Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Measurements
2.3. Statistical Analyses
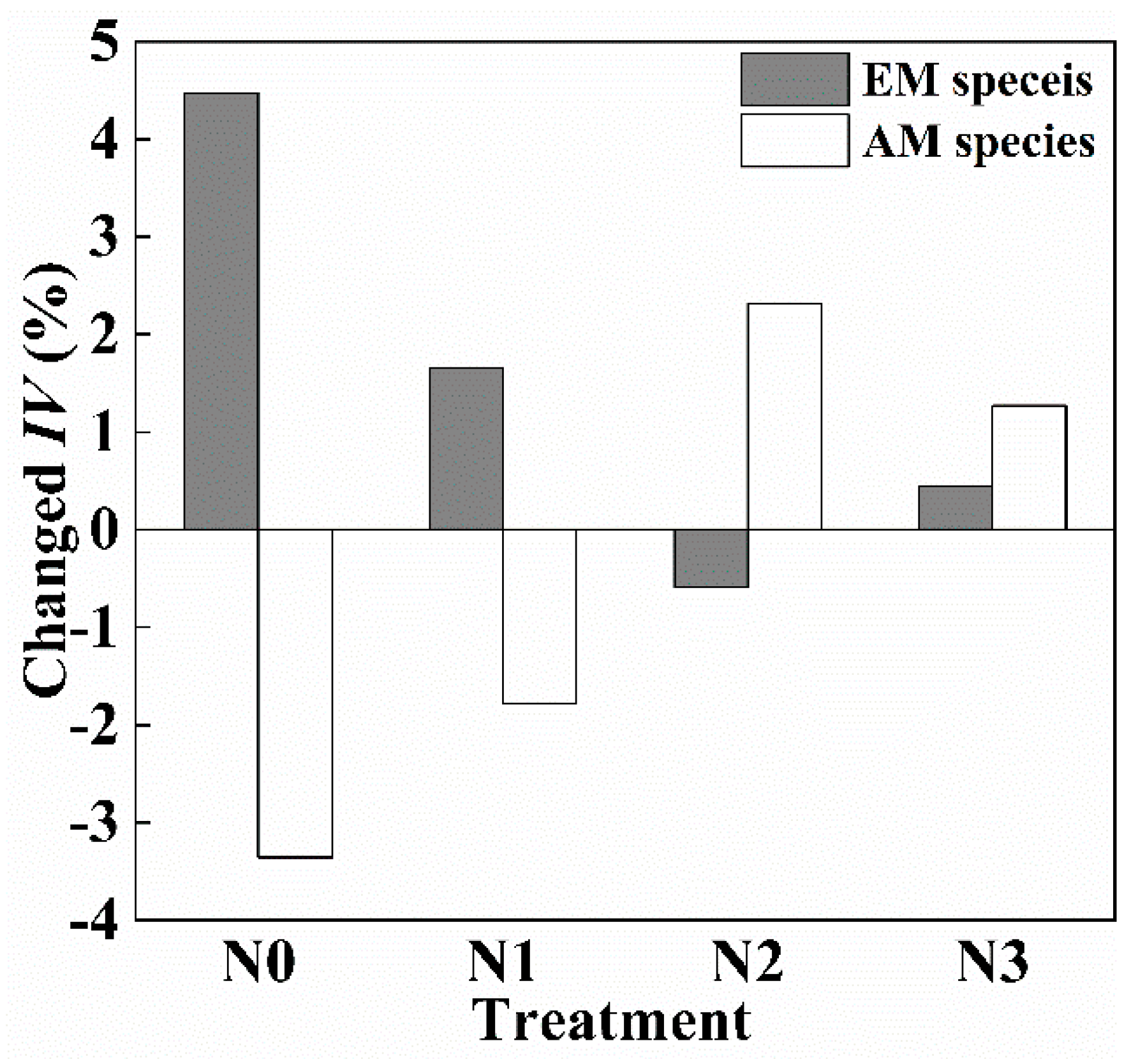
3. Results
3.1. N, P, and the N:P Ratio in the Three Seasons Under Ambient (without N Addition) Conditions
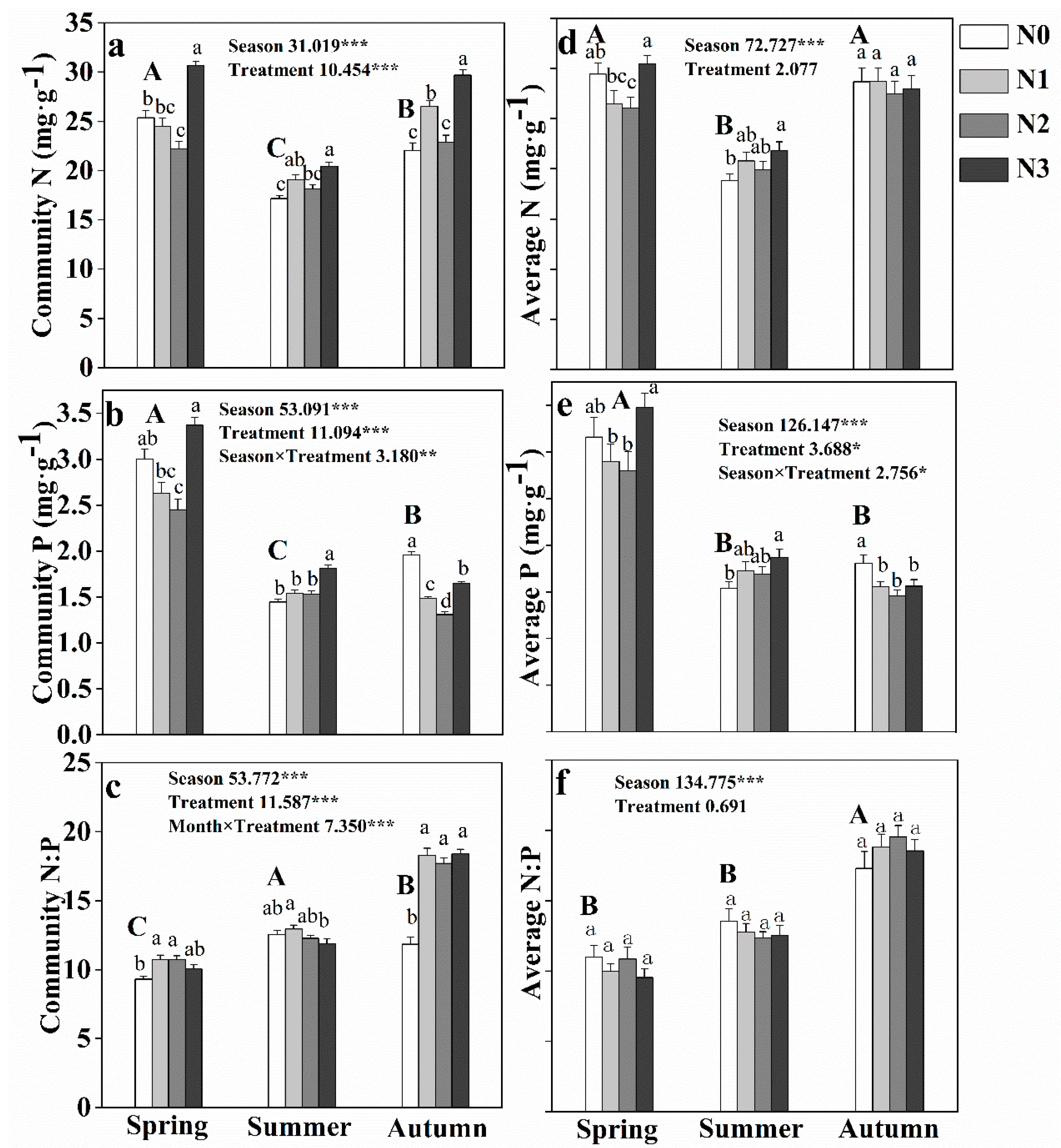
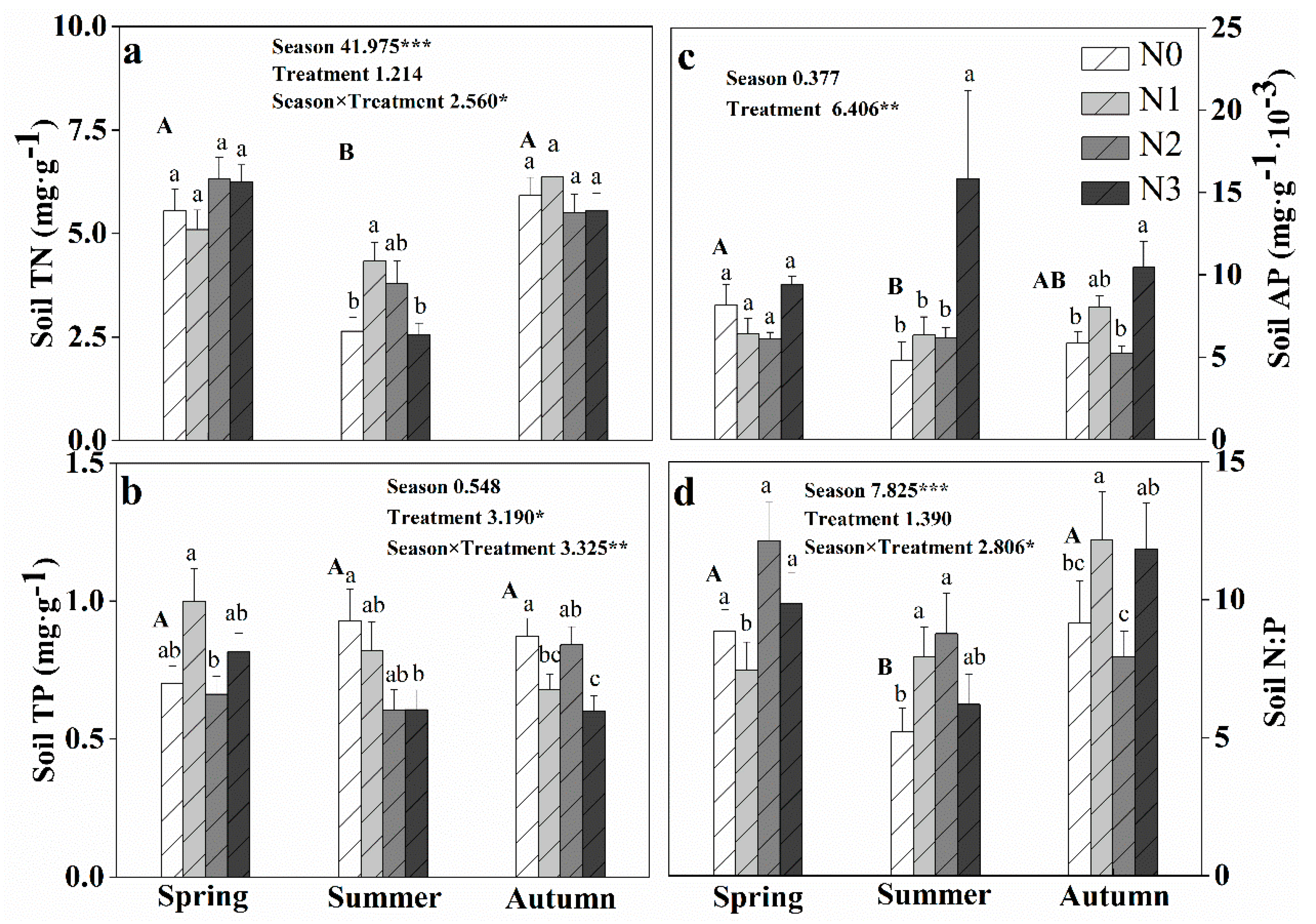
3.2. Effect of N Addition on Community and Soil N:P Stoichiometry during Different Seasons
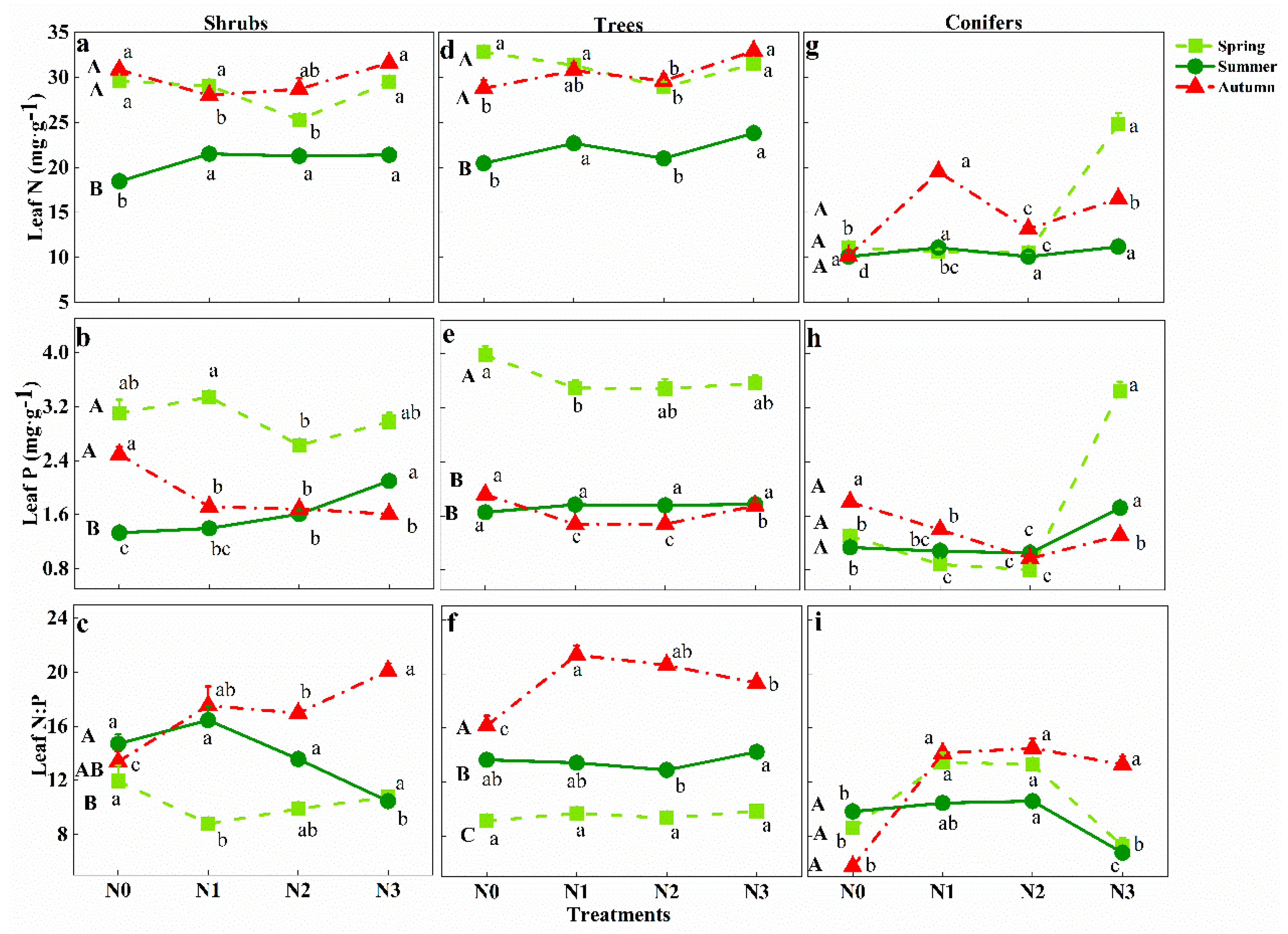
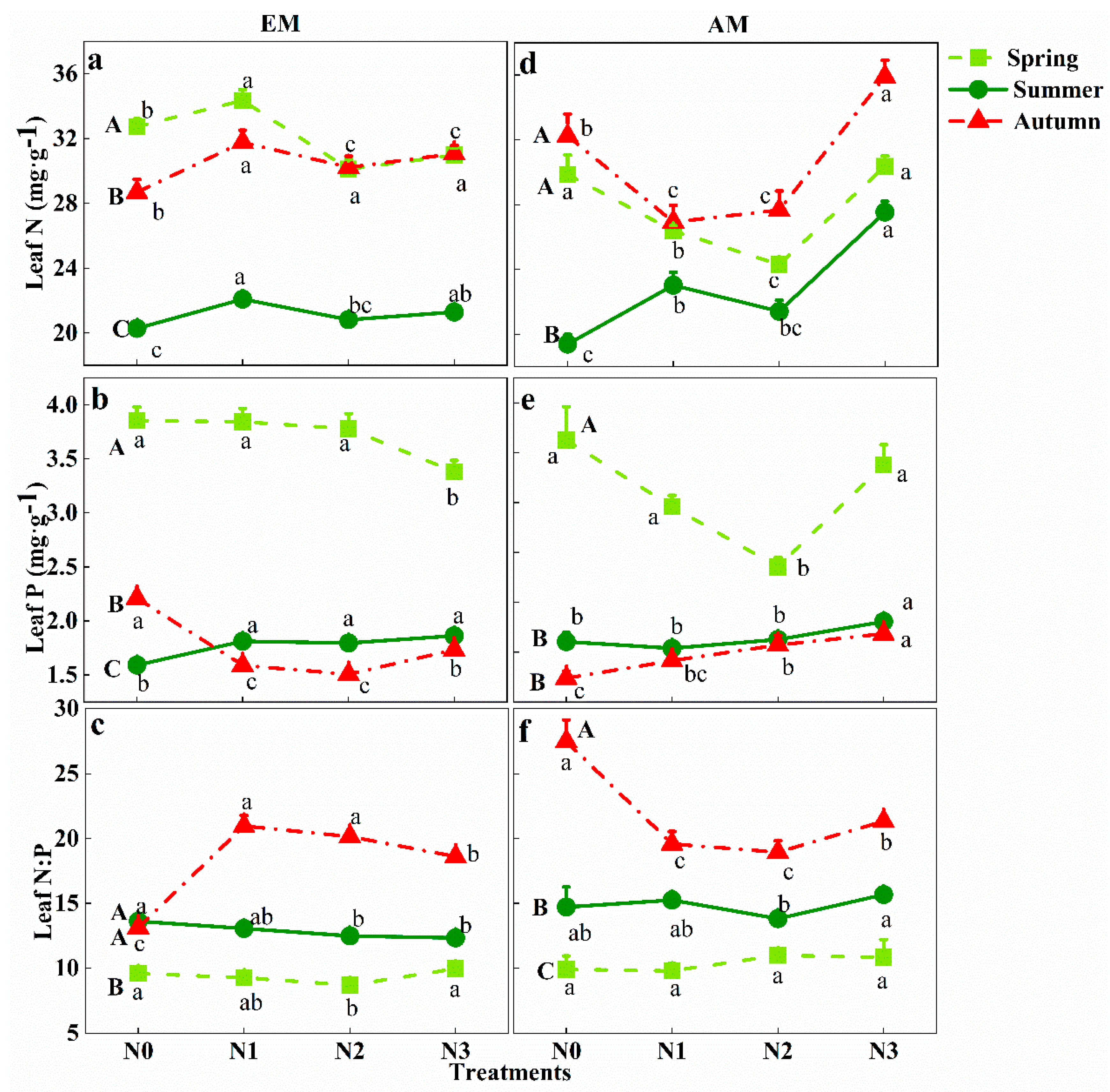
3.3. Response of Leaf N:P Stoichiometry in Different Groups to Seasonal Change and N Addition
4. Discussion
4.1. Seasonal Change in Leaf N:P Stoichiometry
4.2. Seasonal Variation in the Leaf N:P Stoichiometry Response to N Addition
4.3. Status of Nutrient Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, G.K.; Emmett, B.A.; Britton, A.J.; Caporn, S.J.M.; Dise, N.B.; Helliwell, R.; Jones, L.; Leake, J.R.; Leith, I.D.; Sheppard, L.J.; et al. Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob. Chang. Biol. 2012, 18, 1197–1215. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.F.; McNeil, B.E.; Lovett, G.M.; Canham, C.D.; Driscoll, C.T.; Rustad, L.E.; Denny, E.; Hallett, R.A.; Arthur, M.A.; Boggs, J.L.; et al. Do nutrient limitation patterns shift from nitrogen toward phosphorus with increasing nitrogen deposition across the northeastern United States? Ecosystems 2012, 15, 940–957. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Ross, D.J.; Coomes, D.A.; Richardson, S.J.; Smale, M.C.; Dahlgren, R.A. N and P in new zealand soil chronosequences and relationships with foliar N and P. Biogeochemistry 2005, 75, 305–328. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Sardans, J.; Rivas-ubach, A.; Janssens, I.A. The human-induced imbalance between C, N and P in earth’s life system. Glob. Chang. Biol. 2012, 18, 3–6. [Google Scholar] [CrossRef]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sistla, S.A.; Zhang, Y.H.; Lü, X.T.; Han, X.G. Hierarchical responses of plant stoichiometry to nitrogen deposition and mowing in a temperate steppe. Plant Soil 2014, 382, 175–187. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Currie, W.S.; Nadelhoffer, K.J.; Martin, M.E.; McDowell, W.H.; Melillo, J.M.; Steudler, P. Ecosystem response to 15 years of chronic nitrogen additions at the harvard forest lter, massachusetts, USA. For. Ecol. Manag. 2004, 196, 7–28. [Google Scholar] [CrossRef]
- Mayor, J.R.; Wright, S.J.; Turner, B.L.; Austin, A. Species-specific responses of foliar nutrients to long-term nitrogen and phosphorus additions in a lowland tropical forest. J. Ecol. 2014, 102, 36–44. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystem. Bioscience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Goswami, S.; Fisk, M.C.; Vadeboncoeur, M.A.; Garrison-Johnston, M.; Yanai, R.D.; Fahey, T.J. Phosphorus limitation of aboveground production in northern hardwood forests. Ecology 2018, 99, 438–449. [Google Scholar] [CrossRef]
- Davidson, E.A.; de Carvalho, C.J.; Figueira, A.M.; Ishida, F.Y.; Ometto, J.P.; Nardoto, G.B.; Saba, R.T.; Hayashi, S.N.; Leal, E.C.; Vieira, I.C.; et al. Recuperation of nitrogen cycling in amazonian forests following agricultural abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Chapin, F.S.; Vitousek, P.M.; Van Cleve, K. The nature of nutrient limitation in plant communities. Am. Nat. 1986, 127, 48–58. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Townsend, A.R.; Cleveland, C.C.; Asner, G.P.; Bustamamte, M.M.C. Controls over foliar N:P ratios in tropical forests. Ecology 2007, 88, 107–118. [Google Scholar] [CrossRef]
- Fife, D.N.; Nambiar, E.K.S.; Saur, E. Retranslocation of foliar nutrients in evergreen tree species planted in a mediterranean environment. Tree Physiol. 2008, 28, 187–196. [Google Scholar] [CrossRef]
- Wang, M.; Moore, T.R. Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar]
- Zhang, H.Y.; Lu, X.T.; Hartmann, H.; Keller, A.; Han, X.G.; Trumbore, S.; Phillips, R.P. Foliar nutrient resorption differs between arbuscular mycorrhizal and ectomycorrhizal trees at local and global scales. Glob. Ecol. Biogeogr. 2018, 27, 875–885. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Z.Q.; Liu, J.L.; Gu, J.C.; Guo, D.L. Fine root morphology of twenty hardwood species in maoershan natural secondary forest northeastern China. J. Plant Ecol. 2008, 32, 1217–1226. [Google Scholar]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.J. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Aronsson, A.; Elowson, S.; Persson, T. Effects of irrigation and fertilization on mineral nutrients in scots pine needles. Ecol. Bull. 1980, 32, 219–228. [Google Scholar]
- Son, Y.; Lee, I.K.; Ryu, S.R. Nitrogen and phosphorus dynamics in foliage and twig of pitch pine and japanese larch plantations in relation to fertilization. J. Plant Nutr. 2000, 23, 697–710. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Reich, P.B.; Uhl, C.; Walters, M.B.; Ellsworth, D.S. Leaf lifespan as a determinant of leaf structure and function among 23 amazonian tree species. Oecologia 1991, 86, 16–24. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef]
- Perring, M.P.; Hedin, L.O.; Levin, S.A.; McGroddy, M.; de Mazancourt, C. Increased plant growth from nitrogen addition should conserve phosphorus in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 1971–1976. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Booth, R.E.; Leake, J.R.; Read, D.J.; Grime, J.P.; Lee, J.A. Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol. 2003, 161, 279–290. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Long, M.; Wu, H.H.; Smith, M.D.; La Pierre, K.J.; Lü, X.T.; Zhang, H.Y.; Han, X.G.; Yu, Q. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 2016, 408, 475–484. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.; Elser, J.J.; He, N.; Wu, H.; Zhang, G.; Wu, J.; Bai, Y.; Han, X. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, E.K.S.; Fife, D.N. Nutrient retranslocation in temperate conifers. Tree Physiol. 1991, 9, 185–207. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Read, D.J.; Lewis, D.H.; Fitter, A.H.; Alexander, I.J.; Battley, E.H. Mycorrhizas in ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal symbiosis. Q. Rev. Biol. 2008, 3, 273–281. [Google Scholar]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like activity of humic substances in Fagus sylvaticae forests. New Phytol. 2001, 151, 647–657. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like activities of humic substances in different forest ecosystems. New Phytol. 2002, 155, 393–402. [Google Scholar] [CrossRef]
- Gilbert, J.B.; Lawesj, H. Agricultural, botanical, and chemical results of experiments on the mixed herbage of permanent meadow, conducted for more than twenty years in succession on the same land. Part i. Philos. Trans. R. Soc. Lond. 1880, 171, 289–416. [Google Scholar]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T.A. Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetland. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, H.Q.; Liu, J.Y.; Wang, S.Q.; Liu, M.L.; Pan, S.F.; Shi, X.Z. Pools and distributions of soil phosphorus in China. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
| Plots | Treatments | Average N Addition (kg·ha−1·yr−1) | Repeat | Density (trees·ha−1) | Mean Breast Diameter (cm) | Slope Aspect | Slope Degree |
|---|---|---|---|---|---|---|---|
| 1 | N0 | 0 | N0-1 | 1325 | 10.70 | W | <6° |
| 2 | N0 | 0 | N0-2 | 1625 | 8.60 | W | <6° |
| 3 | N1 | 30 | N1-1 | 1575 | 8.46 | W | <6° |
| 4 | N3 | 120 | N3-1 | 2925 | 7.68 | W | <6° |
| 5 | N3 | 120 | N3-2 | 1825 | 9.48 | W | <6° |
| 6 | N3 | 120 | N3-3 | 1100 | 14.02 | W | <6° |
| 7 | N0 | 0 | N0-3 | 1650 | 9.43 | W | <6° |
| 8 | N1 | 30 | N1-2 | 1550 | 11.47 | W | <6° |
| 9 | N1 | 30 | N1-3 | 1575 | 10.41 | W | <6° |
| 10 | N2 | 60 | N2-1 | 2300 | 7.62 | W | <6° |
| 11 | N2 | 60 | N2-2 | 1800 | 11.44 | W | <6° |
| 12 | N2 | 60 | N2-3 | 1525 | 11.45 | W | <6° |
| Scientific Name of Species | Life Form | Ectomycorrhizal (EM) or Arbuscular Mycorrhizal (AM) | The important value (%) under Control Group in 2014 | Uncollected Samples in Different Treatments | ||
|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | ||||
| Pinus koraiensis | Conifer | EM | 27.67 | N3 | ||
| Acer mono | Tree | EM | 11.19 | N1 | ||
| Corylus mandshurica | Shrub | EM | 9.60 | |||
| Tilia amurensis | Tree | EM | 8.04 | N1 | N1 | |
| Betula costata | Tree | EM | 7.05 | N1 N2 N3 | N0 N1 N2 | |
| Ulmus laciniata | Tree | EM | 5.66 | |||
| Ulmus japonica | Tree | EM | 4.13 | N2 | ||
| Acer tegmentosum | Tree | AM | 3.75 | N1 | N0 N1 N2 N3 | |
| Fraxinus mandshurica | Tree | AM | 3.03 | N1 N2 N3 | N1 N2 | |
| Prunus padus | Tree | AM | 1.98 | |||
| Syringa reticulate var. mandshurica | Tree | AM | 1.54 | |||
| Acer ukurunduense | Tree | EM | 1.19 | N1 | N1 | |
| Euonymus pauciflorus | Shrub | AM | 1.13 | |||
| Picea koraiensis | Conifer | EM | 0 | N2 | ||
| Deutzia gladata | Shrub | AM | 0 | |||
| Lonicera chrysantha | Shrub | AM | 0 | |||
| Ribes mandschuricum | Shrub | AM | 0 | |||
| Stoichiometry and Seasons | N | P | N:P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring | Summer | Autumn | Spring | Summer | Autumn | |
| Han et al. [39] | 20.2 | 1.46 | 16.3 | ||||||
| (China) | + * | − | + * | + * | + | + * | − * | − * | + |
| Reich and Oleksyn [7] | 20.1 | 1.77 | 13.8 | ||||||
| (World) | + * | − | + * | + * | − * | + | − * | − | + * |
| Elser et al. [38] | 20.6 | 1.99 | 12.7 | ||||||
| (World) | + * | − * | + * | + * | − * | − | − | + | + * |
| Different Groups | Factors | Season | Treatment | Group | Season × Treatment | Season × Group | Treatment × Group | Season × Treatment × Group |
|---|---|---|---|---|---|---|---|---|
| Broadleaves vs. conifers | N | 53.480 *** | 6.269 *** | 408.073 *** | 2.857 * | 8.434 *** | 2.220 * | |
| P | 25.034 *** | 20.112 *** | 134.599 *** | 6.762 *** | 18.939 *** | 9.209 *** | 3.286 ** | |
| N:P | 33.11 *** | 21.895 ** | 39.339 *** | 11.868 ** | 14.017 ** | 9.716 ** | 3.817 ** | |
| Trees vs. Shrubs | N | 106.743 *** | 4.307 ** | 5.477 * | ||||
| P | 104.666 *** | 2.415 | 0.906 | 2.458 * | 3.691 * | |||
| N:P | 131.018 *** | 3.085 * | 0.005 | 2.581 * | ||||
| EM vs. AM | N | 80.135 *** | 7.201 *** | 1.092 | 8.889 *** | 7.089 *** | ||
| P | 142.893 *** | 1.639 | 17.898 *** | 2.250 ** | ||||
| N:P | 127.291 *** | 0.433 | 14.060 *** | 2.798 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Mao, H.; Jin, G. Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China. Forests 2019, 10, 257. https://doi.org/10.3390/f10030257
Yang D, Mao H, Jin G. Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China. Forests. 2019; 10(3):257. https://doi.org/10.3390/f10030257
Chicago/Turabian StyleYang, Dongxing, Hongrui Mao, and Guangze Jin. 2019. "Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China" Forests 10, no. 3: 257. https://doi.org/10.3390/f10030257
APA StyleYang, D., Mao, H., & Jin, G. (2019). Divergent Responses of Foliar N:P Stoichiometry During Different Seasons to Nitrogen Deposition in an Old-Growth Temperate Forest, Northeast China. Forests, 10(3), 257. https://doi.org/10.3390/f10030257





