Abstract
The continuous need for simplified, minimally invasive restorative procedures with a high precision has led to the advancement of highly filled flowable resin-based materials. These materials present excellent initial outcomes in various clinical applications, including the injection molding technique. Given that several clinical reports present signs of wear and staining, this systematic review aims to investigate the mechanical and optical properties of highly filled flowable composite resins. A comprehensive literature research was conducted to identify relevant studies from the PubMed, the Cochrane Library, and Scopus databases. Data extraction and screening was performed by two independent evaluators. Both in vitro studies and clinical trials were included. A total of thirty-one studies were included in this review. A total of 27 in vitro studies investigated highly filled flowable composite resins independently, or in comparison with conventional composite resins, traditional flowable composites, bulk-fill flowable composites, glass ionomer cements, and compomers. Additionally, four randomized controlled clinical trials (RCTs) compared highly filled flowable composite resins with their conventional counterparts. Highly filled flowable composite resins exhibit adequate optical properties. Despite their significant improvements, their mechanical properties remain inferior to those of medium-viscosity composite resins. These materials demonstrate a favorable initial performance in the injection molding technique. Based on a limited number of RCTs, these materials demonstrate an adequate performance in class I and II restorations; however these findings should be interpreted with caution. The reported drawbacks in laboratory studies may contraindicate their clinical application in extensive cavities, load-bearing areas, and in cases of excessive tooth wear and parafunctional activity. A careful clinical case selection is strongly recommended.
1. Introduction
Conventional, medium-viscosity composite resins are the most commonly used materials for both anterior and posterior restorations [1,2]. Conservation of tooth structure, an adequate color adaptability, satisfactory mechanical properties, and the lower cost compared to indirect restorative counterparts are listed as the benefits of these biomaterials [3,4]. However, their inferior rheological characteristics result in marginal defects, due to a poor adaptation to cavity walls and voids between increments [5]. To confine these drawbacks, first-generation flowable composites were launched in the 1990s, which contained 20–25% less filler loading compared to medium-viscosity composite resins [6,7]. Their low viscosity was conducive to ease of use, improved handling and wetting properties, a good adaptability to cavity walls, and restricted air bubble entrapment in their mass [8,9]. On the other hand, their mechanical and optical properties were impaired by their reduced filler content [10], limiting their use to only as class V restorative materials, liners, pit and fissure sealants, and as materials for marginal restoration repair [6]. Their high volumetric polymerization shrinkage rate of approximately 5% proved to be an additional disadvantage of traditional flowable composite resins [5].
In response to clinicians’ demands for simplicity and the durability of final restorations, dental material manufacturers developed novel flowable composites, suitable for a wide range of clinical cases. Their indications for use include the treatment of tooth wear and tooth erosion, correction of tooth shape and size, smile rejuvenation, and cuspal restoration, without the need for capping layers [11,12,13,14]. Their advanced formulation includes a higher filler loading, optimum filler size, the incorporation of refined monomers, and the pre-treatment of filler particles. Manufacturers suggest that these modifications enhance the materials’ physical and mechanical properties [11]. Simultaneously, superior marginal adaptation is maintained [15]. Their higher filler content—ranging from approximately 61% to 71% by weight—enables them to withstand high occlusal loads [16,17]. Until recently, these statements have not been completely verified. In the literature, this type of flowable composite resin is primarily reported as highly filled flowable composite resin [18,19,20]. The terms “next-generation composites” [21] and “injectable composites” [22] are often used interchangeably, which can lead to confusion.
The use of highly filled flowable composite resins as stand-alone restorative materials—particularly in the context of the injection molding technique—has gained significant attention in recent years [23,24,25,26]. Introduced by Terry and Powers in 2014, the injection molding technique is described as a novel restorative technique that transforms an analog or digital wax-up into a final composite resin restoration, with minimal or no tooth preparation [23,24]. One or more transparent silicone indices based on a wax-up enable the passive insertion of the restorative material, minimizing the risk of restoration distortion. The accurate reproduction of anatomical contours is reported as the main advantage of this technique [27]. Nowadays, the injection molding technique, utilizing highly filled flowable composite resins, is used in a wide range of clinical cases, including single-tooth restorations, tooth recontouring, the replacement of missing teeth with direct composite resin-bonded fixed dental prostheses, and full-mouth rehabilitation—with or without alteration of the vertical dimension [23,24,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. When minimal tooth structure loss is evident, highly filled flowable composite resins have performed optimally [24,34,36,44]. However, several clinical cases reported signs of staining on the material’s surface and/or at the tooth–material interface, minor chippings, and surface voids [30,43]. These observations highlight the inferior performance of highly filled flowable composite resins compared to other commonly used restorative materials.
This systematic review aims to address the following question: Do highly filled flowable composite resins exhibit adequate mechanical and optical properties and a good clinical performance? The use of these materials in novel restorative techniques and their promotion by manufacturers as sole restorative materials highlight the need to understand both their potential and limitations. This knowledge is crucial for appropriate case selection, thereby ensuring the long-term durability of these materials.
2. Materials and Methods
A systematic review was conducted to assess the mechanical properties, optical characteristics, and clinical performance of highly filled flowable composite resins. This report followed the guidelines of the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [51]. The present systematic review was registered on the PROSPERO platform (ID number: 1063216). The PICO framework was adapted to formulate the research question and facilitate the selection process (Figure 1).
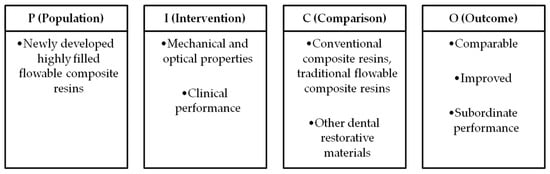
Figure 1.
PICO framework of systematic reviews.
2.1. Sources of Information and Search Strategy
A systematic literature search was conducted using the following electronic databases: MEDLINE via PubMed, Scopus, and the Cochrane Library. A manual search of reference listings has been applied. The search covered publications from the databases’ inception through to February 2025. The synthesis of information from diverse sources, including qualitative and quantitative studies, has been performed. The search strategy is presented in Table 1.

Table 1.
Database search strategy and identification of studies.
2.2. Eligibility Criteria
The search results were filtered by language, and studies in English were eligible. This review included randomized controlled clinical trials and in vitro studies. Laboratory studies that incorporated at least one highly filled flowable composite resin in their methodology were included. Clinical trials in progress and clinical trials not using conventional composite resins as controls were excluded. Studies that exclusively evaluated polymerization shrinkage, depth of cure, and the degree of conversion of highly filled flowable composite resins—without simultaneously evaluating mechanical and optical properties—were excluded at the stage of full-text screening. This review also excluded case reports, case series, review articles, short communications, patents, and conference proceedings.
2.3. Data Extraction, Screening, and Charting
Screening was conducted by two independent reviewers (K.T. and C.R.) in two phases—first by title and abstract and then by full text—based on the pre-established eligibility criteria. In the presence of a discrepancy, screening was conducted by a third reviewer (E.P.) until consensus was reached. Automated screening using AI-powered tools was performed for studies originating from the PubMed and Scopus databases [52]. Studies retrieved from the Cochrane Library database were screened manually. Short communications, conference proceedings, and clinical trials in progress were excluded after manual screening. The reference lists of all the included articles were manually searched to identify further eligible studies.
Data charting was conducted by two examiners. The first examiner (K.T.) extracted the relevant data from the eligible studies, while the second examiner (C.R.) verified its accuracy. The results are organized chronologically in tables. Tables for in vitro studies include the author and year of publication, dental materials used, tested parameters, and key findings. Tables for randomized controlled clinical trials present the author and year of publication, objectives, materials used, sample size, follow-up intervals, evaluation criteria, and observed outcomes.
2.4. Risk of Bias Assessment
The Johanna Briggs Institute (JBI) critical appraisal tools were used to assess the trustworthiness and relevance of the included studies. More precisely, the JBI Critical Appraisal Checklist for Randomized Controlled Clinical Trials (RCTs) was used to evaluate the RCTs [53], and the JBI Checklist for Quasi-Experimental Studies was used for the evaluation of the included in vitro studies [54]. The JBI SUMARI (System for the Unified Management, Assessment, and Review of Information) (https://sumari.jbi.global/, accessed on 25 March 2025) software platform was used to export summary tables following the completion of the critical appraisal. Two independent reviewers (K.T. and C.R.) conducted the assessments of the eligible studies. Discrepancies were resolved through discussion, until a consensus was reached, thereby enhancing the reliability and validity of the systematic review’s key findings.
3. Results
A flow chart illustrating the number of studies identified and screened is shown in Figure 2.
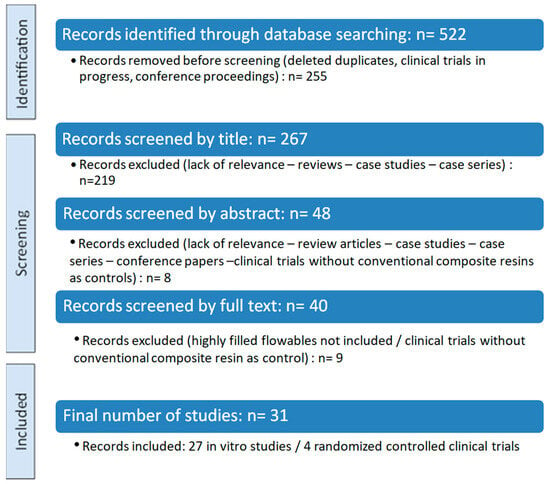
Figure 2.
PRISMA flow diagram.
Out of 522 records identified, 31 studies were included in this systematic review after full-text screening. The studies were divided into in vitro studies and randomized controlled clinical trials, followed by a synthesis of their key finding. A summary of the eligible studies, including their research type and investigated outcomes, is presented in Table 2.

Table 2.
Summary of eligible studies based on research type and investigated parameters.
3.1. In Vitro Studies on Highly Filled Flowable Composite Resins
This review included 27 in vitro studies [17,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. Each study evaluated one or multiple material characteristics. The in vitro studies were subcategorized into two groups: the first group included 15 in vitro studies that incorporated conventional composite resins in their methodology [17,55,56,57,58,59,60,61,62,63,64,65,66,67,68], and the second group consisted of 12 in vitro studies that examined either a single highly filled flowable composite resin or highly filled flowable composite resins alongside traditional flowable composites, bulk-fill flowable composites, glass ionomer cements, and compomers [69,70,71,72,73,74,75,76,77,78,79,80]. Detailed descriptions of the studies’ characteristics and qualitative findings are presented in Table 3 and Table 4.

Table 3.
In vitro studies incorporating conventional composite resins into their methodological framework.

Table 4.
In vitro studies conducted without the incorporation of conventional composite resins in their methodology.
3.1.1. Optical Properties and Color Stability of Highly Filled Flowable Composite Resins
The findings on optical properties indicate that highly filled flowable composite resins exhibit an acceptable color stability, supporting their potential use in esthetically demanding restorations. Initially, Nair et al. demonstrated that a highly filled flowable composite resin performed inferiorly compared to nanofilled and nanohybrid conventional composites [55]. Owing to recent developments in highly filled flowable composites, no statistically significant differences were found in color stability among highly filled flowable composite resins, a bulk-fill flowable composite, and a conventional nanofilled composite resin [56]. Interestingly, Tüter Bayrakrat et al. reported that conventional composite resins had lower fluorescence (ΔEFI) and color adjustment (ΔECP) levels compared to highly filled flowables in class V cavities [62]. Color changes due to toothbrushing abrasion remained within acceptable thresholds when highly filled flowables were compared with traditional and self-adhesive flowables [69].
3.1.2. Surface Characteristics of Highly Filled Flowable Composite Resins
Highly filled flowable composite resins exhibited statistically comparable surface roughness values (arithmetic average of the absolute values of the surface height deviations measured from the mean line, Ra) to those of conventional nanohybrid composites [60,64,65]. In a study by Elsahn et al. in 2023, G-aenial Universal Injectable exhibited lower Ra values, even when compared to a CAD/CAM resin-based material, when used as thin occlusal veneers [74]. Vulović et al. demonstrated that highly filled flowable composite resins, consisting of ultra-fine barium or strontium fillers combined with full-coverage silane technology (G-aenial Universal Injectable, GC, and G-aenial Universal Flo, GC) exhibited lower surface roughness values compared to other highly filled flowables [76,80]. Surface roughness scores were affected by both polishing systems and the acidic challenges implemented in the in vitro methodological protocols of the studies [75,76,80]. Regarding surface gloss, G-aenial Universal Injectable performed favorably compared to other traditional flowables available on the market [69].
3.1.3. Mechanical Characteristics of Highly Filled Flowable Composite Resins
While notable improvements in microhardness, flexural strength, and wear resistance have been reported for highly filled flowable composite resins, divergent results across studies raise concerns regarding their performance. Multiple in vitro studies reported that highly filled flowable restorative materials exhibited an inferior Vickers microhardness compared to conventional microfilled composites, nanofilled composites, bulk-fill composite resins, and CAD/CAM resin-based materials [55,57,59,65,74]. Conversely, when compared with traditional flowable composites, highly filled flowable restorative materials exhibited superior hardness values [73]. Among several highly filled flowable resin composites, G-aenial Universal Injectable presented the highest surface hardness values [80]. Controversial findings were observed regarding the elastic modulus of highly filled flowable composite resins. Imai et al. demonstrated lower elastic modulus values for highly filled flowable composite resins compared to microhybrid and nanofilled conventional composite resins [17], while Degirmenci et al. and Rajabi et al. showed higher flexural strength and elastic modulus values for highly filled flowable materials compared to microhybrid and nanohybrid conventional composites [57,66]. In a recent study, no differences in flexural strength values were observed between highly filled flowables and a nanohybrid conventional composite resin [65]. Highly filled flowable composites outperformed traditional flowables and bulk-fill flowable composites in terms of flexural strength and elastic modulus [72].
Regarding wear resistance, controversial findings have once again been reported. Turk et al. demonstrated that conventional nanohybrid and nanofilled composite resins outperformed flowable composite resins in terms of wear volume loss and maximum wear depths [61]. However, G-aenial Universal Injectable by GC showed a lower wear volume loss than that of a CAD/CAM milled resin-based material when used as 1 mm thin occlusal veneers [74]. Recent data reveal a significant similarity of wear patters between highly filled flowable composites and conventional composite resins. Therefore, several researchers have supported the use of highly filled flowable composite resins in occlusal, load-bearing areas [64,66].
3.2. Randomized Controlled Clinical Trials on Highly Filled Flowable Composite Resins
To date, only four randomized controlled clinical trials have assessed the clinical performance of highly filled flowable resin-based materials compared to conventional composite resins [81,82,83,84]. Three studies focused on the clinical performance of highly filled flowable resin composites in class I and II restorations of varying sizes [81,83,84], while one investigated non-cavitated cervical lesions [82]. Regarding function and esthetics, both materials performed equally well. Concerning marginal adaptation and surface gloss, highly filled flowable composite resins have shown superior performance relative to controls [82,84]. The clinical trials are summarized in Table 5.

Table 5.
Randomized controlled clinical trials with highly filled flowable composite resins.
3.3. Risk of Bias of Included Studies
The risk of bias of the in vitro studies and randomized controlled clinical trials was assessed by two reviewers (K.T. and C.R) independently, based on a fixed categorization relative to the design. The two groups of in vitro studies were evaluated separately. The results are shown in Figure 3, Figure 4, and Figure 5 respectively.
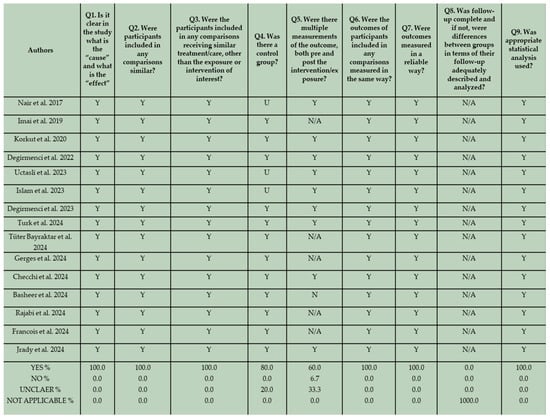
Figure 3.
JBI Critical Appraisal results for the first group of in vitro studies that incorporated conventional composite resins in their methodological framework [17,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
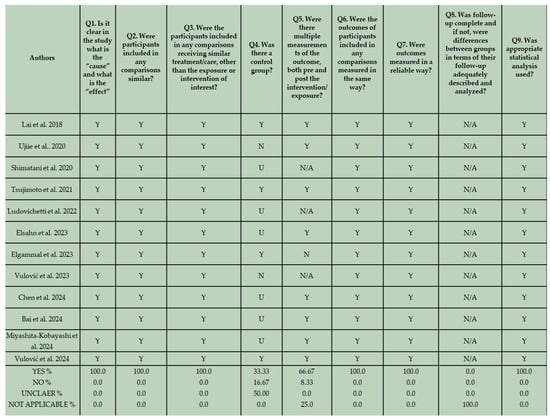
Figure 4.
JBI Critical Appraisal results for the second group of in vitro studies that do not employ conventional composite resins in their methodological framework [69,70,71,72,73,74,75,76,77,78,79,80].
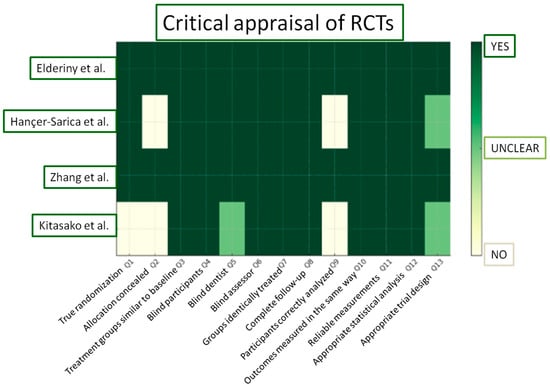
Figure 5.
JBI Critical Appraisal results for the included RCTs [81,82,83,84].
In terms of reporting quality, no bias related to temporal precedence was identified, since all the studies clearly presented “cause” and “effect” variables. Furthermore, there was no indication of bias related to confounding factors and the administration of interventions. This is based on the fact that in all the in vitro studies, specimens were identically prepared and received similar care, aside from the exposure to the intervention. Regarding the potential of risk of bias related to selection and allocation, 80% of the eligible studies that incorporate conventional composite resins in their methodological design, and 33.33% of the included studies that did not employ conventional composite resins in their methodology, provided well-defined control groups. In several studies, an unclear assessment of control groups is evident. Although control groups could be identified based on the formulated null hypothesis, they were not adequately described in the manuscripts. Additionally, not all eligible in vitro studies incorporated both pre- and post-intervention measurements of the examined outcome in their methodological design. This may be attributed to the nature of the design and should not be interpreted as indicative of a high risk of bias. For instance, wear and volume loss can only be assessed after wear simulation. In two studies no pre-intervention measurements were conducted. In these studies surface roughness, hardness, and gloss were assessed after thermocycling and after immersion in different beverages [65,75]. Nevertheless, due to the implementation of standardized and reliable measurement techniques, the risk of bias related to the assessment, detection, and measurement of the outcome appeared low. A bias related to participant retention is not applicable in this context, as all laboratory specimens were accessible for assessment. Lastly, the validity of the applied statistical analysis proved to be high for all the in vitro studies, demonstrating adherence to well-defined analytical standards.
In this section, according to the JBI Checklist for Randomized Controlled Clinical Trials, the studies were evaluated using the scoring criteria “yes”, “no”, “unclear”, “not applicable”, based on the data extracted after the study evaluation. Enlarging on the risk of bias, the study of Kitasako et al. displayed the most significant drawbacks [81]. Although dice were used for randomization, assessors proceeded to modifications of the randomization process when more than one restoration was required for a single patient. Furthermore, no information on allocation concealment was provided in two out of four clinical trials [81,84]. In the study of Kitasako et al. [81], even though participants unavailable for further evaluation were well-defined, they were censored from the total count of restorations initially inserted and consequently from the statistical analysis. Two clinical trials demonstrated an overall low risk of bias [82,83].
4. Discussion
The results of this review should be interpreted with caution. Variations were observed among the studies examining one highly filled flowable material, as well as across studies evaluating different highly filled flowable composite resins or different restorative materials (e.g., highly filled flowable materials, traditional flowable materials, bulk-fill materials, compomers, glass ionomer cements, and conventional composite resins). These discrepancies present a multifactorial and interdependent etiological pattern.
4.1. Factors Influencing Optical and Mechanical Properties of Dental Biomaterials
The mechanical, physical, optical, and surface characteristics of dental materials are predominantly related to their unique composition. The structure of the organic matrix, the composition of inorganic filler particles, the filler-to-resin matrix ratio, and the silanization of the filler components are key factors that affect their properties. The material’s composition significantly affects its further properties, such as the degree of conversion, water sorption, and solubility [17,59,85,86]. Beyond the material’s composition, finishing and polishing procedures, methodological variations, sample fabrication techniques, storage and testing conditions, and external aggravating stimuli (colorant solutions, acidic and abrasive challenges) can significantly affect the laboratory performance of dental materials [56,57,58,60,66,75,76,80].
4.1.1. The Predominant Effect of Inorganic Filler Content on Optical Properties and Microhardness
The optical performance of composite resins is influenced by factors such as surface roughness, gloss, and hardness [87]. Furthermore, surface roughness is affected by filler content, filler type and size, the surface area occupied by filler particles, the degree of conversion, filler–matrix interactions, the silane coupling agents, and ultimately, the material’s hardness [88,89,90]. While colorant solutions and abrasive challenges negatively affect the microhardness and color stability of highly filled flowable resin composites [55], variances in microhardness values may be attributed to the type and distribution of fillers. For instance, the strontium glass fillers found in G-aenial Universal Flo are associated with inferior physical properties and a more complex attachment to the organic matrix than the zirconia and silica fillers incorporated in conventional composite resins [55,91]. A uniform filler distribution contributes to the enhancement of physical and optical properties [59]. The filler size in G-aenial Universal Flo by GC (highly filled flowable) ranges from 16 to 200 nm, whereas Filtek Z350XT (conventional composite resin) contains filler particles ranging from 4 to 20 nm in size [92,93]. These variations may explain the differences in the mechanical properties between these materials.
The optical performance of composite resins is related to translucency, opalescence, chroma, and the refractive indices of both monomers and fillers [94]. Differences in filler type and inorganic composition can significantly affect translucency [95,96]. This fact is endorsed by Bai et al. in 2024, who demonstrated that G-aenial Universal Injectable (GC) exhibited a higher translucency when compared to Filtek Supreme Flowable (3M ESPE), likely due to the imperfect refractive index of its zirconia fillers [78]. G-aenial Universal Injectable, filled with barium glass, displayed the highest translucency and opalescence values when compared with G-aenial Universal Flo, which is filled with strontium glass, and G-aenial Anterior, a microhybrid conventional resin composite, filled with strontium glass and lanthanoid fluoride [60]. Furthermore, the abrasive hardness of polishing systems is directly related to translucency values [97]. Diamond particles, presenting a higher abrasive hardness than aluminum oxide particles, result in an extended abrasion of the organic matrix and increased filler protrusion. This fact partially explains the variations in translucency even within the same material [98,99]. It should be noted that while a higher filler content improves strength and wear resistance, it ultimately reduces translucency and increases opacity [100,101,102,103].
4.1.2. Influence of Inorganic Filler Content on Surface Characteristics
The filler’s loading, shape, and size could partially explain variations in surface roughness and gloss values among composite resins [90,104]. Lower filler loading, an irregular filler shape, a heterogeneous filler composition, and larger average particle sizes are inextricably linked to an increased surface roughness and decreased surface gloss. This is validated by the findings of Miyashita–Kobayashi et al., who compared the surface roughness and gloss of two highly filled flowable composite resins and found differences among them, with a material consisting of supra-nanospherical fillers and an average particle size of 0.2 μm exhibiting a superior surface gloss and smoother surfaces compared to a material containing irregularly shaped fillers and an average particle size of 0.8 μm [79]. Beautifil Injectable X consists of bioactive surface pre-reacted glass ionomer (S-PRG) fillers with an average particle size of 0.8 μm. Conversely, G-aenial Universal Flo and G-aenial Universal Injectable consist of strontium and barium glass fillers, respectively, along with silica particles, forming homogeneous filler patterns. In contrast, Tetric EvoFlow and Filtek Supreme Flowable Restorative incorporate Ytterbium trifluoride (YBF3) and a mixture of three to four diverse filler types, leading to a heterogeneous composition that negatively impacts surface roughness values [76]. At this point, it is essential to critically assess that most in vitro studies analyze the surface roughness using a single height parameter (Ra value). However, the inclusion of spatial, functional, or hybrid (e.g., developed interfacial area ratio, Sdr) parameters can offer a greater insight into the surface texture of previously analyzed highly filled flowable composite resins [105].
4.1.3. The Interaction Between Inorganic Filler Content, Organic Matrix Composition, and the Oral Environment in the Mechanical Performance of Dental Biomaterials
Mechanical properties—particularly wear resistance—depend on the filler type, shape, and size; interfiller spacing; and the type of filler pre-treatment [85,106]. Additionally, characteristics such as the degree of conversion, resistance to hydrolytic degradation, water sorption, and finishing and polishing procedures play a pivotal role in wear resistance and flexural strength [6,10,15,17,70,107,108,109]. The smaller particle size of several highly filled flowables results in lower friction coefficients and restricted internal shear stresses at the polymer matrix, potentially explaining the favorable wear resistance among several highly filled flowable composite resins and bulk-fill flowables [15,70,110]. Moreover, the small interparticle spacing among small-sized fillers may form a protective barrier to the resin matrix. The lower volumetric wear of G-aenial Universal Injectable compared to a resin-based CAD/CAM material in 1 mm thin veneers should be interpreted with caution. Manufacturers recommend a minimum thickness of 1.5 mm for this CAD/CAM material [111], highlighting notable limitations in the experimental design of the included study. The increased filler content and the high concentration of Bis-GMA monomers in conventional composite resins improve their mechanical and physical properties [112,113], thereby justifying the superior wear performance of conventional composite resins compared to highly filled flowable composite resins. The finding that G-aenial Universal Flo (GC) demonstrated significantly comparable flexural strength values to those of a nanofilled composite resin suggests that filler loading alone does not enhance mechanical strength. Different monomers have unique molecular weights and viscosities, directly influencing the material’s mechanical behavior [114].
Upon exposure to oral conditions, dimensional changes, degradation, and weakening of the bond between organic and inorganic components occur [17,115]. A higher filler content is related to reduced water sorption, due to the increased hydrophobicity of the material [116,117]. However, a higher filler content may lead to increased water sorption, due to the greater surface area available for water uptake. Different types of monomers and their quantitative allocation in the organic matrix influence the water sorption behavior of biomaterials [118,119]. A higher Bis-GMA and TEGDMA content provides greater susceptibility to hydrolytic degradation compared to UDMA monomers [120,121]. TEGDMA absorbs more water than Bis-GMA due to its greater flexibility and intermolecular spacing [122,123,124]. The water sorption promotes the hydrolysis of the coupling agent, weakening the filler–matrix bond [125]. By leveling up the amount of nano-sized fillers and creating a homogeneous distribution of inorganic particles, the internal volume available for water infiltration is reduced. This fact partially explains the lower water sorption values of various conventional composite resins compared to highly filled flowables. Nevertheless, disparities are present among different highly filled flowables, primarily due to variations in their organic and inorganic composition. For example, Beautifil Injectable X by Shofu exhibits higher water sorption and degradation levels than G–Aenial Universal Injectable under acidic conditions. This can be attributed to the higher quantity of Bis–GMA and TEGDMA in its organic matrix and the presence of fluorosilicate glass fillers (S-PRG fillers) [14]. This filler type exhibits a higher susceptibility to degradation by weak acids [88,126].
4.2. Limitations of the In Vitro Studies and Randomized Controlled Clinical Trials
Systematic reviews provide an essential input into clinically relevant queries by compiling, analyzing, and synthesizing evidence from a wide range of eligible studies. By following rigorous methodologies, comprehensive and critically appraised findings are produced, leading to evidence-based conclusions [127]. Despite their strengths, certain limitations may arise. More precisely, careful consideration should be given to the methodological quality of the eligible studies, as the study design parameters—sample size calculation, sample preparation, presence of control groups, observational conditions, and calibration of devices—have a considerable impact on the outcome of the study and the reliability of its findings [128]. Therefore, controversial results in in vitro studies should not be attributed solely to material-dependent factors but also to methodology-dependent variables and testing conditions. A typical example of inconsistency among in vitro studies refers to findings related to wear resistance, flexural strength, and elastic modulus. For instance, Degirmenci et al. and Rajabi et al. reported higher elastic modulus and flexural strength values of highly filled flowable composite resins compared to conventional composite resins [57,66]. Furthermore, Rajabi et al. and Checchi et al. showed a lower wear volume loss for highly filled flowable composite resins [64,66]. In contrast, Turk et al. and Imai et al. reported opposite findings, underscoring the variability in outcomes across studies [17,61]. Interestingly, Basheer et al. demonstrated similar flexural strength values among highly filled flowable composite resins and conventional composites but a higher elastic modulus for conventional composite materials [65]. Focusing on flexural strength, notable differences in specimen preparation across studies include variations in the polymerization process (e.g., light-curing duration and differences in the specific surfaces subjected to polymerization), storage process (e.g., storage in artificial saliva, in dark and dry environments, or in distilled water), and testing conditions (e.g., the presence or absence of thermocycling or prior immersion in various liquids). Regarding observations on wear resistance, discrepancies among studies may be attributed to several methodology-related factors such as differences in sample preparation (cylindrical vs. rectangular specimen shapes), sample conditioning (dry storage, storage in distilled water, or incubation for 24 h at 37 °C), variations in chewing simulation parameters ranging from 50.000 to 240.000 chewing cycles, differences in antagonist simulators (stainless steel balls, magnesium silicate-based balls, steatite balls), variations in the radius of the antagonist (ranging from 2.4 mm to 6 mm in diameter), and differences in the devices used for the assessment of wear (optical surface laser scanner, confocal laser scanning microscope, digital scanner without pre-wear baseline measurements). When newly introduced stand-alone restorative materials need to be evaluated, conventional nanofilled or nanohybrid composite resins—rather than traditional flowable composites—should serve as controls. Given that laboratory studies cannot simulate the complex oral environment to a great extent, in vitro studies are placed at the lowest level of the evidence-based medicine hierarchy [129]. Dental materials should perform optimally in the complex and variable oral environment, where masticatory forces, occlusal and dietary habits, temperature fluctuations, the formation of biofilms, enzyme activity, and salivary flow are constantly present. The interaction of these factors with dental materials may alter their physical, mechanical, and optical properties. Therefore, randomized controlled clinical trials provide stronger evidence regarding materials’ performance. Unfortunately, the number of eligible randomized controlled clinical trials is predictably low, and the trials are not readily comparable, since different restorative procedures were applied. For instance, one clinical trial investigated the clinical performance of highly filled flowable composite resins in cervical non-carious lesions [79], while others evaluated their clinical performance in class I and II cavities, in mid-size and extensive posterior restorations, ranging from occlusal caries to overlay replacement [78,80,81]. A high risk of bias arising from the randomization and allocation process is present in the clinical trial of Kitasako et al. in 2016 [81], where dice were used for randomization, and allocation concealment was not ensured. In contrast, the other three clinical trials used automated random sequence generators to minimize randomization bias. Additionally, the same clinical trial lacked a sample size calculation, resulting in a low statistical power. All four RCTs had limited sample sizes. Hançer Sarıca et al. in 2025 included 259 class II restorations at baseline [84], whereas a relatively smaller amount of participants was present in the other three clinical trials. Dropouts further reduced the number of restorations evaluated after baseline. Despite these drawbacks, no bias regarding the outcome measurement was detected, as acceptable evaluation criteria (FDI criteria and modified USPHS criteria) were employed. Limitations involving randomization, sample size, dropout rates, and short term follow-up periods must be taken into consideration before drawing definite conclusions [130].
It should not be overlooked that the clinical performance of composite resin materials in the oral environment is influenced by multiple factors, including cavity size, tooth position, the restorative techniques applied (rubber dam isolation, the polishing systems employed), the patient’s profile (e.g., caries risk, the presence of parafunctional habits), and the operator’s skills. By minimizing the risk factors, clinical failures such as fractures, secondary caries, and esthetic complications can be significantly reduced [131].
Emphasis should be given to the establishment of well-designed clinical trials in order to unfold the full potential of these novel restorative materials. Additionally, it would have been valuable to assess the behavior of highly filled flowable resin-based materials in oral conditions by conducting in situ studies, which may reveal a potential connection between optical parameters, mechanical properties, surface characteristics, saliva, and the oral microbiome.
5. Conclusions
Highly filled flowable resin composites display excellent optical properties in laboratory studies, supporting their clinical use as esthetic restorative materials in the anterior region. Based on the results of this systematic review, they also appear to be suitable for minimally invasive restorations in the posterior region. Given the limited number of randomized controlled trials and the notable limitations of the included in vitro studies, the clinical application of these materials in load-bearing areas and in patients with significant tooth wear or heavy parafunctional activity should be avoided.
Author Contributions
Conceptualization, K.T., E.P. (Eftychia Pappa), and E.P. (Efstratios Papazoglou); methodology, K.T. and C.R.; validation, K.T., M.F. and C.R.; formal analysis, K.T.; investigation, K.T.; data curation, K.T.; writing—original draft preparation, K.T.; writing—review and editing, E.P. (Eftychia Pappa), C.R. and E.P. (Efstratios Papazoglou); visualization, K.T.; supervision, E.P. (Eftychia Pappa), C.R. and E.P. (Efstratios Papazoglou); project administration, K.T., E.P. (Eftychia Pappa), M.F., C.R. and E.P. (Efstratios Papazoglou) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H.F. An update on the reasons for placement and replacement of direct restorations. J. Dent. 2018, 72, 1–7. [Google Scholar] [CrossRef]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. B Eng. 2022, 230, 109495. [Google Scholar] [CrossRef]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled resin composite properties and clinical performance: A review. Oper. Dent. 2018, 43, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.D.A.R.; Silva, L.F.V.D.; Silva, P.F.D.; Silva, A.L.F.E. Color matching and color recovery in large composite restorations using single-shade or universal composites. Braz. Dent. J. 2024, 35, e245665. [Google Scholar] [CrossRef] [PubMed]
- Vouvoudi, E.C. Overviews on the Progress of Flowable Dental Polymeric Composites: Their Composition, Polymerization Process, Flowability and Radiopacity Aspects. Polymers 2022, 14, 4182. [Google Scholar] [CrossRef]
- Baroudi, K.; Rodrigues, J.C. Flowable Resin Composites: A Systematic Review and Clinical Considerations. J. Clin. Diagn. Res. 2015, 9, 18–24. [Google Scholar] [CrossRef]
- Bayne, S.C.; Thompson, J.Y.; Swift, E.J.; Stamatiades, P.; Wilkerson, M. A characterization of first-generation flowable composites. J. Am. Dent. Assoc. 1998, 129, 567–577. [Google Scholar] [CrossRef]
- Olmez, A.; Oztas, N.; Bodur, H. The effect of flowable resin composite on microleakage and internal voids in class II composite restorations. Oper. Dent. 2004, 29, 713–719. [Google Scholar]
- Hervás-García, A.; Martínez-Lozano, M.A.; Cabanes-Vila, J.; Barjau-Escribano, A.; Fos-Galve, P. Composite resins. A review of the materials and clinical indications. Med. Oral. Patol. Oral. Cir. Bucal. 2006, 11, 215–220. [Google Scholar]
- Mirică, I.-C.; Furtos, G.; Bâldea, B.; Lucaciu, O.; Ilea, A.; Moldovan, M.; Câmpian, R.-S. Influence of Filler Loading on the Mechanical Properties of Flowable Resin Composites. Materials 2020, 13, 1477. [Google Scholar] [CrossRef]
- G-aenial Universal Injectable. Available online: https://www.gc.dental/europe/sites/europe.gc.dental/files/products/downloads/gaenialuniversalinjectable/ifu/IFU_G-aenial_Universal_Injectable_W.pdf (accessed on 5 September 2024).
- G-aenial Universal Flo. Available online: https://www.gc.dental/europe/sites/europe.gc.dental/files/products/downloads/gaenialuniversalflo/ifu/IFU_G-aenial_Universal_Flo_W.pdf (accessed on 5 September 2024).
- Clearfil Majesty ES Flow. Available online: https://www.kuraraynoritake.com/world/product/composites/pdf/majesty_es_flow_brochure.pdf (accessed on 5 September 2024).
- Beautifil Injectable X: Safety Data Sheet. Available online: https://www.shofu.com.sg/wp-content/uploads/2020/05/SDS_BEAUTIFIL-Injectable-XVer.2.pdf (accessed on 15 February 2025).
- Sumino, N.; Tsubota, K.; Takamizawa, T.; Shiratsuchi, K.; Miyazaki, M.; Latta, M.A. Comparison of the wear and flexural characteristics of flowable resin composites for posterior lesions. Acta Odontol. Scand. 2013, 71, 820–827. [Google Scholar] [CrossRef]
- Badr, C.; Spagnuolo, G.; Amenta, F.; Khairallah, C.; Mahdi, S.S.; Daher, E.; Battineni, G.; Baba, N.Z.; Zogheib, T.; Qasim, S.S.B.; et al. A Two-Year Comparative Evaluation of the Clinical Performance of a nanohybrid composite resin versus a Flowable Composite Resin. J. Funct. Biomater. 2021, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Takamizawa, T.; Sugimura, R.; Tsujimoto, A.; Ishii, R.; Kawazu, M.; Saito, T.; Miyazaki, M. Interrelation among the handling, mechanical, and wear properties of the newly developed flowable resin composites. J. Mech. Behav. Biomed. Mater. 2019, 89, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.D.; Meral, E.; Gurgan, S. Does a Self-adhesive Flowable Resin Composite Perform Similarly to Highly Filled and Conventional Flowable Resin Composites in Occlusal Cavities? A 2-year Follow-up Study. J. Adhes. Dent. 2021, 23, 497–503. [Google Scholar] [CrossRef]
- Baldi, A.; Scattina, A.; Ferrero, G.; Comba, A.; Alovisi, M.; Pasqualini, D.; Peroni, L.; Muggeo, M.; Germanetti, F.; Scotti, N. Highly-filled flowable composite in deep margin elevation: FEA study obtained from a microCT real model. Dent. Mater. 2022, 38, 94–107. [Google Scholar] [CrossRef]
- Sagsoz, O.; Ilday, N.O.; Karatas, O.; Cayabatmaz, M.; Parlak, H.; Olmez, M.H.; Demirbuga, S. The bond strength of highly filled flowable composites placed in two different configuration factors. J. Conserv. Dent. 2016, 19, 21–25. [Google Scholar] [CrossRef]
- The Next Generation of Flowable Restoratives. Available online: https://www.nature.com/articles/sj.bdj.2012.915 (accessed on 15 February 2025).
- Farghal, N.S.; Awadalkreem, F.; Dasnadi, S.P.; Habush, S.; Hatab, N.A.; Harhash, A. Staining susceptibility and the effect of different stain removal techniques on the optical properties of injectable composite resins. Front. Oral. Health 2025, 6, 1556155. [Google Scholar] [CrossRef]
- Terry, D.; Powers, J. Using injectable resin composite: Part one. Int. Dent. Afr. 2014, 5, 52–62. [Google Scholar]
- Geštakovski, D. The injectable composite resin technique: Minimally invasive reconstruction of esthetics and function. Clinical case report with 2-year follow-up. Quintessence Int. 2019, 50, 712–719. [Google Scholar] [CrossRef]
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive dentistry: Current concepts and clinical considerations. J. Esthet. Restor. Dent. 2021, 33, 51–68. [Google Scholar] [CrossRef]
- Blasi, A.; Alnassar, T.; Chiche, G. Injectable technique for direct provisional restoration. J. Esthet. Restor. Dent. 2018, 30, 85–88. [Google Scholar] [CrossRef]
- Kouri, V.; Moldovani, D.; Papazoglou, E. Accuracy of Direct Composite Veneers via Injectable Resin Composite and Silicone Matrices in Comparison to Diagnostic Wax-Up. J. Funct. Biomater. 2023, 14, 32. [Google Scholar] [CrossRef]
- Coachman, C.; De Arbeloa, L.; Mahn, G.; Sulaiman, T.A.; Mahn, E. An Improved Direct Injection Technique with Flowable Composites. A Digital Workflow Case Report. Oper. Dent. 2020, 45, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Tichy, A.; Motoyama, Y.; Mizutani, K.; Lai, W.J.; Kanno, Z.; Tagami, J.; Nakajima, M. Post-orthodontic recontouring of anterior teeth using composite injection technique with a digital workflow. J. Esthet. Restor. Dent. 2020, 32, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Ypei Gia, N.R.; Sampaio, C.S.; Higashi, C.; Sakamoto, A.; Hirata, R. The injectable resin composite restorative technique: A case report. J. Esthet. Restor. Dent. 2021, 33, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Bretón Brinkmann, J.; Albanchez-González, M.I.; Lobato Peña, D.M.; García Gil, I.; Suárez García, M.J.; Peláez Rico, J. Improvement of aesthetics in a patient with tetracycline stains using the injectable composite resin technique: Case report with 24-month follow-up. Br. Dent. J. 2020, 229, 774–778. [Google Scholar] [CrossRef]
- Hosaka, K.; Tichy, A.; Hasegawa, Y.; Motoyama, Y.; Kanazawa, M.; Tagami, J.; Nakajima, M. Replacing mandibular central incisors with a direct resin-bonded fixed dental prosthesis by using a bilayering composite resin injection technique with a digital workflow: A dental technique. J. Prosthet. Dent. 2021, 126, 150–154. [Google Scholar] [CrossRef]
- Ljubičić, M.; Živković, M. Multidisciplinary approach in treatment of spacing: Orthodontic treatment and partial ve-neers using the injectable composite resin technique. Serbian Dent. J. 2021, 68, 39–44. [Google Scholar] [CrossRef]
- Geštakovski, D. The injectable composite resin technique: Biocopy of a natural tooth–Advantages of digital planning. Int. J. Esthet. Dent. 2021, 16, 280–299. [Google Scholar]
- Hulac, S.; Kois, J.C. Managing the transition to a complex full mouth rehabilitation utilizing injectable composite. J. Esthet. Restor. Dent. 2023, 35, 796–802. [Google Scholar] [CrossRef]
- Peumans, Μ. Geštakovski, D.; Mattiussi, J.; Karagiannopoulos, K. Injection moulding technique with injectable composites: Quick fix or long-lasting solution? Int. Dent. Afr. 2023, 13, 14–22. [Google Scholar]
- Hosaka, K.; Tichy, A.; Yamauti, M.; Watanabe, K.; Kamoi, K.; Yonekura, K.; Foxton, R.; Nakajima, M. Digitally Guided Direct Composite Injection Technique with a Bi-layer Clear Mini-Index for the Management of Extensive Occlusal Caries in a Pediatric Patient: A Case Report. J. Adhes. Dent. 2023, 25, 211–218. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Yang, X.; Gao, J.; Yu, H. Technique to restore the midline space of central incisors using a two-in-one template: A clinical report. J. Prosthodont. 2023, 32, 375–381. [Google Scholar] [CrossRef]
- Villafuerte, K.R.V.; Obeid, A.T.; de Oliveira, N.A. Injectable Resin Technique as a Restorative Alternative in a Patient with a Cleft Lip and Palate: A Case Report. Medicina 2023, 59, 849. [Google Scholar] [CrossRef]
- Healy, M. Injectable composites in modern practice. J. Ir. Dent. Assoc. 2023, 69, 197–198. [Google Scholar] [CrossRef]
- Watanabe, K.; Tichy, A.; Kamoi, K.; Hiasa, M.; Yonekura, K.; Tanaka, E.; Nakajima, M.; Hosaka, K. Restoration of a Microdont Using the Resin Composite Injection Technique with a Fully Digital Workflow: A Flexible 3D-printed Index with a Stabilization Holder. Oper. Dent. 2023, 48, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.; Wu, J.; Luo, T.; Sun, M.; Yu, H. Three-dimensionally printed template with an interproximal isolation design guide for consecutive closure of multiple diastema with injectable resin composite. J. Esthet. Restor. Dent. 2024, 36, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Rafeie, N.; Sampaio, C.S.; Hirata, R. Transitioning from injectable resin composite restorations to resin composite CAD/CAM veneers: A clinical report. J. Esthet. Restor. Dent. 2024, 36, 1221–1227. [Google Scholar] [CrossRef]
- Muslimah, D.F.; Hasegawa, Y.; Antonin, T.; Richard, F.; Hosaka, K. Composite Injection Technique with a Digital Workflow: A Pragmatic Approach for a Protruding Central Incisor Restoration. Cureus 2024, 16, e58712. [Google Scholar] [CrossRef]
- Branzan, R.; Taraboanta, I.; Tanasa, A.M.; Stoleriu, S.; Ghiorghe, A.C.; Pancu, G.; Georgescu, A.; Andra Taraboanta-Gamen, A.; Andrian, S. The use of flowable composite injection technique in a case of sever tooth wear. A case report. Int. J. Med. Dent. 2024, 28, 48–54. [Google Scholar]
- Watanabe, K.; Tanaka, E.; Kamoi, K.; Tichy, A.; Shiba, T.; Yonerakura, K.; Nakajima, M.; Han, R.; Hosaka, K. A dual composite resin injection molding technique with 3D-printed flexible indices for biomimetic replacement of a missing mandibular lateral incisor. J. Prosthodont. Res. 2024, 68, 667–671. [Google Scholar] [CrossRef]
- Rathod, P.; Patel, A.; Mankar, N.; Chandak, M.; Ikhar, A. Enhancing Aesthetics and Functionality of the Teeth Using Injectable Composite Resin Technique. Cureus 2024, 16, e59974. [Google Scholar] [CrossRef]
- Alyahya, Y.; Alrebdi, A.; Farah, R.I.; Albazei, S.S.F. Esthetic Rehabilitation of Congenitally Peg-Shaped Lateral Incisors Using the Injectable Composite Resin Technique: A Clinical Report. J. Pharm. Bioallied Sci. 2024, 16, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, J.; Anniwaer, A.; Huang, C. Esthetic rehabilitation of labial tooth defects caused by caries of the anterior teeth using a composite resin injection technique with veneer-shaped 3D printing indices. J. Prosthodont. Res. 2025, 69, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, D.; Valeri, C.; Quinzi, V.; Schneider Moser, U.; Marzo, G. Advancing Orthodontic Aesthetics: Exploring the Potential of Injectable Composite Resin Techniques for Enhanced Smile Transformations. Dent. J. 2025, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The revised JBI critical appraisal tool for the assessment of risk of bias quasi-experimental studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef]
- Nair, S.R.; Niranjan, N.T.; Jayasheel, A.; Suryakanth, D.B. Comparative Evaluation of Colour Stability and Surface Hardness of Methacrylate Based Flowable and Packable Composite -In vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC51–ZC54. [Google Scholar] [CrossRef]
- Korkut, B.; Haciali, C. Color Stability of Flowable Composites in Different Viscosities. Clin. Exp. Health Sci. 2020, 10, 191–198. [Google Scholar] [CrossRef]
- Degirmenci, A.; Degirmenci, B.U.; Salameh, M. Long-Term Effect of Acidic Beverages on Dental Injectable Composite Resin: Microhardness, Surface Roughness, Elastic Modulus, and Flexural Strength Patterns. Strength. Mater. 2022, 54, 331–343. [Google Scholar] [CrossRef]
- Uctasli, M.; Garoushi, S.; Uctasli, M.; Vallittu, P.K.; Lassila, L. A comparative assessment of color stability among various commercial resin composites. BMC Oral. Health 2023, 23, 789. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Nassar, M.; Elsayed, M.A.; Jameel, D.B.; Ahmad, T.T.; Rahman, M.M. In Vitro Optical and Physical Stability of Resin Composite Materials with Different Filler Characteristics. Polymers 2023, 15, 2121. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, A.; Pehlivan, I.E.; Degirmenci, B.U. Effects of polishing procedures on optical parameters and surface roughness of composite resins with different viscosities. Dent. Mater. J. 2023, 42, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Turk, S.; Erden Kayalidere, E.; Celik, E.U.; Yasa, B. In vitro wear resistance of conventional and flowable composites containing various filler types after thermomechanical loading. J. Esthet. Restor. Dent. 2024, 36, 643–651. [Google Scholar] [CrossRef]
- Tüter Bayraktar, E.; Kızıl Öztürk, E.; Saygılı, C.C.; Türkmen, C.; Korkut, B. Fluorescence and color adjustment potentials of paste-type and flowable resin composites in cervical restorations. Clin. Oral. Investig. 2024, 28, 649. [Google Scholar] [CrossRef]
- Gerges, P.; Labib, M.; Nabih, S.; Moussa, M. Fracture resistance of injectable resin composite versus packable resin composite in class II cavities: An in vitro study. J. Stomatol. 2024, 77, 153–160. [Google Scholar] [CrossRef]
- Checchi, V.; Generali, L.; Corciolani, L.; Breschi, L.; Mazzitelli, C.; Maravic, T. Wear and roughness analysis of two highly filled flowable composites. Odontology 2024, 113, 724–733. [Google Scholar] [CrossRef]
- Basheer, R.R.; Hasanain, F.A.; Abuelenain, D.A. Evaluating flexure properties, hardness, roughness and microleakage of high-strength injectable dental composite: An in vitro study. BMC Oral. Health 2024, 24, 546. [Google Scholar] [CrossRef]
- Rajabi, H.; Denny, M.; Karagiannopoulos, K.; Petridis, H. Comparison of Flexural Strength and Wear of Injectable, Flowable and Paste Composite Resins. Materials 2024, 17, 4749. [Google Scholar] [CrossRef]
- Francois, P.; Attal, J.P.; Fasham, T.; Troizier-Cheyne, M.; Gouze, H.; Abdel-Gawad, S.; Le Goff, S.; Dursun, E.; Ceinos, R. Flexural Properties, Wear Resistance, and Microstructural Analysis of Highly Filled Flowable Resin Composites. Oper. Dent. 2024, 49, 597–607. [Google Scholar] [CrossRef]
- Jrady, A.; Ragab, H.; Algahtani, F.N.; Osman, E. In vitro study on the impact of various polishing systems and coffee staining on the color stability of bleach-shaded resin composite. BMC Oral. Health 2024, 24, 712. [Google Scholar] [CrossRef]
- Lai, G.; Zhao, L.; Wang, J.; Kunzelmann, K.H. Surface properties and color stability of dental flowable composites influenced by simulated toothbrushing. Dent. Mater. J. 2018, 37, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsujimoto, A.; Barkmeier, W.W.; Jurado, C.A.; Villalobos-Tinoco, J.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Comparison of occlusal wear between bulk-fill and conventional flowable resin composites. Am. J. Dent. 2020, 33, 74–78. [Google Scholar] [PubMed]
- Shimatani, Y.; Tsujimoto, A.; Barkmeier, W.W.; Fischer, N.G.; Nagura, Y.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Simulated Cuspal Deflection and Flexural Properties of Bulk-Fill and Conventional Flowable Resin Composites. Oper. Dent. 2020, 45, 537–546. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Irie, M.; Teixeira, E.C.N.; Jurado, C.A.; Maruo, Y.; Nishigawa, G.; Matsumoto, T.; Garcia-Godoy, F. Relationships between Flexural and Bonding Properties, Marginal Adaptation, and Polymerization Shrinkage in Flowable Composite Restorations for Dental Application. Polymers 2021, 13, 2613. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Lucchi, P.; Zambon, G.; Pezzato, L.; Bertolini, R.; Zerman, N.; Stellini, E.; Mazzoleni, S. Depth of Cure, Hardness, Roughness and Filler Dimension of Bulk-Fill Flowable, Conventional Flowable and High-Strength Universal Injectable Composites: An In Vitro Study. Nanomaterials 2022, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Elsahn, N.A.; El-Damanhoury, H.M.; Shirazi, Z.; Saleh, A.R.M. Surface Properties and Wear Resistance of Injectable and Computer-Aided Design/Computer Aided Manufacturing-Milled Resin Composite Thin Occlusal Veneers. Eur. J. Dent. 2023, 17, 663–672. [Google Scholar] [CrossRef]
- Elgammal, Y.A.; Temirek, M.M.; Hassanein, O.E.; Abdelaziz, M.M. The Effect of Different Finishing and Polishing Systems on Surface Properties of New Flowable Bulk-fill Resin Composite. J. Contemp. Dent. Pract. 2023, 24, 587–594. [Google Scholar] [CrossRef]
- Vulović, S.; Stašić, J.N.; Ilić, J.; Todorović, M.; Jevremović, D.; Milić-Lemić, A. Effect of different finishing and polishing procedures on surface roughness and microbial adhesion on highly-filled composites for injectable mold technique. J. Esthet. Restor. Dent. 2023, 35, 917–926. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Xu, M.; Zhou, T.; Loh, Y.M.; Wang, C.; Pow, E.H.N.; Tsoi, J.K.H. The mechanical, wear, antibacterial properties and biocompatibility of injectable restorative materials under wet challenge. J. Dent. 2024, 146, 105025. [Google Scholar] [CrossRef]
- Bai, X.; Chen, Y.; Zhou, T.; Pow, E.H.N.; Tsoi, J.K.H. The chemical and optical stability evaluation of injectable restorative materials under wet challenge. J. Dent. 2024, 146, 105031. [Google Scholar] [CrossRef] [PubMed]
- Miyashita-Kobayashi, A.; Haruyama, A.; Nakamura, K.; Wu, C.-Y.; Kuroiwa, A.; Yoshinari, N.; Kameyama, A. Changes in Gloss Alteration, Surface Roughness, and Color of Direct Dental Restorative Materials after Professional Dental Prophylaxis. J. Funct. Biomater. 2024, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Vulović, S.; Blatz, M.B.; Bukorović, J.; Živković, N.; Todorović, A.; Vencl, A.; Milić Lemić, A. Effect of acidic media on surface characteristics of highly filled flowable resin-based composites: An in vitro study. J. Esthet. Restor. Dent. 2024, 37, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kitasako, Y.; Sadr, A.; Burrow, M.F.; Tagami, J. Thirty-six month clinical evaluation of a highly filled flowable composite for direct posterior restorations. Aust. Dent. J. 2016, 61, 366–373. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Hua, L.; Guan, R.; Hou, B. Randomized controlled clinical trial of a highly filled flowable composite in non-carious cervical lesions: 3-year results. Clin. Oral. Investig. 2021, 25, 5955–5965. [Google Scholar] [CrossRef]
- Elderiny, H.M.; Khallaf, Y.S.; Akah, M.M.; Hassanein, O.E. Clinical Evaluation of Bioactive Injectable Resin Composite vs Conventional Nanohybrid Composite in Posterior Restorations: An 18-Month Randomized Controlled Clinical Trial. J. Contemp. Dent. Pract. 2024, 25, 794–802. [Google Scholar] [CrossRef]
- Hançer Sarıca, S.; Arslan, S.; Balkaya, H. Comparison of the 2-year clinical performances of class II restorations using different restorative materials. Clin. Oral. Investig. 2025, 29, 128. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Sun, H.; Liu, Y.; Liu, W.; Su, B.; Li, S. The Development of Filler Morphology in Dental Resin Composites: A Review. Materials 2021, 14, 5612. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Yang, J.; Chen, H.; Jiang, X. Micromechanical interlocking structure at the filler/resin interface for dental composites: A review. Int. J. Oral. Sci. 2023, 15, 21. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.C.; Hur, B.; Park, J.K. Surface roughness and color stability of various composite resins. J. Korean Acad. Conserv. Dent. 2007, 32, 542–549. [Google Scholar] [CrossRef]
- Niyomsujarit, N.; Worahan, A.; Chaichalothorn, M. Effects of cyclic acid challenge on the surface roughness of various flowable resin composites. M. Dent. J. 2021, 41, 187–196. [Google Scholar]
- Draughn, R.A.; Harrison, A. Relationship between abrasive wear and microstructure of composite resins. J. Prosthet. Dent. 1978, 40, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Marghalani, H.Y. Effect of filler particles on surface roughness of experimental composite series. J. Appl. Oral. Sci. 2010, 18, 59–67. [Google Scholar] [CrossRef]
- Abuelenain, D.A.; Neel, E.A.A.; Al-Dharrab, A. Surface and mechanical properties of different dental composites. Austin J. Dent. 2015, 2, 1019. [Google Scholar]
- Beautifil Flow Plus: Safety Data Sheet. Available online: https://www.shofu.com/wp-content/uploads/Beautifil-Flow-Plus-SDS-US-Version-11.pdf (accessed on 5 September 2024).
- Filtek Z350XT. Technical Product Guide. Available online: https://multimedia.3m.com/mws/media/1363105O/3m-filtek-z350-xt-universal-restorative-tpp-la-apac.pdf (accessed on 15 February 2025).
- Oivanen, M.; Keulemans, F.; Garoushi, S.; Vallittu, P.K.; Lassila, L. The effect of refractive index of fillers and polymer matrix on translucency and color matching of dental resin composite. Biomater. Investig. Dent. 2021, 8, 48–53. [Google Scholar] [CrossRef]
- Yu, B.; Lee, Y.K. Differences in color, translucency and fluorescence between flowable and universal resin composites. J. Dent. 2008, 36, 840–846. [Google Scholar] [CrossRef]
- Paolone, G.; Baldani, S.; De Masi, N.; Mandurino, M.; Collivasone, G.; Scotti, N.; Gherlone, E.; Cantatore, G. Translucency of bulk-fill composite materials: A systematic review. J. Esthet. Restor. Dent. 2024, 36, 995–1009. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, B.S.; Rhee, S.H.; Yang, H.C.; Powers, J.M. Color and translucency of A2 shade resin composites after curing, polishing and thermocycling. Oper. Dent. 2005, 30, 436–442. [Google Scholar]
- Soliman, H.A.N.; Elkholany, N.R.; Hamama, H.H.; El-Sharkawy, F.M.; Mahmoud, S.H.; Comisi, J.C. Effect of Different Polishing Systems on the Surface Roughness and Gloss of Novel Nanohybrid Resin Composites. Eur. J. Dent. 2021, 15, 259–265. [Google Scholar] [CrossRef]
- Jefferies, S.R. Abrasive finishing and polishing in restorative dentistry: A state-of-the-art review. Dent. Clin. North. Am. 2007, 51, 379–397. [Google Scholar] [CrossRef]
- Karadas, M. The effect of different beverages on the color and translucency of flowable composites. Scanning 2016, 38, 701–709. [Google Scholar] [CrossRef]
- Kim, K.H.; Ong, J.L.; Okuno, O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. J. Prosthet. Dent. 2002, 87, 642–649. [Google Scholar] [CrossRef]
- Lee, Y.K. Influence of filler on the difference between the transmitted and reflected colors of experimental resin composites. Dent. Mater. 2008, 24, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Cidreira Boaro, L.C.; Pereira Lopes, D.; de Souza, A.S.C.; Lie Nakano, E.; Ayala Perez, M.D.; Pfeifer, C.S.; Gonçalves, F. Clinical performance and chemical-physical properties of bulk fill composites resin—A systematic review and meta-analysis. Dent. Mater. 2019, 35, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yang, S.M.; Xu, Y.X.; Wang, X.Y. Surface roughness and gloss alteration of polished resin composites with various filler types after simulated toothbrush abrasion. J. Dent. Sci. 2023, 18, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, P.; Reizer, R.; Wieczorowski, M. Functional Importance of Surface Texture Parameters. Materials 2021, 14, 5326. [Google Scholar] [CrossRef]
- Chen, F.; Sun, L.; Luo, H.; Yu, P.; Lin, J. Influence of filler types on wear and surface hardness of composite resin restorations. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231193524. [Google Scholar] [CrossRef]
- Turssi, C.P.; Ferracane, J.L.; Ferracane, L.L. Wear and fatigue behavior of nano-structured dental resin composites. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 196–203. [Google Scholar] [CrossRef]
- Finlay, N.; Hahnel, S.; Dowling, A.H.; Fleming, G.J.P. The in vitro wear behavior of experimental resin-based composites derived from a commercial formulation. Dent. Mater. 2013, 29, 365–374. [Google Scholar] [CrossRef]
- Osiewicz, M.A.; Werner, A.; Roeters, F.J.M.; Kleverlaan, C.J. Wear of direct resin composites and teeth: Considerations for oral rehabilitation. EurJ Oral. Sci. 2019, 127, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Reichl, F.X.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods–A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Cerasmart. Instructions for Use. Available online: https://www.gc.dental/america/sites/america.gc.dental/files/products/downloads/cerasmart/ifu/cerasmart-ifu.pdf (accessed on 15 February 2025).
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- McCabe, J.F.; Rusby, S. Water absorption, dimensional change and radial pressure in resin matrix dental restorative materials. Biomaterials. 2004, 25, 4001–4007. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater. 2008, 24, 737–743. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part. B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Gajewski, V.E.S. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Kusuma Yulianto, H.D.; Rinastiti, M.; Cune, M.S.; de Haan-Visser, W.; Atema Smit, J.; Busscher, H.J.; van der Mei, H.C. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. 2019, 35, 740–750. [Google Scholar] [CrossRef]
- Imazato, S.; Tarumi, H.; Kato, S.; Ebi, N.; Ehara, A.; Ebisu, S. Water Sorption, Degree of Conversion, and Hydrophobicity Resins containing Bis-GMA and TEGDMA. Dent. Mater. J. 1999, 19, 124–132. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Kopacz, K.; Szynkowska-Jozwik, M.I.; Sokolowski, J.; Bociong, K. An Evaluation of the Hydrolytic Stability of Selected Experimental Dental Matrices and Composites. Materials 2022, 15, 5055. [Google Scholar] [CrossRef]
- Goņalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral. Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Huang, W.; Ren, L.; Cheng, Y.; Xu, M.; Luo, W.; Zhan, D.; Sano, H.; Fu, J. Evaluation of the Color Stability, Water Sorption, and Solubility of Current Resin Composites. Materials 2022, 15, 6710. [Google Scholar] [CrossRef]
- Prott, L.S.; Carrasco-Labra, A.; Gierthmuehlen, P.C.; Blatz, M.B. How to Conduct and Publish Systematic Reviews and Meta-Analyses in Dentistry. J. Esthet. Restor. Dent. 2025, 37, 14–27. [Google Scholar] [CrossRef]
- Pimentel, E.S.; França, F.M.G.; Turssi, C.P.; Basting, R.T.; Vieira-Junior, W.F. Effects of in vitro erosion on surface texture, microhardness, and color stability of resin composite with S-PRG fillers. Clin. Oral. Investig. 2023, 27, 3545–3556. [Google Scholar] [CrossRef]
- McKeever, L. Overview of Study Designs: A Deep Dive Into Research Quality Assessment. Nutr. Clin. Pract. 2021, 36, 569–585. [Google Scholar] [CrossRef]
- Wallace, S.S.; Barak, G.; Truong, G.; Parker, M.W. Hierarchy of Evidence Within the Medical Literature. Hosp. Pediatr. 2022, 12, 745–750. [Google Scholar] [CrossRef]
- Fleming, P.S.; Lynch, C.D.; Pandis, N. Randomized controlled trials in dentistry: Common pitfalls and how to avoid them. J. Dent. 2014, 42, 908–914. [Google Scholar] [CrossRef]
- Moraes, R.R.; Cenci, M.S.; Schneider, L.F.J. Clinical Longevity of Direct Resin Composite Restorations. In Dental Composite Materials for Direct Restorations, 1st ed.; Miletik, V., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 269–288. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).