High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Preparation and Characterization
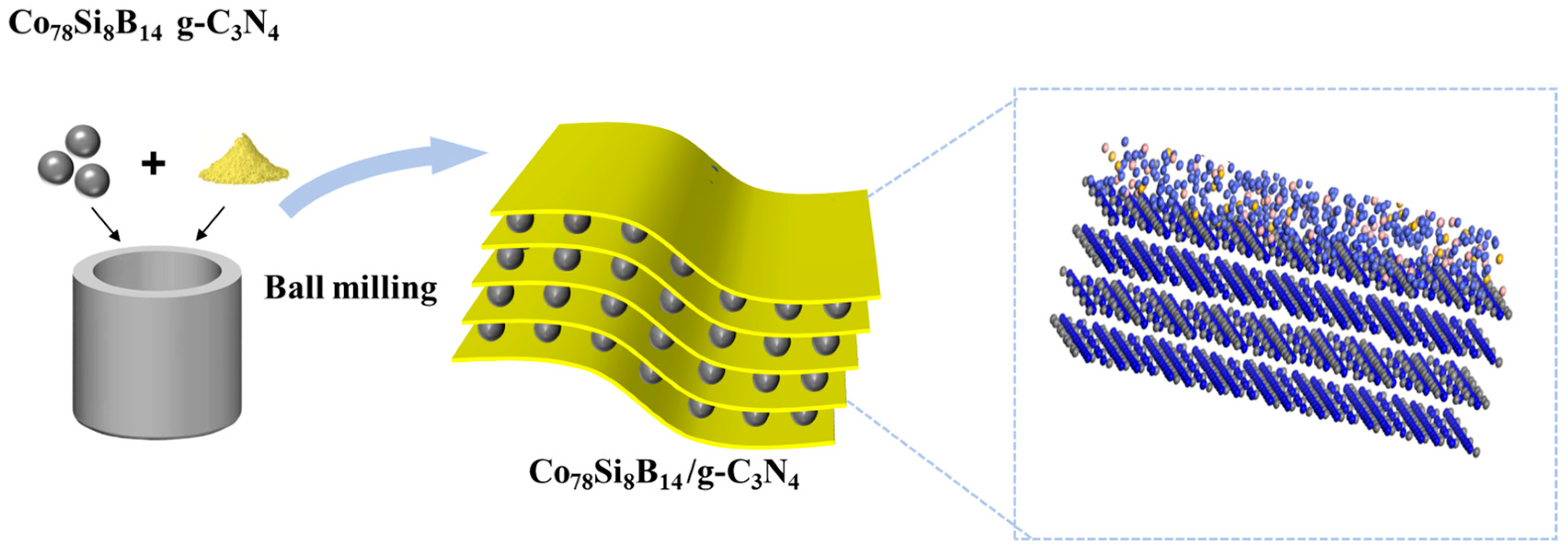
2.2. Preparation of Co78Si8B14/g-C3N4 Catalyst
- (1)
- Preparation of g-C3N4
- (2)
- Preparation of Co78Si8B14 powder
- (3)
- Preparation of Co78Si8B14/g-C3N4 composite catalysts
2.3. Degradation Experiment
2.4. DFT Calculation
3. Results and Discussion
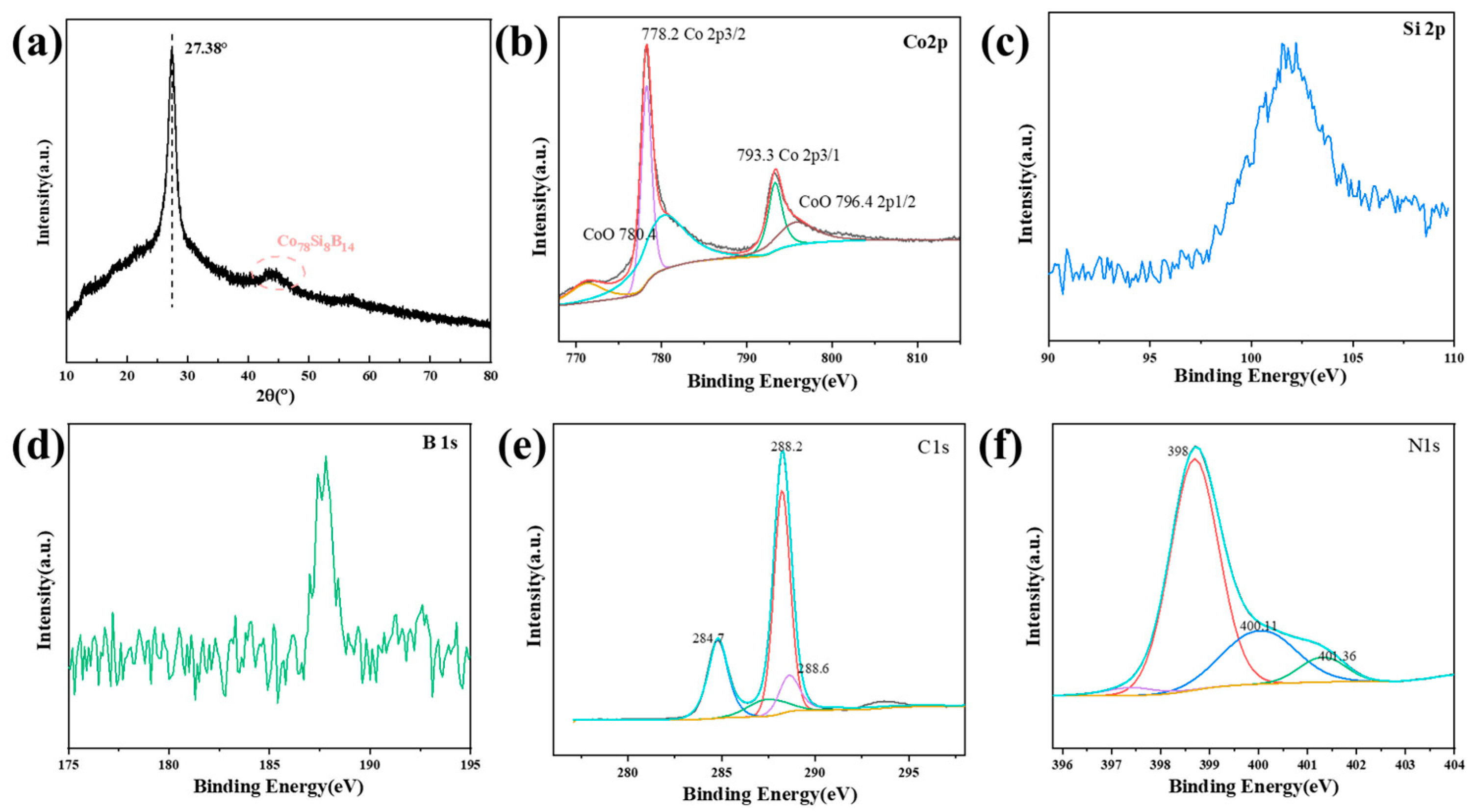
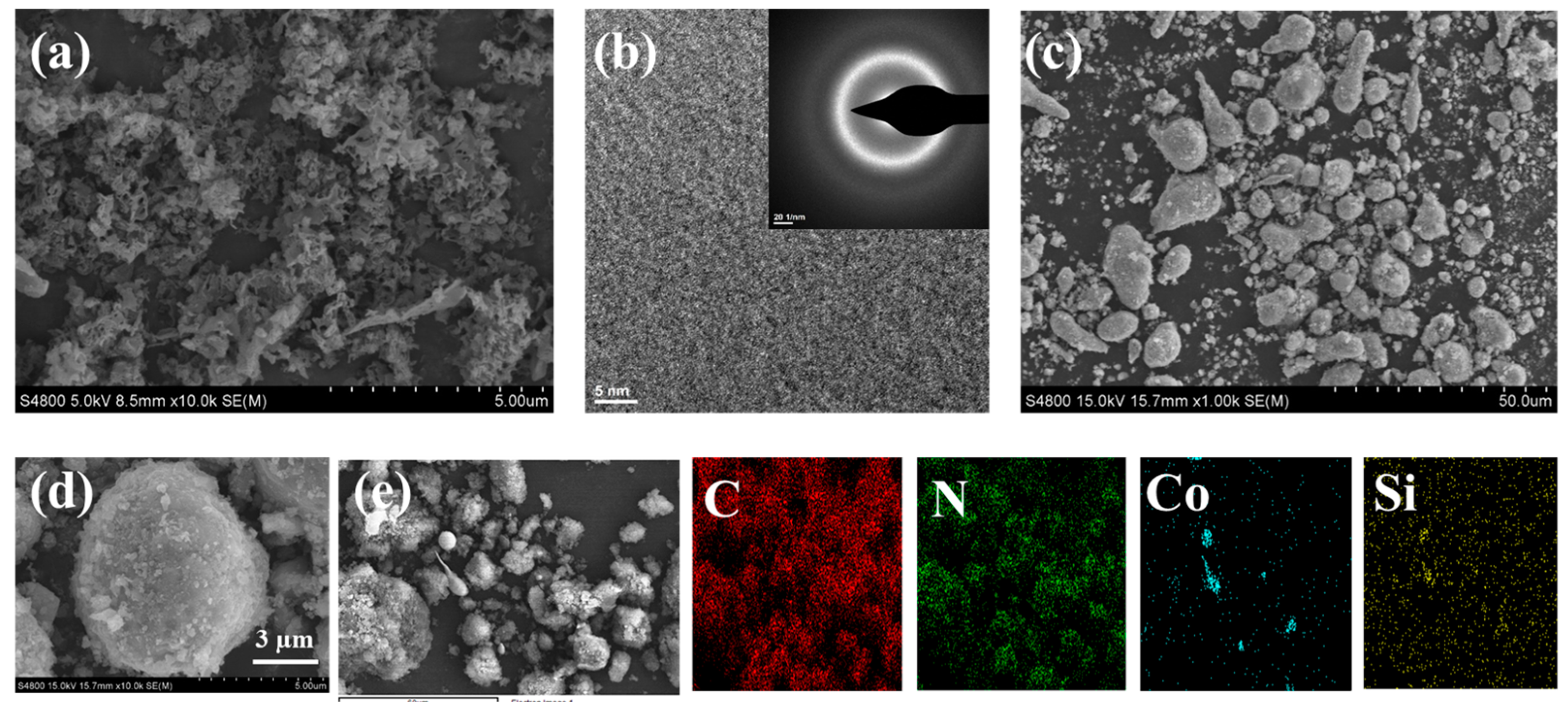
3.1. Structure and Morphology of Co78Si8B14/g-C3N4
3.2. Influence of Operative Parameters on Dye Degradation Analysis
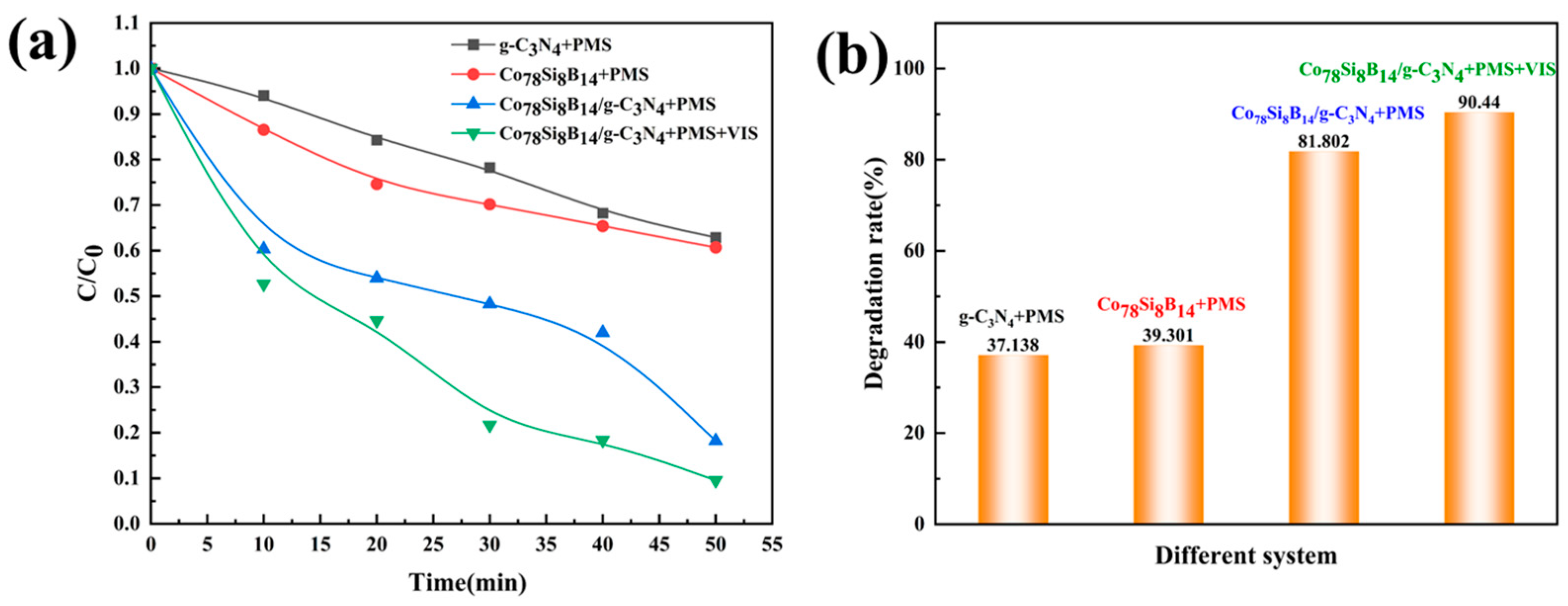
3.2.1. Influence of Systems on Dye Degradation Analysis
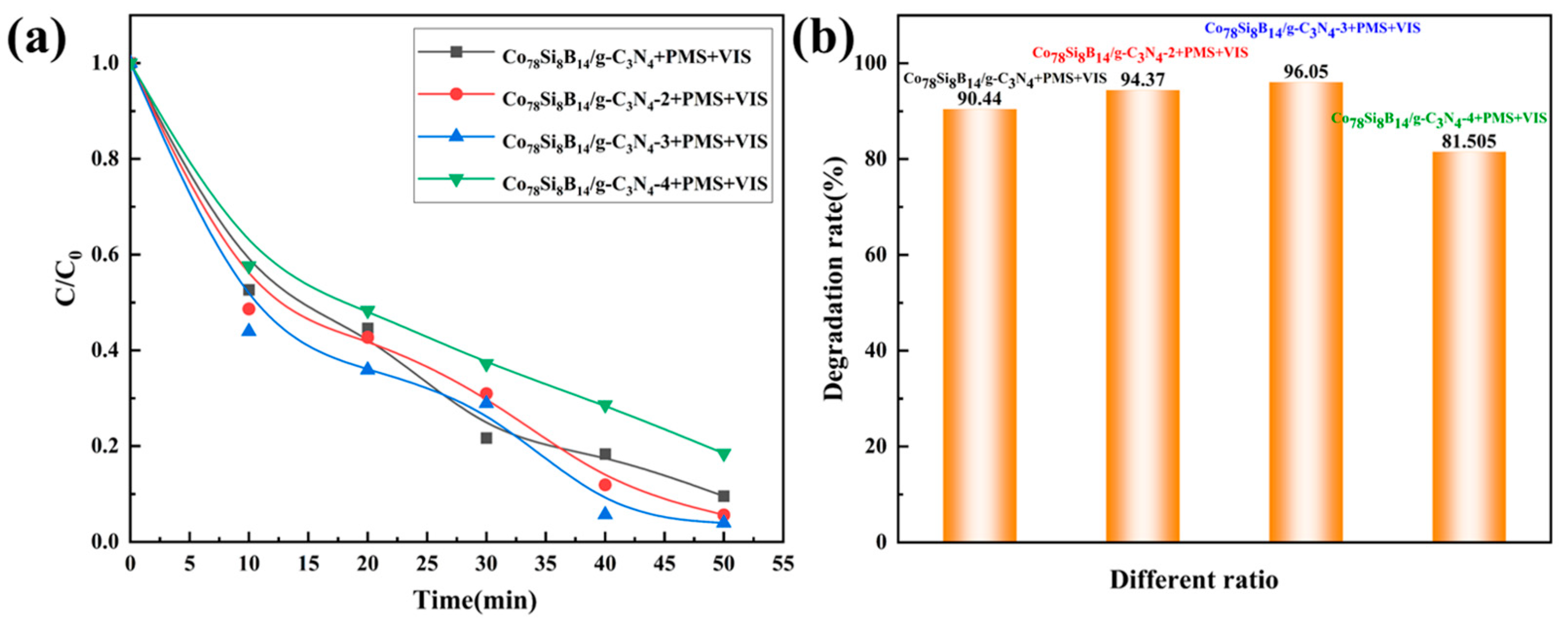
3.2.2. Influence of Different Ratios on the Degradation Performance of Co78Si8B14/g-C3N4 Composite Catalyst
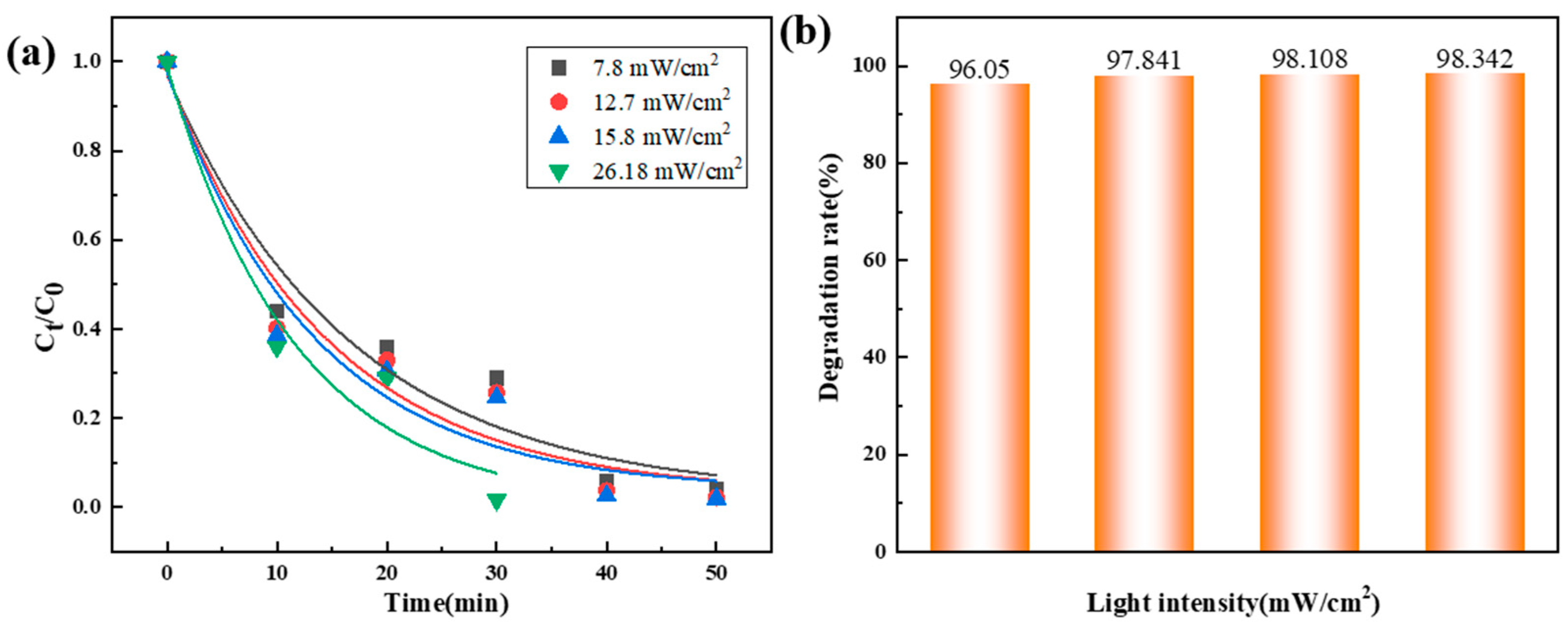
3.2.3. Influence of Light Intensity on the Degradation Performance of Co78Si8B14/g-C3N4 Composite Catalyst
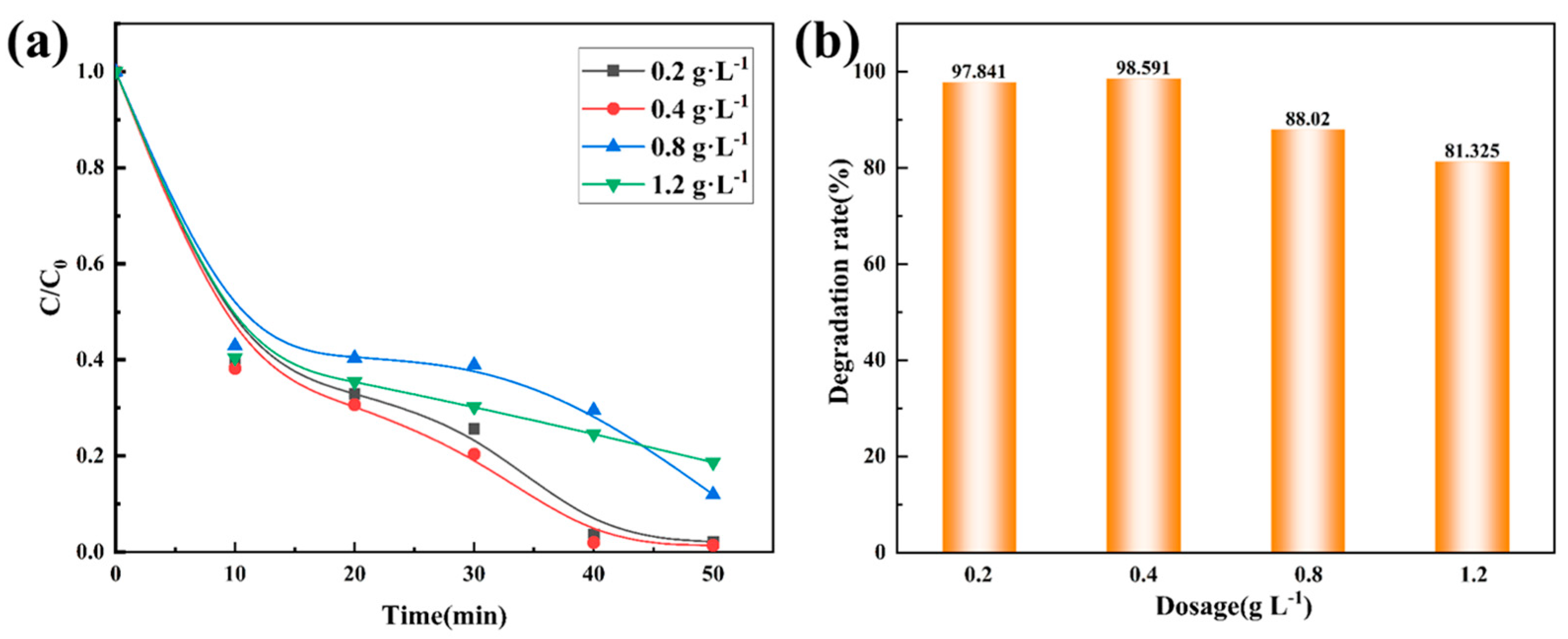
3.2.4. Influence of the Dosage of Co78Si8B14/g-C3N4 Composite Catalyst on Degradation Performance
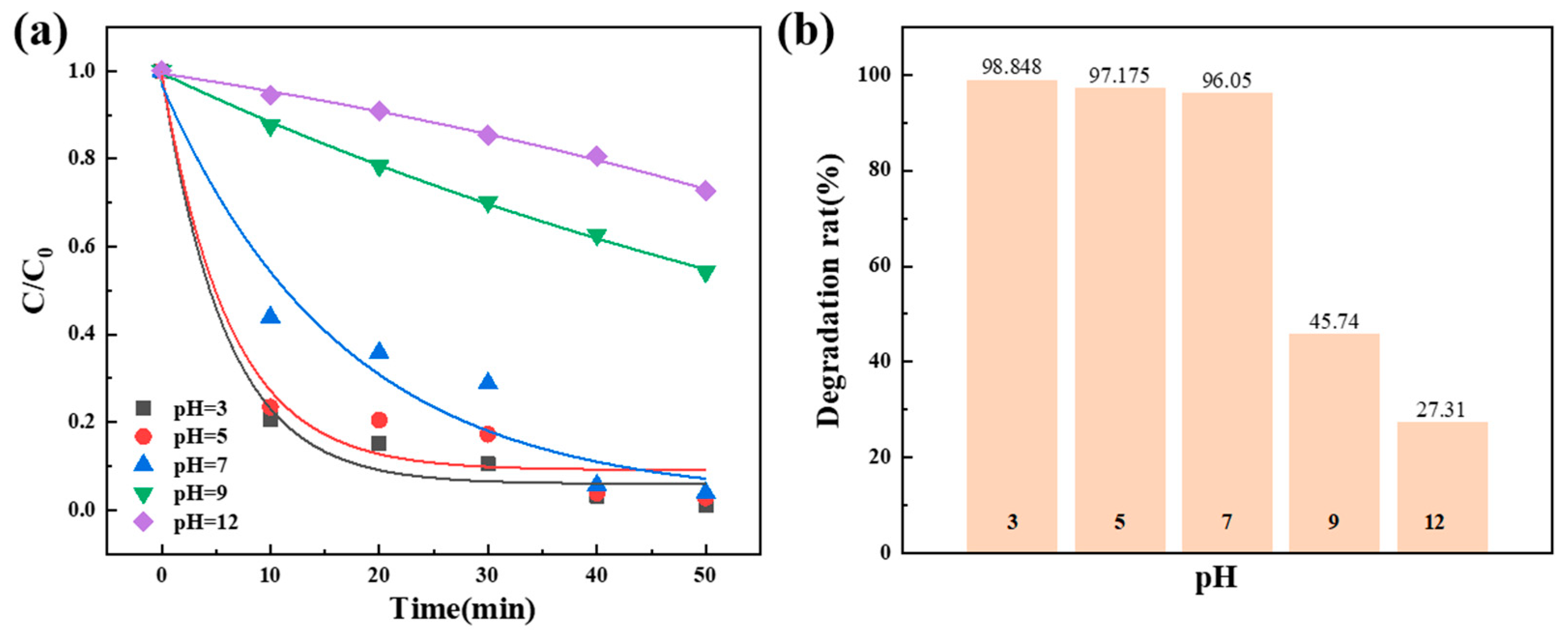
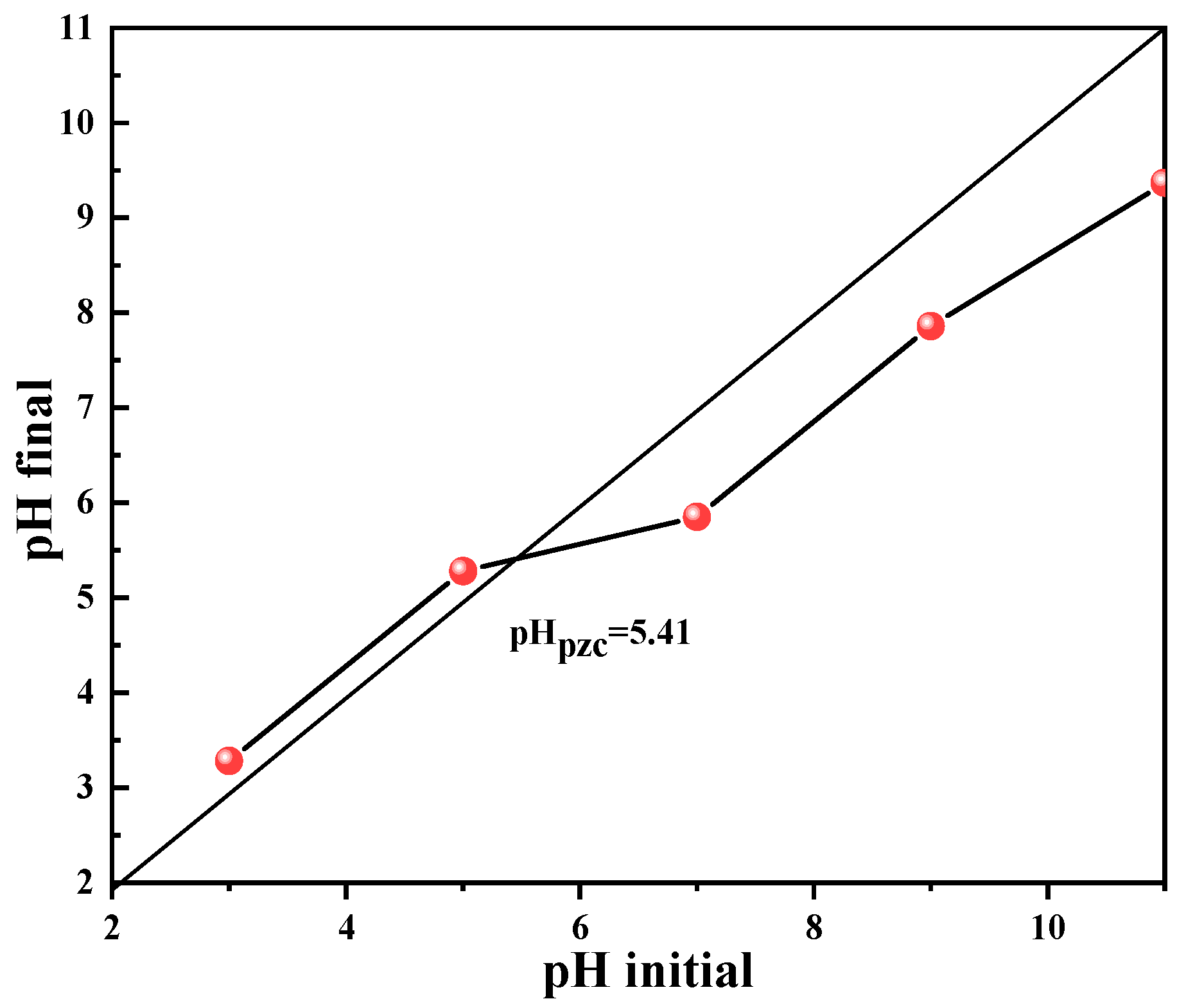
3.2.5. Influence of pH Value on the Degradation Performance of the Dosage of Co78Si8B14/g-C3N4 Composite Catalyst
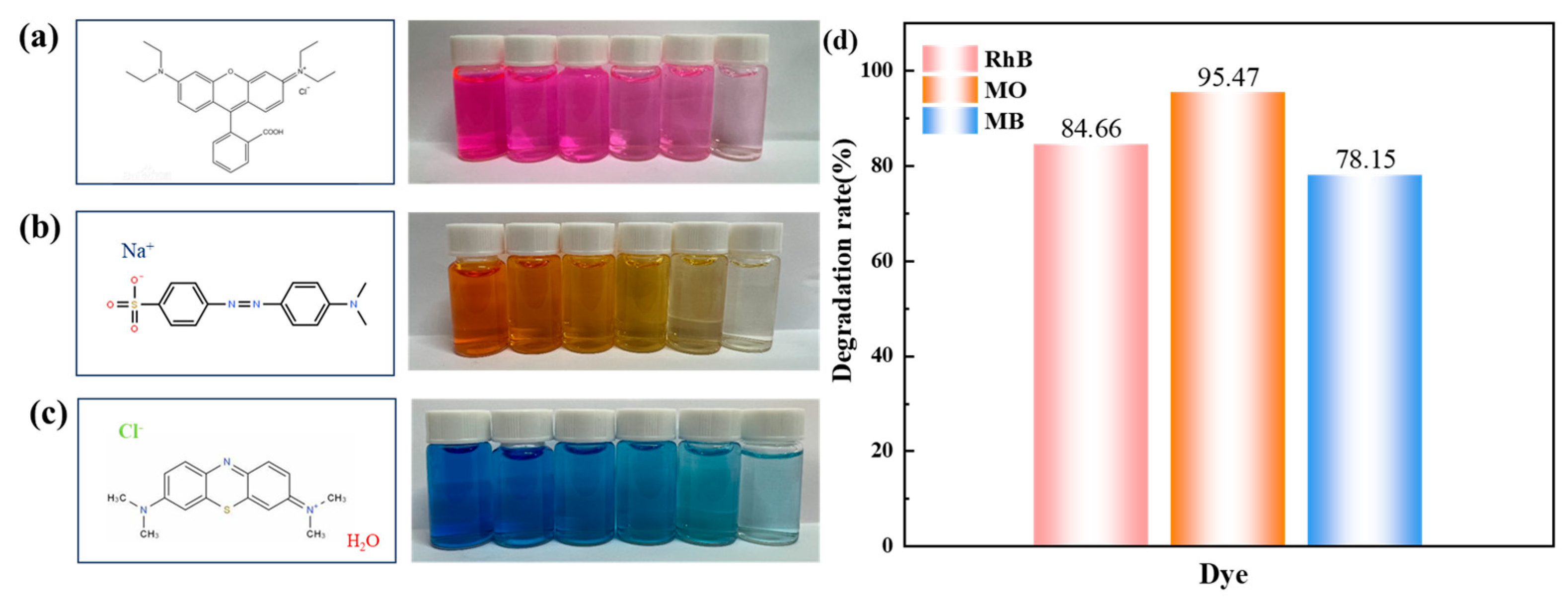
3.2.6. Degradation Performance of Other Dyes
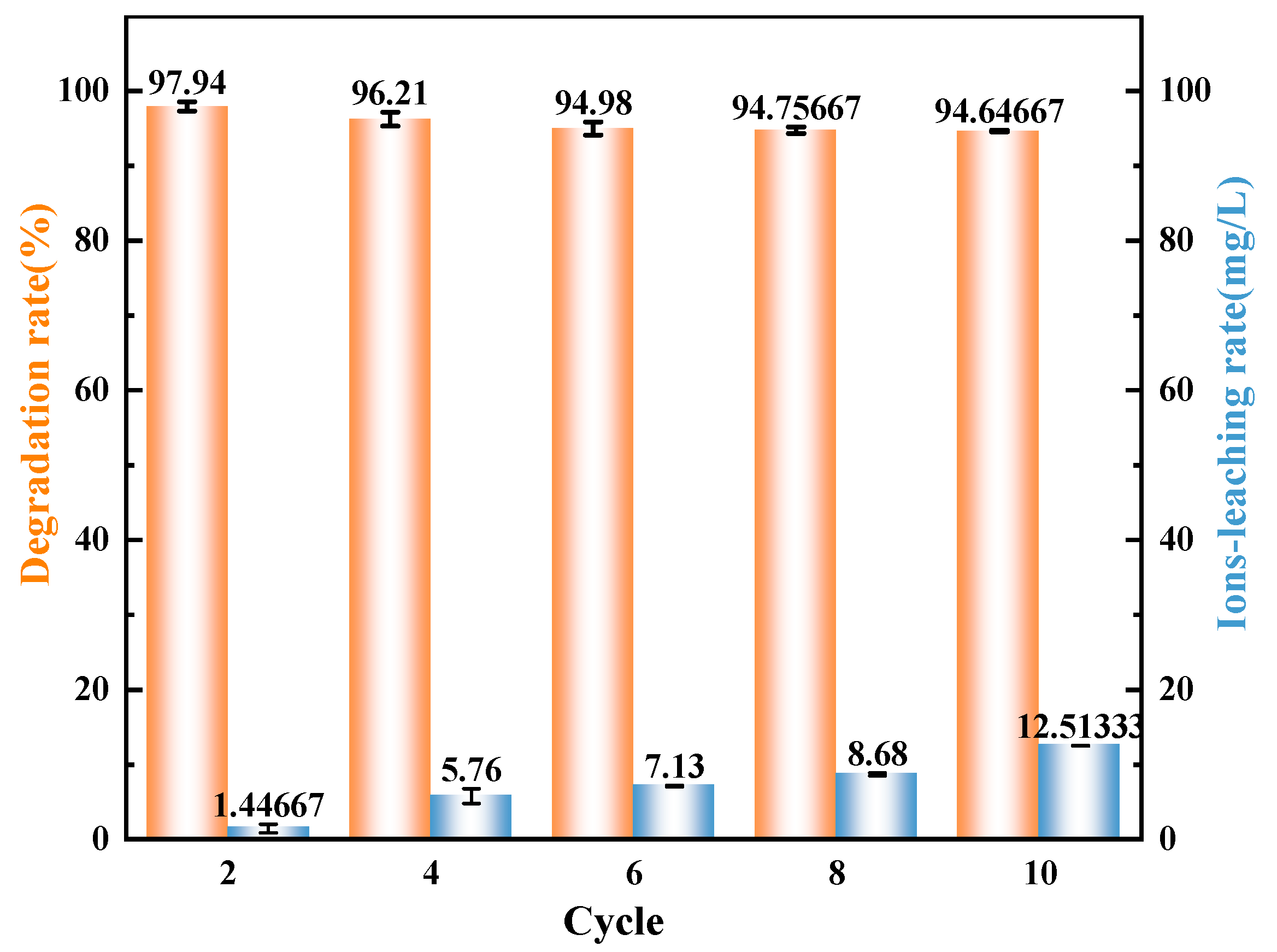
3.3. Cycle Test
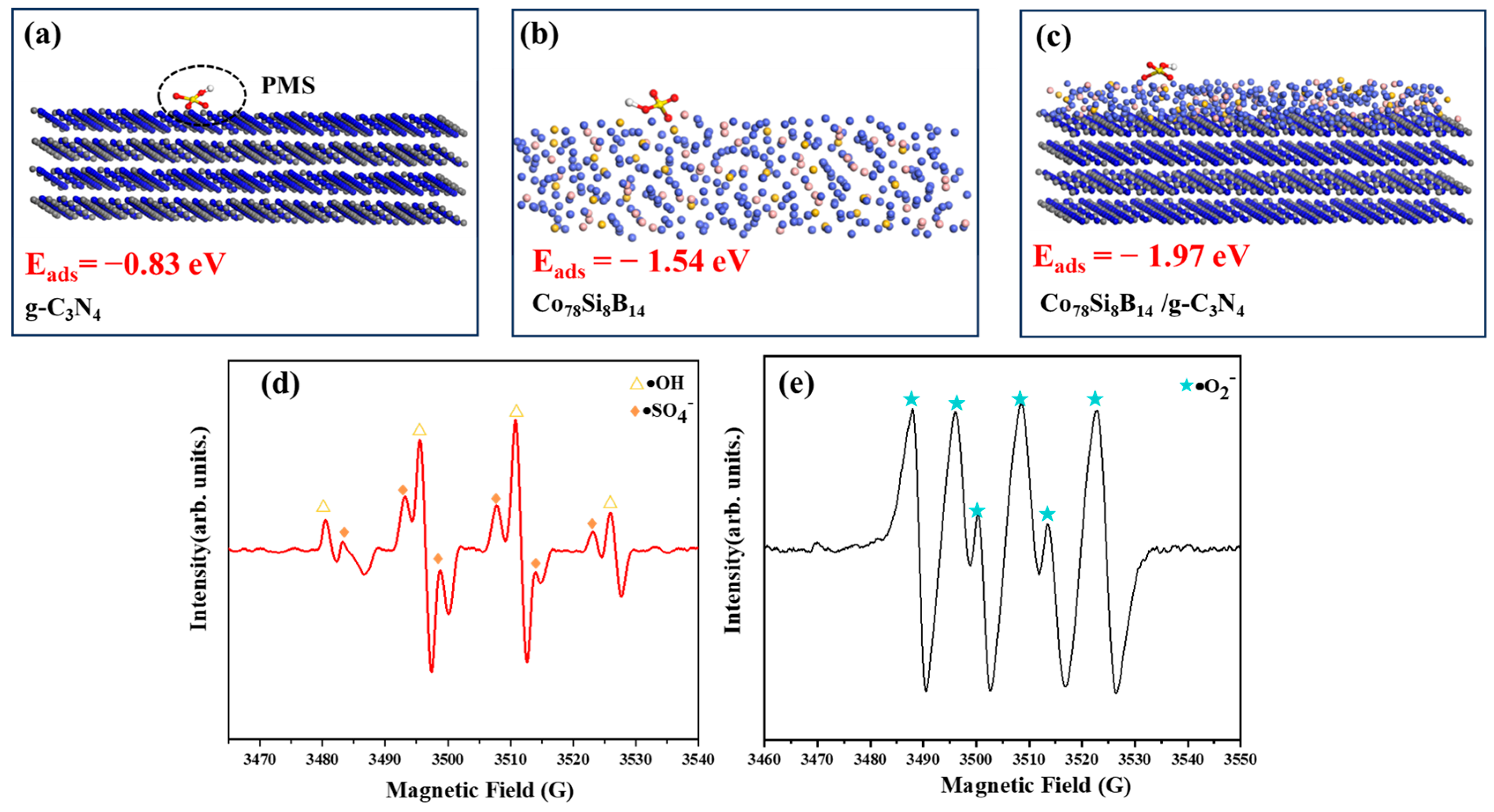
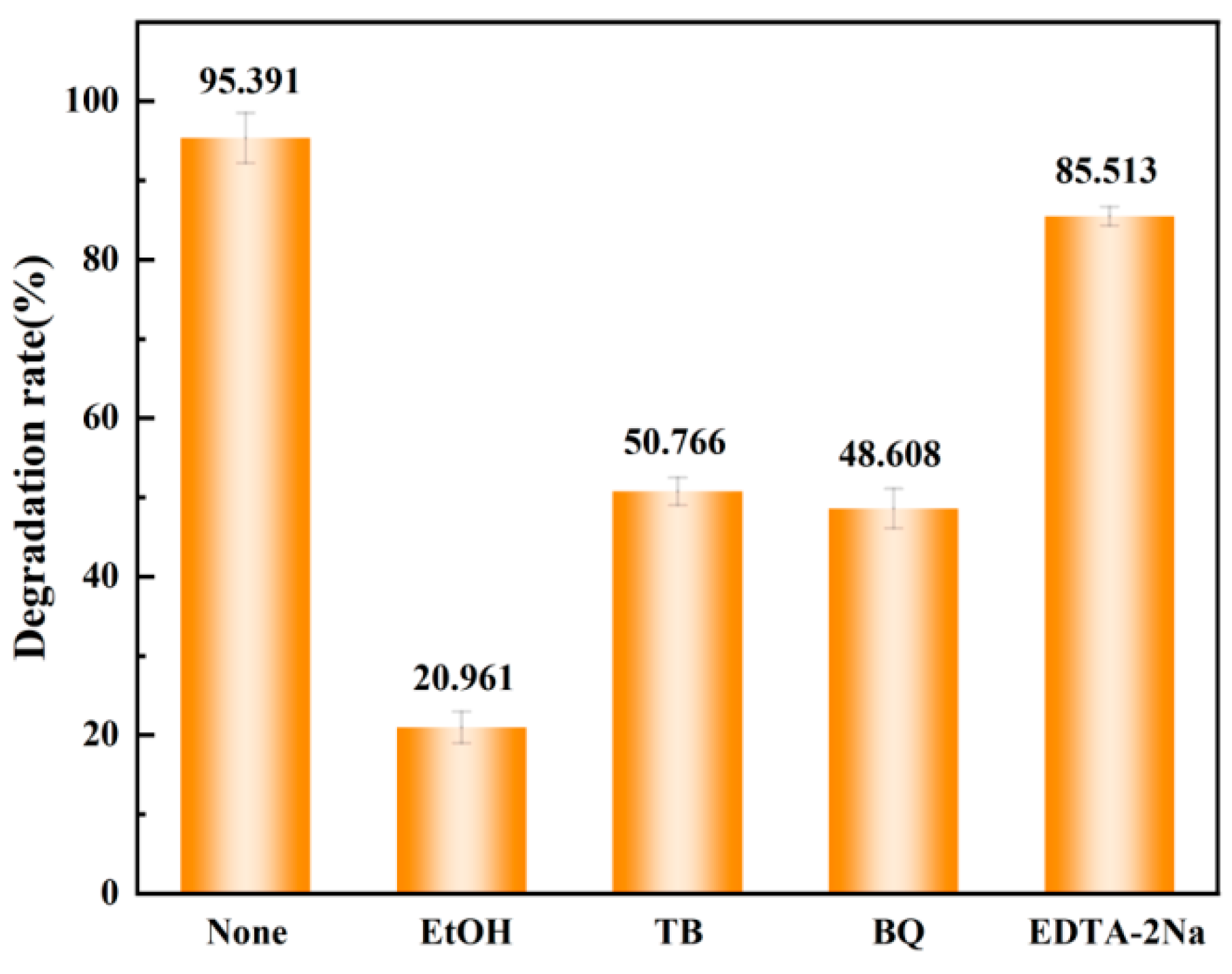
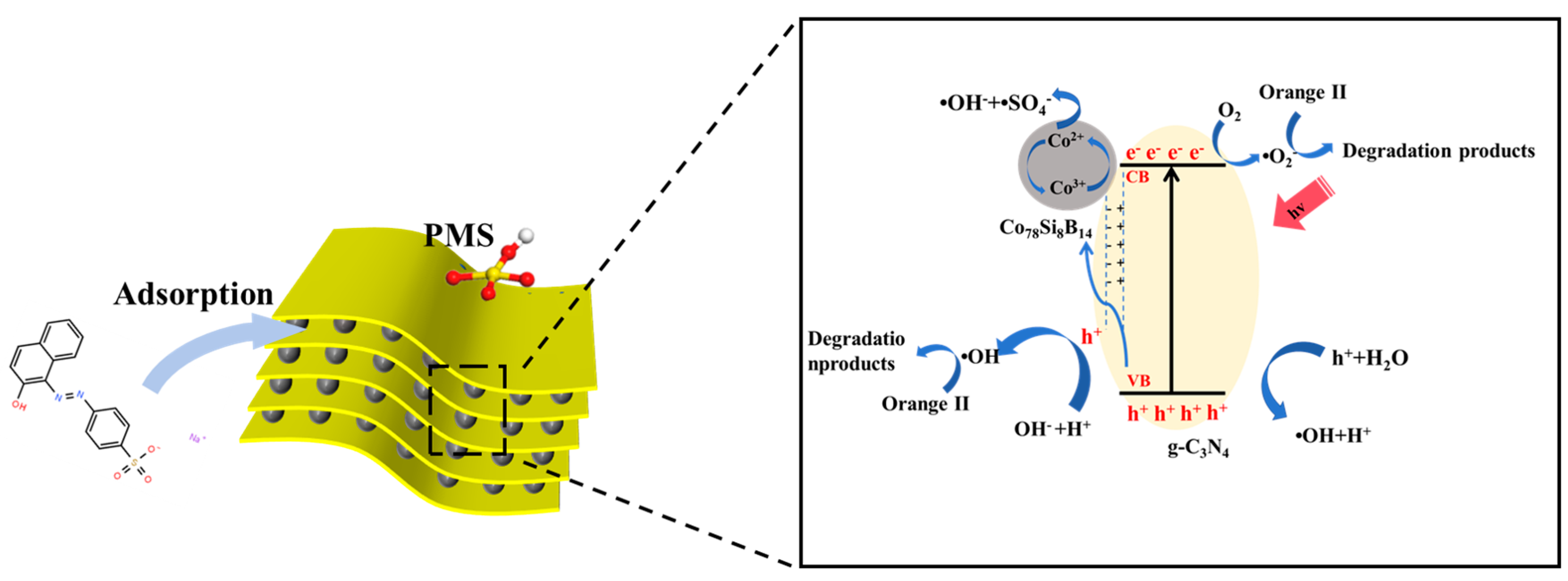
3.4. Degradation Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Tian, S.; Feng, Y.; Zhao, S.; Li, X.; Wang, S.; He, Z. Recent advances of photocatalytic coupling technologies for wastewater treatment. Chin. J. Catal. 2023, 54, 88–136. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, G.; Han, Z.; Li, H.; Chi, S.; Hu, G.; Zhao, X. Interfacial anchoring cobalt species mediated advanced oxidation: Degradation performance and mechanism of organic pollutants. J. Colloid Interface Sci. 2025, 679, 67–78. [Google Scholar] [CrossRef]
- Si, J.; Lu, S.; Luan, H.; Tong, T.; Wang, J.; Xu, G.; Lv, J.; Yao, K. Minor Cu modification endows inactive industrial FeSiBNbCu metallic glass with robust azo dye degradation activity. Appl. Surf. Sci. 2025, 689, 162511. [Google Scholar] [CrossRef]
- Cisneros, R.L.; Espinoza, A.G.; Litter, M.I. Photodegradation of an azo dye of the textile industry. Chemosphere 2002, 48, 393–399. [Google Scholar] [CrossRef]
- Hou, M.; Li, F.; Liu, X.; Wang, X.; Wan, H. The effect of substituent groups on the reductive degradation of azo dyes by zerovalent iron. J. Hazard. Mater. 2007, 145, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Tang, K.H.D.; Chin, B.L.F.; Chan, Y.H.; Yiin, C.L.; Cheah, K.W.; Chai, Y.H.; et al. Technologies for removing pharmaceuticals and personal care products (PPCPs) from aqueous solutions: Recent advances, performances, challenges and recommendations for improvements. J. Mol. Liq. 2023, 374, 121144. [Google Scholar] [CrossRef]
- Manikandan, V.; Ganesan, S.; Devanesan, S.; Kim, W.; Mythili, R.; Song, K.S. Tailored spherical-sea urchin-like MnO2-AC/PTA nanocomposites for superior photocatalytic degradation of BPA and Orange II dye, along with bacteria Inactivation: Mechanistic insights. Process. Saf. Environ. Prot. 2024, 184, 1332–1343. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Spray-Deposited TiO2 Layers on Aluminum Foil for Sustainable Water Remediation. Crystals 2024, 14, 875. [Google Scholar] [CrossRef]
- Bandara, J.; Nadtochenko, V.; Kiwi, J.; Pulgarin, C. Dynamics of oxidant addition as a parameter in the modelling of dye mineralization (Orange II) via advanced oxidation technologies. Water Sci. Technol. 1997, 35, 87–93. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Xiao, F.; Postole, G.; Zhao, H.; Zhao, G. Recent advances and trends of heterogeneous electro-Fenton process for wastewater treatment-review. Chin. Chem. Lett. 2022, 33, 653–662. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Wang, S.; Chen, W.; Zhao, Y.; Li, B. Microenvironment modulation of Fe single atoms in porous g-C3N4 by introducing −SOx groups for enhanced photo-Fenton reactions. Chem. Eng. J. 2024, 500, 157468. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Gao, Q.; Han, B.; Xia, K.; Zhou, C. Hierarchical flower-like Co2TiO4 nanosheets with unique structural and compositional advantages to boost peroxymonosulfate activation for degradation of organic pollutants. J. Mater. Chem. A 2020, 8, 20953–20962. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, S.; Dai, X.; Dong, B. Application, mechanism and prospects of Fe-based/Fe-biochar catalysts in heterogenous ozonation process: A review. Chemosphere 2023, 319, 138018. [Google Scholar] [CrossRef]
- Wang, A.; Du, M.; Ni, J.; Liu, D.; Pan, Y.; Liang, X.; Liu, D.; Ma, J.; Wang, J.; Wang, W. Enhanced and synergistic catalytic activation by photoexcitation driven S-scheme heterojunction hydrogel interface electric field. Nat. Commun. 2023, 14, 6733. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. Photocatalytic disinfection efficiency of 2D structure graphitic carbon nitride-based nanocomposites: A review. J. Mater. Sci. 2019, 54, 12206–12235. [Google Scholar] [CrossRef]
- Prasad, C.; Madkhali, N.; Govinda, V.; Choi, H.Y.; Bahadur, I.; Sangaraju, S. Recent progress on the development of g-C3N4 based composite material and their photocatalytic application of CO2 reductions. J. Environ. Chem. Eng. 2023, 11, 109727. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Wang, Y.; Chen, Q.; Feng, Z.; Sun, T. Concerted catalytic and photocatalytic degradation of organic pollutants over CuS/g-C3N4 catalysts under light and dark conditions. J. Adv. Res. 2019, 16, 135–143. [Google Scholar] [CrossRef]
- Tan, F.B.; Karadirek, Ş.; Tuna, Ö.; Simsek, E.B. Anchoring of tungsten on g-C3N4 layers towards efficient photocatalytic degradation of sulfadiazine via peroxymonosulfate activation. Diam. Relat. Mater. 2025, 152, 111939. [Google Scholar] [CrossRef]
- Murugan, C.; Bhojanaa, K.B.; Ong, W.-J.; Jothivenkatachalam, K.; Pandikumar, A. Improving hole mobility with the heterojunction of graphitic carbon nitride and titanium dioxide via soft template process in photoelectrocatalytic water splitting. Int. J. Hydrogen Energy 2019, 44, 30885–30898. [Google Scholar] [CrossRef]
- Song, Z.; Yan, C.; Qiu, J.; Liu, C.; Zhu, Y.A.; Wang, B.; Xie, Z.; Chen, G.; Li, K.; Le, Z. Photocatalytic reduction of the uranium (VI) by ultra-thin porous g-C3N4 nanosheets synthesized via microwave-assisted. J. Environ. Chem. Eng. 2024, 12, 113951. [Google Scholar] [CrossRef]
- Li, Y.; Wei, J.; Cui, N.; Li, J.; Ji, W.; Wang, L.; Huo, J.; Yan, W.; Zhang, X.; Zhao, Y.; et al. Atomically dispersed Fe-N5 sites with optimized electronic structure for sustainable wastewater purification via efficient Fenton-like catalysis. Appl. Catal. B Environ. Energy 2024, 358, 124385. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Yang, Q.; Yu, H.-B. Toward atomic-scale understanding of structure-dynamics-properties relations for metallic glasses. Prog. Mater. Sci. 2024, 145, 101311. [Google Scholar] [CrossRef]
- Wang, W.; He, Q.; Yi, Y.; Xiao, Y.; Xiao, X.; Yang, H.; Dong, X. Boosting piezocatalytic activity of graphitic carbon nitride for degrading antibiotics through morphologic regulation and chlorine doping. J. Clean. Prod. 2023, 415, 137818. [Google Scholar] [CrossRef]
- Tang, H.; Tu, J.-P.; Liu, X.-Y.; Zhang, Y.-J.; Huang, S.; Li, W.-Z.; Wang, X.-L.; Gu, C.-D. Self-assembly of Si/honeycomb reduced graphene oxide composite film as a binder-free and flexible anode for Li-ion batteries. J. Mater. Chem. A 2014, 2, 5834–5840. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- An, C.; Zhou, Y.; Chen, C.; Fei, F.; Song, F.; Park, C.; Zhou, J.; Rubahn, H.; Moshchalkov, V.V.; Chen, X.; et al. Long-Range Ordered Amorphous Atomic Chains as Building Blocks of a Superconducting Quasi-One-Dimensional Crystal. Adv. Mater. 2020, 32, 2002352. [Google Scholar] [CrossRef]
- Li, D.; Guo, L.; Li, F.; Huang, J.; Li, J.; Li, M.; Li, C. Synthesis and catalytic behavior of nickel heterogenized in covalent organic frameworks as precatalysts in ethylene oligomerization. Microporous Mesoporous Mater. 2022, 338, 111979. [Google Scholar] [CrossRef]
- Zhang, L.; Ju, L.; Li, X.; Guli, A.; Lyu, C. CoOOH with a highly negative CB band for visible-light-driven photocatalytic degradation of refractory organic pollutants in peroxymonosulfate system: Enhanced performance and multi-path synergetic mechanisms. J. Hazard. Mater. 2023, 460, 132403. [Google Scholar] [CrossRef]
- Zokaee, Z.; Mahmoodi, N.M.; Rahimpour, M.R.; Shariati, A. Synthesis of visible light activated metal-organic framework coated on titania nanocomposite (MIL-53(Al)@TiO2) and dye photodegradation. J. Solid State Chem. 2022, 307, 122747. [Google Scholar] [CrossRef]
- Yousif, M.; Ibrahim, A.H.; Al-Rawi, S.S.; Majeed, A.; Iqbal, M.A.; Kashif, M.; Abidin, Z.U.; Arbaz, M.; Ali, S.; Hussain, S.A.; et al. Visible light assisted photooxidative facile degradation of azo dyes in water using a green method. RSC Adv. 2024, 14, 16138–16149. [Google Scholar] [CrossRef]
- Li, C.-X.; Wang, R.; Sun, W.; Cui, K.; Fu, X.-Z.; Cui, M.; Chen, Y.; Guo, Z.; Liu, Y. Efficient degradation of Rhodamine B by visible-light-driven biomimetic Fe (III) complex/peroxymonosulfate system: The key role of FeV=O. J. Environ. Chem. Eng. 2024, 12, 113288. [Google Scholar] [CrossRef]
- Sadhu, S.P.; Ruparelia, J.P.; Patel, U.D. Homogeneous photocatalytic degradation of azo dye Reactive Black 5 using Fe (III) ions under visible light. Environ. Technol. 2022, 43, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Lv, X.-X.; Shu, A.-L.; Lu, X.-Z.; Chen, X. Insight on the degradation of P-chlorophenol based on the Co-g-C3N4/diatomite composite photo-Fenton process. J. Ind. Eng. Chem. 2024, 136, 305–316. [Google Scholar] [CrossRef]
- Silambarasan, R.; Perisetti, U.S.S.S.; Pavalamalar, S.; Anbalagan, K. Enhanced efficiency of photocatalytically synthesised Co3+/Co2+-incorporated CeO2/SnO2 nanocomposite and supercapacitor studies. RSC Adv. 2024, 14, 4153–4164. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Liu, S.; Zhao, M.; Duan, X.; Wu, H.; Liu, L.; Liu, W.; Li, J.; Ren, S.; et al. Constructing C–O bridged CeO2/g-C3N4 S-scheme heterojunction for methyl orange photodegradation: Experimental and theoretical calculation. J. Environ. Manag. 2023, 335, 117608. [Google Scholar] [CrossRef]
- Jeon, H.; Hoang, D.T.; Kim, G.; Kim, I.Y.; Lee, H.; Hong, S. Enhanced photocatalytic and electrocatalytic properties of IrO2 nanoparticles via Cr and Co ion doping: Insights into surface oxygen defect structures. Appl. Surf. Sci. 2025, 686, 162088. [Google Scholar] [CrossRef]
- Tao, Q.; Bai, Y.; Wang, L.; Feng, T.; Lu, S.; Zhang, A.; Li, K.; Hu, N. Synergistic catalytic degradation of Methotrexate using Ce-based high-entropy metal oxides: Insights from DFT calculations and CWPO performance. Sep. Purif. Technol. 2025, 357, 130130. [Google Scholar] [CrossRef]
- Du, X.; Nie, H.; Qu, Y.; Jia, H.; Liu, Y.; Yin, B. Revisiting the efficacy of COF treatment for dyes in wastewater: A comprehensive review. J. Environ. Chem. Eng. 2025, 13, 115660. [Google Scholar] [CrossRef]
- Padama, A.A.B.; Palmero, M.A.; Shimizu, K.; Chookajorn, T.; Watanabe, S. Machine learning and density functional theory-based analysis of the surface reactivity of high entropy alloys: The case of H atom adsorption on CoCuFeMnNi. Comput. Mater. Sci. 2025, 247, 113480. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Huang, C.-H.; Mao, L.; Shao, B.; Shao, J.; Yan, Z.-Y.; Tang, M.; Zhu, B.-Z. First Direct and Unequivocal Electron Spin Resonance Spin-Trapping Evidence for pH-Dependent Production of Hydroxyl Radicals from Sulfate Radicals. Environ. Sci. Technol. 2020, 54, 14046–14056. [Google Scholar] [CrossRef]
| Sample | Multipoint Specific Surface Area (m2·g−1) |
|---|---|
| g-C3N4 | 65.49 |
| Co78Si8B14 | 0.25 |
| Co78Si8B14/g-C3N4 | 72.47 |
| Sample | k (min−1) | R2 |
|---|---|---|
| g-C3N4 + PMS | 0.0101 | 0.99 |
| Co78Si8B14 + PMS | 0.0084 | 0.95 |
| Co78Si8B14/g-C3N4 + PMS | 0.0264 | 0.92 |
| Co78Si8B14/g-C3N4 + PMS + VIS | 0.0430 | 0.94 |
| Catalyst | Dye | Time | Degradation | Ref. |
|---|---|---|---|---|
| Co78Si8B14/g-C3N4 | Orange II | —— | 90.44% | This work |
| MIL-53(Al)@TiO2 | Methylene Blue | 240 min | 95.00% | [29] |
| AsA | Methyl Orange | 180 min | 96.00% | [30] |
| Ag/ZnO | Methyl Orange | 360 min | 80.92% | [31] |
| Fe(III) | Reactive Black 5 | 60 min | 80.00% | [32] |
| Sample | k (min−1) | R2 |
|---|---|---|
| Co78Si8B14/g-C3N4 + PMS + VIS | 0.0430 | 0.94 |
| Co78Si8B14/g-C3N4-2 + PMS + VIS | 0.0558 | 0.93 |
| Co78Si8B14/g-C3N4-3 + PMS + VIS | 0.0666 | 0.88 |
| Co78Si8B14/g-C3N4-4 + PMS + VIS | 0.0279 | 0.96 |
| Light Intensity (mW·cm−2) | k (min−1) | R2 |
|---|---|---|
| 7.80 | 0.0666 | 0.91 |
| 12.70 | 0.0804 | 0.88 |
| 15.80 | 0.0850 | 0.87 |
| 26.18 | 0.1251 | 0.86 |
| Dosage (g L−1) | k (min−1) | R2 |
|---|---|---|
| 0.2 | 0.0804 | 0.85 |
| 0.4 | 0.0934 | 0.88 |
| 0.8 | 0.0286 | 0.92 |
| 1.2 | 0.0190 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Ma, G.; Zhang, J. High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System. Materials 2025, 18, 1733. https://doi.org/10.3390/ma18081733
Yang Z, Ma G, Zhang J. High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System. Materials. 2025; 18(8):1733. https://doi.org/10.3390/ma18081733
Chicago/Turabian StyleYang, Zhenling, Guofeng Ma, and Jun Zhang. 2025. "High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System" Materials 18, no. 8: 1733. https://doi.org/10.3390/ma18081733
APA StyleYang, Z., Ma, G., & Zhang, J. (2025). High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System. Materials, 18(8), 1733. https://doi.org/10.3390/ma18081733





