol. Manag. 2011, 261, 62–67. [Google Scholar] [CrossRef]
- Bendix, J.; Beck, E.; Bräuning, A.; Makeschin, F.; Mosandl, R.; Scheu, S.; Wilcke, W. (Eds.) Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Springer Science & Business Media: Berlín, Germany, 2013; ISBN 978-3-642-38136-2. [Google Scholar]
- Bendix, J.; Trachte, K.; Cermak, J.; Rollenbeck, R.; Nauß, T. Formation of convective clouds at the foothills of the tropical eastern Andes (South Ecuador). J. Appl. Meteorol. Clim. 2009, 48, 1682–1695. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.E.O.C.; Mamani, M.; Huaraca Huasco, W.; Durand, L.; Salinas, N. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 2010, 16, 3176–3192. [Google Scholar] [CrossRef]
- Moser, G.; Leuschner, C.; Hertel, D.; Graefe, S.; Soethe, N.; Iost, S. Elevation effects on the carbon budget of tropical mountain forests (S Ecuador): The role of the belowground compartment. Glob. Chang. Biol. 2011, 17, 2211–2226. [Google Scholar] [CrossRef]
- Werner, F.A.; Homeier, J. Is tropical montane forest heterogeneity promoted by a resource-driven feedback cycle? Evidence from nutrient relations, herbivory and litter decomposition along a topographical gradient. Funct. Ecol. 2015, 29, 430–440. [Google Scholar] [CrossRef]
- Paulick, S.; Dislich, C.; Homeier, J.; Fischer, R.; Huth, A. The carbon fluxes in different successional stages: Modelling the dynamics of tropical montane forests in South Ecuador. For. Ecosyst. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ohl, C.; Bussmann, R. Recolonization of natural landslides in tropical mountain forests of Southern Ecuador. Feddes Repert. 2004, 115, 248–264. [Google Scholar] [CrossRef]
- Brehm, G.; Homeier, J.; Fiedler, K. Beta diversity of geometrid moths Lepidoptera: (Geometridae) in an Andean montane rainforest. Divers. Distrib. 2003, 9, 351–366. [Google Scholar] [CrossRef]
- Curatola Fernández, G.F.; Obermeier, W.A.; Gerique, A.; López Sandoval, M.F.; Lehnert, L.W.; Thies, B.; Bendix, J. Land cover change in the Andes of Southern Ecuador—Patterns and drivers. Remote Sens. 2015, 7, 2509–2542. [Google Scholar] [CrossRef]
- Homeier, J.; Leuschner, C.; Bräuning, A.; Cumbicus, N.L.; Hertel, D.; Martinson, G.O.; Spannl, S.; Veldkamp, E. Effects of nutrient addition on the productivity of montane forests and implications for the carbon cycle. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Bendix, J., Beck, E., Bräuning, A., Makeschin, F., Mosandl, R., Scheu, S., Wilcke, W., Eds.; Springer Science & Business Media: Berlín, Germany, 2013; ISBN 978-3-642-38136-2. [Google Scholar]
- Dislich, C.; Huth, A. Modelling the impact of shallow landslides on forest structure in tropical montane forests. Ecol. Model. 2012, 239, 40–53. [Google Scholar] [CrossRef]
- Fries, A.; Rollenbeck, R.; Bayer, F.; Gonzalez, V.; Oñate-Valivieso, F.; Peters, T.; Bendix, J. Catchment precipitation processes in the San Francisco valley in southern Ecuador: Combined approach using high-resolution radar images and in situ observations. Meteorol. Atmos. Phys. 2014, 126, 13–29. [Google Scholar] [CrossRef]
- Fries, A.; Rollenbeck, R.; Göttlicher, D.; Nauss, T.; Homeier, J.; Peters, T.; Bendix, J. Thermal structure of a megadiverse Andean mountain ecosystem in southern Ecuador and its regionalization. Erdkunde 2009, 321–335. [Google Scholar] [CrossRef]
- Fries, A.; Rollenbeck, R.; Nauß, T.; Peters, T.; Bendix, J. Near surface air humidity in a megadiverse Andean mountain ecosystem of southern Ecuador and its regionalization. Agric. For. Meteorol. 2012, 152, 17–30. [Google Scholar] [CrossRef]
- Schrumpf, M.; Guggenberger, G.; Valarezo, C.; Zech, W. Tropical montane rain forest soils. Development and nutrient status along an altitudinal gradient in the south Ecuadorian Andes. Erde 2001, 132, 43–59. [Google Scholar]
- Wilcke, W.; Oelmann, Y.; Schmitt, A.; Valarezo, C.; Zech, W.; Homeier, J. Soil properties and tree growth along an altitudinal transect in Ecuadorian tropical montane forest. J. Plant Nutr. Soil Sci. 2008, 171, 220–230. [Google Scholar] [CrossRef]
- Martinson, G.O.; Corre, M.D.; Veldkamp, E. Responses of nitrous oxide fluxes and soil nitrogen cycling to nutrient additions in montane forests along an elevation gradient in southern Ecuador. Biogeochemistry 2013, 112, 625–636. [Google Scholar] [CrossRef]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 2009, 63, 337–345. [Google Scholar] [CrossRef]
- De Graaf, N.R.; Poels, R.L.H.; Van Rompaey, R.S.A.R. Effect of silvicultural treatment on growth and mortality of rainforest in Surinam over long periods. For. Ecol. Manag. 1999, 124, 123–135. [Google Scholar] [CrossRef]
- MAE (Ministerio del Ambiente del Ecuador); FAO (Organización de las Naciones Unidas para la Alimentación y la Agricultura, IT). Propiedades Anatómicas, Físicas y Mecánicas de 93 Especies Forestales; MAE: Quito, Ecuador, 2014; 105p.
- Dawkins, H.C. The refining of mixed forest: A new objective for tropical silviculture. Empire For. Rev. 1955, 34, 188–191. [Google Scholar]
- Louman, B.; Valerio, J.; Jiménez, W. Bases ecológicas. Silvicultura de bosques latifoliados húmedos con énfasis en América Central. Ser. Técn. Man. Técn. 2001, 46, 19–78. [Google Scholar]
- Leuschner, C.; Moser, G.; Hertel, D.; Erasmi, S.; Leitner, D.; Culmsee, H.; Schuldt, B.; Schwendenmann, L. Conversion of tropical moist forest into cacao agroforest: Consequences for carbon pools and annual C sequestration. Agrofor. Syst. 2013, 87, 1173–1187. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics; Olkin, I., Ed.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 19 December 2018).
- Vanclay, J.K. Modelling Forest Growth and Yield: Applications to Mixed Tropical Forests; School of Environmental Science and Management Papers; CAB International: Wallingford, UK, 1994; p. 537. ISBN 0 85198 913 6. [Google Scholar]
- Neill, D. Dinámica de bosques amazónicos: Diez años de registro en parcelas permanentes de la Estación Biológica Jatun Sacha. In Resúmenes del tercer Congreso Ecuatoriano de Botánica; Asanza, M., Neill, D., Sandoval, S., Welling, J., Eds.; FUNBOTÁNICA: Quito, Ecuador, 2000; p. 79. [Google Scholar]
- FAO. Evaluación de los Recursos Forestales Mundiales 2010—Informe Nacional Ecuador; FAO: Roma, Italy, 2010. [Google Scholar]
- Gonzalez-Jaramillo, V.; Fries, A.; Rollenbeck, R.; Paladines, J.; Oñate-Valdivieso, F.; Bendix, J. Assessment of deforestation during the last decades in Ecuador using NOAA-AVHRR satellite data. Erdkunde 2016, 70, 217–235. [Google Scholar] [CrossRef]
- Bräuning, A.; Burchardt, I. Detection of growth dynamics in tree species of a tropical mountain rain forest in southern Ecuador. TRACE-Tree Rings Archaeol. Climatol. Ecol. 2006, 4, 127–131. [Google Scholar]
- Bräuning, A.; Homeier, J.; Cueva, E.; Beck, E.; Günter, S. Growth dynamics of trees in tropical mountain ecosystems. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin, Germany, 2008; pp. 347–359. ISBN 978-3-540-73526-7. [Google Scholar]
- Palacios, W.; Jaramillo, N. Ecological forest species groups in Northeastern Ecuador and their importance for the management of indigenous forest. Lyonia 2005, 6, 55–75. [Google Scholar]
- Baker, T.R.; Swaine, M.D.; Burslem, D.F. Variation in tropical forest growth rates: Combined effects of functional group composition and resource availability. Perspect. Plant Ecol. 2003, 6, 21–36. [Google Scholar] [CrossRef]
- Kariuki, M.; Rolfe, M.; Smith, R.G.B.; Vanclay, J.K.; Kooyman, R.M. Diameter growth performance varies with species functional-group and habitat characteristics in subtropical rainforests. For. Ecol. Manag. 2006, 225, 1–14. [Google Scholar] [CrossRef]
- King, D.A.; Davies, S.J.; Supardi, M.N.; Tan, S. Tree growth is related to light interception and wood density in two mixed dipterocarp forests of Malaysia. Funct. Ecol. 2005, 19, 445–453. [Google Scholar] [CrossRef]
- Bare, M.C.; Ashton, M.S. Growth of native tree species planted in montane reforestation projects in the Colombian and Ecuadorian Andes differs among site and species. New For. 2016, 47, 333–355. [Google Scholar] [CrossRef]
- Bendix, J.; Rollenbeck, R.; Reudenbach, C. Diurnal patterns of rainfall in a tropical Andean valley of southern Ecuador as seen by a vertically pointing K-band Doppler radar. Int. J. Climatol. 2006, 26, 829–846. [Google Scholar] [CrossRef]
- Bauch, J.; Quiros, L.; Noldt, G.; Schmidt, P. Study on the wood anatomy, annual wood increment and intra-annual growth dynamics of Podocarpus oleifolius var. macrostachyus from Costa Rica. J. Appl. Bot. Food Qual. 2012, 80, 19–24. [Google Scholar]
- Montoro Girona, M.; Rossi, S.; Lussier, J.M.; Walsh, D.; Morin, H. Understanding tree growth responses after partial cuttings: A new approach. PLoS ONE 2017, 12, e0172653. [Google Scholar] [CrossRef] [PubMed]
- Montoro Girona, M.; Morin, H.; Lussier, J.M.; Walsh, D. Radial growth response of black spruce stands ten years after experimental shelterwoods and seed-tree cuttings in boreal forest. Forests 2016, 7, 240. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
| Species | Q5 PCT | Q5 R | Q3 PCT | Q3 R | Q2 R | Total PCT’s | Total R |
|---|---|---|---|---|---|---|---|
| Cedrela montana | 20 | 14 | 0 | 1 | 7 | 20 | 22 |
| Podocarpus oleifolius | 1 | 0 | 12 | 7 | 10 | 13 | 17 |
| Tabebuia chrysantha | 46 | 14 | 0 | 2 | 25 | 46 | 41 |
| Ficus citrifolia | 4 | 3 | 0 | 0 | 13 | 4 | 16 |
| Nectandra membranacea | 9 | 8 | 5 | 1 | 27 | 14 | 36 |
| Hyeronima asperifolia | 27 | 10 | 1 | 1 | 15 | 28 | 26 |
| Hyeronima moritziana | 3 | 0 | 16 | 10 | 11 | 19 | 21 |
| Clusia ducuoides | 0 | 0 | 37 | 37 | 14 | 37 | 51 |
| Inga acreana | 16 | 5 | 0 | 0 | 16 | 16 | 21 |
| Total | 197 | 251 | |||||
| 448 | |||||||
| Analyzed Variables | Description | Factor |
|---|---|---|
| Diameter Class | Diametric class of the released and reference trees | Class I: 20.1–30.0 cm DBH |
| Class II: 30.1–40.0 cm DBH | ||
| Class III: 40.1–50.0 cm DBH | ||
| Class IV: >50.0 cm DBH | ||
| Treatments | Removed competitors | Released |
| Non-removed competitors | Reference | |
| Period | Time between initial measurement drive by climatic seasons | Period I: 12 months |
| Period II: 24 months | ||
| Period III: 36 months | ||
| Precipitation | Accumulated monthly precipitation | mm/month |
| Temperature | Monthly average temperature | °C/month |
| Species | Treatment | Treatment × Period | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Released | I | II | III | ||||||||||||
| Control | Released | Control | Released | Control | Released | |||||||||||
| SD | SD | SD | Sd | SD | SD | SD | SD | |||||||||
| Inga acreana | 0.56 | 0.47 | 1.13 | 0.72 | 0.43 | 0.36 | 1.01 | 0.81 | 0.64 | 0.36 | 1.16 | 0.61 | 0.63 | 0.66 | 1.22 | 0.78 |
| Hyeronima asperifolia | 0.35 | 0.36 | 0.62 | 0.54 | 0.26 | 0.34 | 0.44 | 0.58 | 0.41 | 0.39 | 0.67 | 0.46 | 0.38 | 0.34 | 0.76 | 0.53 |
| Cedrela montana | 1.16 | 1.21 | 0.87 | 1.24 | 1.20 | 1.23 | 0.93 | 1.38 | 1.25 | 1.02 | 0.98 | 1.55 | 1.03 | 1.32 | 0.70 | 0.63 |
| Tabebuia chrysantha | 0.27 | 0.34 | 0.21 | 0.41 | 0.16 | 0.21 | 0.28 | 0.62 | 0.32 | 0.32 | 0.21 | 0.28 | 0.33 | 0.45 | 0.12 | 0.15 |
| Podocarpus oleifolius | 0.15 | 0.26 | 0.14 | 0.46 | 0.09 | 0.19 | 0.19 | 0.68 | 0.16 | 0.32 | 0.19 | 0.35 | 0.21 | 0.26 | 0.05 | 0.13 |
| Nectandra membranacea | 0.44 | 0.62 | 0.26 | 0.32 | 0.25 | 0.30 | 0.26 | 0.28 | 0.34 | 0.43 | 0.25 | 0.30 | 0.76 | 0.90 | 0.27 | 0.38 |
| Clusia ducuoides | 0.10 | 0.16 | 0.10 | 0.21 | 0.11 | 0.15 | 0.10 | 0.15 | 0.06 | 0.10 | 0.07 | 0.18 | 0.12 | 0.20 | 0.13 | 0.29 |
| Hyeronima moritziana | 0.12 | 0.23 | 0.17 | 0.22 | 0.10 | 0.20 | 0.12 | 0.16 | 0.03 | 0.06 | 0.19 | 0.27 | 0.25 | 0.31 | 0.20 | 0.20 |
| Ficus citrifolia | 1.11 | 1.04 | 1.09 | 1.22 | 1.12 | 0.92 | 1.06 | 1.21 | 1.31 | 1.37 | 1.15 | 1.32 | 0.87 | 0.69 | 1.07 | 1.18 |
| Species | Periods | Class 1 | Class 2 | Class 3 | Class 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Released | Control | Released | Control | Released | Control | Released | ||
| Inga acreana | 1 | 0.43 | 1.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyeronima asperifolia | 0.48 | 0.45 | 0.15 | 0.43 | 0.15 | 0.78 | 0.04 | 0.1 | |
| Cedrela montana | 1.01 | 0.81 | 1.39 | 0.47 | 1.36 | 1.5 | 0 | 0 | |
| Tabebuia chrysantha | 0.28 | 0.72 | 0.14 | 0.11 | 0.15 | 0.07 | 0.08 | 0.23 | |
| Nectandra membranacea | 0.16 | 0.34 | 0.27 | 0.17 | 0.31 | 0.17 | 0 | 0 | |
| Podocarpus oleifolius | 0.17 | 0.12 | 0.07 | 0.02 | 0.03 | 0.42 | 0 | 0 | |
| Ficus subandina | 1.00 | 0.88 | 1.24 | 1.25 | 0 | 0 | 0 | 0 | |
| Clusia ducuoides | 0.13 | 0.15 | 0.06 | 0.08 | 0.13 | 0.08 | 0 | 0 | |
| Hyeronima moritziana | 0.12 | 0.18 | 0.11 | 0.06 | 0 | 0 | 0 | 0 | |
| Inga acreana | 2 | 0.64 | 1.16 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyeronima asperifolia | 0.45 | 0.73 | 0.43 | 0.67 | 0.35 | 0.83 | 0 | 0.46 | |
| Cedrela montana | 1.20 | 0.96 | 1.31 | 0.65 | 1.25 | 1.33 | 0 | 0 | |
| Tabebuia chrysantha | 0.37 | 0.44 | 0.32 | 0.12 | 0.19 | 0.14 | 0.42 | 0.13 | |
| Nectandra membranacea | 0.35 | 0.41 | 0.20 | 0.08 | 0.46 | 0.08 | 0 | 0 | |
| Podocarpus oleifolius | 0.08 | 0.10 | 0.06 | 0.04 | 0.33 | 0.42 | 0 | 0 | |
| Ficus subandina | 1.35 | 0.96 | 1.27 | 1.33 | 0 | 0 | 0 | 0 | |
| Clusia ducuoides | 0.07 | 0.06 | 0.11 | 0.05 | 0 | 0.08 | 0 | 0 | |
| Hyeronima moritziana | 0.05 | 0.25 | 0.03 | 0.13 | 0 | 0 | 0 | 0 | |
| Inga acreana | 3 | 0.63 | 1.22 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyeronima asperifolia | 0.49 | 0.82 | 0.20 | 0.78 | 0.44 | 0.86 | 0 | 0.57 | |
| Cedrela montana | 0.99 | 0.74 | 1.06 | 0.64 | 1 | 0.73 | 0 | 0 | |
| Tabebuia chrysantha | 0.47 | 0.09 | 0.16 | 0.15 | 0.10 | 0.06 | 0.59 | 0.18 | |
| Nectandra membranacea | 0.37 | 0.50 | 0.56 | 0.05 | 1.34 | 0.05 | 0 | 0 | |
| Podocarpus oleifolius | 0.11 | 0.16 | 0.20 | 0 | 0.30 | 0 | 0 | 0 | |
| Ficus subandina | 0.86 | 1.18 | 0.88 | 0.95 | 0 | 0 | 0 | 0 | |
| Clusia ducuoides | 0.13 | 0.15 | 0.09 | 0.05 | 0.14 | 0.18 | 0 | 0 | |
| Hyeronima moritziana | 0.30 | 0.20 | 0.17 | 0.20 | 0 | 0 | 0 | 0 | |
| Species | Release | Precipitation | Temperature | Diametric Class | Release × Diametric Class | Release × Precipitation | Release × Temperature | Diametric Class × Precipitation | Diametric Class × Temperature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch | p | Ch | p | Ch | p | Ch | p | Ch | p | Ch | p | Ch | p | Ch | p | Ch | p | |
| Inga acreana | 14.9 | ≤0.0001 | 0.05 | 0.81 | 0.05 | 0.83 | ─ | ─ | ─ | ─ | 0.18 | 0.66 | 0.99 | 0.31 | ─ | ─ | ─ | ─ |
| Hyeronima asperifolia | 33.5 | ≤0.0001 | 4.1 | 0.04 | 9.8 | 0.001 | 16.7 | 0.0008 | 5.01 | 0.08 | 0.0 | 0.99 | 0.27 | 0.6 | 0.42 | 0.9 | 3.5 | 0.31 |
| Cedrela montana | 6.2 | 0.01 | 15.1 | ≤0.0001 | 31.9 | ≤0.0001 | 4.9 | 0.08 | 1.5 | 0.21 | 1.01 | 0.31 | 2.3 | 0.12 | 4.5 | 0.1 | 5.9 | 0.05 |
| Tabebuia chrysantha | 2.3 | 0.12 | 13.5 | 0.0002 | 1.74 | 0.18 | 26.3 | ≤0.0001 | 4.04 | 0.39 | 1.9 | 0.16 | 0.39 | 0.52 | 13.5 | 0.003 | 0.62 | 0.88 |
| Podocarpus oleifolius | 0.04 | 0.83 | 0.09 | 0.75 | 0.009 | 0.92 | 8.6 | 0.01 | 1.6 | 0.5 | 0.34 | 0.6 | 0.2 | 0.65 | 0.07 | 0.96 | 0.89 | 0.64 |
| Nectandra membranacea | 5.5 | 0.01 | 14.9 | ≤0.0001 | 31.8 | ≤0.0001 | 1.4 | 0.49 | 0.12 | 0.72 | 2.7 | 0.9 | 3.5 | 0.06 | 0.2 | 0.9 | 2.7 | 0.2 |
| Clusia ducuoides | 0.02 | 0.86 | 0.0004 | 0.98 | 1.3 | 0.24 | 2.4 | 0.3 | 0.91 | 0.63 | 0.09 | 0.76 | 1.1 | 0.3 | 2.2 | 0.33 | 4.7 | 0.09 |
| Hyeronima moritziana | 1.7 | 0.19 | 0.0008 | 0.98 | 0.0006 | 0.98 | 2.4 | 0.3 | 0.07 | 0.79 | 0.45 | 0.5 | 3.4 | 0.05 | 0.64 | 0.72 | 0.0002 | 0.99 |
| Ficus citrifolia | 0.007 | 0.93 | 10.1 | 0.001 | 8.8 | 0.002 | 0.46 | 0.49 | 0.12 | 0.72 | 0.19 | 0.65 | 0.17 | 0.67 | 1.9 | 0.16 | 3.3 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, O.; Fries, A.; Hildebrandt, P.; Günter, S.; Mosandl, R. Early Growth Response of Nine Timber Species to Release in a Tropical Mountain Forest of Southern Ecuador. Forests 2019, 10, 254. https://doi.org/10.3390/f10030254
Cabrera O, Fries A, Hildebrandt P, Günter S, Mosandl R. Early Growth Response of Nine Timber Species to Release in a Tropical Mountain Forest of Southern Ecuador. Forests. 2019; 10(3):254. https://doi.org/10.3390/f10030254
Chicago/Turabian StyleCabrera, Omar, Andreas Fries, Patrick Hildebrandt, Sven Günter, and Reinhard Mosandl. 2019. "Early Growth Response of Nine Timber Species to Release in a Tropical Mountain Forest of Southern Ecuador" Forests 10, no. 3: 254. https://doi.org/10.3390/f10030254
APA StyleCabrera, O., Fries, A., Hildebrandt, P., Günter, S., & Mosandl, R. (2019). Early Growth Response of Nine Timber Species to Release in a Tropical Mountain Forest of Southern Ecuador. Forests, 10(3), 254. https://doi.org/10.3390/f10030254






