Abstract
The adsorption properties of water molecules on TiO2 nanotubes (TiO2NT) and the interaction mechanisms between water molecules are studied by first principles calculations. The adsorption preferences of water molecules in molecular or dissociated states on clean and H-terminated TiO2NT are evaluated. Adsorption of OH clusters on (0, 6) and (9, 0) TiO2 nanotubes are first studied. The smallest adsorption energies are −1.163 eV and −1.383 eV, respectively, by examining five different adsorption sites on each type of tube. Eight and six adsorption sites were considered for OH adsorbtion on the H terminated (0, 6) and (9, 0) nanotubes. Water molecules are reformed with the smallest adsorption energy of −4.796 eV on the former and of −5.013 eV on the latter nanotube, respectively. For the adsorption of a single water molecule on TiO2NT, the molecular state shows the strongest adsorption preference with an adsorption energy of −0.660 eV. The adsorption of multiple (two and three) water molecules on TiO2NT is also studied. The calculated results show that the interactions between water molecules greatly affect their adsorption properties. Competition occurs between the molecular and dissociated states. The electronic structures are calculated to clarify the interaction mechanisms between water molecules and TiO2NT. The bonding interactions between H from water and oxygen from TiO2NT may be the reason for the dissociation of water on TiO2NT.
1. Introduction
The demands of energy and the control of environment pollution are factors that inspire us to develop clean and renewable energy sources. Hydrogen is one of the most promising energy carriers due to its high energy density and zero pollution. However, the production of hydrogen with low cost still hinders its application as an energy carrier. Splitting of water on photocatalysts with solar energy is a desirable way to produce hydrogen without pollution and with low-cost. Honda and Fujishima proved photocatalytic hydrogen production from water and demonstrated the decomposition concept using a photo-electrochemical cell [1]. Many studies have been reported in the literature since their pioneering work. Many photocatalysts have been subsequently developed with high quantum efficiency for water splitting under UV and visible light illumination [2,3,4], such as Pt loaded TiO2 particle system [5], Au/TiO2 [6], platinized SrTiO3 single crystals coated with films of NaOH [7], RuO2/TiO2 [8] and NiO/SrTiO3 [9], BaTiO4 loaded with RuO2 [10], K4Nb6O17 [11], and so on. However, photocatalysts with sufficient band gap positions and high chemical stability for practical applications are still under development. With strongly catalytic activity and high stability, TiO2 based semiconductors are one of the promising photocatalysts for water-splitting.
The photogenerated electron-hole pairs of TiO2 react with water and produce hydrogen under illumination. However, the hydrogen production rate is hindered by the quick charge recombination and backward reaction [12]. The hydrogen production efficiency is affected by many factors, such as the incorporating of dopants or other materials that affect charge separation and light harvesting [13]. Generally, the photoactivity of cation-doped titania will be decreased due to the increasing recombination of the photoexcited electron–hole pairs [14]. Nowadays, several photocatalysts have been developed. The metal Ag can enhance the hydrogen production using low-energy UV lamps and maintain the hydrogen production of activity-modified TiO2 [15]. The Ni, Pd, and Pt can significantly improve the photocatalytic production of H2 from water [16]. The metal oxides, such as Ni2O3 and CuO, are also utilized due to their unique plasmon absorption properties using visible light photo-catalysis [17]. Most recently, the photocatalytic activity of Au@Void@TiO2 yolk-shell nanostructures were investigated, in which the thicker and bigger shells displayed higher activity of H2 production [18]. For catalysts of TiO2 in splitting water, one of the important issues is to understand the interaction mechanisms between water molecules and the TiO2 surface in order to improve the photocatalytic activity of TiO2. Many works have studied the interactions between a water molecule and the TiO2 surface [19,20,21,22,23,24,25,26,27,28,29,30]. It was shown experimentally that water molecules mainly adsorb on the clean TiO2(110) surface with a small amount of dissociations [19,20,21]. Controversies exist in the adsorption pathways of water molecules on a TiO2 surface under theoretical works. The selections of the coverage of water molecules, slab depth, and even the calculation methods can affect the calculation results [22,23,24,25,26,27,28,29]. Furthermore, defects and impurities greatly affect the hydrolysis efficiency on TiO2. The load of Au and Pt can greatly decrease the dissociation barrier of water on TiO2 and therefore promote hydrolysis efficiency [30].
The water molecule prefers to adsorb on the top of a five-fold coordinated Ti site with a clean rutile TiO2(110) surface with an adsorption energy of 0.92 eV under 1 ML (Mono-Layer) coverage, which is 0.14 eV higher than that of dissociated state [21,31,32]. However, the estimated adsorption energy of water on TiO2 by density functional theory (DFT) calculations is sensitive to the parameters used in the DFT calculations, which may be the origin of the discrepancy between different works. The adsorption of water on TiO2, especially in the dissociated state, can be significantly enhanced by the presence of fourfold coordinated Ti atoms at the step edge of the surface, due to the easy transfer of charge from the water molecule to the TiO2 [31]. The photocatalytic properties of TiO2 can be affected by the adsorption of hydroxyl, and thus it is important to understand the interaction mechanisms between the water/hydroxyl and the TiO2 surface [33]. There are a variety of distributions of OH groups due to the influence of complex adsorption states of water molecules. Two types of hydroxyls are found to adsorb on the TiO2 surface, which could be classified as thermally unstable and thermally stable (isolate hydroxyl groups) in terms of thermostability [33,34,35,36]. The adsorption bands of these hydroxyls are greatly affected by the crystal structure of TiO2 and the evacuation temperature. The hydroxyls play different roles in the photo-oxidation process [33], but the chemical environment of hydroxyls on the TiO2 surface are still deficient.
Water can adsorb on the TiO2 surface in molecular and dissociated states, and therefore, different hydroxyl clusters can be formed [37]. Herman et al. reported the adsorption of water on the anatase TiO2(101) surface in a molecular state [38], and observed three desorption states in the Temperature Programmed Desorption (TPD) spectra of the multilayer water, adsorbing in sites of 2-fold-coordinated O and 5-fold-coordinated Ti. Some theoretical calculations are consistent with the above experiments in that the water molecule adsorbs in a molecular state on the (101) surface of anatase TiO2 [39], but it will be dissociated on the (001) surface [40,41]. Some theoretical works predicted that water molecules adsorb in rutile TiO2 surface in the dissociated state, and the OH group is bonded in the two-coordinated O and five-coordinated Ti sites [42]. The adsorption states are also affected by the defects in the surface of TiO2. One O vacancy can capture two OH groups [43,44]. The Highest Occupied Molecular Orbital (HOMO) of the dissociated water on the TiO2(101) surface is 0.1 eV higher than that of the molecular state. Therefore, the dissociated state of water on the TiO2 has higher photocatalytic activity than that of the undissociated water [45]. Furthermore, the adsorption energy of a water molecule on a rutile TiO2(110) surface is only slightly affected by the relative sites and orientations of the water molecule. There are repulsive interactions between neighboring OH groups, causing a decrease of the adsorption energy per H atom from 0.56 eV for isolated OH groups to 0.40 eV for neighboring OH pairs. The twofold-coordinated O is the preferred adsorption site even as the coverage increases up to 1 ML [29].
Due to the large surface area and high efficiency of photocatalytic reactions, TiO2 nanotubes (TiO2NT) have been attracted great attention since they were discovered in 1996 [46]. The solar water splitting activity of TiO2NT increases monotonously with the porosity rather than the wall thickness and/or inner diameter [47]. Theoretical calculations show that the formation of OH is the rate-limiting step for the dissociation of the first water molecule on TiO2 nanotube arrays [48]. The adsorption of water on TiO2NT is exothermic in both the molecular and dissociated states, and the molecular adsorption is energetically more favorable [49]. However, the influence of interactions between water molecules on adsorption states of water molecules are still not well understood. The adsorption properties of several water molecules on the TiO2NT are studied in the present work by first principles calculations. The methods used in this paper are briefly described in Section 2, and the results are presented in Section 3. Conclusions are summarized in Section 4.
2. Methodology and Models
The (n, 0) and (0, m) of nanotubes are rolled by the anatase (101) sheet along the [101] and [010] directions, respectively [50]. The numbers of n and m denote the supercell size of the sheet. The sizes of the simulated boxes of the (n, 0) and (0, m) nanotubes (where n = 6 and 9, and m = 3 and 6) are 30.0 × 30.0 × 10.210 Å3 and 30.0 × 30.0 × 3.776 Å3, respectively [51]. The Vienna ab initio simulation package (VASP) was employed to relax the ions and evaluate the total energy and electronic structures [52,53] with PAW(Projector-Augmented Wave)-GGA (Generalized Gradient Approximation) potential [54]. The self-consistent convergences of the total energy difference and forces are chosen as 0.01 meV and 0.01 eV/Å, respectively. All ions of the TiO2 nanotube and adsorbates (water molecule and OH cluster) are allowed to relax to obtain the stable adsorption configurations. The distance between neighboring nanotubes is verified to avoid the interactions between them. The energy cutoff is 400 eV, and 1 × 1 × 2 and 1 × 1 × 8 k-meshes were chosen for the (6, 0) and (0, 3) nanotubes, respectively, to ensure accuracy.
3. Results and Discussion
The adsorption energies of the water molecule and OH cluster on TiO2NT are evaluated via the following definition:
where Ent+a and Ent denote the total energies of the adsorbed water/hydroxyl and clean TiO2NTs, respectively. The Ea is the total energy of the adsorbate (water molecule or OH cluster) evaluated using a 1.0 nm × 1.0 nm ×1.0 nm cell.
Eads = Ent+a − Ent − Ea
3.1. Adsorption of OH on the Clean TiO2NT
The adsorption of OH on TiO2NT was studied. Five adsorption sites, at the inner ring (labeled as “1” and “4”) and the outer ring (labeled as “2” and “3”), and in the center of the tube (labeled as “5”) are considered as shown in Figure 1a,b. Their adsorption energies are listed in Table 1. For the (0, 6) nanotube, the OH prefers to locate at site “3” and saturates the closest Ti atom after full relaxation with an adsorption energy of −1.163 eV, as shown in Figure 1c. The distance between the oxygen of the OH cluster and its closest Ti atom is 1.915 Å. One Ti atom was dragged out about 0.4 Å by the OH cluster. For the (9, 0) nanotube, the adsorption of the OH cluster also had negative adsorption energy. The most stable adsorption of OH cluster was at site “5” with an adsorption energy of −1.383 eV, and the distance between the OH cluster and the closest Ti atom was 2.111 Å, as shown in Figure 1d. Therefore, the OH cluster was strongly adsorbed on the nanotube by saturating the five-fold Ti ions.
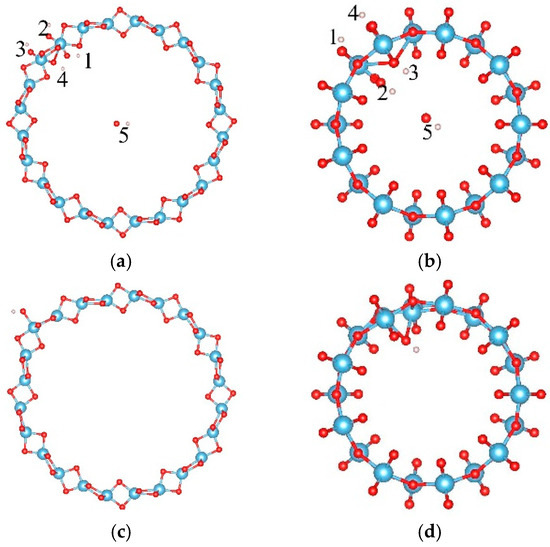
Figure 1.
The adsorption sites of OH on the TiO2 nanotube. (a) The initial adsorption sites of OH on the (0, 6); and (b) (9, 0) nanotube, respectively; (c) the most stable adsorption sites of OH on the (0, 6) nanotube after full relaxations and (d) (9, 0) nanotube, respectively. The blue, red, and white balls denote the Ti, O, and H atoms, respectively.

Table 1.
The adsorption energy of the OH cluster on/in the (0, 6) and (9, 0) nanotubes (eV).
3.2. Adsorption of OH on the Hydrogen Terminated TiO2NT
The dissociation of water will produce OH and H groups. Thus the adsorption properties of the OH group on the H terminated nanotubes were further studied to clarify the interactions between the OH group and H on TiO2NT. Eight and six sites were considered for the adsorptions of the OH on H terminated (0, 6) and (9, 0) nanotubes, respectively. Figure 2 shows the initial adsorption sites, and their adsorption energies after full relaxations are listed in Table 2. For the (0, 6) nanotube, the OH cluster has negative adsorption energy at all considered adsorption sites (around −4.0 eV except at site “8”). The most stable adsorption configuration is when the OH cluster adsorbs on the outer ring of the H terminated (0, 6) nanotube, connecting its O atom with the H atom in the TiO2NT, forming a water-like HO–H group with a bond length and angle between the OH and surfacial H of the nanotube of 1.021 Å and 105.8°, as shown in Figure 3a. However, the OH cluster at site “8” is located at the corner of the simulated cell and does not contact the nanotube. The adsorption energy of −0.777 eV may originate from the calculation error of the energy of the OH cluster, and this error can be ignored in terms of the large adsorption energy of the OH cluster in other sites. For the (9, 0) nanotube, the OH cluster at site “6” shows the largest adsorption energy, indicating the strong bonding interactions of the OH cluster with the nanotube. The strongest adsorption of the OH occurs at site “5” with an adsorption energy of −5.013 eV. At site “5”, the OH cluster is initially located at the center of the nanotube (Figure 2b), and moves toward the inner surface, forming a bond with the H atom on TiO2NT with its O atom with a bond length of 1.052 Å and a H–O–H angle of 109.5°. A water molecule is therefore formed (Figure 3b). Comparing the adsorption energies of OH on clean and H terminated TiO2NTs, one can find that the difference of adsorption energy is mainly caused by the formation of the water molecule. The H termination can greatly affect the adsorption ability of OH on the surface of the nanotube, and therefore, the water-dissociation efficiency will be limited in a H-rich environment due to combinations of OH and H clusters at the surface of the TiO2NT.
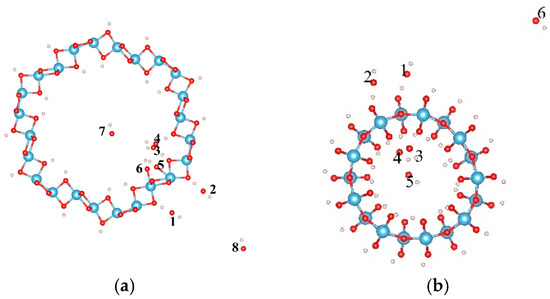
Figure 2.
The initial adsorption sites of OH on the H terminated TiO2 nanotube. (a) (0, 6) and (b) (9, 0). The blue, red, and white balls denote the Ti, O, and H atoms, respectively.

Table 2.
The adsorption energy, Eads, of the OH cluster on the H terminated TiO2 nanotube (eV).
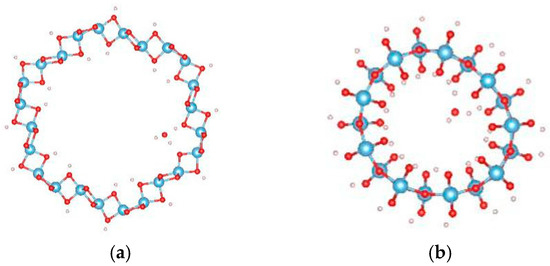
Figure 3.
The most stable adsorption sites of OH on the TiO2 nanotube after full relaxations; (a) (0, 6); and (b) (9, 0). The blue, red, and white balls denote the Ti, O, and H atoms, respectively.
3.3. Adsorption of Water on the Clean TiO2NT
The adsorption configurations were investigated by comparing the stability of a water molecule at different adsorption sites of the TiO2NTs. It was reported that the adsorption energy of a water molecule on the TiO2 surface was almost not affected by the relative site and orientation of the water [45]. Therefore, only several adsorption configurations of a water molecule on TiO2NT are considered in the present work. Because the influence of the radius of TiO2NT on the adsorption energy of a water molecule is insignificant [48], only the adsorption properties of water molecules on the (0, 3) and (6, 0) nanotubes were studied in the following calculations concerning the computation consuming. It was reported that the adsorption of single water molecules on TiO2NT was exothermic and energetically favorable [49]. We also obtained results that the adsorption energies of a single water molecule on the (0, 3) and (6, 0) nanotubes were −0.660 eV and −0.662 eV, respectively. Table 3 shows the adsorption energies of different amounts of water molecules in molecular and dissociated states (denoted as M and D, respectively) on the (0, 3) and (6, 0) nanotubes.

Table 3.
The adsorption energies of water molecules on the (0, 3) and (6, 0) nanotubes (eV).
For the adsorption of a single water molecule on the (0, 3) nanotube, five adsorption sites were considered as shown in Figure 4, and the calculated adsorption energies are listed in Table 3. The five sites have similar adsorption energies, varying in a 0.2 eV energy range. The smallest adsorption energy is −0.660 eV with the relaxed configuration shown in Figure 5a. The oxygen atom of water molecule saturates the five-fold Ti, and the angle between H–O–H is slightly increased to 109.7° by the attraction of the neighbouring oxygen atoms in the nanotube. To further study the stability of this adsorption configuration, the OH and H species of water molecules are initially separated as shown in Figure 5b. After full relaxation, the OH and H species combinated together to the water molecule (as shown in Figure 5c) and the total energy of this configuration is almost identical to that of the most stable adsorption configuration. This result indicates that the single water molecule prefers to adsorb in the molecular state compared to the dissociated mode on the TiO2NT.
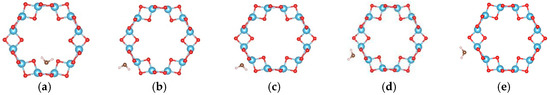
Figure 4.
The initial adsorption sites of water molecules on (0, 3) TiO2 nanotubes. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively. The indices of (a–e) denote the different adsorption sites of a water molecule on the TiO2 nanotube.
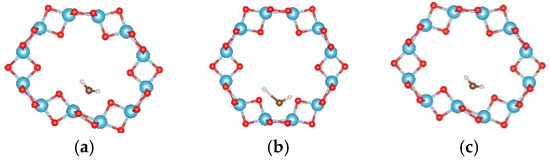
Figure 5.
The configurations of a water molecule adsorbed on the (0, 3) TiO2 nanotube. (a) The relaxed molecular state; (b) initial; and (c) relaxed configurations of dissociated states. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively.
In order to study the interactions between water molecules, the adsorption of two and three water molecules were studied using a 1 × 1 × 3 supercell of the (0, 3) nanotube, and the adsorption configurations are shown in Figure 6 and Figure 7, respectively. Three and two adsorption configurations for the former and latter cases were considered, respectively. The smallest adsorption energies are −1.128 eV (−0.564 eV/H2O) for the former and −1.838 eV (−0.613 eV/H2O) for the latter cases. In the multi-water adsorption systems, the relative stabilities between the molecular and dissociated states were also studied by dissociating one water molecule and keeping the others in their adsorption sites as shown in Figure 8. For two water molecule adsorbed system, the initially dissociated OH and H re-combinated together to the molecular state, however, they do not reform a water molecule in the three water molecule adsorbed system. The total energy of the system with two molecular and one dissociated water is 0.032 eV lower than that with three water molecules, indicating that interactions between water molecules prevent the re-combination of the dissociated water molecule.
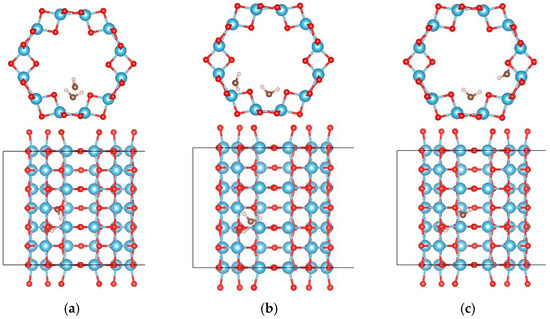
Figure 6.
The configurations of two water molecules adsorbed on (0, 3) TiO2 nanotubes. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively. The indices of (a–c) denote the different adsorption sites of a water molecule on the TiO2 nanotube.
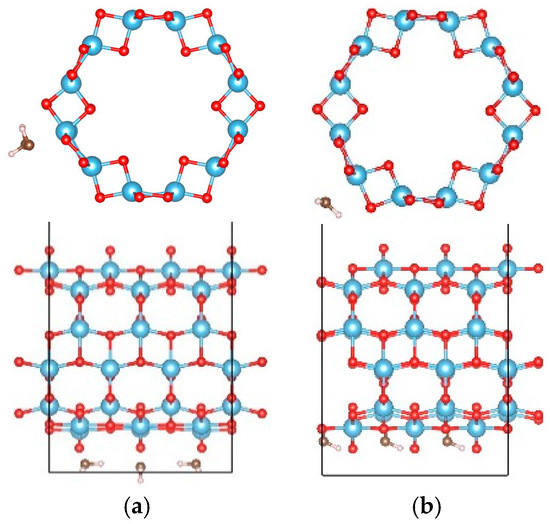
Figure 7.
The configurations of three water molecules adsorbed on the (0, 3) TiO2 nanotubes. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively. The indices of (a,b) denote the different adsorption sites of a water molecule on the TiO2 nanotube.
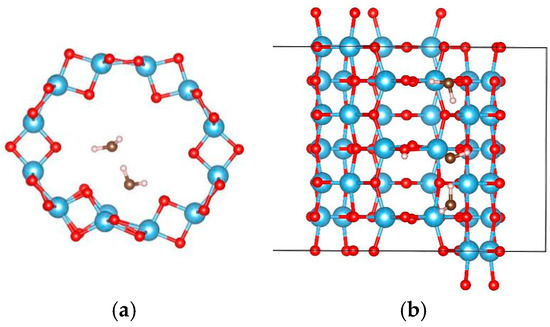
Figure 8.
The relaxed configurations of dissociated water molecules adsorbed on the (0, 3) TiO2 nanotubes. (a) Two water molecules and (b) three water molecules. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively.
For the (6, 0) nanotube, only one adsorption site for the single water molecule adsorption was studied considering the fact that the adsorption behavior of water is less sensitive to the diameter of the tube [48]. Similar adsorption energies when the water molecule adsorbs at different sites on the (0, 3) nanotube were shown in the above calculations. The estimated adsorption energy of one water molecule on the (6, 0) nanotube is −0.662 eV, which is very close to that of a water molecule adsorbed on the (0, 3) nanotube. The total energy of a single water molecule adsorption on the (6, 0) nanotube in the molecular state (Figure 9a) is about 0.02 eV lower than that in the dissociated state (Figure 9b). In order to clarify the interactions between water molecules, seven different adsorption configurations in a two water molecule adsorbed (6, 0) nanotube are studied, as shown in Figure 10. It also shows about 0.2 eV difference in total energies between the different adsorption configurations. The smallest adsorption energy of the two water adsorbed (6, 0) nanotube is −1.277 eV, which is about 0.15 eV smaller than that of the two water molecules adsorbed on the (0, 3) nanotube. The total energy of the (6, 0) nanotube with one dissociated and one molecular water is about 0.01 eV higher than that of the adsorption of two water molecules in the molecular state, as shown in Figure 9c,d. It is similar to the adsorption of a water molecule on the (0, 3) nanotube. Due to the almost identical adsorption characteristics of water and the OH cluster on the (0, 3) and (6, 0) nanotubes, the adsorption of three water molecules on the (6, 0) nanotube is not considered in the present study. It should be pointed out that although the relative stabilities of dissociated or molecular states are really complex due to the large number of possible adsorption configurations for multiple water molecules on the nanotube, the interactions between water molecules and the nanotube are the key factor determining the adsorption characteristics.
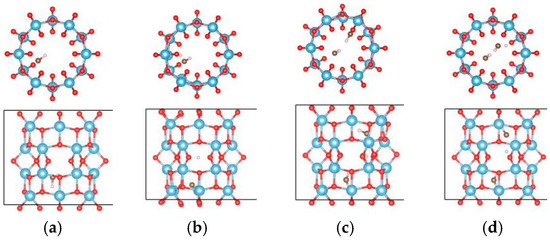
Figure 9.
The relaxed configurations of water molecules adsorbed on the (6, 0) TiO2 nanotubes. (a) One molecular state; (b) one dissociated state; (c) two molecular states; and (d) one molecular state and dissociated state. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively.
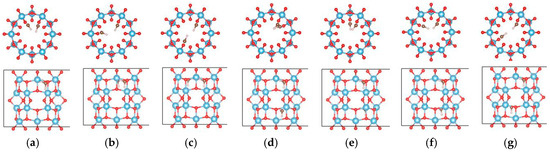
Figure 10.
The initial adsorption sites of water molecules on the (6, 0) TiO2 nanotubes. The blue, red, brown, and white balls denote the Ti, O in TiO2NT, O in water, and H atoms, respectively. The indices (a–g) denote the different adsorption sites of a water molecule on the TiO2 nanotube.
3.4. Electronic Structures of Water Adsorbed Nanotubes
The Bader charge analysis was employed to illustrate the charge distributions of atoms in water adsorbed TiO2NT [55]. For the clean (0, 3) nanotube, the charges of two-fold and three-fold oxygen atoms are 6.86 e and 7.04 e, respectively, and the value is 2.11 e for Ti, indicating that Ti donates electrons to the oxygen atoms. For the two water molecule adsorbed system (Figure 11a), the Bader charges of the indexed ‘Ti’ and ‘O3’ atoms of the nanotube are 2.06 e and 6.88 e, respectively. The total Bader charges of two water molecules ‘H3–O1–H’ and ‘H1–O2–H2’ are 7.93 e and 7.91 e, respectively. Therefore, a small amount of electrons transfer from the water molecule, especially the H1–O2–H2 molecule, to the nanotube.
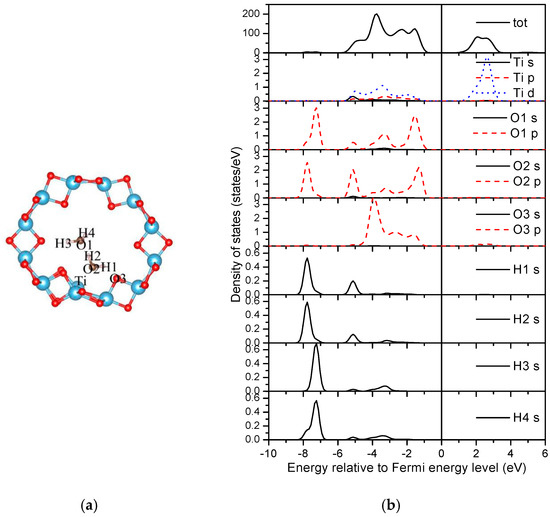
Figure 11.
Density of states of the two water molecule adsorbed (0, 3) nanotube. (a) Structure; (b) DOSs.
For the three water adsorbed nanotube in an energetically stable state (Figure 12a), the Bader charges of the indexed ‘Ti’ and ‘O4’ of the nanotube are 2.04 e and 7.19 e, respectively. The total Bader charges of the H1–O1–H2, H3–O–H4, and H5–O3–H6 ‘molecules’ are 7.94 e, 7.87 e, and 7.91 e, respectively. Specifically, the Bader charge of H4 is about 0.06 e less than that of H3, which may imply a stronger bonding interaction between H4 and its neighboring oxygen atoms. The Bader charge of H2 neighbored by two oxygen atoms is about 0.03 e smaller than that of H1 with one neighboring oxygen atom, causing less charge transfer with respect to H2. The total Bader charge of the H3–O2–H4 ‘molecule’ is the smallest between the considered three water molecules due to the dissociation of H. The “H–O–H–O–H” cluster will then be formed after the dissociation to stabilize the adsorption.
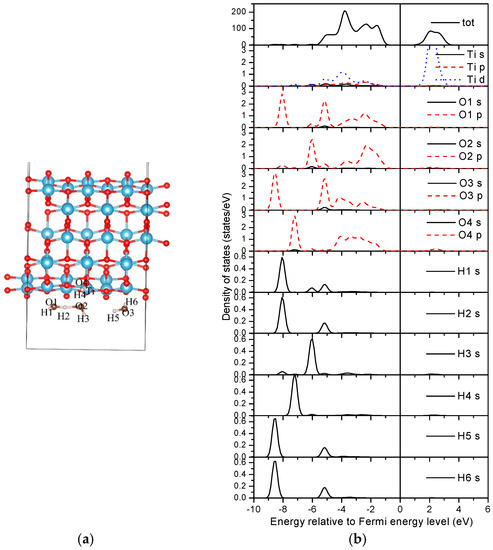
Figure 12.
Density of states of a three water molecule adsorbed (0, 3) nanotube. (a) Structure; (b) DOSs.
The densities of states (DOSs) are further studied to reveal the interactions between water molecules and the TiO2 nanotube. Figure 11b and Figure 12b shown the DOSs of the most stable adsorption configurations of two and three water molecules on the (0, 3) nanotube (Figure 8). It is worth noting that the DFT calculations underestimate the band-gap of the oxides as shown in our calculations. For the two water molecule adsorbed system as shown in Figure 11b, the bonding peaks of H 1s are mainly concentrated in the energy region of (−7.0, −8.0) eV, in which they strongly overlap with the oxygen atoms from the neighboring water (O2 p orbitals) causing strong bonding interactions. No overlaps between O3 from the nanotube and hydrogen from the water can be detected, implying a vanished weak interaction between them. The O p orbitals from water molecules overlap with the Ti d orbitals in the energy region of (−5.5, −1.0) eV. Therefore, the adsorptions of water on TiO2NT are mainly controlled through the bonding interactions between the oxygen atom in the water molecule and the Ti atom from TiO2NT. For the three water molecule adsorbed system, the total DOS of the adsorbed nanotube are similar with that of the two water molecule adsorbed nanotube as shown in Figure 12b. The bonding peaks of the dissociation hydrogen atom (H4) are mainly distributed in the energy region of (−6.6, −7.6) eV, and forms bonds with the oxygen atom (O4) from TiO2NT only. Other hydrogen atoms do not escape from the water, i.e., they mainly bond with their neighboring oxygen atoms in water molecules. The ‘Ti’ also slightly bonds with oxygen atoms from water molecules. Therefore, the bonding interactions between the hydrogen from water molecule and the oxygen from the nanotube may be helpful for the water dissociation on TiO2NT.
The light irradiation and isoelectronic point are important parameters to evaluate the photocatalysis. Presently, the optical properties, such as the adsorption coefficient, are calculated to evaluate the influence of light on the materials. Most recently, we have calculated the adsorption coefficient of nanotubes along and perpendicular to the tube axis, and achieved similar results with experiments [56]. Our calculations show that the large adsorption coefficients occur in the high energy area (~5.0 eV). The O–H may be easy to dissociate under light irradiation with high energy. Recently, Huang et al. studied the electrochemical phase diagrams combining the density functional calculations and the thermodynamics results [57], in which the experimental chemical potentials for ions in solution were employed. The electrode potentials for the oxidation of water to O2 and the reduction of H+ to H2 were located between the Valence Band Maximum (VBM) and Conduction Band Minimum (CBM) of TiO2 under most of pH values. The electronic structures of the nanotube are similar with the bulk TiO2, and therefore, the TiO2NT should be a promising photocatalyst, as shown by experimental results.
4. Conclusions
First principles calculations are carried out to study the adsorption properties of water molecules on TiO2NT. Firstly, the adsorption of OH clusters on clean and H-terminated TiO2NT are studied. The smallest adsorption energy is about −1.0 eV for OH cluster adsorption on the clean tube and −5.0 eV on the H-terminated tube, respectively. A water molecule will be easily formed for the adsorption of an OH cluster on clean and H-terminated TiO2NT. The adsorption properties of single and multiple water molecules on TiO2NT are investigated. The single and two water molecules prefer to adsorb in the molecular state, while the dissociated state is more preferable for the adsorption of three water molecules, according to the total energy calculations. Water molecules tend to dissociate into OH and H. The H then bonds with the oxygen from the nanotube, and the OH cluster will form a ‘H–O–H–O–H’ cluster. Therefore, the interactions between molecules can greatly affect the adsorption of water on the TiO2 nanotube and may bring about the dissociation of water.
Acknowledgments
This work was supported by the National Basic Research Programme of China, Grant No. 2016YFB0701301, Natural Science Foundation of Shandong, China, Grant No. ZR2014EMM013, No. ZR2014EMQ009, and the Fundamental Research Funds for the Central Universities Grant No. HIT. KITP. 2014030. Simulations were performed using HPC resources in CAS Shenyang Super-computing Center.
Author Contributions
Yan Song conceived and designed the experiments; Jianhong Dai performed the experiments; Yan Song and Jianhong Dai analyzed the data; Jianhong Dai wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W. Photochemical Splitting of Water for Hydrogen Production by Photocatalysis: A Review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from Photo-catalytic Water Splitting Process: A Review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Takeuchi, M.; Tsujimaru, K.; Anpo, M.; Thomas, J.M. Photocatalysis for New Energy Production Recent Advances in Photocatalytic Water Splitting Reactions for Hydrogen Production. Catal. Today 2007, 122, 51–61. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry. Science 1980, 207, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Corma, A.; Garcia, H. Titania Supported Gold Nanoparticles as Photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.T.; Somorjai, G.A. Photocatalytic Hydrogen Production from Water on Pt-free SrTiO3 in Alkali Hydroxide Solutions. Nature 1980, 285, 559–560. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Reactions of Water with Carbon and Ethylene over Illuminated Pt/TiO2. Chem. Phys. Lett. 1980, 70, 131–134. [Google Scholar]
- Domen, K.; Naito, S.; Soma, M.; Onishi, T.; Tamaru, K. Photocatalytic Decomposition of Water Vapour on an NiO–SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. 1980, 12, 543–544. [Google Scholar] [CrossRef]
- Inoue, Y.; Niiyama, T.; Asai, Y.; Sato, K. Stable Photocatalytic Activity of BaTi4O9 Combined with Ruthenium Oxide for Decomposition of Water. J. Chem. Soc. Chem. Commun. 1992, 65, 579–580. [Google Scholar] [CrossRef]
- Kudo, A.; Tanaka, A.; Domen, K.; Maruya, K.; Aika, K.; Onishi, T. Photocatalytic Decomposition of Water over NiO–K4Nb6O17 Catalyst. J. Catal. 1988, 111, 67–76. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water–splitting using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Szymanski, P.; El-Sayed, M.A. Some Recent Developments in Photoelectrochemical Water Splitting using Nanostructured TiO2: A Short Review. Theor. Chem. Acc. 2012, 131, 1–12. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Kannekanti, L.; Jakkidi, K.R.; Venkata, P.S.M.; Durga, K.V.; Machiraju, S. Continuous Hydrogen Production Activity over Finely Dispersed Ag2O/TiO2 Catalysts from Methanol: Water Mixtures under Solar Irradiation: A Atructure–activity Correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar]
- Wu, N.-L.; Lee, M.-S. Enhanced TiO2 Photocatalysis by Cu in Hydrogen Production from Aqueous Methanol Solution. Int. J. Hydrogen Energy 2004, 29, 1601–1605. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lee, Y.J.; Joo, J.B.; Yin, Y.D.; Zaera, F. Evaluation of the Effective Photoexcitation Distances in the Photocatalytic Production of H2 from Water using Au@Void@TiO2 Yolk–shell nanostructures. ACS Energy Lett. 2016, 1, 52–56. [Google Scholar] [CrossRef]
- Brinkley, D.; Dietrich, M.; Engel, T.; Engel, T.; Farrall, P.; Gantner, G.; Schafer, A.; Szuchmacher, A. A Modulated Molecular Beam Study of the Extent of H2O Dissociation on TiO2(110). Surf. Sci. 1998, 395, 292–306. [Google Scholar] [CrossRef]
- Henderson, M.A.; Epling, W.S.; Peden, C.H.F.; Perkins, C.L. Insights into Photoexcited Electron Scavenging Processes on TiO2 Obtained from Studies of the Reaction of O2 with OH Groups Adsorbed at Electronic Defects on TiO2(110). J. Phys. Chem. B 2003, 107, 534–545. [Google Scholar] [CrossRef]
- Allegretti, F.; O’Brien, S.; Polcik, M.; Sayago, D.I.; Woodruff, D.P. Adsorption Bond Length for H2O on TiO2(110): A Key Parameter for Theoretical understanding. Phys. Rev. Lett. 2005, 95, 226104. [Google Scholar] [CrossRef] [PubMed]
- Lindan, P.J.D.; Harrison, N.M.; Holender, M.J. Mixed Dissociative and Molecular Adsorption of Water on the Rutile (110) Surface. Phys. Rev. Lett. 1998, 80, 762–765. [Google Scholar] [CrossRef]
- Stefanovich, E.V.; Truong, T.N. Ab initio Study of Water Adsorption on TiO2(110): Molecular Adsorption versus Dissociative Chemisorption. Chem. Phys. Lett. 1999, 299, 623–629. [Google Scholar] [CrossRef]
- Langel, W. Car–Parrinello Simulation of H2O Dissociation on Rutile. Surf. Sci. 2002, 496, 141–150. [Google Scholar] [CrossRef]
- Zhang, C.; Lindan, P.J.D. Multilayer water adsorption on rutile TiO2(110): A first-principles study. J. Chem. Phys. 2003, 118, 4620–4630. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Zhang, C. Exothermic Water Dissociation on the Rutile TiO2(110) Surface. Phys. Rev. B 2005, 72, 075439. [Google Scholar] [CrossRef]
- Harris, L.A.; Quong, A.A. Molecular Chemisorption as the Theoretically Preferred Pathway for Water Adsorption on Ideal Rutile TiO2(110). Phys. Rev. Lett. 2004, 93, 086105. [Google Scholar] [CrossRef] [PubMed]
- Kamisaka, H.; Yamashita, K. The Surface Stress of the (110) and (100) Surfaces of Rutile and the Effect of Water Adsorbents. Surf. Sci. 2007, 601, 4824–4836. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Meyer, B.; Marx, D. Composition, Structure, and Stability of the Rutile TiO2(110) Surface: Oxygen Depletion, Hydroxylation, Hydrogen Migration, and Water Ddsorption. Phys. Rev. B 2009, 79, 115410. [Google Scholar] [CrossRef]
- Peng, S.F.; Ho, J.J. The adsorption and Dissociation of H2O on TiO2(110) and M/TiO2(110) (M = Pt, Au) Surfaces–A Computational Investigation. Int. J. Hydrogen Energy 2010, 35, 1530–1536. [Google Scholar] [CrossRef]
- Hong, F.; Ni, Y.H.; Xu, W.J.; Yan, Y.F. Origin of Enhanced Water Adsorption at Step Edge on Rutile TiO2(110) surface. J. Chem. Phys. 2012, 137, 114707. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, A.; Valentin, C.D.; Selloni, A. O2 Interaction and Reactivity on a Model Hydroxylated Rutile(110) Surface. J. Phys. Chem. B 2005, 109, 20963–20967. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.X.; Long, J.L.; Gu, Q.; Zhang, W.X.; Ruan, R.S.; Li, Z.H.; Wang, X.X. In situ IR Study of Surface Hydroxyl Species of Dehydrated TiO2: Towards Understanding Pivotal Surface Processes of TiO2 Photocatalytic Oxidation of Toluene. Phys. Chem. Chem. Phys. 2012, 14, 9468–9474. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.J.C. Infrared Studies of the Surface Hydroxyl Group on Titanium Dioxide, and of the Chemisorption of Carbon Monoxide and Carbon Dioxide. J. Phys. Chem. 1961, 65, 746–753. [Google Scholar] [CrossRef]
- Lin, H.X.; Deng, W.H.; Long, J.L.; Wang, X.X. In situ IR study of Surface Hydroxyl and Photocatalytic Oxidation of Toluene of Rutile TiO2. Spectrosc. Spectr. Anal. 2014, 34, 1229–1233. [Google Scholar]
- Lin, H.X.; Wang, X.X.; Fu, X.Z. Properties and Distributionof the Surface Hydroxyl Groups of TiO2. Prog. Chem. 2007, 19, 665–670. [Google Scholar]
- Vittadini, A.; Casarin, M.; Selloni, A. Chemistry of and on TiO2-anatase Surfaces by DFT Calculations: A Partial Review. Theor. Chem. Acc. 2007, 117, 663–671. [Google Scholar] [CrossRef]
- Herman, G.S.; Dohnálek, Z.; Ruzycki, N.; Diebold, U. Experimental Investigation of the Interaction of Water and Methanol with Anatase-TiO2(101). J. Phys. Chem. B 2003, 107, 2788–2795. [Google Scholar] [CrossRef]
- Vittadni, A.; Selloni, A.; Rotzinger, F.P. Structure and Energetics of Water Adsorbed at TiO2 Anatase 101 and 001 Surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957. [Google Scholar] [CrossRef]
- Bredow, T.; Jug, K. Theoretical Inverstigation of Water–adsorption at Rutile and Anatase Surfaces. Surf. Sci. 1995, 327, 398–408. [Google Scholar] [CrossRef]
- Selloni, A.; Vittadini, A.; Gratzel, M. The adsorption of Small Molecules on the TiO2 Anatase (101) Surface by First-principles Molecular Dynamics. Surf. Sci. 1998, 402, 219–222. [Google Scholar] [CrossRef]
- Henderson, M.A. Structural Sensitivity in the Dissociation of Water on TiO2 Single-Crystal Surfaces. Langmuir 1996, 12, 5093–5098. [Google Scholar] [CrossRef]
- Henrich, V.E.; Dresselhaus, G.; Zeiger, H.J. Chemisorbed Phases of H2O on TiO2 and SrTiO3. Solid State Commun. 1977, 24, 623–626. [Google Scholar] [CrossRef]
- Wang, L.Q.; Baer, D.R.; Engelhard, M.H.; Shultz, A.N. The Adsorption of Liquid and Vapor Water on TiO2(110) Surfaces: The Role of Defects. Surf. Sci. 1995, 344, 237–250. [Google Scholar] [CrossRef]
- Sun, H.J.; Mowbray, D.J.; Migani, A.; Zhao, J.; Petek, H.; Rubio, A. Comparing Quasiparticle H2O Level Alignment on Anatase and Rutile TiO2. ACS Catal. 2015, 5, 4242–4254. [Google Scholar] [CrossRef]
- Hoyer, P. Formation of a Titanium Dioxide Nanotube Array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Liang, S.Z.; He, J.F.; Sun, Z.H.; Liu, Q.H.; Jiang, Y.; Cheng, H.; He, B.; Xie, Z.; Wei, S.Q. Improving Photoelectrochemical Water Splitting Activity of TiO2 Nanotube Arrays by tuning Geometrical Parameters. J. Phys. Chem. C 2012, 116, 9049–9053. [Google Scholar] [CrossRef]
- Meng, Q.-Q.; Wang, J.-G.; Xie, Q.; Dong, H.-Q.; Li, X.-N. Water Splitting on TiO2 Nanotube Arrays. Catal. Today 2011, 165, 145–149. [Google Scholar] [CrossRef]
- Liu, H.; Tan, K. Adsorption of Water on Single-walled TiO2 Nanotube: A DFT Investigation. Comput. Theor. Chem. 2012, 991, 98–101. [Google Scholar] [CrossRef]
- Bandura, A.V.; Evaresto, R.A. From Anatase (101) Surface to TiO2 Nanotubes: Rolling Procedure and First principles LCAO Calculations. Surf. Sci. 2009, 603, L117–L120. [Google Scholar] [CrossRef]
- Dai, J.H.; Song, Y. First Principles Calculations on the Hydrogen Atom Passivation of TiO2 Nanotube. RSC Adv. 2016, 6, 19190–19198. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for ab initio Total-energy Calculations using a Plane-wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [PubMed]
- Dai, J.H.; Song, Y. Electronic Structure Controlling of Assembly and Optical Properties of TiO2 Nanotube Arrays. ChemistrySelect 2016, 1, 3661–3666. [Google Scholar] [CrossRef]
- Huang, L.F.; Rondinelli, J.M. Electrochemical Phase Diagrams for Ti Oxides from Density Functional Calculations. Phys. Rev. B 2015, 92, 245126. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).