Decreased Temperature with Increasing Elevation Decreases the End-Season Leaf-to-Wood Reallocation of Resources in Deciduous Betula ermanii Cham. Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling
2.3. Analysis of NSC
2.4. Analysis of Nitrogen, Phosphorus and Potassium
2.5. Methods for Evaluating Resource Remobilization
2.6. Data Analysis
3. Results
3.1. NSC Concentration
3.2. N Concentration
3.3. P Concentration
3.4. K Concentration
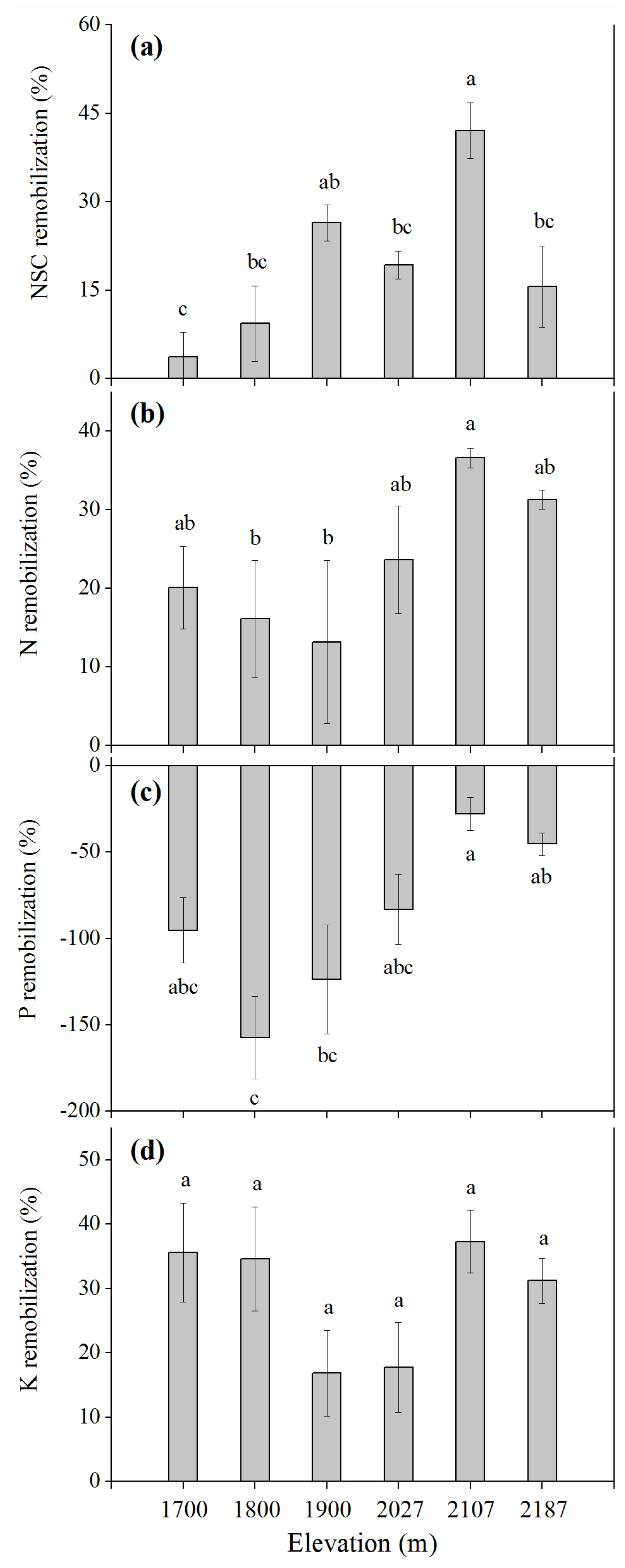
3.5. Elevational Effects on Resource Remobilization
4. Discussion
4.1. Tissue- and Resource-Dependent Reallocation or Accumulation
4.2. Elevational Effects on Resource Remobilization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Yang, J. Effects of elevation and microsite on growth of Pinus cembra in the subalpine zone of the Austrian Alps. Annu. For. Sci. 2004, 61, 319–325. [Google Scholar] [CrossRef]
- Bhattacharya, A. Changing Climate and Resource use Efficiency in Plants; Academic Press: London, UK, 2018. [Google Scholar]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Cherbuy, B.; Joffre, R.; Gillon, D.; Rambal, S. Internal remobilization of carbohydrates, lipids, nitrogen and phosphorus in the Mediterranean evergreen oak Quercus ilex. Tree Physiol. 2001, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.; Schulze, E.; Mooney, H. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Li, Y.; Lan, G.; Xia, Y. Rubber Trees demonstrate a clear retranslocation under seasonal drought and cold stresses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Millard, P. Ecophysiology of the internal cycling of nitrogen for tree growth. Zeitschrift für Pflanzenernährung Bodenkunde 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Marchi, S.; Sebastiani, L.; Gucci, R.; Tognetti, R. Changes in sink-source relationships during shoot development in olive. J. Am. Soc. Hortic. Sci. 2005, 130, 631–637. [Google Scholar] [CrossRef]
- Marchi, S.; Sebastiani, L.; Gucci, R.; Tognetti, R. Sink-source transition in peach leaves during shoot development. J. Am. Soc. Hortic. Sci. 2005, 130, 928–935. [Google Scholar] [CrossRef]
- Millard, P.; Hester, A.; Wendler, R.; Baillie, G. Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct. Ecol. 2001, 15, 535–543. [Google Scholar] [CrossRef]
- Millard, P.; Wendler, R.; Hepburn, A.; Smith, A. Variations in the amino acid composition of xylem sap of Betula pendula Roth. trees due to remobilization of stored N in the spring. Plant Cell Environ. 1998, 21, 715–722. [Google Scholar] [CrossRef]
- Millard, P.; Proe, M.F. Storage and internal cycling of nitrogen in relation to seasonal growth of Sitka spruce. Tree Physiol. 1992, 10, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Pastor, J.; McClaugherty, C.A.; Richardson, C.J. Nutrient-use efficiency: A litterfall index, a model, and a test along a nutrient-availability gradient in North Carolina peatlands. Am. Nat. 1995, 145, 1–21. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Chapin, F.S. Environmental and physiological factors governing nutrient resorption efficiency in barley. Oecologia 1992, 90, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.M.S.B.; Maruyama, H.; Nishida, S.; Tawaraya, K.; Wasaki, J. Landrace of japonica rice, Akamai exhibits enhanced root growth and efficient leaf phosphorus remobilization in response to limited phosphorus availability. Plant Soil 2017, 414, 327–338. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- González-Zurdo, P.; Escudero, A.; Mediavilla, S. N resorption efficiency and proficiency in response to winter cold in three evergreen species. Plant Soil 2015, 394, 87–98. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrient Resorption. In Plant Cell Death Processes; Academic Press: San Diego, CA, USA, 2004; pp. 215–226. [Google Scholar]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: the case of drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef]
- Kutbay, H.; Ok, T. Foliar N and P resorption and nutrient levels along an elevational gradient in Juniperus oxycedrus L. subsp. macrocarpa (Sibth. & Sm.) Ball. Ann. For. Sci. 2003, 60, 449–454. [Google Scholar]
- Du, H.; Liu, J.; Li, M.-H.; Büntgen, U.; Yang, Y.; Wang, L.; Wu, Z.; He, H.S. Warming-induced upward migration of the alpine treeline in the Changbai Mountains, northeast China. Glob. Chang. Biol. 2018, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; He, H.; Liu, K.; Du, H.; Wu, Z.; Zhao, Y.; Jin, H. Typhoon diverged forest succession from natural trajectory in the treeline ecotone of the Changbai Mountains, Northeast China. For. Ecol. Manag. 2018, 407, 75–83. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, A.; He, H.; Yu, F.; Tognetti, R.; Cherubini, P.; Wang, X.; Li, M.H. Evergreen Quercus aquifolioides remobilizes more soluble carbon components but less N and P from leaves to shoots than deciduous Betula ermanii at the end-season. iForest 2018, 11, 517–525. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Q.; Liu, J.; Zhou, W.; Qi, L.; Wang, X.; Zhou, L.; Dai, L. Formation mechanisms of the alpine Erman’s birch (Betula ermanii) treeline on Changbai Mountain in Northeast China. Trees 2014, 28, 935–947. [Google Scholar] [CrossRef]
- Liu, Q.-J.; Li, X.-R.; Ma, Z.-Q.; Takeuchi, N. Monitoring forest dynamics using satellite imagery—A case study in the natural reserve of Changbai Mountain in China. For. Ecol. Manag. 2005, 210, 25–37. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Kräuchi, N. Growth responses of Picea abies and Larix decidua to elevation in subalpine areas of Tyrol, Austria. Can. J. For. Res. 2003, 33, 653–662. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.-F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef]
- Yamaguchi, D.P.; Nakaji, T.; Hiura, T.; Hikosaka, K. Effect of seasonal change and experimental warming on the temperature dependence of photosynthesis in the canopy leaves of Quercus serrata. Tree Physiol. 2016, 36, 1283–1295. [Google Scholar] [CrossRef]
- Li, M.; Xiao, W.-F.; Shi, P.; Wang, S.-G.; Zhong, Y.-D.; Liu, X.-L.; Wang, X.-D.; Cai, X.-H.; Shi, Z.-M. Nitrogen and carbon source–sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Yan, C.; Luo, W.; Wang, R.; Han, X.; Jiang, Y.; Li, M.-H. Responses and sensitivity of N, P and mobile carbohydrates of dominant species to increased water, N and P availability in semi-arid grasslands in northern China. J. Plant Ecol. 2017, 10, 486–496. [Google Scholar] [CrossRef]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Karlsson, P.S.; Weih, W. The significance of resorption of leaf resources for shoot growth in evergreen and deciduous woody plants from a subarctic environment. Oikos 1998, 81, 567–575. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, E.K.S.; Fife, D.N. Nutrient retranslocation in temperate conifers. Tree Physiol. 1991, 9, 185–207. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Gessler, A.; Treydte, K. The fate and age of carbon—Insights into the storage and remobilization dynamics in trees. New Phytol. 2016, 209, 1338–1340. [Google Scholar] [CrossRef]
- Körner, C. The nutritional status of plants from high altitudes. A worldwide comparison. Oecologia 1989, 81, 379–391. [Google Scholar] [CrossRef]
- Chung, H.; Muraoka, H.; Nakamura, M.; Han, S.; Muller, O.; Son, Y. Experimental warming studies on tree species and forest ecosystems: A literature review. J. Plant Res. 2013, 126, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; Edwards, N.T.; Walker, A.V.; O’Hara, K.H.; Campion, C.M.; Hanson, P.J. Forest phenology and a warmer climate—growing season extension in relation to climatic provenance. Glob. Chang. Biol. 2012, 18, 2008–2025. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, T.; Zhang, Y. Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. Eur. J. For. Res. 2012, 131, 811–819. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.-C. Sugars as signaling molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Morin, X.; Améglio, T.; Ahas, R.; Kurz-Besson, C.; Lanta, V.; Lebourgeois, F.; Miglietta, F.; Chuine, I. Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol. 2007, 27, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Furze, M.E.; Trumbore, S.; Hartmann, H. Detours on the phloem sugar highway: Stem carbon storage and remobilization. Curr. Opin. Plant Biol. 2018, 43, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Oleksyn, J.; Modrzýnski, J.; Tjoelker, M.G.; Z·ytkowiak, R.; Reich, P.B.; Karolewski, P. Growth and physiology of Picea abies populations from elevational transects: common garden evidence for altitudinal ecotypes and cold adaptation. Funct. Ecol. 1998, 12, 573–590. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin, Genmary, 1999. [Google Scholar]
- Li, M.; Kräuchi, N.; Dobbertin, M. Biomass distribution of different-aged needles in young and old Pinus cembra trees at highland and lowland sites. Trees 2006, 20, 611–618. [Google Scholar] [CrossRef]
- Wang, A.; Wang, X.; Tognetti, R.; Lei, J.-P.; Pan, H.-L.; Liu, X.-L.; Jiang, Y.; Wang, X.-Y.; He, P.; Yu, F.-H.; et al. Elevation alters carbon and nutrient concentrations and stoichiometry in Quercus aquifolioides in southwestern China. Sci. Total Environ. 2018, 622, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
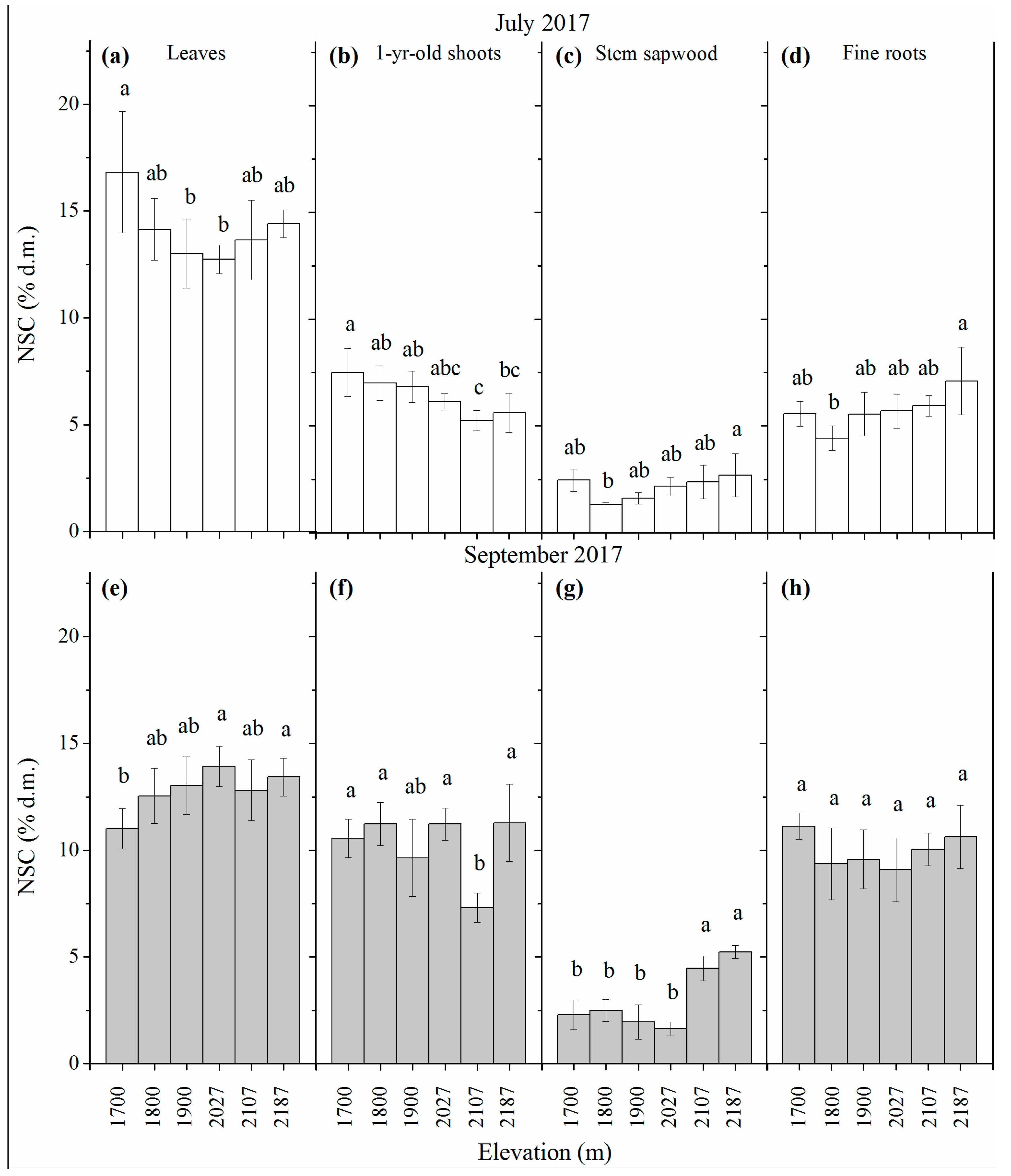
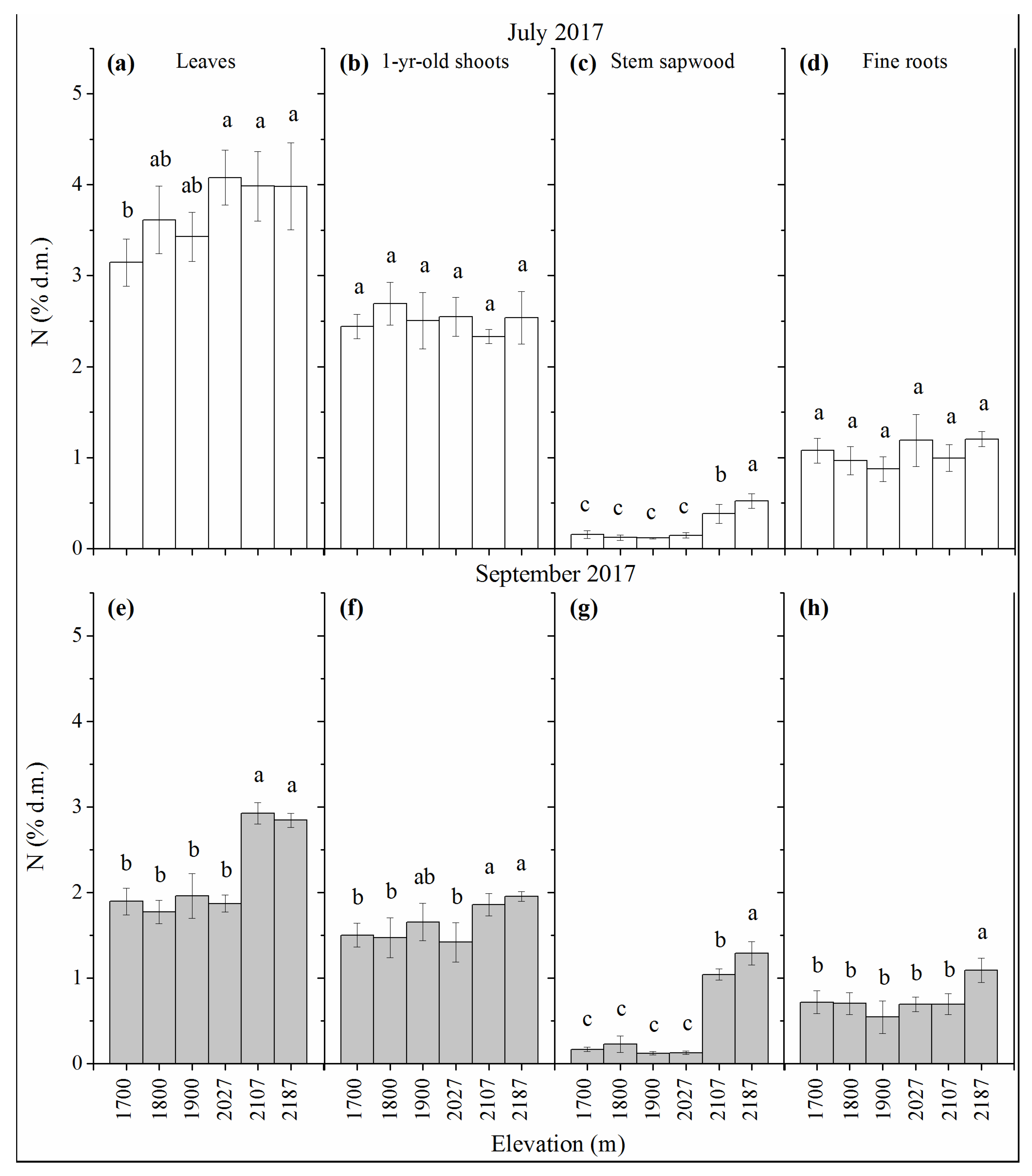
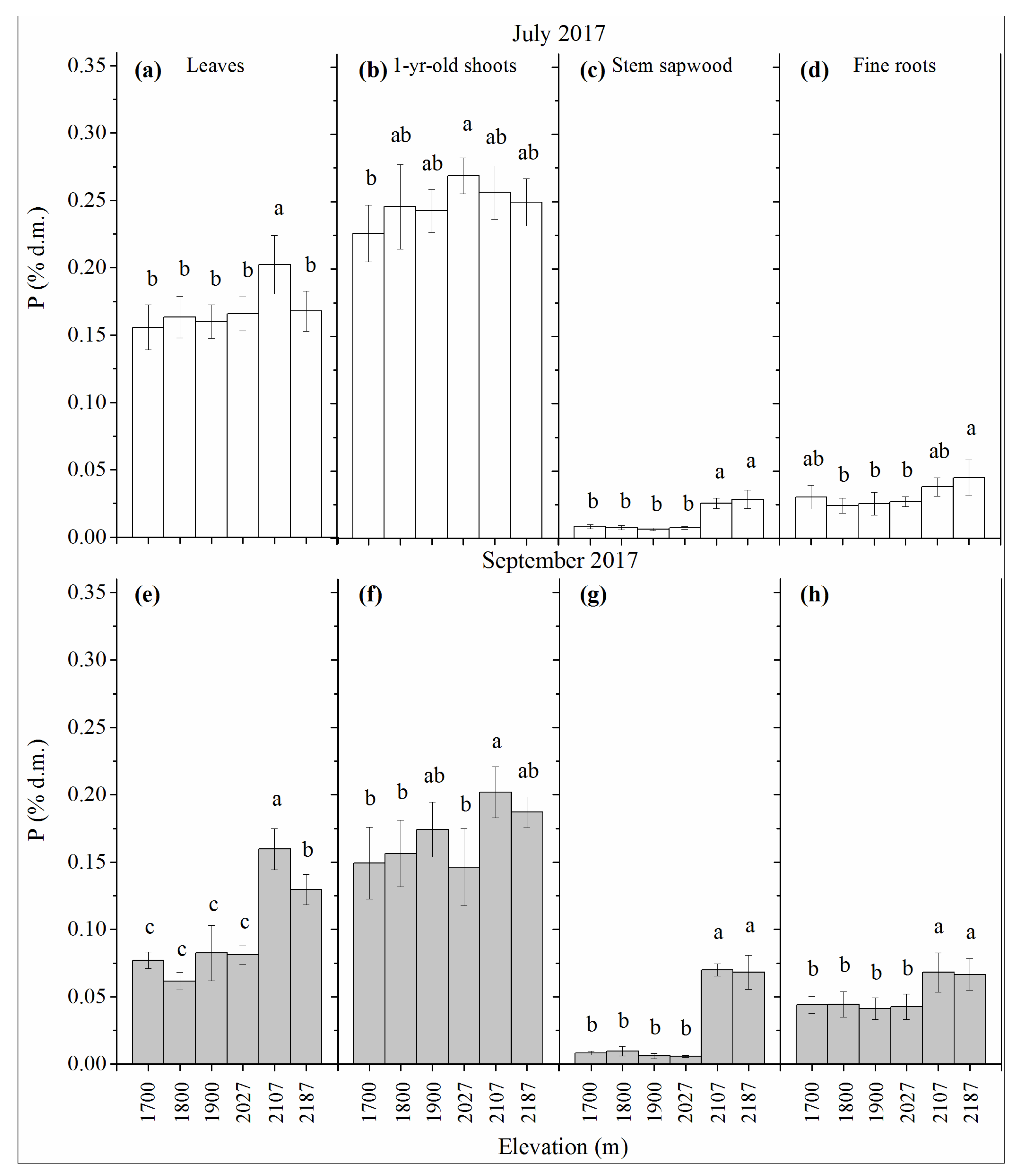
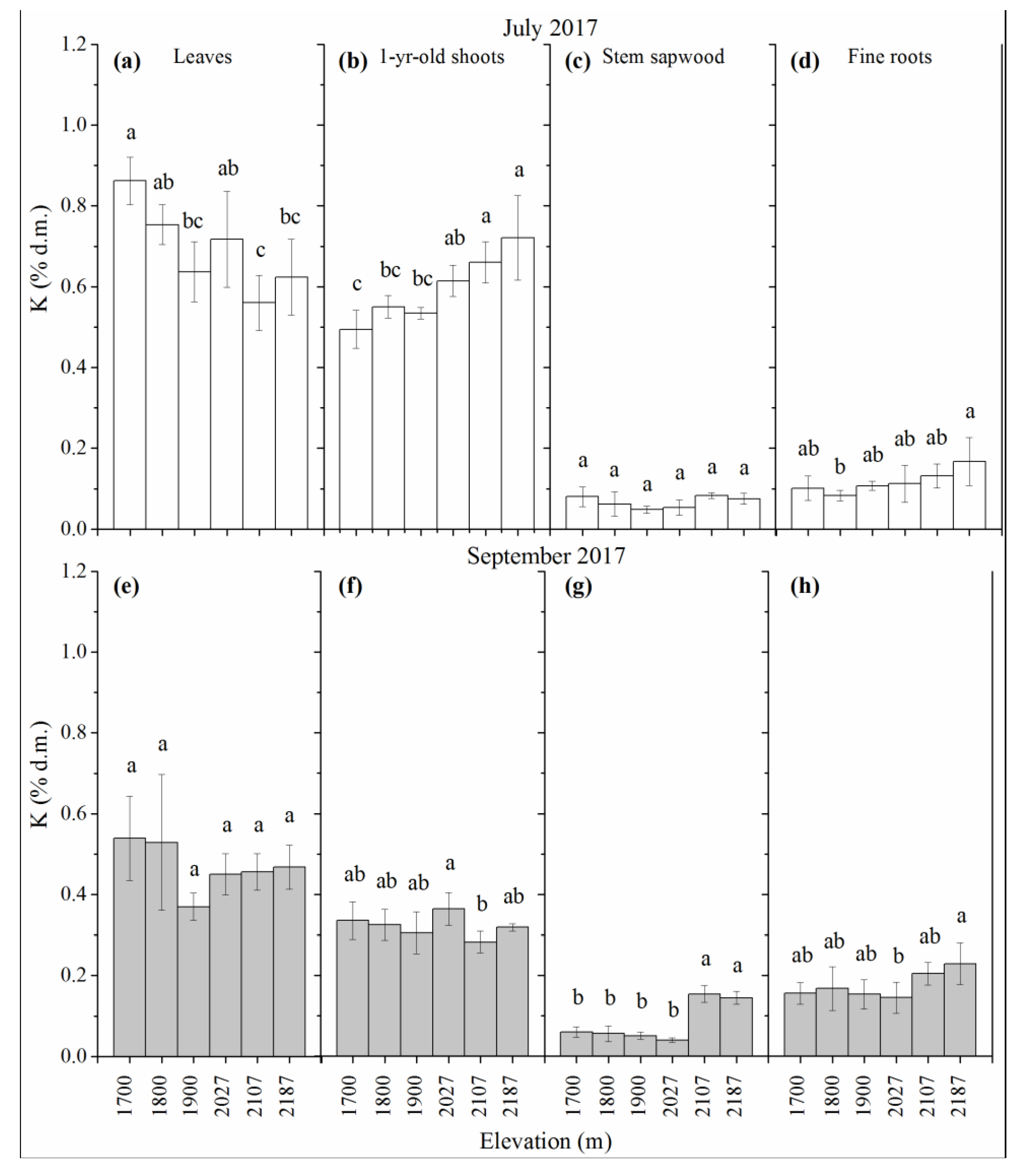

| Site No. | Elevation | Average | Average | Slope Exposure |
|---|---|---|---|---|
| (m a.s.l.) | DBH (cm) | Height (m) | ||
| 1 | 2187 | 1.0 ± 0.1 a | 0.9 ± 0.1 | West |
| 2 | 2107 | 1.1 ± 0.1 a | 1.5 ± 0.1 | West |
| 3 | 2027 | 4.7 ± 0.4 | 4.2 ± 0.1 | West |
| 4 | 1900 | 9.4 ± 1.0 | 9.3 ± 0.4 | West |
| 5 | 1800 | 9.2 ± 0.7 | 9.2 ± 0.5 | West |
| 6 | 1700 | 10.9 ± 0.7 | 11.2 ± 0.8 | West |
| NSC | Nitrogen | Phosphorus | Potassium | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Leaves | ||||||||
| Elevations (E) | 0.42 | 0.829 | 20.15 | <0.001 | 25.57 | <0.001 | 7.21 | <0.001 |
| Time (T) | 27.80 | <0.001 | 478.52 | <0.001 | 554.96 | <0.001 | 125.46 | <0.001 |
| E × T | 14.82 | <0.001 | 7.19 | <0.001 | 11.61 | <0.001 | 2.74 | 0.043 |
| 1-year-old-shoots | ||||||||
| Elevations (E) | 8.25 | <0.001 | 2.11 | 0.098 | 3.96 | 0.009 | 8.42 | <0.001 |
| Time (T) | 253.11 | <0.001 | 301.44 | <0.001 | 222.21 | <0.001 | 465.96 | <0.001 |
| E × T | 5.77 | 0.001 | 5.77 | 0.001 | 3.53 | 0.016 | 9.37 | <0.001 |
| Stem sapwood | ||||||||
| Elevations (E) | 23.45 | <0.001 | 206.63 | <0.001 | 215.03 | <0.001 | 22.26 | <0.001 |
| Time (T) | 36.69 | <0.001 | 306.85 | <0.001 | 113.94 | <0.001 | 28.97 | <0.001 |
| E × T | 11.69 | <0.001 | 101.70 | <0.001 | 49.27 | <0.001 | 28.55 | <0.001 |
| Fine roots | ||||||||
| Elevations (E) | 3.63 | 0.014 | 7.13 | <0.001 | 10.60 | <0.001 | 5.88 | 0.001 |
| Time (T) | 232.16 | <0.001 | 84.80 | <0.001 | 72.13 | <0.001 | 37.25 | <0.001 |
| E × T | 1.50 | 0.228 | 2.28 | 0.079 | 1.21 | 0.333 | 0.62 | 0.688 |
| R NSC | R Nitrogen | R Phosphorus | R Potassium | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Elevations (E) | 8.67 | <0.001 | 17.20 | <0.001 | 10.72 | <0.001 | 3.49 | 0.016 |
| Tissue types (T) | 287.57 | <0.001 | 42.36 | <0.001 | 79.04 | <0.001 | 84.44 | <0.001 |
| E × T | 5.73 | <0.001 | 2.50 | 0.005 | 3.22 | <0.001 | 2.50 | 0.005 |
| Elevation (m a.s.l.) | NSC (g) | N (g) | P (g) | K (g) |
|---|---|---|---|---|
| Leaves | ||||
| 2187 | −1.4 ± 0.4a | −5.8 ± 1.5b | −4.3 ± 0.8b | −5.3 ± 1.9a |
| 2107 | −3.0 ± 1.3a | −12.9 ± 2.7a | −10.5 ± 2.8a | −4.7 ± 3.6a |
| 1-year-old shoots | ||||
| 2187 | 31.1 ± 8.3a | −7.0 ± 2.3a | −7.6 ± 2.2a | −17.4 ± 4.0a |
| 2107 | 26.6 ± 9.9a | −17.1 ± 7.0a | −18.2 ± 8.1a | −43.7 ± 16.3a |
| Stem sapwood | ||||
| 2187 | 63.0 ± 21.2b | 77.5 ± 10.8b | 76.7 ± 20.1b | 49.8 ± 7.6b |
| 2107 | 240.4 ± 72.6a | 391.5 ± 65.1a | 369.6 ± 50.5a | 193.4 ± 43.3a |
| Fine roots | ||||
| 2187 | 22.0 ± 6.7b | −4.1 ± 2.2b | 22.3 ± 8.8a | 17.0 ± 4.7a |
| 2107 | 85.1 ± 15.1a | −36.7 ± 13.1a | 102.7 ± 34.7a | 59.3 ± 26.5a |
| Trees across the Entire Transect | Trees below the Alpine Treeline | Trees above the Alpine Treeline | |
|---|---|---|---|
| Non-structural carbohydrates (NSC) | 8 | 9 | 6 |
| Nitrogen (N) | 40 | 47 | 27 |
| Phosphorus (P) | 42 | 52 | 22 |
| Potassium (K) | 31 | 36 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, Y.; Li, M.-H.; Liu, K.; Dang, Y.-C.; Han, H.-D.; He, H.S. Decreased Temperature with Increasing Elevation Decreases the End-Season Leaf-to-Wood Reallocation of Resources in Deciduous Betula ermanii Cham. Trees. Forests 2019, 10, 166. https://doi.org/10.3390/f10020166
Cong Y, Li M-H, Liu K, Dang Y-C, Han H-D, He HS. Decreased Temperature with Increasing Elevation Decreases the End-Season Leaf-to-Wood Reallocation of Resources in Deciduous Betula ermanii Cham. Trees. Forests. 2019; 10(2):166. https://doi.org/10.3390/f10020166
Chicago/Turabian StyleCong, Yu, Mai-He Li, Kai Liu, Yong-Cai Dang, Hu-Dong Han, and Hong S. He. 2019. "Decreased Temperature with Increasing Elevation Decreases the End-Season Leaf-to-Wood Reallocation of Resources in Deciduous Betula ermanii Cham. Trees" Forests 10, no. 2: 166. https://doi.org/10.3390/f10020166
APA StyleCong, Y., Li, M.-H., Liu, K., Dang, Y.-C., Han, H.-D., & He, H. S. (2019). Decreased Temperature with Increasing Elevation Decreases the End-Season Leaf-to-Wood Reallocation of Resources in Deciduous Betula ermanii Cham. Trees. Forests, 10(2), 166. https://doi.org/10.3390/f10020166





