Abstract
In order to elucidate the high-temperature reaction process of solid waste-based high belite sulphoaluminate cement containing residual gypsum in clinker (NHBSAC) and obtain the formation laws of each mineral in clinker, this article studied its high-temperature reaction kinetics. Through QXRD analysis and numerical fitting methods, the formation of C4A3, β-C2S, and CaSO4 in clinker under different calcination systems was quantitatively characterized, the corresponding high-temperature reaction kinetics models were established, and the reaction activation energies of each mineral were obtained. The results indicate that the content of C4A3 and β-C2S increases with the prolongation of holding time and the increase in calcination temperature, while CaSO4 is continuously consumed. Under the control mechanism of solid-state reaction, the formation and consumption of minerals follow the kinetic equation. C4A3 and β-C2S satisfy the D4 equation under diffusion mechanism control, and CaSO4 satisfies the R3 equation under interface chemical reaction mechanism control. The activation energy required for mineral formation varies with different temperature ranges. The activation energies required to form C4A3 at 1200–1225 °C, 1225–1275 °C, and 1275–1300 °C are 166.28 kJ/mol, 83.14 kJ/mol, and 36.58 kJ/mol, respectively. The activation energies required to form β-C2S at 1200–1225 °C and 1225–1300 °C are 374.13 kJ/mol and 66.51 kJ/mol, respectively. This study is beneficial for achieving flexible control of the mineral composition of NHBSAC clinker, providing a theoretical basis and practical experience for the preparation of low-carbon cement and the optimization design of its mineral composition.
1. Introduction
Cement is one of the basic raw materials widely used in the construction industry and plays an important role in social and economic development. Among them, Portland cement (OPC) and sulphoaluminate cement (SAC) are the most commonly used and technologically mature cement materials. However, the production process of OPC requires a large amount of mineral resources and causes certain environmental pollution, making the contradiction between energy, resources, and environment increasingly prominent [1,2,3,4]. Compared with OPC, the preparation of SAC significantly reduces the amount of limestone and CO2 emissions, but it requires substantial amounts of high-quality bauxite and gypsum resources [5,6,7]. In view of this, the research and development of low-carbon cement has become a hot topic [8,9].
High belite sulphoaluminate cement (HBSAC) is a low-carbon cement optimized from SAC, with a composition of 37–47% C4A3, 46–56% β-C2S, and 5–9% C4AF. Compared to SAC, its higher content of β-C2S further reduces the use of calcium, while its lower content of C4A3 reduces the use of aluminum and sulfur, allowing low-quality raw materials to be used for the preparation of HBSAC. At present, significant progress has been made in the study of HBSAC in the following areas: One is the feasibility study of using solid waste to prepare HBSAC. The solid waste materials that have been used to prepare HBSAC include aluminum silicate solid waste such as fly ash [10], tailings [11], coal gangue [12,13], and red mud [14], as well as calcium sulfur solid waste such as phosphogypsum [15], solid sulfur ash [16], and lithium mica slag [17]. The second is the study of the HBSAC calcination system. Under laboratory conditions, the calcination temperature of HBSAC is usually within the range of 1250–1350 °C, and the holding time is within the range of 20–60 min. Under industrial experimental conditions, the calcination system varies due to differences in production equipment and processes. The calcination temperature is generally around 1300 °C, while the holding time may differ due to differences in clinker production. During the study process, some experts and scholars have also reached some beneficial conclusions by improving the calcination system. Shen et al. [18] and Bullerjahn [19] successfully prepared HBSAC clinker containing C5S2 through the secondary calcination method. In this study, the “two-stage calcination” refers to reheating the clinker at 1100–1200 °C on the basis of the original calcination system. Li et al. [20] successfully prepared HBSAC using microwave sintering under a calcination system of 1150 °C for 10 min. The third aspect is the study of hydration properties. At present, the study conclusions on the hydration properties of HBSAC are basically consistent, with C4A3 hydration dominating in the early stage and β-C2S hydration dominating in the later stage. Due to the increased β-C2S content in the HBSAC clinker system, while ensuring rapid early strength development, it also solves the problem of slow later strength development in SAC.
In recent years, in order to understand the formation rules of HBSAC minerals, optimize their mineral composition, and ultimately improve their properties, experts and scholars have conducted in-depth exploration of HBSAC. Research has found that the high-temperature reaction processes of various minerals in clinkers often play a critical role in the final properties of clinkers. At present, research on mineral formation mainly adopts three methods. The first method is to qualitatively analyze the clinker minerals under different calcination systems to obtain their formation laws. Su et al. [11] used XRD analysis to obtain the formation rules of clinker minerals under different calcination systems and ultimately determined the temperature range for the formation of each mineral in the clinker. The second method is to analyze the BSE images of minerals under different calcination systems in order to understand the formation process of minerals at high temperatures. Liu et al. [21] studied the formation process and reaction mechanism of C5S2 by analyzing its BSE images under different calcination regimes. The results indicate that the formation process of C5S2 is roughly the reaction of SiO2 and CaO to generate CS (calcium silicate), which is further converted into C2S (dicalcium silicate) and combines with unconverted CS to form C5S2, and the entire process is controlled by a diffusion mechanism. The third method is to construct dynamic models of each mineral, analyze the kinetic parameters of each mineral, and then grasp its formation laws. In terms of study on C4A3, Ma et al. [22] successfully synthesized C4A3 single ore through the chemical reagent method and constructed its formation kinetics model. The results indicate that the formation process of C4A3 single ore is controlled by the interfacial chemical reaction mechanism, and the formation activation energy is 198.01 kJ/mol. It is worth noting that Li et al. [23] also obtained C4A3 single ore using the chemical reagent method but measured its formation activation energy to be 234 kJ/mol. Geng [24] successfully prepared C4A3 using solid waste as raw material. The results indicate that C4A3 is controlled by a diffusion mechanism and conforms to the Ginstling equation, with an activation energy of 85.63 kJ/mol. In terms of study on β-C2S, Xu et al. [25] investigated the high-temperature reaction kinetics of β-C2S, and the results showed that under the diffusion control mechanism, the formation process of β-C2S follows the D4 equation. This is consistent with the conclusion drawn by Hao [26] through numerical simulation to explore the reaction kinetics of β-C2S. In addition, Hao obtained an activation energy of 121.5 kJ/mol for β-C2S formation through simulation. However, current study results mainly focus on single minerals, and there is little study on the high-temperature reaction kinetics of multi-mineral synergistic systems in clinker.
Our team used industrial solid wastes such as petroleum coke ash (PCA), fly ash (FA), carbide slag (CS), and bauxite (BX) to prepare a high belite sulphoaluminate cement containing residual gypsum in clinker (NHBSAC). In order to further investigate the high-temperature reaction process of various minerals in clinker and reveal the formation laws of each mineral. We used methods such as QXRD analysis and numerical fitting to study the high-temperature reaction kinetics of various minerals in order to obtain the kinetic parameters of each mineral, clarify their formation laws under different calcination regimes, establish corresponding high-temperature reaction kinetics models, and provide a theoretical basis and practical experience for the preparation of low-carbon cement and the optimization design of its mineral composition.
2. Experimental
2.1. Experimental Materials
NHBSAC was prepared from solid wastes such as PCA, FA, CS, and BX. Among them, PCA was purchased from Sinopec Qingdao Petrochemical Co., Ltd., Qingdao, China; FA was purchased from Henan Jinrun New Materials Co., Ltd., Zhengzhou, China; CS was purchased from Qingdao Haiwan Chemical Co., Ltd., Qingdao, China; and BX was purchased from Henan Borun Casting Materials Co., Ltd., Gongyi, China. The chemical composition and mineral composition of the aforesaid raw materials are shown in Table 1 and Figure 1.

Table 1.
Main chemical composition of raw materials.
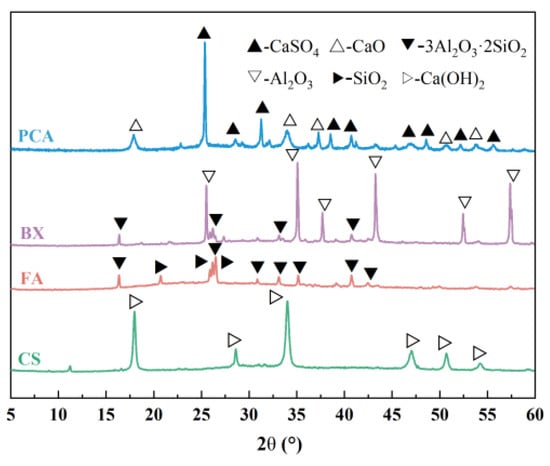
Figure 1.
XRD patterns of raw materials.
2.2. Experimental Scheme
In order to reveal the reaction laws of clinker minerals at high temperatures, the high-temperature reaction kinetics mechanisms of each mineral were explored. In this study, a total of 20 experimental schemes were designed, with calcination temperatures set at 1200 °C, 1225 °C, 1250 °C, 1275 °C, and 1300 °C and holding times set at 15 min, 30 min, 45 min, and 60 min, respectively. Each scheme was obtained by combining different holding times and calcination temperatures. Table 2 shows the design of the experimental scheme and the proportion of raw materials.

Table 2.
Experimental schemes for each group and the proportion of raw materials.
2.3. Cement Preparation Process
The preparation process of NHBSAC mainly follows the two grinding and one burning process, including grinding and molding, preheating and sintering, and cooling and regrinding, as shown in Figure 2. It is worth noting that this study adopted the “preheating and sintering” technology, which can not only more realistically simulate the industrial production process of “preheater → precalciner → rotary kiln” but also effectively improve the calcination efficiency of clinker.
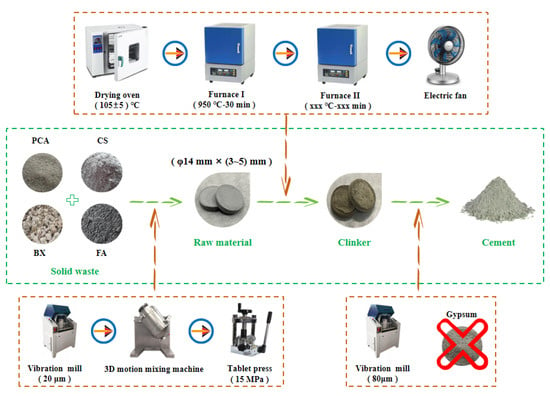
Figure 2.
Preparation process of NHBSAC.
2.4. Test Methods
- (1)
- Loss on ignition: The determination method refers to the combustion difference method in the “Methods for Chemical Analysis of Cement” (GB/T 176-2008, China) [27].
- (2)
- Chemical composition: A Model 1800 X-ray fluorescence spectrometer from Shimadzu, Kyoto, Japan, was utilized to analyze. The testing parameters included an Rh target X-ray tube (the voltage and current of the tube are 40 kV and 80 mA), a 4 kW thin window, and a scanning speed of 300°/min.
- (3)
- Mineral composition: A D8 Advance X-ray diffractometer from Bruker, Karlsruhe, Germany, was utilized to analyze. The testing parameters included the following: a Cu target; Kα ray; voltage and current of the tube at 40 kV and 40 mA; qualitative and quantitative analyses with residence times of 0.05 s and 0.5 s; a scanning range of 5° to 60° for the 2θ angle; and a step width of 0.02°. The quantitative analysis of clinker minerals was conducted using FullProf 2020.6 software. The crystallographic data of the relevant minerals applied in Rietveld refinement are presented below: PDF#88-0812 for C5S2, PDF#71-0969 for C4A3, PDF#86-0398 for β-C2S, PDF#99-0010 for CaSO4, and PDF#77-0442 for TiO2.
3. Results and Discussion
3.1. Mineral Composition of Cement Clinker
3.1.1. Qualitative Analysis
According to the experimental schemes designed in Table 2, clinkers were calcined and then subjected to qualitative analysis. The results are shown in Figure 3. From this figure, it can be seen that the clinker under all conditions contains the target minerals C4A3, β-C2S, and CaSO4. Furthermore, as the calcination temperature rises and the holding time prolongs, the content of C4A3 and β-C2S continues to increase, and the content of CaSO4 continues to decrease. However, it should be noticed that when the temperature is not higher than 1225 °C, regardless of the holding time, intermediate mineral C5S2 will appear in the clinker minerals. When the temperature is higher than 1225 °C, C5S2 will disappear.
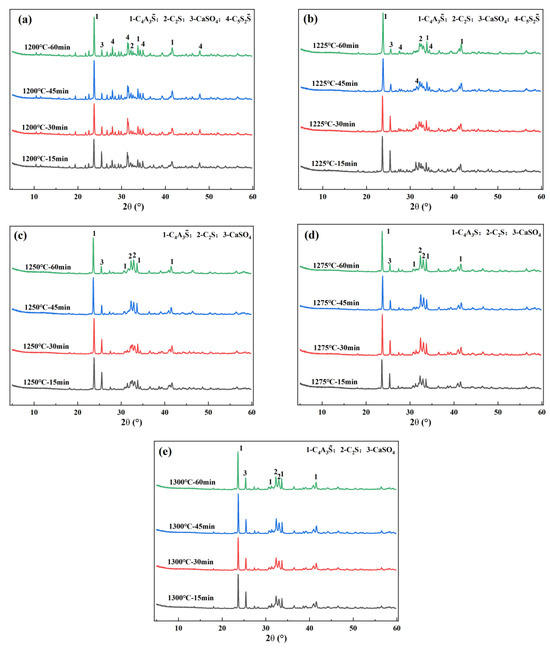
Figure 3.
XRD analysis of clinkers under different calcination systems: (a) 1200 °C; (b) 1225 °C; (c) 1250 °C; (d) 1275 °C; and (e) 1300 °C.
3.1.2. Quantitative Analysis
During the calcination process, amorphous phases will form in the clinker. Nevertheless, traditional crystal analysis techniques such as XRD are difficult to effectively characterize amorphous phases, which leads to deviations between the results of quantitative analysis and the actual values. In order to remove the impact of the amorphous phase, the internal standard method [28] was used for quantitative analysis. This method introduces a crystal with a known content that does not affect other minerals to assist analysis. In this study, TiO2 was adopted as the internal standard substance; its content in cement–TiO2 is 15%. The actual content of clinker minerals obtained through the internal standard method is shown in Table 3. The analysis results of the cement–TiO2 mixture under different calcination systems are shown in Figure 4. Due to limited space, only representative analysis results were presented.

Table 3.
Actual content of clinker minerals obtained by internal standard method analysis.
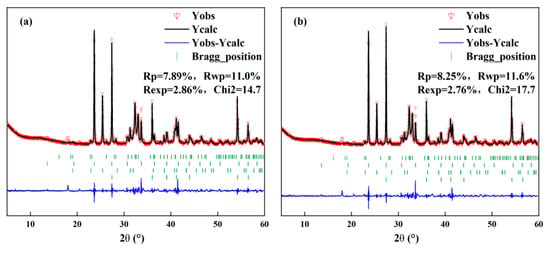
Figure 4.
Analysis result of cement–TiO2 mixture under different calcination systems: (a) 1300 °C for 15 min and (b) 1300 °C for 30 min.
3.2. High-Temperature Reaction Kinetics of C4A3
3.2.1. Conversion Rate of C4A3
The conversion rate of mineral refers to the degree of mineral formation in clinkers; it can be described by the ratio of the mineral’s actual content to its designed content [29], as shown in Equation (1).
where α—conversion rate (%); wn—the actual content of clinker mineral (%); and Wn—design content of clinker minerals (%).
As shown in Table 3, the content of C4A3 increases with the increase in calcination temperature and holding time. This is because the increase in calcination temperature and holding time is beneficial for promoting the reaction of CaSO4 with CaO and Al2O3, thereby accelerating the formation of C4A3. The formation process of C4A3 is shown in Equation (2).
In this study, the designed content of C4A3 is 35%. By using Equation (1) and the data in Table 3, the conversion rates of C4A3 under different calcination systems can be obtained, and the results are listed in Table 4.

Table 4.
Conversion rates of C4A3 under different calcination systems (%).
3.2.2. Kinetic Model of the C4A3 Formation
When preparing NHBSAC, the calcination temperature is relatively low and the liquid phase generated is less, so the high-temperature reaction is mainly a solid-state reaction. Table 5 shows several common control mechanisms and kinetic equations [30,31] of solid-state reactions. The kinetic equations in Table 5 can be represented by Equation (3).
where t—calcination time; α—reaction conversion rate (%); and K—reaction rate constant.

Table 5.
Control mechanisms and kinetic equations of solid-state reaction [30,31].
By substituting the conversion rate into the above kinetic equation and fitting the relationship between α and t, it can be clearly seen that the diffusion-controlled kinetic equation provides a more accurate description of the C4A3 formation. The reason for this might lie in the effect of Ca2+ diffusion on the process of mineral formation. The fitting relationship between α and t of C4A3 under different reaction models is shown in Figure 5. In Table 5, D4 is the most suitable kinetic equation; as demonstrated in Figure 5d, it provides a relatively good quantitative explanation for the formation of C4A3 under different calcination systems. Correspondingly, the sphere model (Ginstling) can represent the formation kinetic model of C4A3 [32]. This conclusion is consistent with Geng’s study on the preparation of C4A3 from solid waste [24], but different from Ma’s study [22], possibly because Ma’s study was based on pure chemical reagents.
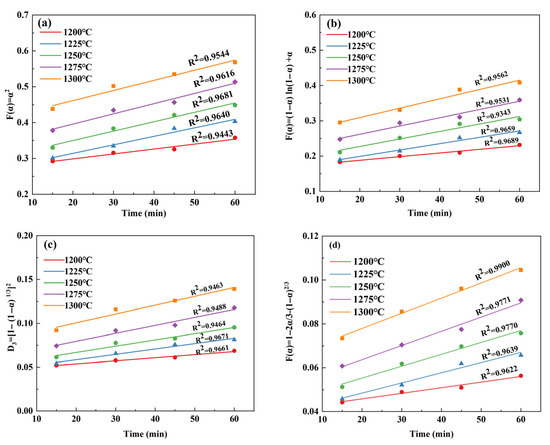
Figure 5.
Fitted curves of α and t for C4A3 at different reaction models: (a) flat model; (b) cylindrical model; (c) spherical model (Jander); and (d) spherical model (Ginstling).
3.2.3. Kinetic Parameters of C4A3
Activation energy refers to the energy barrier or threshold that needs to be overcome for a certain reaction to occur. The logarithmic equation of the Arrhenius formula can be used to calculate the activation energy required for cement clinker formation, as shown in Equation (4). The specific method is to establish a linear fitted relation for -LnK in relation to 1/T and calculate the slope of the fitting line.
where A—prefactor; K—reaction rate constant (s−1); R—ideal gas constant (8.314 × 10−3 kJ/mol); Ea—activation energy (kJ/mol); and T—reaction temperature (°C).
The reaction rate constants of C4A3 at different calcination temperatures are shown in Table 6, and they are the slopes of the respective lines in Figure 5. From Table 6, it can be seen that as the temperature continues to increase, the reaction rate constant shows an increasing trend.

Table 6.
Reaction rate constants of C4A3 under various temperatures.
Based on the data in Table 6, the linear fitted relation between -LnK and 1/T was established as shown in Figure 6a. As shown in this figure, the value of R2 is relatively low; it is not reasonable to directly use the formula to calculate the activation energy. Therefore, the temperature range should be divided into three sections, and their activation energies should be calculated separately. In addition, due to the limited number of data points in the ranges of 1200–1225 °C and 1275–1300 °C, we have added the -LnK values corresponding to the intermediate temperature points and made a fitting curve as shown in Figure 6b, and the results are shown in Table 7. From this table, it can be seen that as the temperature increases, the activation energy required for C4A3 formation continuously decreases and is significantly lower than that (198.01 kJ/mol, 234 kJ/mol) when Ma and Li prepared C4A3 using pure chemical reagents [22,23]. This result indicates that our study demonstrates significant advantages in the field of low-energy preparation.
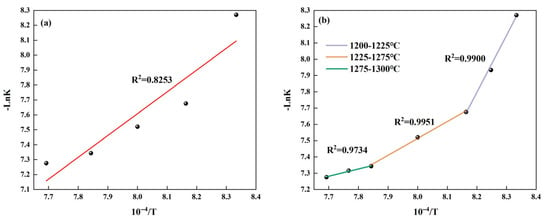
Figure 6.
The fitting relationship between -LnK and 1/T during the formation process of C4A3: (a) fitting relationship at 1200–1300 °C and (b) fitting relationship for different temperature ranges.

Table 7.
Activation energy of C4A3 formation in different temperature ranges.
3.3. High-Temperature Reaction Kinetics of β-C2S
3.3.1. Conversion Rate of β-C2S
As shown in Table 3, the content of β-C2S also increases with the increase in calcination temperature and the prolongation of holding time. During the reaction process, due to the relatively low formation temperature of β-C2S, it has not yet reached the level of liquid phase formation. Therefore, its formation process can be regarded as a solid-state reaction, and its formation process is shown in Equation (5).
In this study, the designed content of β-C2S is 45%. By using Equation (1) and the data in Table 3, the conversion rates of β-C2S under different calcination systems can be obtained, and the results are listed in Table 8.

Table 8.
Conversion rates of β-C2S under different calcination systems (%).
3.3.2. Kinetic Model of the β-C2S Formation
It is similar to that of C4A3. By substituting the conversion rate into the above kinetic equation and fitting the relationship between α and t, it can be clearly seen that the diffusion-controlled kinetic equation provides a more accurate description of the β-C2S formation, as shown in Figure 7. This finding aligns with the study findings of Xu [25] and Hao [26] regarding β-C2S.
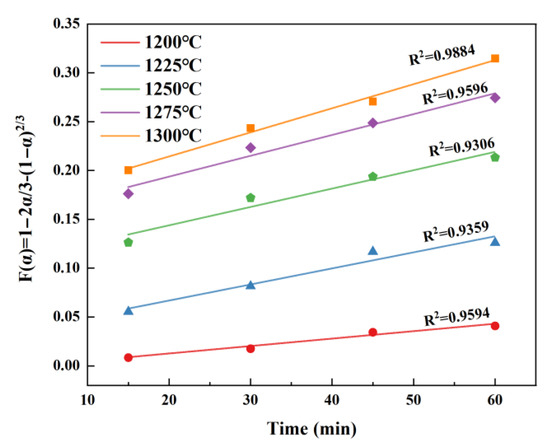
Figure 7.
Fitted curves of α and t for β-C2S at different temperatures.
3.3.3. Kinetic Parameters of β-C2S
The reaction rate constants of β-C2S at different calcination temperatures are shown in Table 9, and they are the slopes of the respective lines in Figure 7. From this table, it can be seen that as the temperature continues to increase, the reaction rate constant also shows an increasing trend.

Table 9.
Reaction rate constants of β-C2S under various temperatures.
Based on the data in Table 9, the linear fitted relation between -LnK and 1/T was established as shown in Figure 8a. As shown in this figure, the value of R2 is relatively low; it is not reasonable to directly use the formula to calculate the activation energy. Therefore, the temperature range should be divided into two sections, and their activation energies should be calculated separately. In addition, due to the limited number of data points in the range of 1200–1225 °C, we have added the -LnK values corresponding to the intermediate temperature points and made a fitting curve as shown in Figure 8b. The results are shown in Table 10. From this table, it can be seen that the activation energy is higher when the temperature range is 1200–1225 °C. This is because the relatively low temperature results in relatively small molecular kinetic energy, thus requiring a higher activation energy. When the temperature gradually increases to the range of 1225–1300 °C, its activation energy is significantly lower than the activation energy (121.5 kJ/mol) obtained by Hao using numerical simulation [26]. This demonstrates that our study holds an edge in low-energy preparation methods.
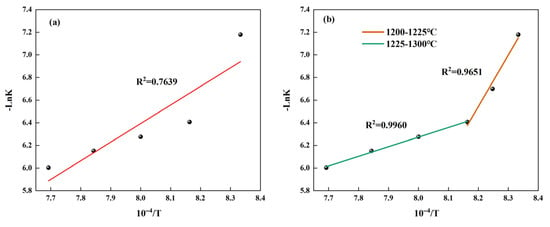
Figure 8.
The fitting relationship between -LnK and 1/T during the formation process of β-C2S: (a) fitting relationship at 1200–1300 °C and (b) fitting relationship for different temperature ranges.

Table 10.
Activation energy of β-C2S formation in different temperature ranges.
3.4. High-Temperature Reaction Kinetics of CaSO4
3.4.1. Conversion Rate of CaSO4
The consumption rate of CaSO4 refers to the degree of CaSO4 consumption in clinkers; it can be described by the ratio between content differences in clinker minerals and raw materials, as shown in Equation (6).
where w′—actual content of CaSO4 in clinker minerals (%); α′—consumption rate of CaSO4 (%); and Wr—content of CaSO4 in raw mineral (%).
In this study, the designed content of CaSO4 has been determined to be 15%, and the content of CaSO4 in the raw materials ranges from 38.69 to 44.15%. By using Equation (6) and the data in Table 3, the consumption rates of CaSO4 under different calcination systems can be obtained, and the results are listed in Table 11.

Table 11.
Consumption rates of CaSO4 under different calcination systems (%).
3.4.2. Kinetic Model of the CaSO4 Formation
It is similar to that of C4A3 and β-C2S. By substituting the data from Table 11 into the kinetic equation in Table 5 for validation, it is not difficult to find that the R3 equation under interfacial chemical reactions control can better reflect the consumption of CaSO4, as shown in Figure 9.
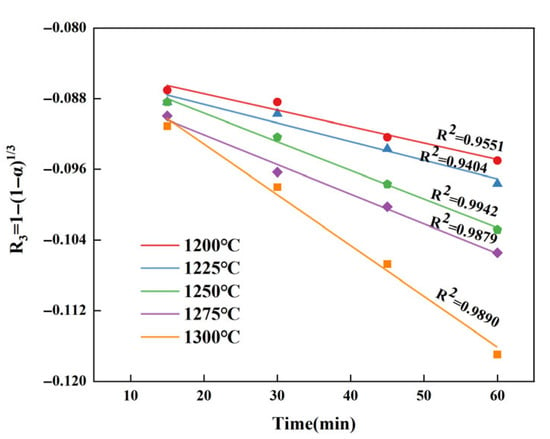
Figure 9.
Fitted curves of α′ and t for CaSO4 at different temperatures.
3.4.3. Kinetic Parameters of CaSO4
The reaction rate constants of CaSO4 at different calcination temperatures are shown in Table 12, and they are the slopes of the respective lines in Figure 9. From this table, it can be seen that as the temperature continues to increase, the reaction rate constant also shows an increasing trend.

Table 12.
Reaction rate constants of CaSO4 under various temperatures.
4. Conclusions
In order to further elucidate the calcination process of the new solid waste-based HBSAC clinker, this article starts from the perspective of high-temperature reaction kinetics and uses QXRD quantitative analysis and numerical fitting analysis methods. The reaction laws of C4A3, β-C2S, and CaSO4 were explored, a high-temperature kinetic model of the minerals was established, and the kinetic parameters of each mineral were obtained. This study provides a certain reference for the development of low-carbon cement, and its main conclusions are as follows:
- (1)
- For NHBASC, the C4A3 and β-C2S content increases with prolonged holding times and higher calcination temperatures, while CaSO4 continues to be consumed. Within the temperature range of 1200–1300 °C, the conversion rates of C4A3 and β-C2S both increase with the increase in calcination temperature and the prolongation of holding time, but the conversion rate of β-C2S decreases when the temperature is too high. Overall, the conversion rates of β-C2S are higher than that of C4A3, indicating that β-C2S reacts more thoroughly within this temperature range. The above mineral reaction laws provide a certain basis for optimizing the calcination system, enabling precise design of the clinker mineral composition through the synergistic adjustment of calcination temperature and holding time.
- (2)
- The formation of C4A3 and β-C2S is influenced by diffusion mechanisms, and they both satisfy the Glinstling equation. The activation energy required for mineral formation varies in different temperature ranges. The activation energies required to form C4A3 at 1200–1225 °C, 1225–1275 °C, and 1275–1300 °C are 166.28 kJ/mol, 83.14 kJ/mol, and 36.58 kJ/mol, respectively. The activation energies required to form β-C2S at 1200–1225 °C and 1225–1300 °C are 374.13 kJ/mol and 66.51 kJ/mol, respectively. Overall, the activation energy required for mineral formation is relatively low, indicating that solid waste-based NHBSAC embodies the advantages of low carbon and energy saving, which meets the development needs of green building materials.
- (3)
- The consumption of CaSO4 is controlled by the interfacial chemical reaction mechanism, satisfying the R3 equation. By obtaining the reaction mechanism and kinetic parameters of CaSO4, it can be known that the reaction rates of CaSO4 increase with the increase in calcination temperature and the extension of holding time. Therefore, in the process of clinker preparation, the holding time should not be too long to avoid affecting the residual CaSO4 content in NHBSAC.
This study mainly conducted theoretical exploration on the formation laws and kinetic reaction parameters of minerals. The next step will use the above theoretical results to guide the optimization and matching of mineral composition, prepare corresponding clinker, and conduct actual properties testing and analysis on the obtained clinker.
Author Contributions
Writing—review and editing, supervision, and funding acquisition, D.S.; writing—review and editing, validation, and conceptualization, M.Y.; validation, X.L.; validation, H.T.; investigation, Y.H.; investigation, J.W.; writing—original draft, software, and methodology, F.D.; supervision, D.X.; supervision, investigation, and funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 52208265) and the Natural Science Foundation of Shandong Province (Grant No. ZR2022QE185).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to resolve spelling and grammatical errors. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Chemical Formula | Chemical Name | Mineral Name |
| 4CaO·2SiO2·CaSO4 | Calcium Sulfosilicate | Ternesite | |
| 3CaO·3Al2O3·CaSO4 | Calcium Sulphoaluminate | Ye’elimite | |
| C4AF | 4CaO·Al2O3·Fe2O3 | Tetracalcium Aluminoferrite | Brownmillerite |
| β-C2S | 2CaO·SiO2 | Dicalcium Silicate | Belite |
References
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Kanavaris, F.; Das, B.B.; Idrees, M. Decarbonising cement and concrete production: Strategies, challenges and pathways for sustainable development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Yan, C.; Liu, L.; Zhao, J.; Gao, J. Potential application of all-solid waste belite-ye’elimite cement in high early-strength pavement concrete: Strength formation, characterization, and benefit evaluation. Case Stud. Constr. Mater. 2025, 22, e04185. [Google Scholar] [CrossRef]
- Ige, O.E.; Olanrewaju, O.A.; Duffy, K.J.; Obiora, C. A review of the effectiveness of Life Cycle Assessment for gauging environmental impacts from cement production. J. Clean. Prod. 2021, 324, 129213. [Google Scholar] [CrossRef]
- Huo, G.; Jiang, X.; Sun, X.; Li, H.; Shi, H. Performance of high-belite calcium sulfoaluminate cement subjected to hydrochloric acid and sulfuric acid. Front. Mater. 2024, 10, 1282919. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Q.; Zhou, W.; Gong, X.; Chen, J.; Huang, C.; Ma, G.; Liu, X.; Chang, X. Effect of calcium sulfate type and dosage on the properties of high-belite sulfoaluminate cement. J. Sustain. Cem. Mater. 2024, 13, 829–840. [Google Scholar] [CrossRef]
- Yoon, H.; Seo, J.; Kim, S.; Lee, H.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, D.; Zhang, D.; Cui, J.; Wang, W.; Liu, L. Development of high-early-strength low-carbon engineered cementitious composites with calcium sulfoaluminate cement incorporating high-volume fly ash. Case Stud. Constr. Mater. 2023, 18, e01959. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Su, D.; Yue, G.; Li, Q.; Guo, Y.; Gao, S.; Wang, L. Research on the preparation and properties of high belite sulphoaluminate cement (HBSAC) based on various industrial solid wastes. Materials 2019, 12, 1510. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Ren, H.; Mao, R.; Wu, H.; Liang, X.; Zhou, J.; Zhang, Z. Preparation and properties of phosphogypsum-based calcined coal gangue composite cementitious materials. Case Stud. Constr. Mater. 2024, 21, e03963. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Telesca, A.; Matschei, T.; Marroccoli, M. Study of eco-friendly belite-calcium sulfoaluminate cements obtained from special wastes. Appl. Sci. 2020, 10, 8650. [Google Scholar] [CrossRef]
- Galluccio, S.; Beirau, T.; Pöllmann, H. Maximization of the reuse of industrial residues for the production of eco-friendly CSA-belite clinker. Constr. Build. Mater. 2019, 208, 250–257. [Google Scholar] [CrossRef]
- Li, F.W. Research of Preparing Sulphate Aluminium Cement Using Calcium and Aluminum Residue and Low Grade Bauxite. Ph.D. Thesis, Nanchang University, Nanchang, China, 2012. (In Chinese). [Google Scholar]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Li, H.; Agrawal, D.K.; Cheng, J.; Silsbee, M.R. Microwave sintering of sulphoaluminate cement with utility wastes. Cem. Concr. Res. 2001, 31, 1257–1261. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Ren, X.; Ye, J.; Zhang, J.; Qian, J. Formation, structure, and thermal stability evolution of ternesite based on a single-stage sintering process. Cem. Concr. Res. 2021, 147, 106519. [Google Scholar] [CrossRef]
- Ma, S.H.; Shen, X.D.; Huang, Y.P.; Zhong, B.Q. Preparation and formation mechanism of calcium sulphoaluminate. J. Chin. Ceram. Soc. 2008, 36, 78–81. (In Chinese) [Google Scholar]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of calcium sulfoaluminate formation from tricalcium aluminate, calcium sulfate and calcium oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Geng, Y.J. Study on Reaction Kinetics, Hydration Kinetics and Properties of Sulphoaluminate Cement Prepared from Petroleum Coke Desulfurization slag. Ph.D. Thesis, Qingdao University of Technology, Qingdao, China, 2018. (In Chinese). [Google Scholar]
- Xu, L.J.; Jian, M.F. Kinetic parameters determination of dicalcium silicate sintering process. J. Chin. Ceram. Soc. 2011, 30, 710–713. (In Chinese) [Google Scholar]
- Hao, X.Y.; Jian, M.F. Study on mathematical model of dicalcium silicate sintering process. Inorg. Chem. Ind. 2010, 42, 15–17. [Google Scholar]
- GB/T 176-2008; Methods for Chemical Analysis of Cement. Standards Press of China: Beijing, China, 2008.
- Canbek, O.; Erdoğan, S.T. Influence of production parameters on calcium sulfoaluminate cements. Constr. Build. Mater. 2020, 239, 117866. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Wang, K.; Shah, S.P. Research progress in advanced nanomechanical characterization of cement-based materials. Cem. Concr. Compos. 2018, 94, 277–295. [Google Scholar] [CrossRef]
- Berrio, A.; Tobón, J.I.; De la Torre, A. Kinetic model for ye’elimite polymorphs formation during clinkering production of CSA cement. Constr. Build. Mater. 2022, 321, 126336. [Google Scholar] [CrossRef]
- Huang, X.; Shi, F.; Wang, G.; Yu, J.; Ma, S.; Li, W. Kinetics and mechanism of ternesite formation from dicalcium silicate and calcium sulfate dihydrate. Materials 2022, 15, 2626. [Google Scholar] [CrossRef]
- Luciano, G.; Svoboda, R. Simulation and non-linear optimization of kinetic models for solid-state processes. Model. Simul. Mater. Sci. Eng. 2024, 32, 035014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).