Abstract
Tungsten (W), a rare metal, is categorized as a Critical and Strategic Raw Material (CRM) by the European Union (EU), with the highest economic importance of all selected CRMs since 2014. Tungsten and its derivatives are extracted from their commercial raw materials, mainly wolframite [(Fe,Mn)WO4] and scheelite (CaWO4) ores. Subsequently to mining and mineral processing, the W ore is submitted to thermal treatment and hydrometallurgy under aggressive conditions (high pressure and temperature), which are usually applied for the extraction of tungsten compounds. This paper aims to investigate a thermal route for scheelite processing using various selected chemical agents, resulting in a W-bearing material that is capable of being leached under softer conditions. In this context, a thermodynamic study of the interaction between FeWO4, MnWO4 and CaWO4 and various chemical reagents is described. The thermochemical calculations and data modeling show that, among other considerations, the reaction of CaWO4 with magnesium chloride (MgCl2) can lead to the formation of magnesium tungsten oxide (MgWO4), which appears to be more easily leachable than CaWO4. Experimental tests of the reaction of scheelite with MgCl2 appear to validate the thermodynamic predictions with satisfactory process kinetics at temperatures from 725 to 775 °C.
1. Introduction
Energy transition, the manufacturing of smart materials, diverse advanced industries and the development of highly innovative technologies, require increasingly large amounts of several raw materials (mainly metallic raw materials). According to European (EU) assessments [1], a good number of these raw materials are considered to be Critical and Strategic Raw Materials (CRMs). Such a designation results from their great economic importance, combined with a high supply risk. The number of CRMs for the EU has grown from 14 CRMs in 2011 to 27 in 2017, and was augmented thereafter to reach 34 CRMs in 2023. Please note that copper and nickel do not meet the critical thresholds, but they are included in CRMs as Strategic Raw Materials.
France, like other EU countries, is strongly dependent on CRMs for its industrialization and support of emerging technologies. Efforts to enable the sustainable supply of these materials from domestic and international sources are currently ongoing. National scientific programs, including the Priority Research Program and Equipments (PEPR), under the management of the French National Research Agency, ANR, launch initiatives to consolidate the country’s scientific and technological base. One of the PEPR programs (The Subsoil (resources) exploratory PEPR), entitled “Innovative and sustainable Technologies platform—InnovTech”, aims to develop new approaches and technology to reduce energy consumption and waste in different stages of metal extraction. The project is coordinated by the BRGM (The French Geological Survey Office) and the co-leader is the GeoRessources laboratory at the “Université de Lorraine” (France). One of the targeted metals of this program is tungsten (W) contained in scheelite skarns from the “French Massif Central” deposit. Please note that tungsten has the highest economic importance among all of the selected CRMs.
Tungsten (meaning heavy stone in Swedish) is also known as wolfram (from wolframite, said to be named from wolf rahm or spumi lupi, because the ore interfered with the smelting of tin and was supposed to devour the tin) [2]. Tungsten has the highest melting point “m.p.” (3422 °C) of all metals, boils around 5555 °C and is a heavy metal with a specific gravity of 19.3. Tungsten is one of the transition and refractory metals and belongs, along with chromium and molybdenum, to group 6 on the periodic table. All of these elements are known to have diverse valences, which, in common compounds, range mainly from +2 to +6.
Tungsten belongs to the rare elements, with an earth’s crust abundance of 1.25 mg/kg, but it is widespread within all continents. Tungsten occurs in wolframite [(Fe,Mn)WO4], scheelite (CaWO4), hubnerite (MnWO4) and ferberite (FeWO4) [2].
As described in Reference [3], the amount of tungsten consumed in the EU is used for a wide range of applications, which are as follows: 67% of tungsten is used for the manufacturing of tungsten carbides, 11% is used for the manufacturing of W-metal, 11% is used for the manufacturing of chemicals, 8% is used for the manufacturing of tungsten-based steel applications and the rest (3% of W) is used for the manufacturing of other tungsten alloys. The repartition of tungsten-use in the EU in various applications is shown in Figure 1 [3]. Mill and cutting tools, mining and construction and wear tools, with 33%, 23% and 18%, respectively, share the main applications of tungsten-bearing materials. The remaining material, about a quarter of tungsten’s end uses, is distributed across other applications, as shown in Figure 1.
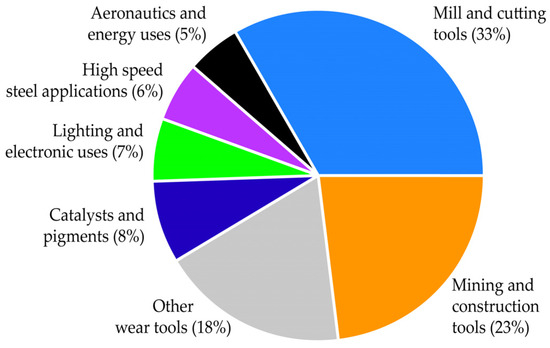
Figure 1.
Tungsten-use in various applications in the European Union.
According to the available data [4], the world mine production of tungsten is estimated at 78,000 tons in 2023 (Figure 2). The mine production of tungsten is dominated by China, providing more than 80% of the global W-production. As far as the European Union is concerned, the main mining production of tungsten is concentrated mostly in Austria, Portugal and Spain.
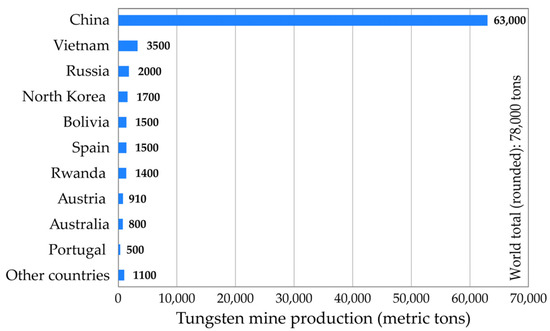
Figure 2.
World mine production of tungsten in 2023.
Although tungsten resources and reserves are scattered across many countries, China remains prevalent [4], with nearly 52% of the world’s reserves, estimated at 4.4 × 106·tons (Figure 3). Australia and Russia hold second and third place, with their W-reserves estimated at around 13% and 9%, respectively.
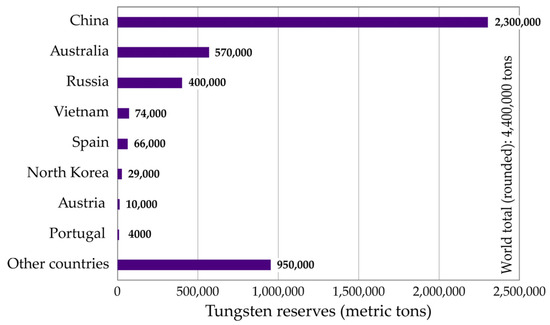
Figure 3.
World reserves of tungsten.
As mentioned above, the main tungsten ores of industrial importance are scheelite (CaWO4) and wolframite [(Fe,Mn)WO4), the latter being a solid solution of the isomorphic minerals, namely ferberite and hubnerite. Scheelite and wolframite account for 70% and 30% of the world resources, respectively. In general, most deposits have low WO3 content, which can, at most, reach 2% WO3 for richer W-ores; however, the extractive metallurgy of tungsten requires concentrates with around 65% WO3 content.
Therefore, the ores must first go through physical enrichment processes that are often complicated due to their complex elemental, chemical and mineralogical compositions [5]. All of the known mineral processing methods, as well as the development of advanced flow-sheets for the treatment of scheelite ores, resulting in a W-concentrate of metallurgical grade, were summarized in Reference [5].
However, the real challenge of the tungsten industry is the metallurgical extraction of tungsten intermediates, such as ammonium paratungstate (APT), with a chemical formula of (NH4)10[H2W12O42]·4H2O, being the most important precursor for the majority of tungsten products. Figure 4 [6] groups simplified schemes for treatments of low- and high-grade scheelite and wolframite concentrates, resulting in APT manufacturing. In general, concentrates undergo a pretreatment (thermal and/or acidic) to remove impurities (S, As, P, organic carbon, etc.) that would be difficult and expensive to remove later. The hydrochloric acid digestion around 90 °C is industrially employed, mainly for the high-grade scheelite concentrates [6,7], producing pure tungstic acid (H2WO4 or WO3·H2O) and/or APT after dissolving H2WO4 into concentrated NH3·H2O.
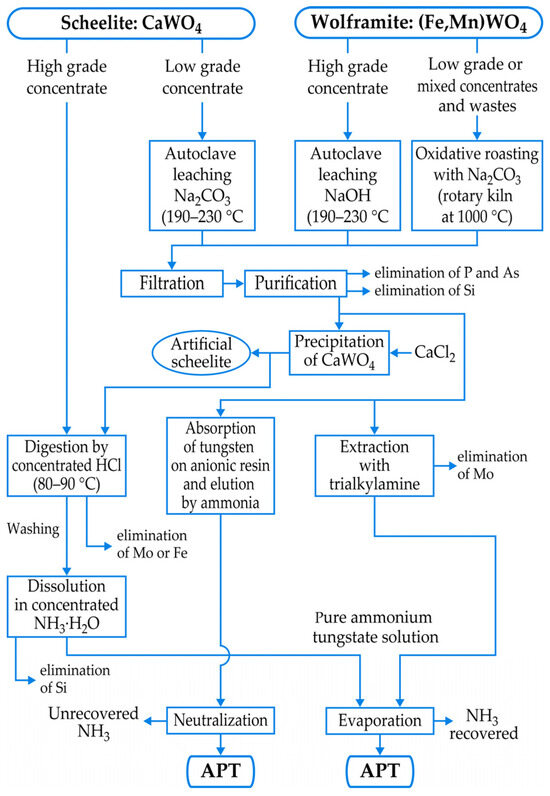
Figure 4.
Treatments of tungsten concentrates.
The most current commercial process by soda/caustic soda digestion (high-pressure and high-temperature processes mainly applied), followed by solvent extraction or an ion exchange step, can accommodate a variability of raw materials. However, high reagent consumption, the absence of recycling the aqueous solutions and chemical reagents, as well as discharge of high-salinity wastewater for solvent extraction and ion exchange steps, are some of the process drawbacks [7]. Novel laboratory routes for improving the existing processes, leading to better leaching and efficiency recovery of tungsten from its concentrates, are well-documented in Reference [7].
There are also some thermochemical insights and experimental laboratory works describing new thermal alternative methods to replace the current industrial processes for tungsten extraction from its bearing materials. An early study [8] predicted the reactions of gangue minerals (SiO2, Ca5(PO4)3F, SnO2, FeS2, MoS2 and FeAsS) with CaCO3 and Ca3WO6, resulting in mixed compounds which nature depends on for the oxygen partial pressure in the selected system. Xu et al. [9] performed a thermodynamic analysis of leaching of the thermally synthetized Ca3WO6 in NH3-(NH4)2CO3-H2O solution. Their experimental results, in which they simultaneously applied grinding and leaching, indicated an almost full dissolution of Ca3WO6 at a temperature close to 40 °C by forming (NH4)2WO4(aq) and solid CaCO3. Another scientific approach [10] provided a leaching yield of MgWO4 that was higher than 98% in a solution of NaOH at 90 °C, after thermal synthesis of magnesium tungstate from scheelite using MgCl2.
Recently [11], a carbo-thermic reduction in the presence of calcium sulfate was applied to a wolframite–scheelite mixed ore containing tin as cassiterite (SnO2). About 99% of tin is separated by volatilization as SnS. Wolframite is converted to CaWO4 and Ca2(Fe,Mn)WO6, which are easily digested by the mixed sulphuric–phosphoric acid solution with a leaching efficiency of tungsten of around 99%. The kinetics of chlorination of a scheelite–wolframite concentrate with chlorine and sulphur dioxide are investigated early [12]. They identified WO2Cl2, FeCl2, FeCl3, S2, FeS, CaSO4 and CaCl2 as the main reaction products. In a similar study, Menéndez et al. [13] performed the physicochemical characterization and chlorination of several natural wolframite (MnxFe1−xWO4) samples with chlorine and sulphur dioxide. Under the best conditions, they announced an extraction extent of tungsten that was close to 86%. Fuga et al. [14] studied the chlorination kinetics of a MnWO4 reaction with chlorine between 650 and 950 °C, and they identified WO2Cl2 and MnCl2 as the main reaction products.
The aim of this paper is to provide a comprehensive thermochemical assessment of the interaction between tungsten-bearing minerals and diverse chemical reagents. After predicting the thermodynamic reactivity of tungsten compounds towards selected chemical agents, special attention was paid to the modeling of the equilibrium composition of various tungsten-containing systems at chosen temperatures. Furthermore, experimental tests of the reactions of scheelite with calcium carbonate (CaCO3) and calcium chloride (MgCl2) are carried out to verify the validity of the thermodynamic predictions.
2. Phase Diagrams of the CaO-WO3 and MgO-WO3 Systems
Both calcium and magnesium oxides and their mixture are largely used in the manufacturing of basic refractory bricks due to their chemical and mechanical performance, as well as their high melting points (2613 °C for CaO and 2852 °C for MgO). In addition, the ternary and quaternary phase diagram systems, CaO-MgO-SiO2, CaO-MgO-SiO2-Al2O3 and many others, are the basis of extractive metallurgy (especially in slag formation) and in the synthesis of glass and glass–ceramic materials.
In the context of this study, it is interesting to examine the available binary phase diagrams of CaO(MgO)-WO3 systems. In the CaO-WO3 phase diagram depicted in Figure 5a, there are two well-defined and stable compounds, CaWO4 and Ca3WO6 (3CaO·WO3), which melt congruently at about 1580 °C and 2250 °C, respectively. According to this diagram, both compounds do not show any crystalline structural change, whilst the tungsten (VI) oxide (WO3) crystal structure is temperature-dependent. Furthermore, it is orthorhombic at 600–745 °C and it is transformed into a tetrahedral state beyond this temperature.
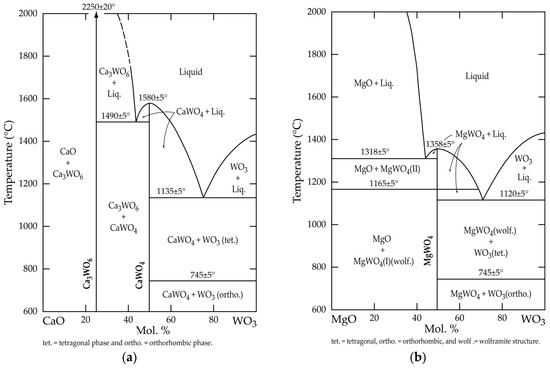
Figure 5.
Phase diagram of (a) CaO-WO3; (b) MgO-WO3 system (adapted from Reference [15]).
The MgO-WO3 phase diagram presented in Figure 5b demonstrates the formation of a single compound, namely magnesium tungstate (MgWO4), which is stable up to its melting point (ca. 1385 °C); however, it undergoes a reversible structural phase transition at around 1165 °C. The features of this transformation are described elsewhere [16].
These observations prove that, in addition to scheelite, two other compounds that are thermally stable, at least at T < 1350 °C, must be taken into account in the studied systems. Hence, this statement will be used for the thermodynamic investigation of the interaction between scheelite and the selected chemical reagents.
3. Thermodynamic Elements of the Interaction Between (Fe, Mn, Ca) Tungstate and Various Chemical Reagents
As mentioned in the Section 1, one of the aims of this study is to thermally transform tungsten-bearing materials (FeWO4, MnWO4 and CaWO4) into less-common compounds (such as Ca3WO6 and MgWO4) that are more likely to be digested by current chemical reagents under mild conditions. HSC Chemistry Software® (version 5.1) [17], as well other available data [18], are used for the thermodynamic predictions and assessment of the selected system. Although there are other types of computer software and databases for thermodynamic predictions (such as FactSage, Thermo-Calc Software, etc.), HSC Chemistry appears to be the most widely used. The used methodology, as well as the precautions taken into account during the thermochemical calculations, were mentioned in a recent study [19]. The thermodynamic data (enthalpy (H), entropy (S) and heat capacity ©) of some chemical species are sometimes given in a low and narrow range of temperatures, and HSC data extrapolation at high temperatures may lead to errors. As an example linked to this study showed, the thermodynamic data for CaWO4 and Ca3WO6 are available up to 1000 K (727 °C). However, as shown in Figure 5a, these well-defined compounds are thermally stable at temperatures well above 727 °C and do not undergo any structural and/or transition changes prior to melting. Hence, the thermochemical data for both compounds can be extrapolated without error in the temperature range of interest of this investigation (lower than or equal to 1100 °C).
The first step for an envisaged process is to check the thermodynamic reactivity of input chemicals by evaluating the standard free Gibbs energy changes (ΔG°), computed by the HSC thermochemical database, for foreseen chemical reactions. Figure 6a–c plots the evolution of ΔG° versus temperature for the reactions of FeWO4, MnWO4 and CaWO4 with selected chemical reagents, as described in Equations (1)–(19). There can certainly also be intermediate reactions, such as the formation of FeCO3, MnCO3, Fe(HO)2 and Mn(OH)2, but in the practical temperature of interest (most likely higher than 600 °C), these compounds are dissociated in respective oxides (FeO and MnO, in absence of oxygen) and their respective gases (CO2(g) and H2O(g)). Likewise, other possible reactions should lead to the formation of more complex intermediate phases, but the thermodynamic data are often lacking. However, as the ΔG° is a state function, the final ΔG° value does not depend on the intermediate paths of the system transformation, but only on the initial and final state of the system.
1/3FeWO4(s) + CaO(s) → 1/3Ca3WO6(s) + 1/3FeO(s)
1/3FeWO4(s) + Ca(OH)2(s) → 1/3Ca3WO6(s) + 1/3FeO(s) + H2O(g)
1/3FeWO4(s) + CaCO3(s) → 1/3Ca3WO6(s) + 1/3FeO(s) + CO2(g)
1/2FeWO4(s) + NaOH(s,l) → 1/2Na2WO4(s,l) + 1/2FeO(s) + 1/2H2O(g)
FeWO4(s) + Na2CO3(s,l) → Na2WO4(s,l) + FeO(s) + CO2(g)
FeWO4(s) + MgCl2(s,l) → MgWO4(s) + FeCl2(s,l,g)
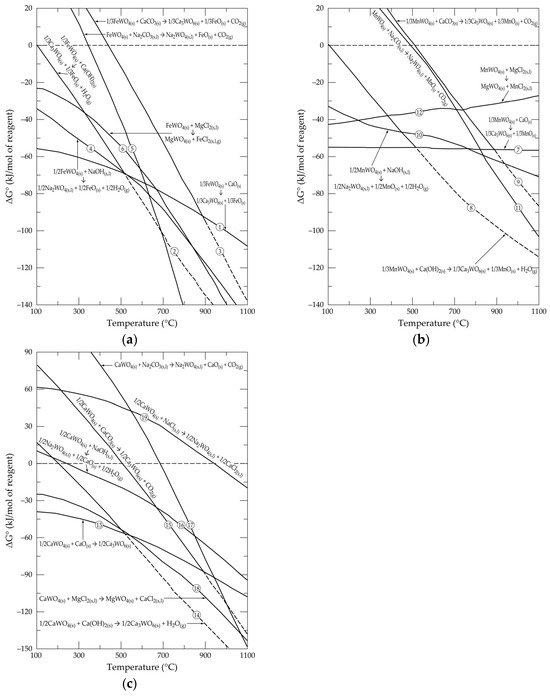
Figure 6.
Evolution of standard free energy changes as a function of temperature for the reactions of selected chemical reagents with (a) FeWO4; (b) MnWO4; (c) CaWO4.
As shown in Figure 6a, the thermodynamic reactivity of FeWO4, with respect to CaO, Ca(OH)2, NaOH and MgCl2, seems to be high (negative values of ΔG°) from around 100 °C. At a higher temperature (T > 500 °C), all of the considered forward reactions (Equations (1)–(6)) are spontaneous. Similarly, the reactions of MnWO4 (Equations (7)–(12)) with the selected reagents occur spontaneously, starting at least from temperatures higher than 500 °C (Figure 6b).
1/3MnWO4(s) + CaO(s) → 1/3Ca3WO6(s) + 1/3MnO(s)
1/3MnWO4(s) + Ca(OH)2(s) → 1/3Ca3WO6(s) + 1/3MnO(s) + H2O(g)
1/3MnWO4(s) + CaCO3(s) → 1/3Ca3WO6(s) + 1/3MnO(s) + CO2(g)
1/2MnWO4(s) + NaOH(s,l) → 1/2Na2WO4(s,l) + 1/2MnO(s) + 1/2H2O(g)
MnWO4(s) + Na2CO3(s,l) → Na2WO4(s,l) + MnO(s) + CO2(g)
MnWO4(s) + MgCl2(s,l) → MgWO4(s) + MnCl2(s,l)
1/2CaWO4(s) + CaO(s) → 1/2Ca3WO6(s)
1/2CaWO4(s) + Ca(OH)2(s) → 1/2Ca3WO6(s) + H2O(g)
1/2CaWO4(s) + CaCO3(s) → 1/2Ca3WO6(s) + CO2(g)
1/2CaWO4(s) + NaOH(s,l) → 1/2Na2WO4(s,l) + 1/2CaO(s) + 1/2H2O(g)
CaWO4(s) + Na2CO3(s,l) → Na2WO4(s,l) + CaO(s) + CO2(g)
CaWO4(s) + MgCl2(s,l) → MgWO4(s) + CaCl2(s,l)
1/2CaWO4(s) + NaCl(s,l) → 1/2Na2WO4(s,l) + 1/2CaCl2(s,l)
Only the reactions of CaWO4 with CaO and MgCl2 (Equations (13) and (18)) are thermodynamically feasible at a low temperature (Figure 6c). For some reactions, such as that with Na2CO3 (Equation (17)) and NaCl (Equation (19)), it is necessary to exceed 700 and 950 °C, respectively, in order for the reactions to occur in the desired direction.
Processes based on the roasting of wolframite and scheelite with alkali compounds (especially with Na2CO3) are known in the current tungsten extraction processes [6]. Thus, among the chemical processes based on the reactions mentioned above, attempts have been made for those based on the treatment with calcite (CaCO3) and magnesium chloride (MgCl2). It is therefore interesting to follow the chemical equilibria between the tungsten compounds and these chosen chemical reagents.
4. Calculation of the Equilibrium Composition in the (Fe,Mn,Ca)-W-C-O Systems
The HSC software® 5.1 also offers the facility to calculate the equilibrium composition of a multicomponent system. The equilibrium composition is calculated using the GIBBS-solver, which uses the Gibbs energy minimization method [17]. Thus, a chemical system seeks the minimum change in Gibbs free energy (ΔG = 0) at the equilibrium state. For a simple system, the equilibrium constant approach is used to find the equilibrium compositions; however, in complex systems with a large number of constituents distributed in different phases (solid–liquid–gas), the calculation of the equilibrium composition is a difficult one. The GIBBS solver module of the HSC allows us to calculate the equilibrium composition as a function of the amount of species introduced at the chosen temperature and pressure. Although there are many options for the result presentation, the obtained data are often plotted as an evolution of the equilibrium composition and as a function of temperature.
Figure 7a shows the evolution of the molar composition of the Fe-Ca-W-C-O system by inputting 1.00 and 3.00 kmol FeWO4 and CaCO3, respectively, as starting compounds. To simplify the system, the calculation is made in the absence of oxygen. The species’ equilibrium composition evolves from 100 °C in the following two steps: the first step is up to 450 °C, with a rapid decrease in the amount of FeWO4 and CaCO3 and an increase in the amount of CaWO4, FeO and CO2(g). Please note that the curves representing CaWO4, FeO and CO2 overlap up to 450 °C. This suggests that iron in the ferberite is substituted by calcium-generating scheelite. The second step (T > 450 °C) is characterized by the apparition of a new chemical species (Ca3WO6) reaching the value of 1.00 kmol at around 725 °C, while the amount of CaWO4 tends towards zero at about 725 °C. From this temperature, the equilibrium composition of the system consists of 1.00 kmol Ca3WO6, 1.00 kmol FeO and 3.00 kmol CO2(g), which is consistent with the overall reaction described by Equation (3). Similar observations are valid for the Mn-Ca-W-C-O system (Figure 7b), but the equilibrium evolution is shifted towards higher temperatures. The final equilibrium composition at temperatures near 900 °C is consistent with the stoichiometry of the reaction given in Equation (9).
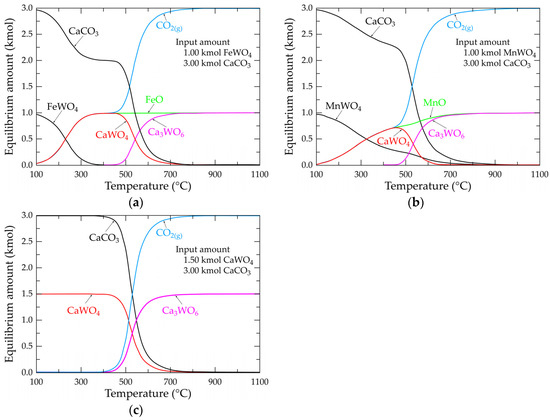
Figure 7.
Evolution of the equilibrium composition as a function of temperature of the main selected species for systems (a) Fe-W-Ca-C-O; (b) Mn-W-Ca-C-O; (c) W-Ca-C-O.
The simpler presentation is the W-Ca-C-O system, shown in Figure 7c. The evolution of the equilibrium composition starts at temperatures approaching 400 °C and ends at those near 800 °C, with an equilibrium composition of Ca3WO6 + CO2(g) and with a molar ratio of Ca3WO6/CO2(g) = 2, which is in agreement with the stoichiometry of the reaction given in Equation (15).
5. Calculation of the Equilibrium Composition in the Ca-W-Mg-O-Cl System
In the previous section, the equilibrium amount of main constituents in the Ca-W-Mg-O-Cl system was calculated at temperatures up to 1100 °C. Calculations are performed in the absence of oxygen atmosphere. Although the reaction of CaWO4 with MgCl2 (Equation (18)) requires an equimolar amount of CaWO4 and MgCl2, it was pertinent for modeling the equilibrium amount by varying the MgCl2 input from 0.50 to 1.50 kmol, while the amount of CaWO4 was kept constant and equal to 1.00 kmol. The obtained equilibrium diagrams are shown in Figure 8. Using 0.50 kmol MgCl2 in the starting materials (Figure 8a) and for temperatures lower than 800 °C, the equilibrium amount of the system is composed of equimolar quantities (0.50 kmol) of CaWO4, MgWO4 and CaCl2. Beyond 800 °C, two main species (Ca3WO6 and WO2Cl2(g)) appear and their amounts increase with the temperature. According to these observations, the likely reaction, thus producing these species, is that described by Equation (20), indicating that CaWO4 and CaCl2 are incompatible from a thermodynamic point of view. The calculation with 0.75 kmol MgCl2 (Figure 8b) also indicates the presence of these two species (Ca3WO6 and WO2Cl2(g)), but at lower relative amounts. At higher temperatures, magnesium oxide also belongs to the composition of the studied system. The equilibrium composition diagram with 1.00 kmol MgCl2 is predominated by an equimolar amount of MgWO4 and CaCl2 which is consistent with the stoichiometry of Equation (18).
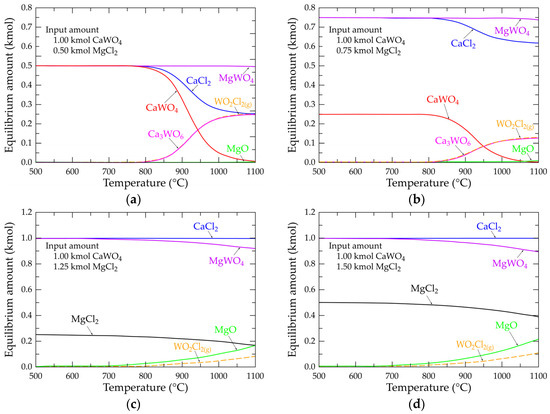
Figure 8.
Evolution of the equilibrium composition as a function of temperature of the main selected species for systems Ca-W-Mg-O-Cl at (a) 0.50 kmol MgCl2; (b) 0.75 kmol MgCl2; (c) 1.25 kmol MgCl2; (d) 1.50 kmol MgCl2.
Figure 8c,d shows the equilibrium compositions of the Ca-W-Mg-O-Cl system at 1.25 and 1.50 kmol MgCl2, respectively. The equilibrium quantities of CaCl2 remain constant (ca. 1.00 kmol) at all of the considered temperatures. However, the equilibrium amount of MgWO4 decreases with the temperature, and is more pronounced when 1.50 kmol MgCl2 is used in the system (Figure 8d). Additionally, equilibrium amounts of WO2Cl2(g) and MgO increase significantly at temperatures above 800 °C. From these calculations and according to Equation (21), one may deduce that magnesium chloride can react with scheelite, thus generating calcium chloride and magnesium oxide. It is a spontaneous reaction, as is the case with a ΔG° value at 775 °C of −8.30 kJ/mol. The synthesis of tungsten oxychloride (WO2Cl2(g)) can also be described by Equation (22), but this reaction path seems improbable, as its ΔG° value at 775 °C is 72.17 kJ/mol.
The synthesis of metal oxychlorides, such as chromium oxychloride (CrO2Cl2(g)), during the oxychlorination of chromite (Fe,Mg)(Cr,Al,Fe)2O4 with a gaseous Cl2 + O2 mixture, is reported in previous works [20,21,22]; however, due to the high oxidation capacity of Cl2(g) + O2(g), chromium(III) is oxidized to chromium(VI), as seen in Equations (23) and (24), while in the case of scheelite, tungsten is already in a hexavalent state. Furthermore, the synthesis of WO2Cl2(g), using CaWO4 and MgCl2 as starting materials, does not seem to be described in the literature.
2CaWO4(s) + CaCl2(s,l) → Ca3WO6(s) + WO2Cl2(g)
1/2CaWO4(s) + MgCl2(s,l) → 1/2CaCl2(s,l) + 1/2WO2Cl2(g) + MgO(s)
MgWO4(s) + MgCl2(s,l) → WO2Cl2(g) + 2MgO(s)
1/2FeCrIII2O4(s) + Cl2(g) + 3/8O2(g) → 1/4Fe2O3(s) + CrVIO2Cl2(g)
1/2MgCrIII2O4(s) + Cl2(g) + 1/4O2(g) → 1/2MgO(s) + CrVIO2Cl2(g)
6. Experimental Insights of Thermal Reactions of CaWO4 with CaCO3 and MgCl2
6.1. Materials and Expermental Procedure
Powdered analytical grade CaCO3, CaWO4 and MgCl2 samples are used is the study. Mixtures of CaWO4 + CaCO3 and CaWO4 + MgCl2 at defined proportions are produced by dry grinding. The elemental composition and crystallinity of the first two samples, as well as the solid reaction products, are determined using an energy dispersive spectroscopy electron microscopy (EDS-SEM, HITACHI S-4800, Hitachi Ltd., Tokyo, Japan) and X-ray diffraction (XRD, Bruker D8 Advance device, Bruker, Karlsruhe, Germany), respectively. No MgCl2 analysis was performed due to the highly hygroscopic nature of MgCl2. The experimental study results were achieved using the setup shown in Figure 9. The main piece of equipment is a tubular resistant furnace with the ability to reach 1500 °C. The working reactor is made of quartz capable of withstanding temperatures as high as 1300 °C in the absence of corrosive atmospheres. When the desired temperature is reached and stabilized under nitrogen flow, the alumina support containing the sample (approximately 1.8 g) is placed in the center of the furnace and the planned treatment time is allowed to elapse.
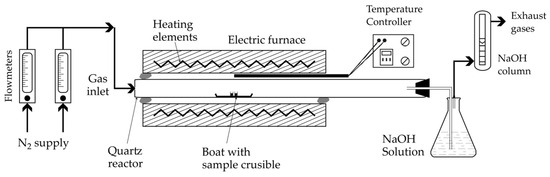
Figure 9.
Schematic presentation of the experimental setup used for thermal tests.
6.2. Reaction of CaWO4 with CaCO3
The formation of Ca3WO6 in the W-Ca-C-O system is demonstrated fairly well with thermodynamic assessments. To verify the reliability of thermodynamic calculation, a co-processing of pure CaWO4 and CaCO3 that have 15% more CaCO3 than the stoichiometric molar amount required by Equation (15) is performed in N2 gas flow at 850 °C, and the solid product obtained after a 120 min of treatment is examined with the XRD technique. Additionally, tests of thermal treatment of only CaCO3, in N2 and at various temperatures, show that at 850 °C for a duration time of 30 and 60 min, about 50% and 91% of CaCO3 are, respectively, decomposed into CaO, according Equation (25).
CaCO3(s) → CaO (s) + CO2(g)
The analysis of the XRD diffractogram of the (CaWO4 + CaCO3) treatment product (Figure 10) confirms the presence of the well-crystallized Ca3WO6 with its main XRD peaks (d = 2.84 Å, 4.01 Å and 4.70 Å). However, the initial constituent (CaWO4) is still the predominant phase (d = 3.11 Å, 4.76 Å and 1.93 Å). In addition, CaO and Ca(OH)2 (the latter being the product of the interaction of CaO with moisture during sample preparation) are also identified in the thermal treatment sample. This finding indicates that although the reactions of CaCO3 (CaO) with CaWO4 are spontaneous processes (at least at T > 600 °C: Equations (13) and (15) and Figure 7c), temperatures higher than 850 °C and/or after an extended time of treatment should be applied for complete conversion of CaWO3 into Ca3WO6. One may conclude that the reactions’ kinetics are the limiting steps of the overall process. Further experimental tests have to be performed to obtain insight into the reaction mechanism and kinetics.
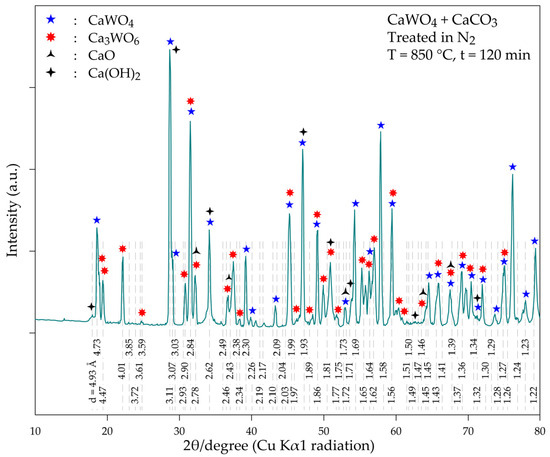
Figure 10.
XRD diffractogram of the product obtained during the thermal treatment of a mixture of CaWO4 + CaCO3 at 850 °C for 120 min.
6.3. Reaction of CaWO4 with MgCl2
To validate the thermochemical calculation in the CaWO4-MgCl2 system, one of the preliminary thermal tests of the CaWO4 + MgCl2 mixture (MgCl2/CaWO4 = 1.25: molar ratio) is carried out at 775 °C under nitrogen atmosphere for 2 h. This temperature is chosen for at least three reasons, such as the following (i) to follow the thermodynamic predictions; (ii) to insure homogenous mixing between the CaWO4 solid and MgCl2 liquid (as the m.p. of MgCl2 is 714 °C [2]); (iii) to avoid the loss of MgCl2(l) by evaporation, as this is expected at a temperature higher than 800 °C. The product of the thermal treatment is then washed with deionized water in order to eliminate the chlorides (MgCl2 and CaCl2), which cause problems for SEM-EDS and XRD sample preparation and analysis. Chlorides are hygroscopic (easily converted to (Mg,Ca)Cl2·6H2O, thus absorbing atmospheric moisture), making their handling and correct consistent analysis difficult. The solid mass after filtration and drying is examined using the XRD and SEM-EDS techniques.
As shown in Figure 11, the magnesium tungstate phase (MgWO4) is well-crystallized with main peaks (d = 4.69 Å, 3.71 Å, 2.93 Å, 2.90 Å and 3.61 Å) and many other diffraction peaks, which predominate the obtained X-ray diffractogram. CaWO4 appears to be transformed into MgWO4. Although some diffraction peaks of CaWO4 is found overlapping with those of MgWO4, the presence of CaWO4 in the product is doubtful or occur as minor phases (i.e., if even present). Several characteristic X-ray peaks of magnesium oxide (MgO) are also revealed in the treatment product, although they are of low intensity. Its presence in the treatment residue may be explained by the reaction given above in Equation (21). Although pure nitrogen is used for experimental testing, traces of oxygen and/or moisture in the system can also result in the conversion of MgCl2 and MgO.
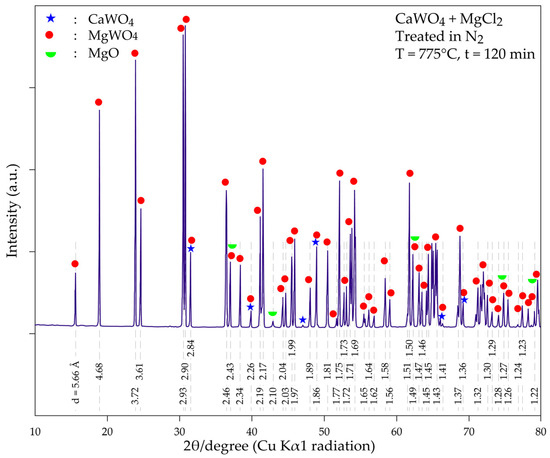
Figure 11.
XRD diffractogram of the product obtained during the thermal treatment of a mixture of CaWO4 + MgCl2 at 775 °C for 120 min.
SEM-EDS results of the treated product are reported in Figure 12a,b. Its morphology shows homogenous particles with sizes less than 10 µm (Figure 12a). Furthermore, another minor particle (spot n° 1) is observed. The general EDS spectrum (red spectrum in Figure 12b) clearly shows the presence of MgWO4, while the analysis of spot n°1 reveals the presence of MgO. These observations confirm the results obtained by XRD analyses; however, the presence of calcium (as CaWO4) is not revealed by SEM-EDS, which can be explained as either a complete transformation of CaWO4 into MgWO4 or the unreacted CaWO4 located in the core of the coarse particles, which is not accessible by the EDS probe.
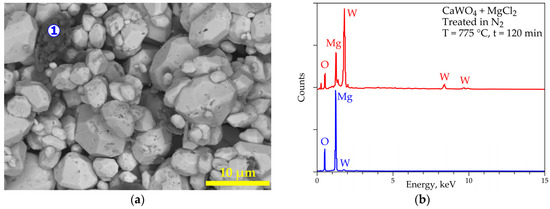
Figure 12.
SEM-EDS results of the (CaWO4 + MgCl2) sample treated at 775 °C for 120 min in nitrogen atmosphere with spot “1” indicating EDS microanalysis. (a) General view (backscattered electron “BSE” micrograph) of the obtained sample. (b) EDS analysis of spot “1” (bleu spectrum); red spectrum represents overall EDS results of other homogenous particles.
To have an accurate idea of the kinetics of the CaWO4-MgCl2 interaction, a set of experimental tests from 725 °C to 775 °C and a 7.5 to 120 min residence time are carried out. As in the previous case, the composition of the obtained products is systematically monitored using the XRD and SEM-EDS techniques.
The XRD patterns for a short-time treatment (7.5 and 30 min) of the resulting products are shown in Figure 13a,b. It is of note that, even at 725 °C, MgWO4 is the predominant phase when the sample is treated for 7.5 min. Additionally, typical peaks of CaWO4 are observed, although they are of low intensity. Similar observations are valid for the treatment at 775 °C (Figure 13b). This finding suggests that the kinetic reactivity of MgCl2-CaWO4 is high in the temperature range studied, confirming the thermodynamic predictions discussed earlier.
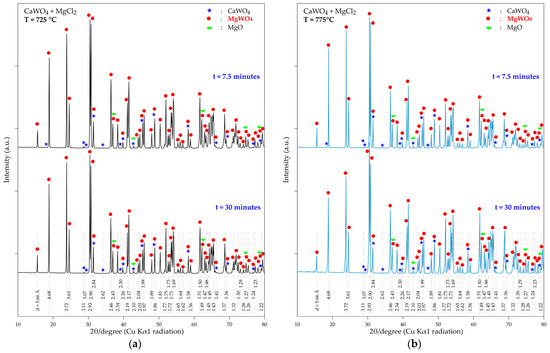
Figure 13.
XRD patterns of the product obtained during the thermal treatment a mixture of CaWO4 + MgCl2 for 7.5 and 30 min at (a) 725 °C; (b) 775 °C.
In a recent study [19], it is suggested that MgCl2 can be used for the extraction of tin from cassiterite (SnO2) materials via the formation of SnCl4(g) at around 800 °C. Inferentially, magnesium chloride should also be used for the removal of tin from tungsten ores and concentrates containing SnO2 as an impurity prior to the hydrometallurgical extraction of tungsten.
7. Conclusions
This study provides some thermodynamic analysis and a modeling approach for the possible conversion of wolframite, and principally scheelite (CaWO4), into other compounds that are more suitable for subsequent hydrometallurgical processing.
Although treatment of the mineral with CaCO3 seems to be a feasible process thermodynamically, a satisfactory formation of the reaction products is not observed, which may be attributed to kinetic limitations. Thus, higher temperatures may be required for the treatment of scheelite with this chemical reagent.
Magnesium chloride, on the other hand, appears to be a useful chemical reagent for a satisfactory conversion of scheelite to magnesium tungstate (MgWO4). Equilibrium-phase diagrams suggest favorable conditions for the transformation of CaWO4 to MgWO4. Experimental results of the thermal treatment of scheelite are consistent with the thermodynamic predictions at moderate temperatures with acceptable kinetics. The study reveals that relatively lower temperatures (a little higher than 700 °C) are fine for this process; however, temperatures around 800 °C may be ideal due to the necessity involved in the removal of impurities such as tin (cassiterite), which may be associated with scheelite.
Future work will be devoted to thermochemical investigations on more complex systems, followed by extensive experimental tests on wolframite and scheelite concentrates and their mixtures, thus leading to the synthesis of easily leachable tungsten compounds under mild conditions.
Author Contributions
Conceptualization, N.K., F.D., A.Y.F. and E.S.; methodology, N.K., C.K., E.A. and S.D.; software, N.K., A.Y.F., E.A. and E.S.; validation, N.K., E.S., L.F. and Y.F.; formal analysis, F.D., C.K. and S.D.; investigation, N.K., F.D., A.Y.F. and E.S.; resources, N.K., L.F. and Y.F.; data curation, F.D., C.K. and S.D.; writing—original draft preparation, N.K., F.D., E.S. and E.A.; writing—review and editing, N.K., F.D., C.K., A.Y.F., E.A., S.D., E.S., L.F. and Y.F.; visualization, N.K., E.A., L.F. and Y.F.; supervision, N.K. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of the ANR-France 2030 project, “Innovative and sustainable technologies platform” PC5-InnovTech, Grant Agreement No. ANR-22-EXSS-0006, https://anr.fr/ProjetIA-22-EXSS-0006, accessed on 13 January 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Study on the Critical Raw Materials for the EU, 2023 Final Report. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 20 December 2024).
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2014–2015. [Google Scholar]
- 27 TUNGSTEN, 27.1 Overview. Available online: https://scrreen.eu/wp-content/uploads/2023/01/TUNGSTEN_CRM_2020_Factsheets_critical_Final.pdf (accessed on 23 December 2024).
- Tungsten. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-tungsten.pdf (accessed on 23 December 2024).
- Foucaud, Y. Tungsten recovery from a skarn with a low separation contrast: Contribution of molecular modelling in the flotation of calcium minerals. Ph.D. Thesis, Université de Lorraine, Vandœuvre-lès-Nancy, France, 25 October 2019; p. 322. [Google Scholar]
- Pastor, H. Métallurgie et recyclage du tungstène. Procédés. Tech. L’ingénieur 2000, 3, M2378. [Google Scholar] [CrossRef]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Cui, Y.; Li, J. Thermodynamic and XRD analysis of reaction behaviors of gangue minerals in roasting mixture of scheelite and calcium carbonate for Ca3WO6 preparation. Int. J. Refract. Met. Hard Mater. 2016, 60, 82–91. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, K.; Li, X.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z. Leaching of synthetic Ca3WO6 with ammoniacal ammonium carbonate solution under atmospheric pressure: A fundamental study. Hydrometallurgy 2019, 184, 55–66. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Peng, C.; Li, J.; Chen, W. Sequential extraction of tungsten from scheelite through roasting and alkaline leaching. Miner. Eng. 2019, 132, 238–244. [Google Scholar] [CrossRef]
- Ren, H.; Li, J.; Tang, Z.; Zhao, Z.; Chen, X.; Liu, X.; He, L. Sustainable and efficient extracting of tin and tungsten from wolframite-scheelite mixed ore with high tin content. J. Clean. Prod. 2020, 269, 122282. [Google Scholar] [CrossRef]
- Menéndez, C.J.; Tavani, E.L.; Nolasco, E.J. Kinetic study of the isothermal and non-isothermal chlorination of a scheelite–wolframite concentrate with chlorine and sulphur dioxide. Thermochim. Acta 1999, 338, 103–112. [Google Scholar] [CrossRef]
- Menéndez, C.J.; Barone, V.L.; Botto, I.L.; Tavani, E.L. Physicochemical characterization of the chlorination of natural wolframites with chlorine and sulphur dioxide. Miner. Eng. 2007, 20, 1278–1284. [Google Scholar] [CrossRef]
- Fouga, G.G.; Taddeo, R.M.; Bosco, M.V.; Bohé, A.E. Kinetic study of Hubnerite (MnWO4) chlorination. Thermochim. Acta 2012, 536, 30–40. [Google Scholar] [CrossRef]
- Levin, E.M.; Robbins, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists; The American Ceramic Society: Columbus, OH, USA, 1969; Volume 2, pp. 86, 89. [Google Scholar]
- Hildebrandt, V.; Kahlenberg, V.; Krüger, H.; Wagner, S.; Dinu, D.F.; Hofer, T.S.; Tropper, P.; Liedl, K. Structural and computational studies on a quenchable high-temperature polymorph of magnesium tungstate (MgWO4-II). J. Solid State Chem. 2023, 327, 124269. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows, Version 5.1; Outokumpu Research: Pori, Finland, 2002.
- Barin, I. Thermochemical Data of Pure Substances; VCH: Weinheim, Germany, 1989. [Google Scholar]
- Fosu, A.Y.; Demeusy, B.; Diot, F.; Lavonen, T.; Meriläinen, V.; Bartier, D.; Foucaud, Y.; Kanari, N. Selective Extraction of Valuable and Critical Metals in Cassiterite Concentrate by Dry Chlorination, Part I: Thermodynamic and Modelling Perspective. Materials 2024, 17, 4186. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite Part I. Effect of temperature. Thermochim. Acta 2001, 371, 143–154. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite part II. Effect of reactive gases. Thermochim. Acta 2001, 371, 75–86. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Filippov, L.; Shallari, S.; Diot, F.; Patisson, F. Reactivity of Low-Grade Chromite Concentrates Towards Chlorinating Atmospheres. Materials 2020, 13, 4470. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).