Study on the High-Temperature Reaction Kinetics of Solid Waste-Based High Belite Sulphoaluminate Cement Containing Residual Gypsum in Clinker
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Experimental Scheme
2.3. Cement Preparation Process
2.4. Test Methods
- (1)
- Loss on ignition: The determination method refers to the combustion difference method in the “Methods for Chemical Analysis of Cement” (GB/T 176-2008, China) [27].
- (2)
- Chemical composition: A Model 1800 X-ray fluorescence spectrometer from Shimadzu, Kyoto, Japan, was utilized to analyze. The testing parameters included an Rh target X-ray tube (the voltage and current of the tube are 40 kV and 80 mA), a 4 kW thin window, and a scanning speed of 300°/min.
- (3)
- Mineral composition: A D8 Advance X-ray diffractometer from Bruker, Karlsruhe, Germany, was utilized to analyze. The testing parameters included the following: a Cu target; Kα ray; voltage and current of the tube at 40 kV and 40 mA; qualitative and quantitative analyses with residence times of 0.05 s and 0.5 s; a scanning range of 5° to 60° for the 2θ angle; and a step width of 0.02°. The quantitative analysis of clinker minerals was conducted using FullProf 2020.6 software. The crystallographic data of the relevant minerals applied in Rietveld refinement are presented below: PDF#88-0812 for C5S2, PDF#71-0969 for C4A3, PDF#86-0398 for β-C2S, PDF#99-0010 for CaSO4, and PDF#77-0442 for TiO2.
3. Results and Discussion
3.1. Mineral Composition of Cement Clinker
3.1.1. Qualitative Analysis
3.1.2. Quantitative Analysis
3.2. High-Temperature Reaction Kinetics of C4A3
3.2.1. Conversion Rate of C4A3
3.2.2. Kinetic Model of the C4A3 Formation
3.2.3. Kinetic Parameters of C4A3
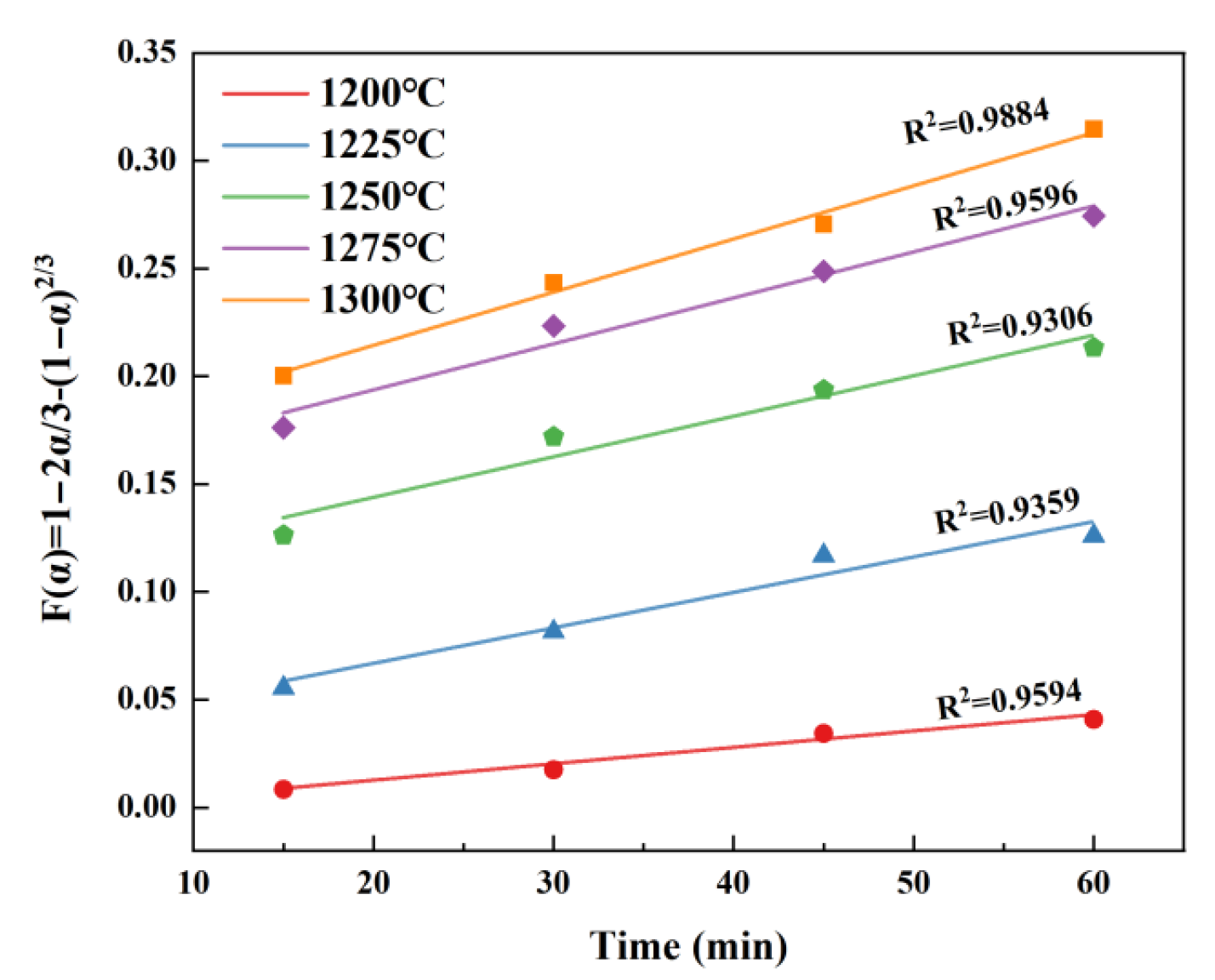
3.3. High-Temperature Reaction Kinetics of β-C2S
3.3.1. Conversion Rate of β-C2S
3.3.2. Kinetic Model of the β-C2S Formation
3.3.3. Kinetic Parameters of β-C2S
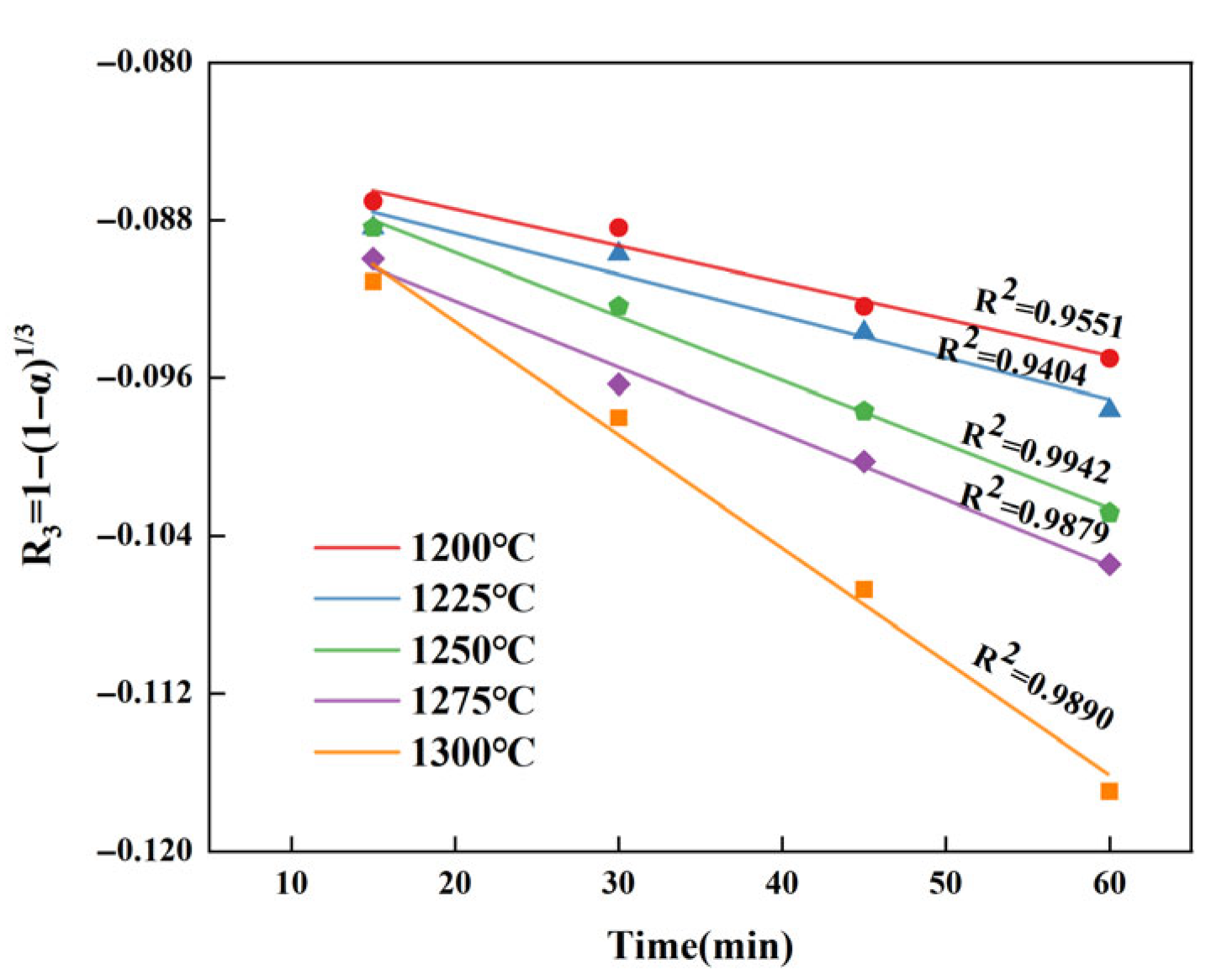
3.4. High-Temperature Reaction Kinetics of CaSO4
3.4.1. Conversion Rate of CaSO4
3.4.2. Kinetic Model of the CaSO4 Formation
3.4.3. Kinetic Parameters of CaSO4
4. Conclusions
- (1)
- For NHBASC, the C4A3 and β-C2S content increases with prolonged holding times and higher calcination temperatures, while CaSO4 continues to be consumed. Within the temperature range of 1200–1300 °C, the conversion rates of C4A3 and β-C2S both increase with the increase in calcination temperature and the prolongation of holding time, but the conversion rate of β-C2S decreases when the temperature is too high. Overall, the conversion rates of β-C2S are higher than that of C4A3, indicating that β-C2S reacts more thoroughly within this temperature range. The above mineral reaction laws provide a certain basis for optimizing the calcination system, enabling precise design of the clinker mineral composition through the synergistic adjustment of calcination temperature and holding time.
- (2)
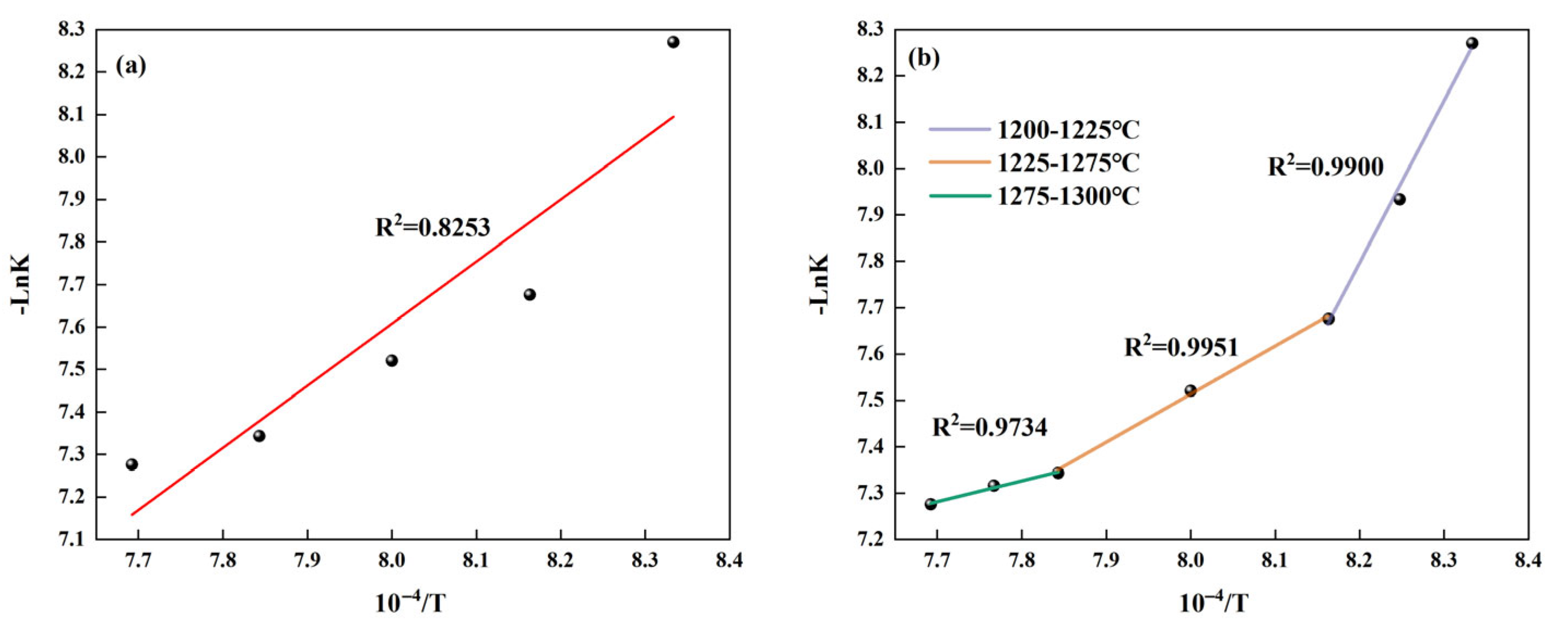
- The formation of C4A3 and β-C2S is influenced by diffusion mechanisms, and they both satisfy the Glinstling equation. The activation energy required for mineral formation varies in different temperature ranges. The activation energies required to form C4A3 at 1200–1225 °C, 1225–1275 °C, and 1275–1300 °C are 166.28 kJ/mol, 83.14 kJ/mol, and 36.58 kJ/mol, respectively. The activation energies required to form β-C2S at 1200–1225 °C and 1225–1300 °C are 374.13 kJ/mol and 66.51 kJ/mol, respectively. Overall, the activation energy required for mineral formation is relatively low, indicating that solid waste-based NHBSAC embodies the advantages of low carbon and energy saving, which meets the development needs of green building materials.
- (3)
- The consumption of CaSO4 is controlled by the interfacial chemical reaction mechanism, satisfying the R3 equation. By obtaining the reaction mechanism and kinetic parameters of CaSO4, it can be known that the reaction rates of CaSO4 increase with the increase in calcination temperature and the extension of holding time. Therefore, in the process of clinker preparation, the holding time should not be too long to avoid affecting the residual CaSO4 content in NHBSAC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| Abbreviation | Chemical Formula | Chemical Name | Mineral Name |
| 4CaO·2SiO2·CaSO4 | Calcium Sulfosilicate | Ternesite | |
| 3CaO·3Al2O3·CaSO4 | Calcium Sulphoaluminate | Ye’elimite | |
| C4AF | 4CaO·Al2O3·Fe2O3 | Tetracalcium Aluminoferrite | Brownmillerite |
| β-C2S | 2CaO·SiO2 | Dicalcium Silicate | Belite |
References
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Kanavaris, F.; Das, B.B.; Idrees, M. Decarbonising cement and concrete production: Strategies, challenges and pathways for sustainable development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Yan, C.; Liu, L.; Zhao, J.; Gao, J. Potential application of all-solid waste belite-ye’elimite cement in high early-strength pavement concrete: Strength formation, characterization, and benefit evaluation. Case Stud. Constr. Mater. 2025, 22, e04185. [Google Scholar] [CrossRef]
- Ige, O.E.; Olanrewaju, O.A.; Duffy, K.J.; Obiora, C. A review of the effectiveness of Life Cycle Assessment for gauging environmental impacts from cement production. J. Clean. Prod. 2021, 324, 129213. [Google Scholar] [CrossRef]
- Huo, G.; Jiang, X.; Sun, X.; Li, H.; Shi, H. Performance of high-belite calcium sulfoaluminate cement subjected to hydrochloric acid and sulfuric acid. Front. Mater. 2024, 10, 1282919. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Q.; Zhou, W.; Gong, X.; Chen, J.; Huang, C.; Ma, G.; Liu, X.; Chang, X. Effect of calcium sulfate type and dosage on the properties of high-belite sulfoaluminate cement. J. Sustain. Cem. Mater. 2024, 13, 829–840. [Google Scholar] [CrossRef]
- Yoon, H.; Seo, J.; Kim, S.; Lee, H.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, D.; Zhang, D.; Cui, J.; Wang, W.; Liu, L. Development of high-early-strength low-carbon engineered cementitious composites with calcium sulfoaluminate cement incorporating high-volume fly ash. Case Stud. Constr. Mater. 2023, 18, e01959. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Su, D.; Yue, G.; Li, Q.; Guo, Y.; Gao, S.; Wang, L. Research on the preparation and properties of high belite sulphoaluminate cement (HBSAC) based on various industrial solid wastes. Materials 2019, 12, 1510. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Ren, H.; Mao, R.; Wu, H.; Liang, X.; Zhou, J.; Zhang, Z. Preparation and properties of phosphogypsum-based calcined coal gangue composite cementitious materials. Case Stud. Constr. Mater. 2024, 21, e03963. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Telesca, A.; Matschei, T.; Marroccoli, M. Study of eco-friendly belite-calcium sulfoaluminate cements obtained from special wastes. Appl. Sci. 2020, 10, 8650. [Google Scholar] [CrossRef]
- Galluccio, S.; Beirau, T.; Pöllmann, H. Maximization of the reuse of industrial residues for the production of eco-friendly CSA-belite clinker. Constr. Build. Mater. 2019, 208, 250–257. [Google Scholar] [CrossRef]
- Li, F.W. Research of Preparing Sulphate Aluminium Cement Using Calcium and Aluminum Residue and Low Grade Bauxite. Ph.D. Thesis, Nanchang University, Nanchang, China, 2012. (In Chinese). [Google Scholar]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Li, H.; Agrawal, D.K.; Cheng, J.; Silsbee, M.R. Microwave sintering of sulphoaluminate cement with utility wastes. Cem. Concr. Res. 2001, 31, 1257–1261. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Ren, X.; Ye, J.; Zhang, J.; Qian, J. Formation, structure, and thermal stability evolution of ternesite based on a single-stage sintering process. Cem. Concr. Res. 2021, 147, 106519. [Google Scholar] [CrossRef]
- Ma, S.H.; Shen, X.D.; Huang, Y.P.; Zhong, B.Q. Preparation and formation mechanism of calcium sulphoaluminate. J. Chin. Ceram. Soc. 2008, 36, 78–81. (In Chinese) [Google Scholar]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of calcium sulfoaluminate formation from tricalcium aluminate, calcium sulfate and calcium oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Geng, Y.J. Study on Reaction Kinetics, Hydration Kinetics and Properties of Sulphoaluminate Cement Prepared from Petroleum Coke Desulfurization slag. Ph.D. Thesis, Qingdao University of Technology, Qingdao, China, 2018. (In Chinese). [Google Scholar]
- Xu, L.J.; Jian, M.F. Kinetic parameters determination of dicalcium silicate sintering process. J. Chin. Ceram. Soc. 2011, 30, 710–713. (In Chinese) [Google Scholar]
- Hao, X.Y.; Jian, M.F. Study on mathematical model of dicalcium silicate sintering process. Inorg. Chem. Ind. 2010, 42, 15–17. [Google Scholar]
- GB/T 176-2008; Methods for Chemical Analysis of Cement. Standards Press of China: Beijing, China, 2008.
- Canbek, O.; Erdoğan, S.T. Influence of production parameters on calcium sulfoaluminate cements. Constr. Build. Mater. 2020, 239, 117866. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Wang, K.; Shah, S.P. Research progress in advanced nanomechanical characterization of cement-based materials. Cem. Concr. Compos. 2018, 94, 277–295. [Google Scholar] [CrossRef]
- Berrio, A.; Tobón, J.I.; De la Torre, A. Kinetic model for ye’elimite polymorphs formation during clinkering production of CSA cement. Constr. Build. Mater. 2022, 321, 126336. [Google Scholar] [CrossRef]
- Huang, X.; Shi, F.; Wang, G.; Yu, J.; Ma, S.; Li, W. Kinetics and mechanism of ternesite formation from dicalcium silicate and calcium sulfate dihydrate. Materials 2022, 15, 2626. [Google Scholar] [CrossRef]
- Luciano, G.; Svoboda, R. Simulation and non-linear optimization of kinetic models for solid-state processes. Model. Simul. Mater. Sci. Eng. 2024, 32, 035014. [Google Scholar] [CrossRef]
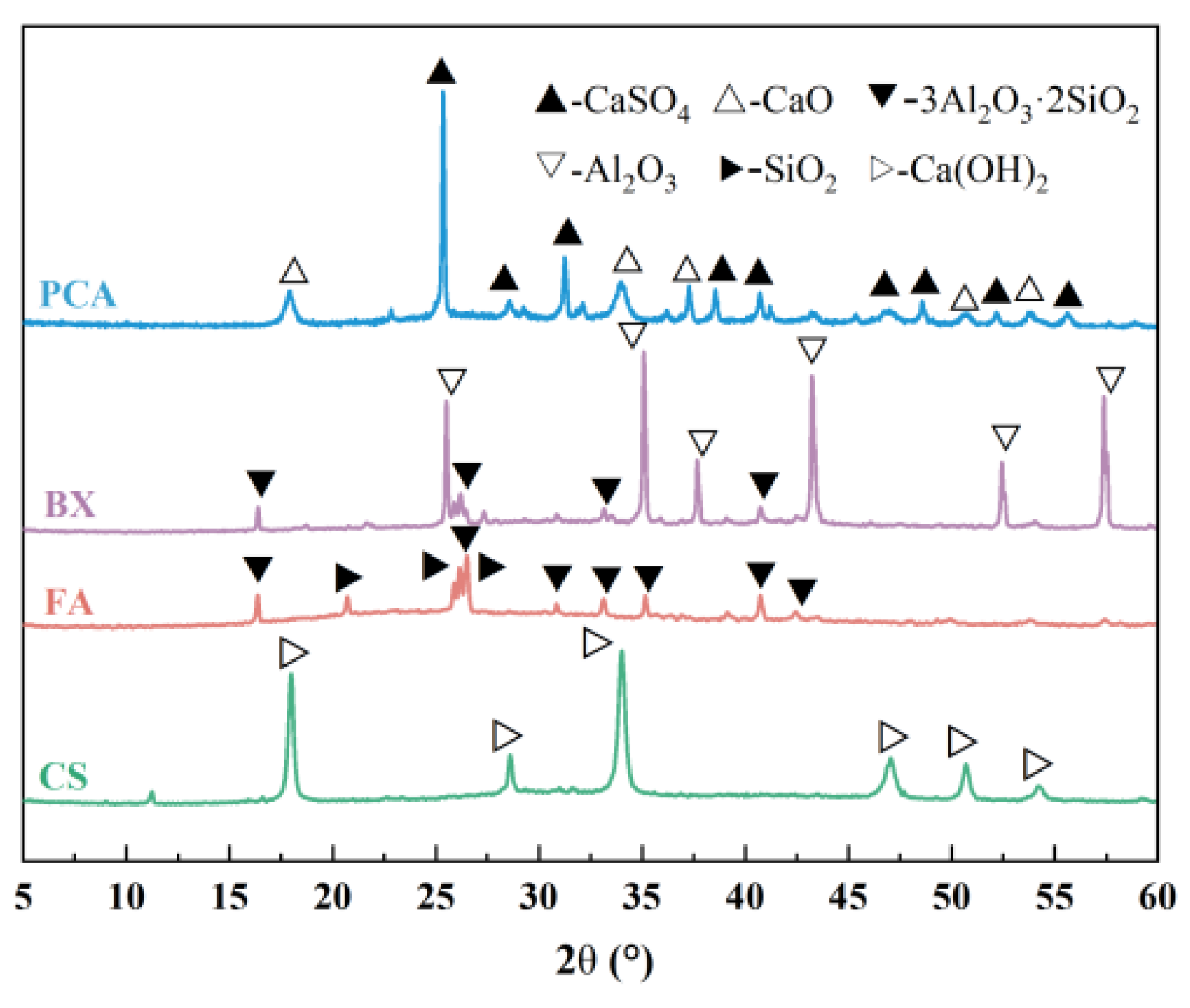
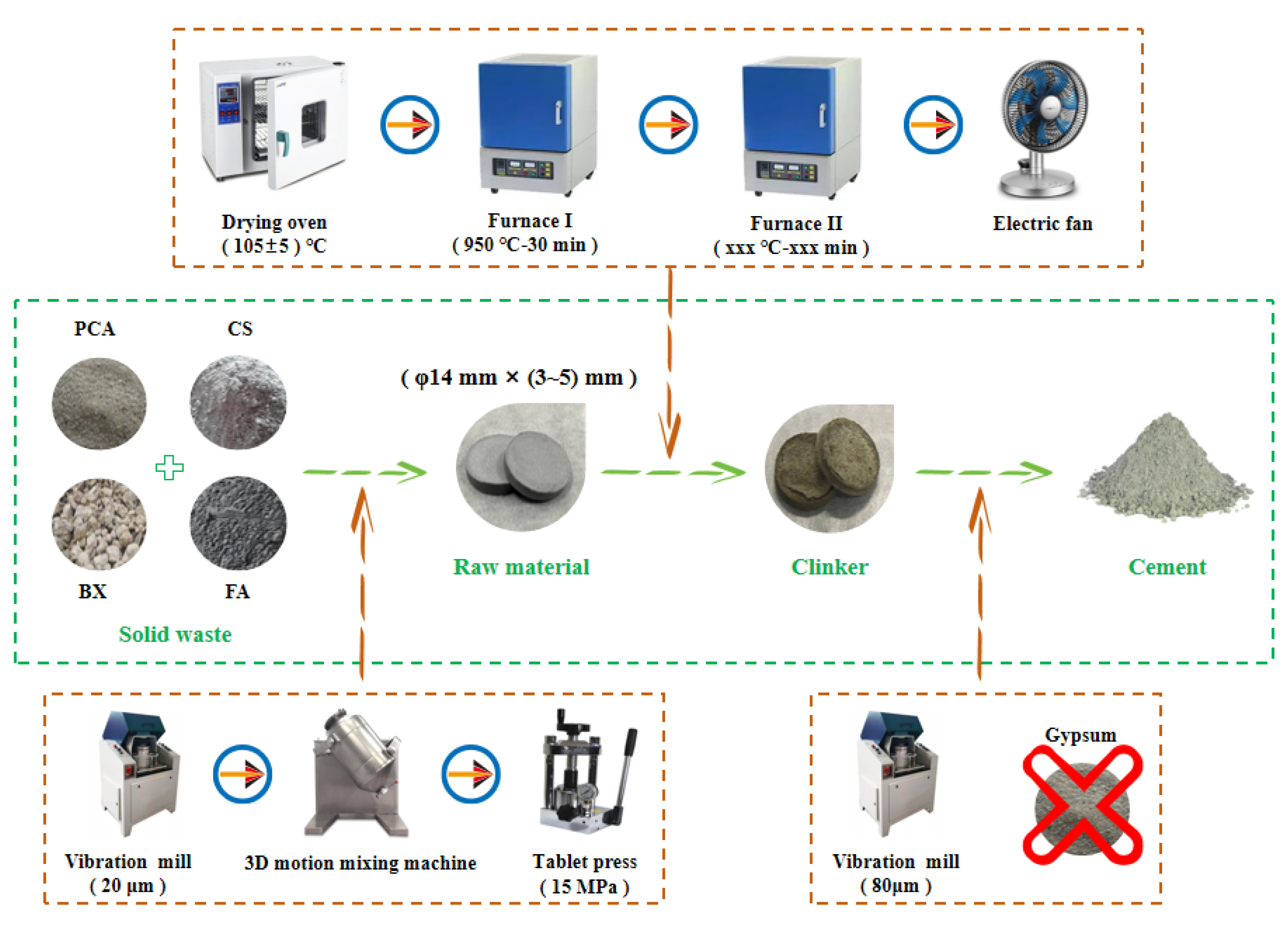

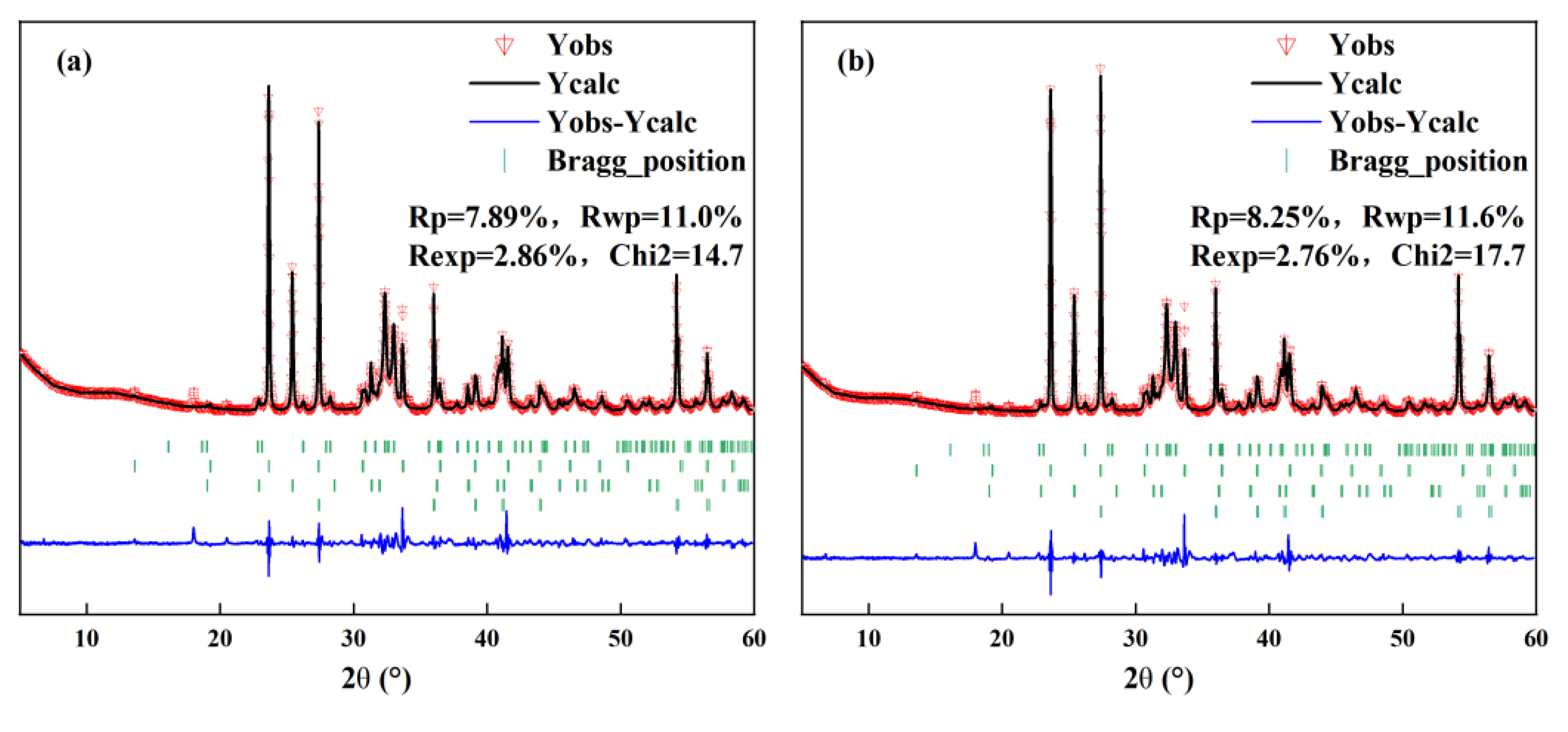
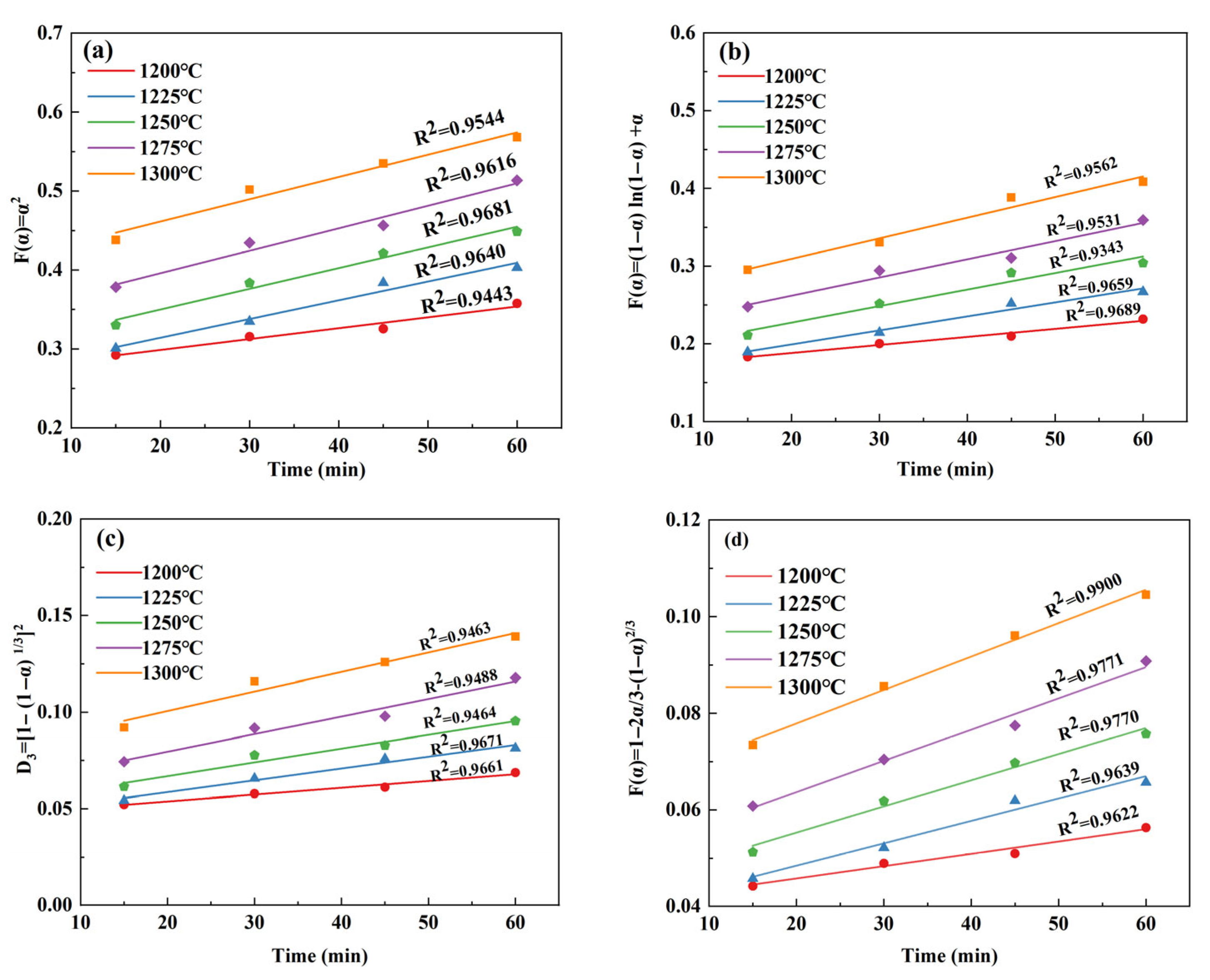

| Raw Material | CaO/% | Al2O3/% | SiO2/% | Fe2O3/% | SO3/% | MgO/% | TiO2/% | Others/% | LOI/% |
|---|---|---|---|---|---|---|---|---|---|
| PCA | 62.36 | 0.45 | 0.85 | 0.24 | 26.09 | 1.07 | 0.00 | 0.36 | 8.58 |
| FA | 3.44 | 30.37 | 52.17 | 5.64 | 0.79 | 0.73 | 1.22 | 3.24 | 2.40 |
| CS | 67.75 | 1.10 | 2.18 | 0.17 | 1.69 | 0.10 | 0.00 | 2.08 | 24.93 |
| BX | 1.07 | 75.09 | 14.83 | 1.53 | 0.18 | 0.48 | 4.50 | 1.62 | 0.70 |
| No. | Calcination Temperature/°C -Holding Time/min | Raw Material/% | |||
|---|---|---|---|---|---|
| PCA | FA | CS | BX | ||
| 1 # | 1200-15 | 45.55 | 16.78 | 28.36 | 9.31 |
| 2 # | 1200-30 | 44.17 | 17.39 | 27.83 | 10.61 |
| 3 # | 1200-45 | 43.92 | 17.50 | 27.21 | 11.37 |
| 4 # | 1200-60 | 43.74 | 17.64 | 26.54 | 12.08 |
| 5 # | 1225-15 | 44.66 | 16.95 | 28.90 | 9.49 |
| 6 # | 1225-30 | 44.41 | 17.21 | 27.71 | 10.67 |
| 7 # | 1225-45 | 44.27 | 17.36 | 26.91 | 11.46 |
| 8 # | 1225-60 | 44.30 | 17.41 | 26.28 | 12.01 |
| 9 # | 1250-15 | 44.87 | 16.81 | 28.91 | 9.41 |
| 10 # | 1250-30 | 44.82 | 16.98 | 27.67 | 10.53 |
| 11 # | 1250-45 | 44.97 | 17.04 | 26.58 | 11.41 |
| 12 # | 1250-60 | 45.24 | 17.01 | 25.85 | 11.90 |
| 13 # | 1275-15 | 47.58 | 16.13 | 27.10 | 9.19 |
| 14 # | 1275-30 | 47.41 | 16.18 | 25.89 | 10.52 |
| 15 # | 1275-45 | 47.32 | 16.26 | 25.04 | 11.38 |
| 16 # | 1275-60 | 47.32 | 16.26 | 24.39 | 12.03 |
| 17 # | 1300-15 | 49.92 | 15.55 | 25.51 | 9.02 |
| 18 # | 1300-30 | 49.61 | 15.60 | 24.49 | 10.30 |
| 19 # | 1300-45 | 49.46 | 15.55 | 23.64 | 11.35 |
| 20 # | 1300-60 | 49.14 | 15.55 | 23.17 | 12.13 |
| NO. | Actual Content of Clinker Minerals/% | NO. | Actual Content of Clinker Minerals/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β-C2S | CaSO4 | β-C2S | CaSO4 | ||||||
| 1 # | 18.92 | 12.22 | 14.35 | 18.39 | 11 # | 22.71 | 43.72 | 13.59 | -- |
| 2 # | 19.66 | 17.13 | 14.25 | 19.53 | 12 # | 23.44 | 44.67 | 13.21 | -- |
| 3 # | 20.06 | 23.04 | 13.97 | 21.96 | 13 # | 21.53 | 42.10 | 14.06 | -- |
| 4 # | 20.93 | 24.78 | 13.78 | 15.23 | 14 # | 22.80 | 44.68 | 13.68 | -- |
| 5 # | 19.19 | 28.10 | 14.25 | 10.32 | 15 # | 23.65 | 45.68 | 13.40 | -- |
| 6 # | 20.25 | 32.63 | 14.16 | 7.17 | 16 # | 25.08 | 46.46 | 13.02 | -- |
| 7 # | 21.69 | 37.10 | 13.87 | 5.79 | 17 # | 23.17 | 40.20 | 14.06 | -- |
| 8 # | 22.22 | 38.03 | 13.59 | 3.55 | 18 # | 24.55 | 41.21 | 13.78 | -- |
| 9 # | 20.11 | 38.07 | 14.25 | -- | 19 # | 25.60 | 42.39 | 12.92 | -- |
| 10 # | 21.67 | 41.82 | 13.97 | -- | 20 # | 26.38 | 43.77 | 12.16 | -- |
| Calcination Temperature/°C | Holding Time/min | |||
|---|---|---|---|---|
| 15 | 30 | 45 | 60 | |
| 1200 | 54.06 | 56.17 | 57.32 | 59.81 |
| 1225 | 54.84 | 57.87 | 61.96 | 63.49 |
| 1250 | 57.45 | 61.92 | 64.89 | 66.98 |
| 1275 | 61.51 | 65.15 | 67.56 | 71.66 |
| 1300 | 66.19 | 70.13 | 73.14 | 75.37 |
| Equation Number | Equation | Model | Control Mechanism |
|---|---|---|---|
| D1 | Flat model | Diffusion control | |
| D2 | Cylindrical model | ||
| D3 | Spherical model (Jander) | ||
| D4 | Spherical model (Ginstling) | ||
| R1 | Spherical model (first-order reaction) | Interface chemical reaction control | |
| R2 | Cylindrical model | ||
| R3 | Spherical model (zero-order reaction) | ||
| R4 | Flat model | ||
| A1 | -- | Nucleation growth control | |
| A2 | -- | ||
| A3 | -- |
| Equation | Temperature/°C | K |
|---|---|---|
| 1200 | 2.56 × 10−4 | |
| 1225 | 4.63 × 10−4 | |
| 1250 | 5.41 × 10−4 | |
| 1275 | 6.47 × 10−4 | |
| 1300 | 6.92 × 10−4 |
| Temperature Ranges/°C | 1200–1225 | 1225–1275 | 1275–1300 |
| Activation Energy/kJ/mol | 166.28 | 83.14 | 36.58 |
| Calcination Temperature/°C | Holding Time/min | |||
|---|---|---|---|---|
| 15 | 30 | 45 | 60 | |
| 1200 | 25.79 | 36.16 | 48.63 | 52.31 |
| 1225 | 59.33 | 68.89 | 78.32 | 80.28 |
| 1250 | 80.36 | 88.28 | 92.30 | 94.30 |
| 1275 | 88.87 | 94.32 | 96.43 | 98.07 |
| 1300 | 84.87 | 87.00 | 89.48 | 92.40 |
| Equation | Temperature/°C | K |
|---|---|---|
| 1200 | 7.62 × 10−4 | |
| 1225 | 1.65 × 10−3 | |
| 1250 | 1.88 × 10−3 | |
| 1275 | 2.13 × 10−3 | |
| 1300 | 2.47 × 10−3 |
| Temperature Ranges/°C | 1200–1225 | 1225–1275 |
| Activation Energy/kJ/mol | 374.13 | 66.51 |
| Calcination Temperature/°C | Holding Time/min | |||
|---|---|---|---|---|
| 15 | 30 | 45 | 60 | |
| 1200 | −28.45 | −28.93 | −30.35 | −31.30 |
| 1225 | −28.93 | −29.40 | −30.82 | −32.24 |
| 1250 | −28.93 | −30.35 | −32.26 | −34.14 |
| 1275 | −29.88 | −31.77 | −33.19 | −35.09 |
| 1300 | −29.90 | −31.30 | −35.56 | −39.35 |
| Equation | Temperature/°C | K |
|---|---|---|
| 1200 | −1.86 × 10−4 | |
| 1225 | −2.11 × 10−4 | |
| 1250 | −3.25 × 10−4 | |
| 1275 | −3.36 × 10−4 | |
| 1300 | −5.75 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Yang, M.; Hao, Y.; Wang, J.; Liu, X.; Tang, H.; Dong, F.; Xing, D.; Kong, W. Study on the High-Temperature Reaction Kinetics of Solid Waste-Based High Belite Sulphoaluminate Cement Containing Residual Gypsum in Clinker. Materials 2025, 18, 3369. https://doi.org/10.3390/ma18143369
Su D, Yang M, Hao Y, Wang J, Liu X, Tang H, Dong F, Xing D, Kong W. Study on the High-Temperature Reaction Kinetics of Solid Waste-Based High Belite Sulphoaluminate Cement Containing Residual Gypsum in Clinker. Materials. 2025; 18(14):3369. https://doi.org/10.3390/ma18143369
Chicago/Turabian StyleSu, Dunlei, Mingxin Yang, Yani Hao, Jiahui Wang, Xin Liu, Haojian Tang, Fengyuan Dong, Dejin Xing, and Weiyi Kong. 2025. "Study on the High-Temperature Reaction Kinetics of Solid Waste-Based High Belite Sulphoaluminate Cement Containing Residual Gypsum in Clinker" Materials 18, no. 14: 3369. https://doi.org/10.3390/ma18143369
APA StyleSu, D., Yang, M., Hao, Y., Wang, J., Liu, X., Tang, H., Dong, F., Xing, D., & Kong, W. (2025). Study on the High-Temperature Reaction Kinetics of Solid Waste-Based High Belite Sulphoaluminate Cement Containing Residual Gypsum in Clinker. Materials, 18(14), 3369. https://doi.org/10.3390/ma18143369






