Abstract
Titanium (Ti) and fluorine (F) have the potential to provide a variety of desirable physical, chemical, mechanical, and biological properties applicable to a broad range of indications. Consequently, Ti- and F-containing glasses and glass ceramics are currently under investigation for use in nuclear, optical, electrochemical, dental, and industrial fields. Accordingly, significant interest exists with respect to understanding the individual and interaction effects that these elements have on material structure and properties to support the accelerated design, development, and deployment of these materials. This review aims to serve as a foundational reference across multiple disciplines, highlighting the fundamental properties and versatility of Ti- and F-containing glasses and glass ceramics. By consolidating our current knowledge of these materials, this broad overview will identify areas in which we can further our understanding to support the a priori prediction and effective design of these systems. Finally, this paper will introduce the potential to improve material design by integrating experimentation, modelling, and computational approaches in a manner commensurate with the principles of the Materials Genome Initiative.
1. Introduction
A major challenge in the design and development of new biomaterials is the complexity associated with attempting to recreate the functions and/or properties of living tissue ex vivo via the incorporation of multiple components (e.g., scaffold, cells, growth factors, and molecular signals) intended to emulate these tissues [1,2]. Despite remarkable discoveries and advances associated with these innovative approaches, the literature contends that this method may, in certain instances, risk over-engineering medical devices, thus limiting their potential translation to clinical use [1]. As a result, an alternative and complimentary design philosophy has emerged in the literature; specifically, it is contended by Place et al. that rather than attempting to engineer complex materials capable of mimicking the intricacy of physiological tissues, we should instead aim to develop synthetic materials that establish key interactions with bodily fluids and cells in ways that unlock the “body’s innate powers of organization and self-repair” [1]. In this regard, new designs for bioactive glasses provide a remarkable opportunity to discover and develop structurally simple yet functionally complex materials capable of promoting a range of desirable materials and host responses [3,4,5]. Bioactive glasses are regarded as robust carrier systems for the controlled and localized delivery of therapeutic metal ions (TMI). These TMIs have been associated with the ability to concurrently adjust the physical and chemical characteristics of glasses while also modulating biological responses, including (but not limited to) antibacterial, anti-inflammatory, osteoinductive, and angiogenic processes [6]. The literature has recently provided excellent reviews on several TMIs [6,7,8]; however, new candidate elements are emerging, and as such, the field is driving exciting new discoveries across an array of applications [9]. Two bioinorganic ions of particular interest for their potential to modulate glass structure and properties, both individually and synergistically, as well as their ability to elicit desirable biological responses, are titanium (Ti) and fluorine (F).
Ti- and F-containing glasses and glass ceramics are being investigated across an array of scientific and industrial applications. Specifically, and with respect to the effects of Ti on the physical and chemical characteristics of such materials, the literature notes that variations in the coordination and valency of Ti ions may be expected to cause structural modifications and local field variations within glass networks [10,11,12]. According to Sun’s Single Bond Strength Criterion and Dietzel’s Field Strength Criterion, Ti is characterized as an intermediate element [13]. This feature provides the unique opportunity to tailor Ti-containing glasses for a range of indications; for instance, the empty or unfilled d-shells of Ti ions may contribute to non-linear polarizabilities, making TiO2 substituted glasses promising candidates for use as nonlinear optical devices [14]. Materials consisting of mixed valence state Ti ions are also probable candidates as cathode materials for photoelectrochemical cells (PECs) [15]. Additionally, TiO2 polymorphous materials are large bandgap semiconductors (3.2 eV) and, due to their high storage capability, cycling stability, and charging/discharging rate, are considered useful for use as electrode materials for lithium-ion batteries in photocatalysis and in solar cells [15,16]. Furthermore, the addition of Ti to glass and glass-ceramic materials has also been shown to increase chemical durability, thermoluminescent sensitivity, thermal stability, and the mechanical properties of glasses and glass ceramics. As a result, these materials are being explored in the nuclear field for use as thermoluminescent dosimeters [17], immobilization of high-level radioactive waste [18], and gamma-ray shielding materials [19].
Contrastingly, the introduction of certain anions, in particular, the halogens and, more specifically, F, into various glass networks has also yielded desirable physical and chemical properties for a range of applications. Fluorine is a network-modifying element and has been shown to replace oxygen ions to form non-bridging oxygens as a result of its similar atomic and ionic radii and electronegativity, thus decreasing network connectivity [20,21]. Fluorine has also been shown to predominantly coordinate with other modifier cations (e.g., Ca, Na), which may lead to the formation of alkali-fluoride-rich regions with enhanced chemical durability [22,23,24]. Thus, understanding the structural role that F may play in various glass networks can inform the design of new materials based on desirable intrinsic (composition and structure) and extrinsic (material performance) parameters. For example, the inclusion of alkaline earth fluorides (e.g., CaF2) in borate, phosphate, and silicate glasses has been shown to improve transparency, decrease liquidus temperatures, and reduce melt viscosity [14,25,26]. It has also been established that alkaline earth fluorides produce highly transparent materials and are, therefore, intended as additives for optical materials used in photonics or as scintillators [14]. Additionally, F-containing materials display enhanced electrochemical properties for use in Li-ion cells due to the strong inductive potential of F with phosphates, for example, in lithium fluorophosphate glasses/glass ceramics [27]. As a consequence of their collective impact on the physical and chemical characteristics of glass networks, glasses and glass ceramics containing both Ti and F are of increasing interest to the community, especially with respect to controlling crystallization kinetics, microstructure, and subsequent material properties [19,20,25,26,28,29,30,31].
Based on their enhanced crystallization kinetics, Ti- and F-containing glasses and glass ceramics are also being investigated for applications in dentistry, ranging from dental fissure sealants with increased chemical durability [32,33] to ceramic materials for restorative applications and as core materials for veneered resin-bonded ceramic restorations. Accordingly, aside from their ability to modulate specific material properties suited to a range of scientific and industrial applications, Ti- and F-containing glasses and glass ceramics may offer synergies vis-à-vis modulating host responses for biomedical indications. For example, F has been shown to be incorporated into the hydroxyapatite lattice through the substitution of hydroxyl groups [34], leading to the formation of fluoridated apatites (e.g., fluorapatite). Fluorapatite has reduced solubility and increased resistance to erosion due to (i) the densification of the crystal lattice, which is associated with increased acid resistance and (ii) fluorapatite’s lower critical pH (4.5) when compared to that of hydroxyapatite (5.5) [34,35,36,37]. Consequently, F is a highly effective anticaries agent since it can reduce the rates of surface dissolution in enamel, as well as enhance remineralization of teeth [34,38,39]. Additional studies have also established that F is bactericidal, inhibiting the metabolism of dental plaque bacteria responsible for demineralization [39]. Furthermore, F, being both small and the most highly electronegative element, has been investigated for its ability to induce a profound pharmacological effect when bound to carbon in small organic molecules. These effects include improved metabolic stability, altered physiochemical properties, and increased binding affinity of certain compounds such as anti-cancer agents (e.g., thymidylate synthase inhibitors such as 5-fluropyrimidines [40]), antidepressants (e.g., fluoxetine [41]), anti-inflammatory agents (e.g., flufenamic acid and diflunisal [40]), and central nervous system drugs (e.g., sevoflurane, triflupromazine, and fluconazole [42]), to a target protein [43,44].
Similarly, the literature contends that Ti may also have potential therapeutic benefits. While the biological role of Ti in humans is not fully understood, there is evidence of its criticality in several biological mechanisms throughout nature. For instance, ascidians are avid accumulators of Ti, which may catalyze, regulate, and stabilize component monomers associated with the synthesis of biological polymers required for tunic formation or wound healing [45]. In recent years, the investigation of natural phenomena such as this has served as a great source of inspiration in the design of synthetic biomaterials [46]. In this case, nature suggests that Ti might assume a role in the construction or repair of soft tissues. Contrastingly, and with respect to hard tissue engineering, in vitro studies have demonstrated that low doses of Ti (~1 ppm) increase osteoblast proliferation and osteoblast phenotype gene expression [47], a feature which may be advantageous in orthopaedics, oral, and maxillofacial applications. Finally, the literature notes that additional biological roles for Ti include deprotonating difficult-to-deprotonate substrates, such as Ti(IV)-bound water molecules [48], due to the powerful Lewis acidity of Ti(IV) as well as the low-potential redox of a Ti4+/Ti3+ couple. These reactions may be beneficial in a metalloenzyme active site, wherein Ti can take part in a wide range of processes and biochemical reactions, such as electron transfer, substrate recognition/binding, and catalysis [45].
Critically, initial reports have demonstrated that Ti may work in synergy with F. For example, in an effort to develop slow-releasing devices for fluoride in dentistry [49], a mechanism for fluoride fixation in enamel has been proposed in the literature in which the fluoride is bound to a polyvalent metal ion in the form of a strong complex. Specifically, McCann [50] examined various metals (Al, Ti, Zr, La, Fe, Be, Sn, Mg, Zn) and discovered that both fluoride uptake and retention may be enhanced when the tooth is pr-treated with any polyvalent metal capable of forming strong fluoride complexes while simultaneously binding to the apatite crystals. Ti ion pre-treatment showed the maximum uptake and retention of F. This, in combination with Ti and its established ability to substitute Ca2+ ions in the apatite lattice, forming a Ti phosphate compound, further increases acid resistance [51]. These data suggest that titanium fluoride complexes are beneficial as a topical treatment in dentistry [51,52,53,54].
While both Ti and F have been demonstrated to elicit desirable material and host responses individually, the literature suggests that there is interest in harnessing the benefits of both elements by incorporating them together in a glass network. The objective of this review is to contextualize the impact that Ti and F may have, both individually and synergistically, in modulating glass structure and properties when included together in the composition a glass or contiguous glass ceramics. As such, this document is intended to provide a timely consolidation of our present understanding in this regard. Specifically, this review is intended to provide a state-of-the-art summary of Ti- and F-containing glass and glass ceramic materials currently under investigation for use in a variety of scientific, industrial, and biomedical indications, including optical devices, cathode materials, radiation shielding materials, and dental restorative materials. A crucial first step in the characterization of such novel materials, prior to assessing indication-specific properties (e.g., optical, nuclear, magnetic, electrical characteristics), is gathering information related to the physical and chemical characterization of such materials so as to understand (i) the fundamental role that each element plays in the network and (ii) how that role may vary depending on the chemical composition of the glass/glass ceramic. These physical and chemical characteristics include but are not limited to material composition, extractable and leachable, structural composition or configuration, thermal properties, density and molar volume, chemical durability, size and morphology, topography, surface chemistry, surface energy, bulk characteristics, and mechanical properties. Accordingly, this paper intends to consolidate existing knowledge of the effects that Ti and F have on the physical, chemical, mechanical, and biological characteristics of glasses and glass ceramics containing both elements with varying chemical compositions. A summary of the experimental tests utilized to study these materials and the associated experimental results will be provided to identify areas in which we can further our knowledge and understanding of Ti- and F-containing glasses and glass ceramics. Finally, this paper will introduce the potential to improve glass material design by integrating experimentation, modelling and computational approaches in a manner that strengthens our ability to support the a priori prediction and effective design of new materials with desirable attributes in a manner consistent with the principles of the Materials Genome Initiative.
2. Methodology
To clearly establish the potential impact and unique contribution of titanium and fluorine on the physical, chemical, mechanical, and biological properties of glasses and glass ceramics, an initial search strategy was completed using the search strings shown in Table 1. ‘Web of Science’ and ‘PubMed’ databases acted as primary sources for peer-reviewed literature.

Table 1.
Standard search parameters for PubMed and Web of Science.
Eligibility of the papers was established in line with the objectives of this work; specifically, the inclusion criteria adhered strictly to (1) glasses and/or glass ceramics that (2) contain both Ti and F and (3) provided confirmation of structure with XRD verification. Papers excluded from this review include those which did not meet the above-mentioned criteria, along with papers investigating slag materials. The associated search results are summarized in Table 2.

Table 2.
Search results based on searches in Table 1.
3. Results
As presented in Table 2, a total of 3217 articles met the search streams summarized in Table 1. Having hand-reviewed each search result, a total of 26 articles were identified as candidates for inclusion in this work. One of these articles [55] could not be reviewed due to translational barriers, thus warranting its exclusion from the current assessment. The total number of relevant papers was therefore reduced from 26 to 25. In addition to these articles, there were seven [56,57,58,59,60,61,62,63] which classified the material under investigation as a ‘glass’ (6) or ‘glass-ceramic’ (1) but did not provide XRD verification to support this claim. Although these articles met other inclusion criteria, they were excluded from this review based on the lack of the provision of XRD data. The final articles were grouped into categories based on the format and chemical composition of the material being described (Figure 1).
Figure 1.
Primary and secondary categories by which each article was categorized.
The material format was divided into (1) verified glasses and (2) verified glass ceramics. A glass can be defined as a “non-crystalline solid exhibiting glass transformation behaviour” or simply as “an amorphous solid” in which the amorphous characteristic is intended to describe atomic disorder as evidenced by an X-ray diffraction (XRD) analysis [13]. Glass ceramics on the other hand can be defined as “inorganic, non-metallic materials prepared by controlled crystallization of glasses via different processing methods” [64]. They contain at least one type of functional crystalline phase, of which the volume fraction may vary from ppm to almost 100%, and a residual glass phase [64]. Accordingly, for the purposes of this review, articles investigating glasses that exhibited signs of crystallinity via XRD analysis but were not deliberately heat treated, were categorized as glasses. Furthermore, articles that studied glass-ceramic materials, but performed experimental tests on both the glass precursor material (prior to heat treatment) and the glass ceramic (after heat treatment), were categorized under glass ceramics. For completeness, the findings derived from testing the glass precursor and those derived from testing the glass ceramic will be discussed separately.
Both material formats were divided and further characterized according to the primary glass-forming element(s) incorporated into each network. Articles investigating glasses/glass-ceramics including only one glass-forming element (e.g., B, Si, P, Ge) in the network were grouped into the (a) single primary glass former category, whereas those that had multiple glass-forming elements (≥2) (e.g., borosilicate, borophosphate, and phosphosilicate) were grouped into the (b) mixed glass formers category. For enhanced clarity, papers that examined multiple glass compositions arising from a baseline glass (i.e., engineered with a single glass-forming element), in particular, those which substituted or otherwise added small amounts of additional network formers [26,30], were grouped based on the baseline glass former.
This paper is structured to review the findings within each of the specified categories based on (1) the materials chemistry as it pertains to the inclusion of Ti and F, and (2) how Ti and F influence the network and material behaviour in terms of physical, chemical, mechanical and biological characteristics. For each article investigating glass materials, specific data such as author, year of publication, area of application, melting process, experimental data collected, and chemical composition has been summarized in Table 3. Table 4 presents the main experimental findings from each article as they relate to Ti and F inclusion, wherein only information related to the scope of this review was included (i.e., the effects of Ti and F on physical, chemical, mechanical, and biological characteristics). Table 5 and Table 6 present the same for articles investigating glass-ceramic materials, respectively.

Table 3.
Summary information on glass articles meeting inclusion criteria.

Table 4.
Key experimental findings on the effects of Ti and F on the properties of glasses summarized from articles meeting inclusion criteria.

Table 5.
Summary information on glass-ceramic articles meeting inclusion criteria.

Table 6.
Key experimental findings on the effects of Ti and F on the properties of glass ceramics summarized from articles meeting inclusion criteria.
The initial search yielded > 3200 articles, which varied over a wide range of indications, including dentistry, orthopaedics, optics, industrial, electrochemistry, nuclear, physics, and mechanical characterization. As shown in Figure 2, the glass materials were most prominently investigated with respect to generalized materials characterization and nuclear indications, while the most common areas of application for glass ceramics were materials characterization and dentistry. Of the 25 articles under review, the majority characterized phosphate networks (10), followed by networks containing mixed glass formers (Si/P (2), Si/B (2), B/P (2)), borate networks (4), silicate networks (4), and F-only networks (1).
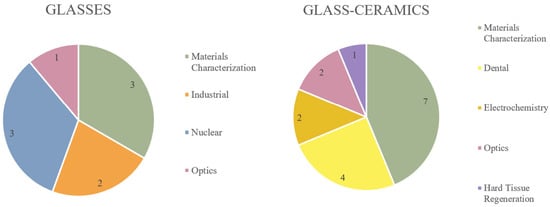
Figure 2.
Areas of indication (and number of articles per area) for articles investigating glasses and glass ceramics.
4. Discussion
The objective of this research was to examine the existing literature as it pertains to the inclusion of both Ti and F in glasses and glass ceramics. Having reviewed > 3200 papers and shortlisting 25 based on the inclusion/exclusion criteria described in the methodology section, the authors conclude that the literature is ambiguous and, at times, contradictory with respect to the effects that Ti and F have on the physical, chemical, mechanical and biological properties of these materials. For clarity, this discussion will be structured such that the findings related to glass materials are discussed first, followed by those related to glass-ceramic materials.
4.1. Glasses
The observed trends (increasing or decreasing) in a selection of experimental findings associated with an increasing content of Ti and/or F in glass materials are presented in Table 7. There are multiple categories in which the literature indicates contrasting trends with respect to the effects of Ti and F on glass structure and properties. Although these contrasting trends are primarily a result of varying glass chemistries, contradicting results exist between glasses with similar compositions. Furthermore, there are several properties where the effects of increasing Ti and F content have not yet been addressed, highlighting large gaps in our knowledge of Ti- and F-containing glass and glass ceramics despite their significant potential across a broad range of applications.

Table 7.
Trends in a selection of experimental findings from articles investigating glass materials.
4.1.1. Coordination Number
As a result of overall glass chemistry, the literature indicates contrasting trends with respect to the influence of Ti on the coordination number of glass-forming elements. For instance, an increase in coordination number (upon the addition of Ti) occurred in networks where the primary glass-forming system was phosphate [18] or borosilicate [19]. Mechanistically, it has been determined from Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and nuclear magnetic resonance (NMR) that Ti may act as a network former in such systems. Specifically in phosphate glass, the addition of TiO2 altered the phosphate network by breaking P=O bonds to form P-O-Ti bonds, which increased cross-linking and strengthened the phosphate chains [18]. For the borosilicate glass, the average coordination number and number of bridging oxygens increased as ZnO was substituted by TiO2. Therefore, as the content of TiO2 increased, there was an increase in interconnection [19].
Contrastingly, the coordination number of the network former decreased with increasing Ti in networks formed exclusively with boron as the glass former. This decreasing trend appears to be consistent in the literature. However, there are only three articles [14,65,66] that provided data on the effects of Ti vis-à-vis network coordination numbers in borate glasses, two investigating the same glass system [65,66]. It was reported that low concentrations of TiO2 (where the Na3AlF6 modifier behaviour was predominant [65]) were associated with the presence of TiO4 units and appeared to promote an increased population of B4 units in borate glasses. Contrastingly, high concentrations of TiO2 were associated with the presence of TiO62− units and increased the population of B3 units [65]. The conversion of B4 to B3 observed in TiO2-rich glasses was likely associated with the Ti coordination change as a result of TiO4/2 + 2B(O,F)4 → Ti(O,F)6 + 2BO3 [65,66]. In a separate borate glass network [14], data derived from FTIR show that as the concentration of TiO2 increased in the glass samples, the intensity of the bands attributed to TiO6 and BO3 increased, while the bands attributed to TiO4 and BO4 decreased in intensity [14]. Although the decreasing trend in coordination number upon the gradual addition of Ti appears to be consistent across these three articles, there are contrasting findings as it relates to the concentration of Ti. Specifically, Anghel, Florian and Bessada found that low concentrations of TiO2 (0–4.4 wt.%/0–10.54 mol%) were associated with an increased presence of B4 units, whereas Lakshmi and Cole reported that TiO2 (0–0.7 mol%) acted as a modifier, increasing the presence of B3 units. Existing literature is conflicted with respect to the role of Ti in the structural modification of glasses. Like many other areas of glass science, this may be due to the individual effects of Ti on the network or, more likely, the interaction effects between Ti and other components. Describing a mechanism by which the interaction effects of network constituents influence material properties will require the development of new experimental approaches with the capacity to identify individual and interaction effects. Further research is necessary to elucidate this role.
Two articles [65,66], both investigating networks in which boron was the primary network-forming element, provided data relating to the effects of F on coordination number. It was observed that increasing the content of F in the network led to a decrease in the coordination amount of boron. In samples with low TiO2 content (e.g., 80T11), the lower concentration of high field strength Ti4+ cations resulted in increased fluorine uptake by boron. This allowed F− ions to substitute O2− ions, leading to the formation of non-bridging oxygens and causing the conversion of B4 units to B3 units [66]. While the findings from both articles are consistent, it is important to note that they share certain limitations. Firstly, both articles studied the same glass compositions and were authored by the same researchers. This lack of variability in the data sources restricts our ability to make accurate predictions about the effects of F (fluorine) across a wide range of concentrations on the structure of glasses with different chemical compositions. To gain a more comprehensive understanding of the impact of fluorine on glass structures, it is crucial to conduct studies encompassing a broader range of glass chemistries. Such investigations should be structured to uncover the nuanced interactions between fluorine and various glass components, allowing for more generalized and robust conclusions. By exploring a wider spectrum of glass compositions, the literature may better establish how fluorine influences glass properties under different chemical scenarios, leading to a more thorough comprehension of its effects on glass structures.
4.1.2. Density and Network Connectivity
The literature indicates varying trends with respect to the effects of increasing Ti content on the density and network connectivity of Ti- and F-containing glasses. For instance, increased density and network connectivity (upon the addition of Ti) occurred in networks where the primary glass-forming system was borophosphate [16], phosphate [18], and borosilicate [19]. Specifically in phosphate and borophosphate glasses, the addition of TiO2 has been shown to strengthen the network by creating cross-links between phosphate and borate chains (P-O-P and B-O-B linkages are substituted by P-O-Ti or B-O-Ti linkages), thus increasing the bulk density [16,18]. Similarly, an increase in the number of bonds per unit area and the cross-linking density was observed with increasing Ti content in borosilicate glasses [19].
Contrastingly, the density and network connectivity decreased with increasing Ti in networks formed exclusively with boron. In one borate glass system [14], density decreased with increasing TiO2 concentration because zinc fluoride was gradually substituted by titanium oxide, which has a comparatively lower density and lighter atomic weight than zinc fluoride [14]. Additionally, it was reported that the addition of glass modifiers, such as Ti4+, into borate networks, may cause an increase in the number of non-bridging oxygen attached to large ring borate groups (e.g., diborate rings, pentaborate rings, di-pentaborate rings, and triborate rings), thus disrupting these ring structures and decreasing the connectivity of the network [65]. This resulted in the formation of smaller non-ring borate groups (e.g., diborate, triborate, and pentaborate) in the network [65]. The findings derived from the literature [14,18,19,65] agree with the reported trends in coordination numbers summarized above, wherein a decrease in coordination number is often associated with a decrease in network connectivity. Only two articles [15,16] reported the effects of Ti addition on the density and network connectivity of a glass with the same glass-forming system, i.e., borophosphate. Contrary to the findings of Guntu et al. [16] discussed above, Rua and Kumar [15] found that an increase in TiO2 content caused an increase in non-bridging oxygens, which induced higher grades of disorder within borophosphate networks and resulted in a decreased network connectivity. These two articles [15,16] observed opposing and contrasting results, which emphasize the difficulties associated with predicting the effects of Ti and F inclusion in certain glass systems.
4.1.3. Glass Transition Temperature
The increasing content of TiO2 in phosphate and borosilicate glass networks has been shown, by means of differential thermal analysis (DTA), to increase glass transition temperature (Tg) [18,19]. Specifically, the thermal stability of Ti-containing phosphate glasses was greater than that of a Ti-free glass sample because the addition of TiO2 was shown to strengthen the network by creating cross-links between phosphate chains [18]. Similarly, in borosilicate glass, as the content of TiO2 increased, more bridging oxygens formed and there was an increase in interconnectivity of the network [19]. This increase in connectivity is regarded as the mechanism underlying the observed increase in Tg and is consistent with the observations noted with respect to coordination number, density, and network connectivity in these systems.
In juxtaposition to phosphate and borosilicate glasses, the addition of TiO2 may cause minor decreases in the Tg in borophosphate glass [15]. Specifically, Rao and Kumar reported that increasing the content of TiO2 up to 0.6 mol% led to a reduction in Tg from 445 to 440 °C. Mechanistically, the decrease in Tg was believed to be a result of the increased number of non-bridging oxygens and decreased network connectivity associated with the addition of TiO2 to the network [15]. The authors further report that at increased addition of TiO2 (at 0.8 mol% and 1 mol%), the glass transition values began to increase to 441 and 442 °C, respectively [15]. However, the observed decrease in Tg may be within the error of the DTA equipment, and so careful consideration should be given to this dataset. Furthermore, the Tg has been shown to decrease upon the addition of TiO2 to borate glass [17]. Regrettably, however, this specific article provided DTA curves for only two glass chemistries: LBA (50Li2O·45B2O3·5Al2O3) and LBA·50LiF2·xTiO2 (wt.%). Therefore, the decrease in Tg observed in the LBA·50LiF2·xTiO2 glass compared to the LBA glass could have been due to the added LiF2 (or a combination of the added LiF2 and TiO2) rather than solely the TiO2. Consequently, this article was excluded from Table 7. To prevent these ambiguities in future research, we should aim to gather baseline data on the effects of gradual Ti and F addition across broad composition ranges using a systematic approach capable of predicting the effects of multiple constituents within a network.
4.1.4. Mechanical Characteristics
There was one article that evaluated the effect of TiO2 addition on the mechanical properties of Ti- and F-containing glasses [16]. As CaF2 was increasingly substituted by TiO2 in a borophosphate glass, Young’s modulus, shear modulus, and bulk modulus were all observed to increase, while the microhardness and Poisson’s ratio decreased [16]. The substitution of divalent Ca2+ ions by Ti4+ ions, as well as the substitution of P-O-P and B-O-B bonds for stronger P-O-Ti and/or B-O-Ti bonds upon the addition of TiO2, led to an increased packing density within the glasses. The increased packing density, also associated with increased material rigidity, was reported to have led to an increase in the various elastic moduli. Additionally, the observed trends in Poisson’s ratio and microhardness upon increasing concentration of TiO2 suggested that the prepared glasses exhibited an increasingly interconnected structure [16]. Surprisingly, little information is available in the literature on the mechanical properties of Ti- and F-containing glasses, suggesting that further research in this area would be of considerable value to the community.
4.2. Glass Ceramics
The observed trends in a selection of experimental findings associated with increasing content of Ti and/or F in glass-ceramic materials are listed in Table 8. Similar to the findings summarized in Table 7 for glass materials, the literature indicates varying trends with respect to the effects of Ti and F on glass-ceramic structure and properties, depending on glass chemistry. Additionally, as shown in Table 8, there are properties for which the effects of Ti and F addition have not yet been addressed in the literature. For a selection of the findings listed in Table 8, the experimental test was performed on the glass-ceramic precursor material (the glass intended to produce a glass ceramic) prior to heat treatment. These results are denoted with an asterisk in Table 8. The following section will be structured to discuss the findings of tests that were performed on the glass-ceramic precursors, followed by those that were performed on the glass ceramics.

Table 8.
Overall trends in a selection of experimental findings from articles investigating glass ceramics.
4.2.1. Glass-Ceramic Precursor
The findings discussed in the following section are derived from experimental tests that were performed on glass-ceramic precursor materials prior to heat treatment.
Coordination Number
Among the 16 articles investigating glass-ceramic materials, one article examined the coordination number of a glass precursor material before heat treatment [26]. In that study, CaF2 was added to a Ti-containing glass with silica as the network-forming element, leading to a decrease in the coordination number of silica [26]. Raman bands from the glass-ceramic precursor were attributed to Si-O stretch vibrations of Qn (n = 1, 2, 3, 4) tetrahedral units. The addition of CaF2 caused a reduction in the fraction of Q4 units, indicating disruption of the SiO2 three-dimensional network. Specifically, F− ions replaced bridging oxygens in =Si-O-Si= with weak =Si-F linkages, weakening the glass network [26]. Since the decrease in coordination number was observed only for the specific compositions T and TF, a continuous trend in coordination number upon the gradual addition of F could not be established. To achieve a deep scientific understanding of fluorine’s influence on glass structures, it is imperative to undertake studies involving a wider array of glass chemistries. Embracing diverse investigations will unveil the intricate interactions between fluorine and various glass components, facilitating more comprehensive and robust evaluations of such materials. By delving into a broader spectrum of glass compositions, we may effectively evaluate how fluorine impacts glass properties within distinct chemical scenarios, fostering a deeper comprehension of its effects on glass structures. Indeed, addressing this complex challenge can be achieved through the enhanced utilization of the design of mixture methodologies and the integration of machine learning techniques. By combining these powerful tools, we can accelerate the pace of research and facilitate a more rapid and comprehensive development of our understanding in this area. The design of mixture methodologies allows for the systematic exploration of a wide range of glass compositions, optimizing the experimental design for efficient data collection. Coupling this with machine learning enables the extraction of valuable patterns and trends from large datasets, guiding researchers towards novel insights and accelerating the discovery process. This synergistic approach holds the potential to revolutionize glass science and propel our understanding of fluorine’s impact on glass structures to new heights.
Glass Transition Temperature
The literature indicates that increasing Ti content has contrasting effects on the Tg of Ti- and F-containing glasses, depending on glass chemistry. An increase in Tg has been reported to occur with increasing Ti content in networks where the primary glass-forming system is borosilicate [31] or silicate [72]. It was found that by increasing the TiO2 content from 1–10 wt.% in a borosilicate network, the Tg and both the crystallization peak temperatures increased (Tg: 705–723 °C, TpI: 874–902 °C, TpII: 938–980 °C) [31]. The increase in these thermal characteristics was believed to be due to the high field strength of the Ti4+ ion, which accelerates the interaction between structural units and results in the formation of linkages between some positive cations, such as Mg+ and K+. This finding is consistent with the increased Tg observed by Shaaban et al. upon the addition of TiO2 in a borosilicate glass [19]. Furthermore, the addition of TiO2 to a glass formed exclusively with silicate significantly increased the glass transition and dilatometric softening point temperatures [72]. While the exact mechanism behind this increase in Tg was not discussed, Takav et al. [72] concluded that the addition of TiO2 to the base composition increased the glass viscosity, thus decreasing the occurrence of crystallization.
In contrast to the above-noted findings, a decrease in Tg was associated with the addition of TiO2 in networks where the primary glass-forming system was phosphosilicate [73]. Mechanistically, the decreased Tg was attributed to the decrease in viscosity that came from gradually adding Ti into the network. It was reported that the addition of Ti4+ ions accelerated the formation of cross-links between phosphate units, thus disrupting the glass network and decreasing the glass viscosity [73]. Additionally, the Tg of a phosphate glass was found to decrease upon the addition of TiO2 [27]. Specifically, when comparing the Tg of the VV sample (Li3V2(PO4)2F3) to the TV sample (Li3TiV(PO4)2F3), that of the TV sample was lower. Because this finding was derived from comparing the Tg of only two different glass compositions (VV and TV), a continuously decreasing trend in Tg upon the gradual addition of Ti could not be identified. To provide a more comprehensive understanding of the effects of Ti on the Tg of glasses, multiple compositions of varying Ti content should be investigated. It can be concluded, however, that the addition of TiO2 to the phosphate glass caused an overall decrease in Tg, contrary to the results found for the phosphate glass previously described by Lu et al. [18].
The addition of F was found to decrease the Tg in both silicate [26] and phosphate [33] glasses. In silicate glass networks, Tg and Tp were both found to decrease upon the addition of CaF2 [26]. With the addition of CaF2, F− ions substituted the bridging oxygens in =Si-O-Si= due to the similar radius between oxygen and fluorine. The replacement of the strong linkage with a pair of non-bridging =Si-F linkages caused a decrease in viscosity, an increased occurrence of crystallization, and a decreased Tg and Tp [26]. The changes that were observed in these parameters upon adding CaF2 were also hypothesized to be due to phase separation in the melts. Specifically, fluorides can be immiscible in silicate melts, resulting in nucleated droplet phase separation that facilitates the occurrence of crystallization [26]. Because the decrease in Tg was observed only by comparing two compositions (T and TF), a continuous trend in Tg upon the gradual addition of F could not be identified. Thus, to provide a more comprehensive understanding of the effects of F on the Tg of glasses, multiple compositions of varying F content should be investigated. Separately, the inclusion of F also resulted in a decreased Tg in phosphate glasses [33]. The mechanism behind this decrease in Tg was not discussed. This presents an excellent opportunity to contribute new knowledge to the Ti- and F-containing glass literature regarding the effect of F on the Tg of phosphate glasses.
Mechanical Characteristics
As mentioned above, it was found that the addition of CaF2 to silicate glass caused a decrease in glass viscosity [26]. Mechanistically, F− acted as a network modifier, weakening the glass structure and replacing the bridging oxygens in =Si-O-Si= due to the similar radius between oxygen and fluorine. Consequently, the formation of weak =Si-F bonds led to the weakening of the polymerization of the glass network and, thus, a decrease in viscosity.
4.2.2. Glass Ceramics
The following section presents findings derived from experimental tests on glass-ceramic materials after undergoing heat treatment.
Coordination Number
In the literature, the influence of titanium (Ti) on the coordination number of the primary glass-forming element(s) in Ti- and F-containing glass ceramics has not been explicitly discussed. However, insights from chemical durability and Raman analyses in a phosphate glass [25] reveal valuable information. It was observed that TiO2 concentrations of up to 0.6 mol% led to a preference for tetrahedral configurations of Ti ions, promoting their active involvement in network formation with increased P-O-Ti bonds in the glass network. Simultaneously, up to 0.6 mol% TiO2 addition, the Raman bands linked to TiO6 units showed a decrease in intensity. Conversely, when the TiO2 concentration was raised from 0.6 to 0.8 mol%, a different trend emerged. The Raman intensity associated with TiO6 structural units increased, while that of TiO4 structural units decreased. These results suggest that within the range of 0.6–0.8 mol% TiO2, Ti ions predominantly formed octahedral configurations, which, akin to Ti3+ ions, may participate in the formation of non-bridging oxygens. Though not explicitly discussed in the article, based on these findings, it can be inferred that Ti addition up to 0.6 mol% enhanced the phosphate coordination number of this glass system, while further addition of TiO2 up to 0.8 mol% decreased the coordination number.
Density
A single article examined the impact of Ti addition on the density and network connectivity of glass ceramics [25]. The introduction of TiO2 (up to 0.6 mol%) in a phosphate glass ceramic resulted in an increased density, while subsequent increases in TiO2 (from 0.6 to 0.8 mol%) were associated with a slight decrease in density. The progressive increase in density up to 0.6 mol% TiO2 indicated a growing structural compactness in the material. This can be attributed to the greater presence of Ti ions in a Ti4+ state, as confirmed by Raman spectroscopy [25]. Notably, up to 0.6 mol% concentration, Ti ions exhibited a preference for tetrahedral positions, fostering more P-O-Ti bonds rather than acting as modifiers that create additional non-bridging oxygen bonds in the glass network. Furthermore, because Ti ions in the Ti4+ state possess a higher field strength compared to the Ti3+ state, thus leading to an overall increase in material compactness, the authors suggested that the increased density up to 0.6 mol% TiO2 was an indication of Ti ions existing primarily in the Ti4+ state in sample T6 (0.6 mol% TiO2).
Glass Transition Temperature
The introduction of Ti into glass-ceramic networks, where the primary glass-forming system is phosphate, has been found to result in an increase in the glass transition temperature (Tg) [25]. Particularly, it was observed that as the concentration of TiO2 was raised up to 0.6 mol%, there was a corresponding increase in Tg [25]. This rise in Tg can be attributed to the augmented cross-link density and closer packing facilitated by the increasing presence of tetrahedral Ti ions, as discussed in the preceding section [25].
Mechanical Characteristics
In the study of glass ceramics, the evaluation of mechanical properties was more prevalent compared to glasses. Table 8 presents the ambiguities and contrasting trends in various characteristics, such as microhardness and viscosity, in relation to the addition of TiO2 to glass ceramics. For instance, the incorporation of TiO2 into a silicate glass ceramic was found to result in an increase in the glass viscosity [72]. Consequently, the glass ceramics containing TiO2 experienced less interruption from crystallization, leading to improved sinterability. This, coupled with the observation of harder crystalline phases precipitating within the material’s microstructure, contributed to an increase in microhardness with the progressive addition of TiO2 [72]. However, it is important to note that the increased TiO2 content also resulted in suppressed crystallinity and a reduced interlocking crystalline arrangement. Consequently, this caused a decrease in fracture toughness and flexural strength in glass ceramics [72].
In a phosphate glass ceramics, it was found that the gradual increase in Ti content (up to 0.6 mol%) caused an increase in Young’s modulus (i.e., 58–62.6 GPa), shear modulus (i.e., 26.1–28.11 GPa), Poisson’s ratio (i.e., 0.111–0.114), and microhardness (i.e., 6.65–7.24 GPa) [25]. Upon the further addition of TiO2 (from 0.6 to 0.8 mol%), there was a decrease in these properties (i.e., 57.2 GPa, 25.7 GPa, 0.113, 6.63 GPa, respectively). This behaviour corresponds well with the density findings reported in the literature [25], wherein the increased density values were indicative of increasing structural compactness and decreasing disorder in the glass network. In support of the above findings, the literature notes that in a less disordered framework, the mechanical loss factor or the coefficient of internal friction of piezoelectric composite oscillators is decreased, leading to an increase in elastic coefficients and micro-hardness of glass materials [25].
Contrary to the increased viscosity observed in silicate glass ceramics with increasing Ti content [72], the viscosity of a phosphosilicate glass ceramic decreased with increasing TiO2 [73]. As previously mentioned, this behaviour was reported to be a result of TiO2 disrupting the glass network and decreasing glass solubility by forming cross-linking Ti4+ units [73,75]. Furthermore, in contrast to the increased microhardness in silicate glass ceramics with increasing Ti content [72], the microhardness of a borosilicate glass ceramic decreased with the addition of Ti [31]. The decreased hardness was believed to be correlated to the formation of the interlocking, dense, blocky microstructure, as evidenced by SEM [31].
Chemical Durability
Although chemical durability/dissolution behaviour was not evaluated in any of the articles investigating glasses, there were three articles that studied this behaviour in phosphate [25] and silicate [30,72] glass ceramics. For instance, in phosphate glass ceramics, the addition of TiO2 (up to 0.6 mol%) caused an increase in chemical durability of the glass ceramic, whereafter further increase in TiO2 (from 0.6 to 0.8 mol%) reportedly led to a slight decrease in chemical durability [25]. The average dissociation rate (DR) of the TiO2-free glass ceramic was 5.37 ((×10−6) g/cm2/min). With increasing content of TiO2 up to 0.6 mol%, the DR decreased to 0.81 ((×10−6) g/cm2/min), whereafter it increased to 1.72 ((×10−6) g/cm2/min) upon the addition of 0.8 mol% TiO2. These results suggested that the addition of TiO2 (up to 0.6 mol%) increased the chemical durability of the glass ceramics. This was believed to be due to the titanium ions forming preferentially into tetrahedral configurations, thus participating in the formation of P-O-Ti bonds in the network rather than forming non-bridging oxygens. The addition of TiO2 had a similar effect on a silicate glass ceramic [30], wherein the composition that included Ti had a higher chemical resistance compared to the composition without Ti. Unfortunately, only two of the four compositions in the study were investigated for their chemical durability. As a result, observing the effects of a gradual increase in Ti content across multiple compositions was not possible, and a continuous trend in chemical durability could not be derived. Furthermore, the increased chemical resistance of the Ti-containing material cannot be fully attributed to the Ti addition, as the comparative glass compositions varied in phosphate content. Consequently, this result was excluded from Table 8. This example is representative of a fundamental challenge in the design of glass networks. In particular, given the almost limitless compositional arrangements that are possible [76], it is fundamentally clear that alternative design strategies be implemented as soon as possible—so as to elucidate the individual and interaction effects of multiple glass constituents on the composition–structure–property relationships within a glass network [77].
In a separate silicate glass-ceramic system, the gradual increase in Ti content was found to have varying effects on the chemical durability of the material [72]. For instance, there was significantly decreased chemical durability for glass-ceramic FC6 (6 wt. ratio TiO2) compared to FC0 (0 wt. ratio TiO2), but further increases in TiO2 leads to the increase in chemical durability of the glass ceramics FC9 (9 wt. ratio TiO2) and FC12 (12 wt. ratio TiO2). It was concluded that glass structure connectivity was improved by the increase in TiO2 content, which, in turn, resulted in increased chemical stability in the residual glass phase. Presumably, the increased chemical stability of the residual glass phase was responsible for the decreased chemical solubility of FC9 and F12.
There were two articles that reported findings on the influence of F on the chemical durability/dissolution properties of Ti- and F-containing glass ceramics [32,33]. Both articles were written by the same primary author and evaluated very similar phosphate glass networks. From analyzing SEM micrographs of the CaF2-containing (CTP-F) and CaF2-free (CTP) glass-ceramic surfaces after etching with acid, it was found that the chemical durability of the CaF2-containing glass ceramic was drastically improved. Specifically, SEM micrographs showed almost no surface alteration in the CTP-F glass ceramic, whereas the CTP glass-ceramic surface was severely etched. The increased chemical durability was attributed to the addition of F, which induced the preferential formation of apatite crystal throughout the network. It was believed that a large number of orthophosphate groups in the glass were used for the formation of these apatite crystals, causing the amount of CaTi4(PO4)6 crystal formed in the glass to decrease. As a result, the residual Ti constituent resided in the glassy phase of the material. Succinctly, the excellent chemical durability of the phosphate glass ceramics was suggested to originate from the microstructure of the glass-ceramic, specifically, the increased crystalline phases and a high content of Ti in the residual glassy phase [32,33].
5. Future Directions
Ti- and F-containing glasses and glass-ceramic materials have applications in industries aimed at addressing challenges in human welfare, national security, and clean energy, yet the current state of the literature confounds our understanding of these materials, specifically with respect to the effects of Ti and F on material structure and properties. Consequently, we are unable to accurately predict the roles of Ti and F (both individually and interactively) in modulating the physical, chemical, mechanical, and biological characteristics of glasses and glass ceramics, hindering the accelerated development of these materials and subsequently delaying their translation to commercial use. Accordingly, further investigation of Ti- and F-containing glasses and glass ceramics is required to support the streamlined discovery, design, and deployment of these materials.
The results summarized in Table 7 and Table 8 reflect the conflicts and gaps in our knowledge relating to the individual roles of Ti and F in glass networks and how they influence various characteristics, such as coordination number, Tg, density and network connectivity, chemical durability, mechanical characteristics, and biological responses. More critically, the literature contains many assumptions on the role that individual elements play on material properties; however, these analyses are absent crucial considerations relating to interaction effects between elements. Specifically, for many of the experimental findings summarized in Table 7 and Table 8, the literature indicates conflicting observations with respect to the influence that increasing Ti or F content has on glass structure and properties. These contrasting results were mostly a result of varying chemical composition; however, opposing trends were identified for glasses/glass ceramics with the same primary constituents. These contradictions make it extremely difficult to anticipate, predict, and optimize the roles of glass constituents (e.g., Ti and F) for a given indication. In cases where there were consistent trends in the effects of Ti and/or F addition in glasses/glass ceramics with the same glass-forming system, the number of articles reporting these results was very few (≤3). Accordingly, there is a lack of consistency and repeatability in the current literature, making it difficult to accurately predict the influence that Ti and/or F may have on glasses and glass ceramics of varying chemical compositions.
Furthermore, for each experimental finding summarized in Table 7 and Table 8, the trends were often only characterized for a limited number of glass chemistries. For example, the glass networks most evaluated were those where the primary glass-forming system was boron or borophosphate. There were no articles that reported findings on the effects of Ti addition on the properties of oxyfluoride silicate glasses. Alternatively, the glass-ceramic networks most evaluated were those where the primary glass-forming system was silicate or phosphate. There were no articles that reported findings on the effects of Ti addition on the properties of F-containing borate glass ceramics. Moreover, the only networks investigated for the effects of F addition (in glasses and glass ceramics) were borate, silicate, and phosphate. Regrettably, the lack of complete data on a range of alternative glass chemistries (e.g., borate, phosphate, silicate, germanium, phosphosilicate, borophosphate, borosilicate) limits our understanding of the effects that Ti and F may have on the physical, chemical, mechanical, and biological properties of a variety of networks. To address this limitation, we should aim to gather baseline data on the composition–structure–property relationships that exist within varying glass chemistries upon the incorporation of Ti and F across wide compositional ranges.
Additionally, there are notable gaps in the types of responses investigated in these reports. For instance, the effect of F on the density and network connectivity, Tg, and mechanical characteristics of Ti- and F-containing glasses was not addressed. For glass-ceramic materials, the effect of F on the density and/or network connectivity was not addressed. Furthermore, the chemical durability/dissolution properties of Ti- and F-containing glasses have not been discussed in the literature, meaning that our ability to predict and control this critical aspect of materials design is highly constrained. The evaluation of glass dissolution behaviour provides insight into the glass structure and how it controls which ions are more readily released into the solution. This information is critical in predicting and understanding the rate and mechanism by which the glass will degrade—a process that must be controlled for a variety of applications, from short-term and long-term medical materials to solar cells. With respect to these materials in medicine, there is a substantial lack of knowledge related to the biological safety and efficacy associated with Ti- and F-containing glasses and glass ceramics. Specifically, there is no information available on the characterization of these materials as they relate to host responses, such as mineralization potential and effect on cellular responses (e.g., angiogenesis, cell viability, antimicrobial, etc.). These types of biological tests are crucial in the design and development of medical devices, specifically in assessing the biocompatibility of a material and its constituents. Overall, this knowledge gap provides an excellent opportunity to investigate the biological properties of Ti- and F-containing glasses and glass ceramics in a manner consistent with enhancing our ability to understand the individual and interaction effects between elements in order to predict material properties.
Although the primary inclusion criterion for this review was the incorporation of both Ti and F in the glasses/glass ceramics under investigation, the overwhelming majority of articles in the literature characterized the effects of only one of these constituents, primarily TiO2, on material properties. Specifically, just two glass articles reported findings on the effect of both Ti and F addition on one material, and these articles were published by the same author [58,59]. There were no glass-ceramic articles that investigated the effects of both Ti and F content within the same system. This is a result of employing trial and error and/or one variable at a time (OVAT) approaches to examining the influence of Ti and/or F substitutions in glass networks. These non-systematic approaches, whilst valuable to the literature, overlook the complexity of multi-component glasses and the likelihood for elements to interact with each other, forming synergistic relationships within the network that may influence material and host responses. These approaches complicate our ability to determine the individual and interaction effects of multiple glass constituents (e.g., Ti and F), especially in systems comprising numerous cations and anions with variable valences and cationic field strengths. As a result of the current literature teachings, it is extremely difficult to anticipate and understand the composition–structure–property relationships that arise from the inclusion of Ti and F (both individually and interactively) across broad compositional ranges. This, in turn, leads to long lead times in the development and commercial use of these materials [78]. Thus, based on the wide breadth of applicability for Ti-and F-containing glasses and glass ceramics, a more comprehensive understanding of these materials would be of extreme value in future materials design and development. As such, an approach that can simultaneously discover the effects of individual factors on material and host responses, as well as the synergistic relationships between interacting constituents, is required.
In this regard, the Materials Genome Initiative (MGI) has advanced a new paradigm for accelerated materials discovery [78]. Specifically, by combining experiment, theory, and computation in a systematic, high-throughput manner, we can strengthen our ability to support the a priori prediction of new materials with desirable physical, chemical, mechanical, and biological characteristics. One approach that is capable of characterizing these materials in a manner commensurate with the MGI is the Design of Mixtures (DoM) statistical modelling approach. The DoM approach has been employed across many industries to allow for unambiguous, systematic evaluations of the individual and interaction effects associated with various mixture components. This approach allows for the development of polynomial equations that indicate the relative influences of components on a given response and ultimately support the optimization of materials to a wide variety of properties via response surface regression methodologies [79,80,81,82,83]. These optimization studies can be used to correlate theoretical predictions to actual experimental results using minimal time and resources. This form of predictive modelling stands in contrast to traditional trial-and-error style approaches for materials discovery and has the potential to accelerate the design of glass and glass-ceramic materials via the simultaneous use of experimental methods and advanced modelling [78]. Specifically, the DoM approach can be employed to produce quantitatively predictive models relating to the composition–structure–property relationships in Ti- and F-containing glasses and glass ceramics with distinct compositions and variable glass-forming systems. By employing a simulation, modelling, and machine learning approach, these theoretical predictions can contribute to diverse digital datasets that can be accessible to researchers across the globe, thus encouraging collaboration and enhanced learning. As such, this program will enable the prediction of preferred material chemistries for a wide range of applications spanning nuclear, optical, electrochemical, dental, and industrial fields. This provides an exciting opportunity to contribute new knowledge to the broad scientific community surrounding the composition–structure–property relationships of glasses and glass ceramics modified with Ti and F.
6. Conclusions
There exist several ambiguities in the literature with respect to the effects that both Ti and F have on the properties of glasses and glass ceramics (individually and interactively). Specifically, the current literature lacks consistency, repeatability, and complete data on a range of glass chemistries and broad compositional ranges of Ti and F. Additionally, there is a discernible lack of information available as it pertains to the characterization of Ti- and F-containing glasses and glass ceramics for use in biomedical indications. Regrettably, the foundation for our existing knowledge of these materials has been based on the use of traditional non-systematic approaches. These approaches confound our understanding, lead to conflicting literature, and complicate our ability to determine the individual and interaction effects of multiple glass constituents. As a result, we are currently unable to accurately predict the effects of Ti and F on the physical, chemical, mechanical, and biological properties of glasses and glass ceramics with varying glass chemistries. This precludes the streamlined discovery, development, and deployment of these materials. To provide a more comprehensive understanding of glass and glass-ceramic systems modified with Ti and F and to support the accelerated design of these materials for use in a variety of indications, a systematic experimental approach, such as the Design of Mixtures approach, is strongly recommended.
Author Contributions
Conceptualization: B.K. and D.B.; Methodology: B.K. and D.B.; Investigation: B.K.; Formal analysis: B.K. and D.B.; Writing—Original Draft: B.K. and D.B.; Writing—Reviewing and Editing: B.K. and D.B.; Visualization: B.K.; Project Administration: B.K. and D.B.; Validation: B.K. and D.B.; Resources: D.B.; Supervision: D.B.; Funding acquisition: D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant number 2022-03371.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We acknowledge the support of the Dalhousie Medical Research Foundation (DMRF).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Brovarone, C.V. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Hoppe, A.; Boccaccini, A.R. Biological Impact of Bioactive Glasses and Their Dissolution Products. Front. Oral Biol. 2015, 17, 22–32. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 1–41. [Google Scholar] [CrossRef]
- Limbach, R.; Karlsson, S.; Scannell, G.; Mathew, R.; Edén, M.; Wondraczek, L. The effect of TiO2 on the structure of Na2O-CaO-SiO2 glasses and its implications for thermal and mechanical properties. J. Non-Cryst. Solids 2017, 471, 6–18. [Google Scholar] [CrossRef]
- Wren, A.; Laffir, F.; Kidari, A.; Towler, M. The structural role of titanium in Ca–Sr–Zn–Si/Ti glasses for medical applications. J. Non-Cryst. Solids 2011, 357, 1021–1026. [Google Scholar] [CrossRef]
- Alajerami, Y.S.M.; Hashim, S.; Hassan, W.M.S.W.; Ramli, A.T. The effect of titanium oxide on the optical properties of lithium potassium borate glass. J. Mol. Struct. 2012, 1026, 159–167. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glass Making; Elsevier Inc.: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Lakshmi, N.R.; Cole, S. Influence of TiO2 ions on Spectroscopic Properties of Oxyfluoride Glasses. Mater. Today Proc. 2019, 18, 192–206. [Google Scholar] [CrossRef]
- Rao, M.; Kumar, G.R. Influence of TiO2 on structural, luminescent and conductivity investigations of CaF2-CaO-Y2O3-B2O3-P2O5 glasses. Optik 2018, 179, 1109–1117. [Google Scholar] [CrossRef]
- Guntu, R.K.; Jahangeer, N.; Rao, C.S. Thermoluminescence, elastic and dielectric investigations of calcium fluoro borophosphate glass composite materials doped by small concentrations of TiO2. Indian J. Phys. 2021, 95, 579–586. [Google Scholar] [CrossRef]
- Ayta, W.; Silva, V.; Cano, N.; Silva, M.; Dantas, N. Thermoluminescence, structural and magnetic properties of a Li2O–B2O3–Al2O3 glass system doped with LiF and TiO2. J. Lumin. 2011, 131, 1002–1006. [Google Scholar] [CrossRef]
- Lu, M.; Wang, F.; Liao, Q.; Chen, K.; Qin, J.; Pan, S. FTIR spectra and thermal properties of TiO2-doped iron phosphate glasses. J. Mol. Struct. 2015, 1081, 187–192. [Google Scholar] [CrossRef]
- Kasumova, R.N.; Bananyarly, S.I. Spectroscopic and Attenuation Shielding Studies on B2O3-SiO2-LiF-ZnO-TiO2 Glasses. Silicon 2005, 41, 339–340. [Google Scholar]
- Kiprianov, A.A.; Karpukhina, N.G. Oxyhalide silicate glasses. Glas. Phys. Chem. 2006, 32, 1–27. [Google Scholar] [CrossRef]
- Stamboulis, A.; Hill, R.G.; Law, R.V. Structural characterization of fluorine containing glasses by 19F, 27Al, 29Si and 31P MAS–NMR spectroscopy. J. Non-Cryst. Solids 2005, 351, 3289–3295. [Google Scholar] [CrossRef]
- Christie, J.K.; Pedone, A.; Menziani, M.C.; Tilocca, A. Fluorine Environment in Bioactive Glasses: Ab Initio Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 2038–2045. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Cortada, M.; Menabue, L.; Menziani, M.C.; Pedone, A.; Segre, U. Elucidation of the Structural Role of Fluorine in Potentially Bioactive Glasses by Experimental and Computational Investigation. J. Phys. Chem. B 2008, 112, 12730–12739. [Google Scholar] [CrossRef]
- Tilocca, A. Models of structure, dynamics and reactivity of bioglasses: A review. J. Mater. Chem. 2010, 20, 6848–6858. [Google Scholar] [CrossRef]
- Krishna, G.M.; Veeraiah, N.; Venkatramaiah, N.; Venkatesan, R. Induced crystallization and physical properties of Li2O–CaF2–P2O5:TiO2 glass system: Part I. Characterization, spectroscopic and elastic properties. J. Alloys Compd. 2008, 450, 477–485. [Google Scholar] [CrossRef]
- Zheng, W.; Cui, J.; Sheng, L.; Chao, H.; Peng, Z.; Shen, C. Effect of complex nucleation agents on preparation and crystallization of CaO-MgO-Al2O3-SiO2 glass-ceramics for float process. J. Non-Cryst. Solids 2016, 450, 6–11. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Michalski, P.P.; Wasiucionek, M.; Garbarczyk, J.E. Synthesis of nanostructured Li3Me2(PO4)2F3 glass-ceramics (Me = V, Fe, Ti). Solid State Ion. 2016, 288, 193–198. [Google Scholar] [CrossRef]
- Schulz, C.; Miehe, G.; Fuess, H.; Wange, P.; Götz, W. X-ray powder diffraction of crystalline phases in phosphate bioglass ceramics. Z. Krist.-Cryst. Mater. 1994, 209, 249–255. [Google Scholar] [CrossRef]
- Antonious, M.S. Effect of additives on the crystallization of barium sulphate. Phosphorus Sulfur Silicon Relat. Elem. 1996, 112, 235–245. [Google Scholar] [CrossRef]
- Guo, X.; Cai, X.; Song, J.; Yang, G.; Yang, H. Crystallization and microstructure of CaO–MgO–Al2O3–SiO2 glass–ceramics containing complex nucleation agents. J. Non-Cryst. Solids 2014, 405, 63–67. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. Influence of TiO2 content on the crystallization and microstructure of machinable glass-ceramics. J. Asian Ceram. Soc. 2016, 4, 55–60. [Google Scholar] [CrossRef]
- Kasuga, T.; Ueno, E.; Obata, A. Preparation of Apatite-Containing Calcium Phosphate Glass-Ceramics. Key Eng. Mater. 2007, 330–332, 157–160. [Google Scholar] [CrossRef]
- Kasuga, T.; Kimata, T.; Obata, A. Preparation of a Calcium Titanium Phosphate Glass-Ceramic with Improved Chemical Durability. J. Am. Ceram. Soc. 2009, 92, 1709–1712. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Featherstone, J. Mechanistic Aspects of the Interactions Between Fluoride and Dental Enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Nasker, P.; Mukherjee, M.; Kant, S.; Tripathy, S.; Sinha, A.; Das, M. Fluorine substituted nano hydroxyapatite: Synthesis, bio-activity and antibacterial response study. Ceram. Int. 2018, 44, 22008–22013. [Google Scholar] [CrossRef]
- Walmsley, A.D.; Walsh, T.F.; Lumley, P.; Burke, F.T.; Shortall, A.C.; Hayes-Hall, R.; Pretty, I. Chapter 8—Restorations of teeth (simple restorations) and preventative dentistry. In Restorative Dentistry, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 73–87. [Google Scholar]
- Lussi, A.; Carvalho, T.S. The Future of Fluorides and Other Protective Agents in Erosion Prevention. Caries Res. 2015, 49 (Suppl. 1), 18–29. [Google Scholar] [CrossRef] [PubMed]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Marquis, R.E. Oral Bacteria. Can. J. Microbiol. 1995, 964, 955–964. [Google Scholar] [CrossRef]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Inkielewicz-Stępniak, I. Impact of fluoxetine on liver damage in rats. Pharmacol. Rep. 2011, 63, 441–447. [Google Scholar] [CrossRef]
- Sun, S.; Adejare, A. Fluorinated molecules as drugs and imaging agents in the CNS. Curr. Top. Med. Chem. 2006, 6, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Zierden, M.R.; Valentine, A.M. Contemplating a role for titanium in organisms. Metallomics 2016, 8, 9–16. [Google Scholar] [CrossRef]
- Katiyar, N.K.; Goel, G.; Hawi, S.; Goel, S. Nature-inspired materials: Emerging trends and prospects. NPG Asia Mater. 2021, 13, 56. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Chrzanowski, W.; Knowles, J.C. Biological performance of titania containing phosphate-based glasses for bone tissue engineering applications. Mater. Sci. Eng. C 2014, 35, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Buettner, K.M.; Valentine, A.M. Bioinorganic Chemistry of Titanium. Chem. Rev. 2012, 112, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Wahengbam, P.; Lee, W.B.; Tikku, A. Role of titanium tetrafluoride (TiF4) in conservative dentistry: A systematic review. J. Conserv. Dent. 2011, 14, 98–102. [Google Scholar] [CrossRef] [PubMed]
- McCann, H. The effect of fluoride complex formation on fluoride uptake and retention in human enamel. Arch. Oral Biol. 1969, 14, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Alexandria, A.K.; Nassur, C.; Nóbrega, C.B.C.; Valença, A.M.G.; Rosalen, P.L.; Maia, L.C. In situ effect of titanium tetrafluoride varnish on enamel demineralization. Braz. Oral Res. 2017, 31, e86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, X.; Shang, J.; Jin, Y.; Zhang, C. Photocatalytic Reduction of CO2 Using Titanium-Substituted and Fluorine-Doped Titanium-Substituted Hydroxyapatite as Photocatalysts. Catal. Lett. 2017, 147, 2706–2713. [Google Scholar] [CrossRef]
- Ergun, C. Effect of Ti ion substitution on the structure of hydroxylapatite. J. Eur. Ceram. Soc. 2008, 28, 2137–2149. [Google Scholar] [CrossRef]
- Hu, A.; Li, M.; Chang, C.; Mao, D. Preparation and characterization of a titanium-substituted hydroxyapatite photocatalyst. J. Mol. Catal. A Chem. 2007, 267, 79–85. [Google Scholar] [CrossRef]
- Woo, H.; Kang, S. A study on the heat treatment effect upon luminous properties of oxy-fluoride glass doped with TiO2. J. Korean Cryst. Growth Cryst. Technol. 2020, 30, 232–236. [Google Scholar]
- Li, J.; Shu, Q.; Chou, K. Structural Study of Glassy CaO–SiO2–CaF2–TiO2 Slags by Raman Spectroscopy and MAS-NMR. ISIJ Int. 2014, 54, 721–727. [Google Scholar] [CrossRef]
- Bogomolova, L.; Krasil’Nikova, N.; Bogdanov, V.; Khalilev, V.; Mitrofanov, V. Electron paramagnetic resonance of transition metal ions in fluorogermanate glasses. J. Non-Cryst. Solids 1995, 188, 130–135. [Google Scholar] [CrossRef]
- Bessada, C.; Anghel, E.M. Nuclear magnetic resonance studies of the Na2B4O7-Na3AlF6-TiO2 system. In Proceedings of the International Symposium on Molten Salt Chemistry and Technology, Shanghai, China, 9–13 October 2001; pp. 65–68. [Google Scholar]
- Ehrt, D.; Leister, M.; Matthai, A. Polyvalent elements iron, tin and titanium in silicate, phosphate and fluoride glasses and melts. Phys. Chem. Glas. 2001, 42, 231–239. [Google Scholar]
- Hong, L.; Qing, Y.; Ning, H. Influence of Fluorine on the Structure and Luminescence Properties of Sm3+ Doped Strontium Titanium Silica Glass. Key Eng. Mater. 2012, 509, 273–278. [Google Scholar] [CrossRef]
- Neelima, G.; Kummara, V.K.; Viswanath, C.D.; Tyagarajan, K.; Ravi, N.; Prasad, T.J. Photoluminescence of terbium doped oxyfluoro-titania-phosphate glasses for green light devices. Ceram. Int. 2018, 44, 15304–15309. [Google Scholar] [CrossRef]
- Chen, W.; Chen, K.; Mao, J. Effect of TiO2 on the Mechanical Properties of the K2O-MgO-SiO2-CaF2 Glass-Ceramics. Key Eng. Mater. 1998, 161–163, 185–190. [Google Scholar] [CrossRef]
- Harris, E. The nature of Ti3+-centers in a zirconium fluoride glass. Phys. Chem. Glas. 1987, 28, 12–114. [Google Scholar]
- Deubener, J.; Allix, M.; Davis, M.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Anghel, E.M.; Florian, P.; Bessada, C. Structural Description of the Na2B4O7-Na3AlF6-TiO2 System. 1. IR and Raman Study of the Solidified Melts. J. Phys. Chem. 2007, 111, 962–967. [Google Scholar] [CrossRef]
- Florian, P.; Anghel, E.M.; Bessada, C. Structural Description of the Na2B4O7-Na3AlF6-TiO2 System. 2. A Multinuclear NMR Approach of Melts and Solids. J. Phys. Chem. B 2007, 111, 968–978. [Google Scholar] [CrossRef]
- Ignat’Eva, L.N.; Polishchuk, S.A.; Antokhina, T.F.; Buznik, V.M. IR Spectroscopic Study of the Structure of Glasses Based on Titanium Oxyfluoride. Glas. Phys. Chem. 2004, 30, 139–141. [Google Scholar] [CrossRef]
- Krishna, G.M.; Veeraiah, N.; Venkatramaiah, N.; Venkatesan, R. Induced crystallization and physical properties of Li2O–CaF2–P2O5:TiO2 glass system: Part II. Electrical, magnetic and optical properties. J. Alloys Compd. 2008, 450, 486–493. [Google Scholar] [CrossRef]
- Krishna, G.M.; Gandhi, Y.; Veeraiah, N. Luminescence spectroscopy of Ti ions in Li2O–CaF2–P2O5 glass ceramics. J. Lumin. 2008, 128, 631–634. [Google Scholar] [CrossRef]
- Yu, X.; Duan, L.; Ni, L.; Wang, Z. Fabrication and luminescence behavior of phosphate glass ceramics co-doped with Er3+ and Yb3+. Opt. Commun. 2012, 285, 3805–3808. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Kruk-Fura, P.E.; Mikoåcajczuk, P.J.; Garbarczyk, J.E. Syntheses and nanocrystallization of NaF–M2O3–P2O5 NASICON-like phosphate glasses (M = V, Ti, Fe). Int. J. Appl. Glas. Sci. 2020, 11, 87–96. [Google Scholar] [CrossRef]
- Takav, P.; Banijamali, S.; Zadeh, A.S.A.H.; Mobasherpour, I. Influence of TiO2 content on phase evolution, microstructure and properties of fluorcanasite glass-ceramics prepared through sintering procedure for dental restoration applications. Ceram. Int. 2018, 44, 7057–7066. [Google Scholar] [CrossRef]
- Fathi, H.M.; Johnson, A. The effect of TiO2 concentration on properties of apatite-mullite glass-ceramics for dental use. Dent. Mater. 2016, 32, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Y. On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials 2004, 25, 3379–3387. [Google Scholar] [CrossRef]
- Brow, W.W.R.; Tallant, D. Spectroscopic Studies of the Structure of Titanophosphate and Calcium Titanophosphate Glasses. Phys. Chem. Glas. 1997, 38, 6. [Google Scholar]
- Zanotto, E.; Coutinho, F. How many non-crystalline solids can be made from all the elements of the periodic table? J. Non-Cryst. Solids 2004, 347, 285–288. [Google Scholar] [CrossRef]
- Maine, E.; Seegopaul, P. Accelerating advanced-materials commercialization. Nat. Mater. 2016, 15, 487–491. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, J.J.; Jackson, N.E.; Webb, M.A.; Chen, L.-Q.; Moore, J.E.; Morgan, D.; Jacobs, R.; Pollock, T.; Schlom, D.G.; Toberer, E.S.; et al. New frontiers for the materials genome initiative. Npj Comput. Mater. 2019, 5, 41. [Google Scholar] [CrossRef]
- Piepel, G.F.; Cornell, J.A. Mixture Experiment Approaches: Examples, Discussion, and Recommendations. J. Qual. Technol. 1994, 26, 177–196. [Google Scholar] [CrossRef]
- Bowden, G.D.; Pichler, B.J.; Maurer, A. A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes. Sci. Rep. 2019, 9, 11370. [Google Scholar] [CrossRef]
- Tye, H. Application of statistical ‘design of experiments’ methods in drug discovery. Drug Discov. Today 2004, 9, 485–491. [Google Scholar] [CrossRef]
- Murray, P.M.; Tyler, S.N.G.; Moseley, J.D. Beyond the Numbers: Charting Chemical Reaction Space. Org. Process. Res. Dev. 2013, 17, 40–46. [Google Scholar] [CrossRef]
- Kehoe, S.; Ardhaoui, M.; Stokes, J. Design of Experiments Study of Hydroxyapatite Synthesis for Orthopaedic Application Using Fractional Factorial Design. J. Mater. Eng. Perform. 2011, 20, 1423–1437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).