Research Progress in Special Engineering Plastic-Based Electrochromic Polymers
Abstract
:1. Introduction
2. Special Engineering Plastic-Based Electrochromic Polymers (SPECPs)
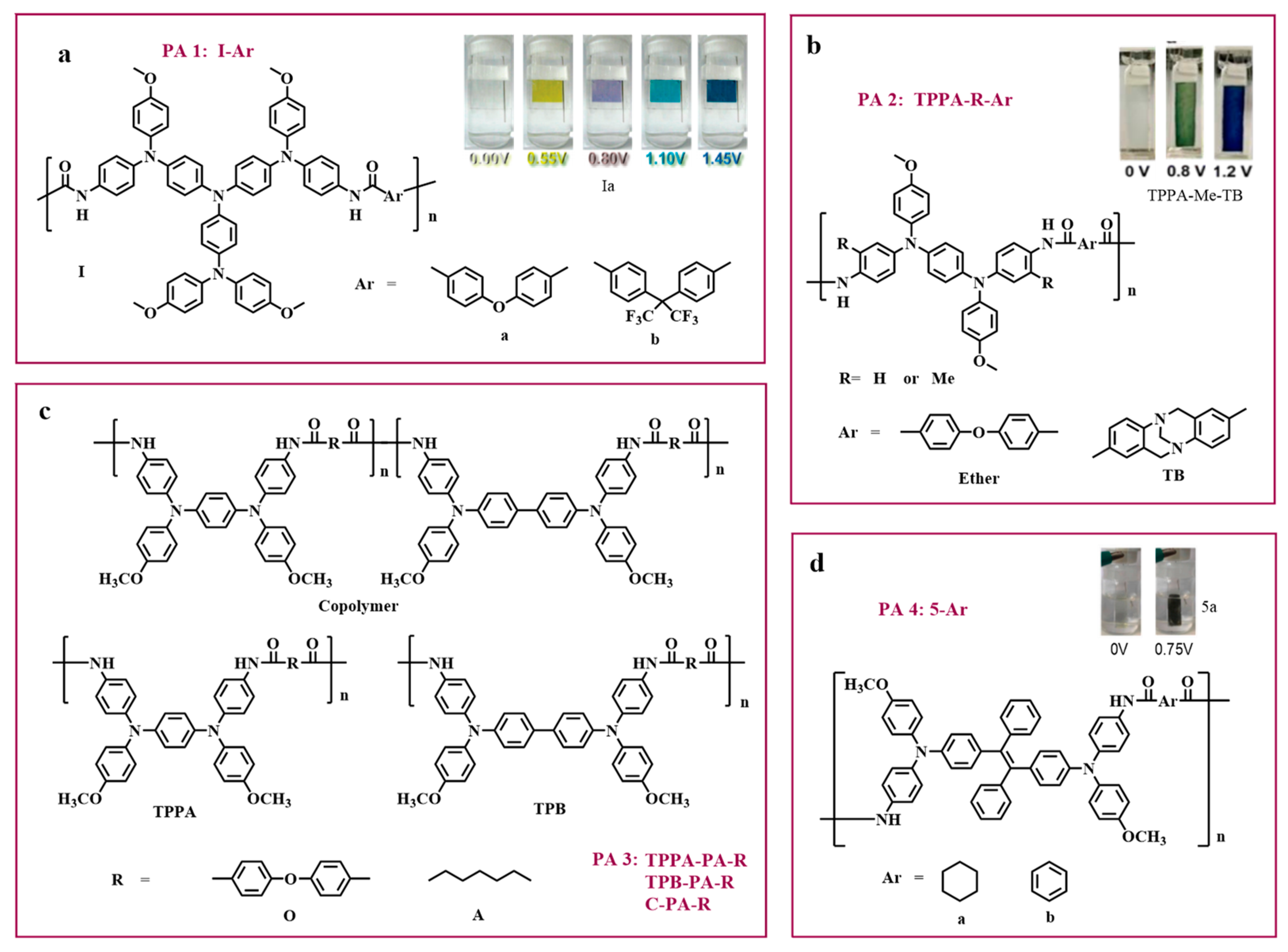
2.1. Polyamide-Imide Type Electrochromic Polymers (PAI–SPECPs)
2.2. Polyimide Type Electrochromic Polymer (PI–ECPs)
2.3. Polyamide-Type Electrochromic Polymers (PA–ECPs)
2.4. Polyarylketone Type Electrochromic Polymers (PAK–ECPs)
2.5. Polyarylsulfone Type Electrochromic Polymers (PAS–ECPs)
2.5.1. PAES–ECPs
2.5.2. PAAS–ECPs
3. Multifunctional Applications of SPECPs
3.1. Memory Device
3.2. Supercapacitor
3.3. Electrofluorochromism
3.4. Infrared Stealth
3.5. Other Functions
4. Challenges and Recommendations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, C.; Jia, A.B.; Zhang, Y.M.; Zhang, S.X. Emerging Electrochromic Materials and Devices for Future Displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Sheng, S.Z.; He, Z.; Wang, R.; Pan, Z.; Zhao, H.Y.; Liu, J.W.; Yu, S.H. Self-Powered Flexible Electrochromic Smart Window. Nano Lett. 2021, 21, 9976–9982. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Wang, Y.; Lin, Y.; Jia, C. Fully Integrated Pressure-Controlled Electrochromic E-skins. J. Mater. Chem. A 2021, 9, 9134–9144. [Google Scholar] [CrossRef]
- Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic Conjugated Polymers for Multifunctional Smart Windows with Integrative Functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Ling, Y.; Li, L.; Liu, J.; Li, K.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Air-Working Electrochromic Artificial Muscles. Adv. Mater. 2023, 2305914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, J.; Sun, F.; Zhou, D.; Cao, G.; Wang, S.; Hu, X.; Ma, J.; Su, F.; Tian, Y.; et al. Self-Driven Ni-Based Electrochromic Devices for Energy-Efficient Smart Windows. Adv. Mater. Technol. 2023, 8, 2201688. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Y.; Zhao, J.; Fan, Z.; Ding, B.; Lee, J.Y.; Zhang, X.; Xuan, Y. Amorphous and Porous Tungsten Oxide Films for Fast-Switching Dual-Band Electrochromic Smart Windows. Adv. Opt. Mater. 2022, 11, 2202115. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, R.; Shao, P.; Zhang, Y.; Wen, R.T. Electrochromic Performance Fading and Restoration in Amorphous TiO2 Thin Films. Adv. Opt. Mater. 2022, 10, 2200903. [Google Scholar] [CrossRef]
- Atak, G.; Bayrak Pehlivan, İ.; Montero, J.; Granqvist, C.G.; Niklasson, G.A. Electrochromic Tungsten Oxide Films Prepared by Sputtering: Optimizing Cycling Durability by Judicious Choice of Deposition Parameters. Electrochim. Acta 2021, 367, 137233. [Google Scholar] [CrossRef]
- Tutel, Y.; Durukan, M.B.; Hacioglu, S.O.; Baskose, U.C.; Toppare, L.; Unalan, H.E. Cobalt-doped MoO3 Thin Films and Dual-band Electrochromic Devices with Excellent Cyclic Stability. Appl. Mater. Today 2023, 35, 101924. [Google Scholar] [CrossRef]
- Kim, G.; Hong, S.; Yoo, S.; Park, J. Solution-Processed All-Solid-State Electrochromic Devices Based on SnO2/NiO doped with Tin. Coatings 2021, 11, 1431. [Google Scholar] [CrossRef]
- Pande, G.K.; Kim, D.Y.; Sun, F.; Pal, R.; Park, J.S. Photocurable Allyl Viologens Exhibiting RGB-to-Black Electrochromic Switching for Versatile Heat-shielding Capability. Sol. Energy Mater. Sol. Cells 2023, 263, 112579. [Google Scholar] [CrossRef]
- Feng, F.; Guo, S.; Ma, D.; Wang, J. An Overview of Electrochromic Devices with Electrolytes Containing Viologens. Sol. Energy Mater. Sol. Cells 2023, 254, 112270. [Google Scholar] [CrossRef]
- Özcan, S.; Kobak, R.Z.; Budak, Ö.; Koca, A.; Bayır, Z.A. Synthesis, Electrochemistry, Spectroelectrochemistry, and Electrochromism of Metallophthalocyanines Substituted with Four (2,4,5-Trimethylphenyl)ethynyl Groups. Electroanalysis 2022, 34, 1610–1620. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Ahmad, R.; Ebralidze, I.I.; Poisson, J.; Easton, E.B.; Zenkina, O.V. Multichromic Monolayer Terpyridine-Based Electrochromic Materials. ACS Appl. Mater. Interfaces 2020, 12, 41749–41757. [Google Scholar] [CrossRef]
- Ghosh, T.; Kandpal, S.; Rani, C.; Bansal, L.; Tanwar, M.; Kumar, R. Multiwavelength Color Switching from Polyaniline-Viologen Bilayer: Inching toward Versatile All-Organic Flexible Electrochromic Device. Adv. Electron. Mater. 2022, 9, 2201042. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Q.; Luo, X.; Ma, H.; Zheng, W.; Yu, J.; Zhang, Z.; Zhang, K.; Qu, K.; Yang, R.; et al. Low-Cost Fabrication of High-Performance Fluorinated Polythiophene-Based Vis–NIR Electrochromic Devices toward Deformable Display and Camouflage. Chem. Mater. 2022, 34, 9923–9933. [Google Scholar] [CrossRef]
- Yang, B.; Ma, D.; Zheng, E.; Wang, J. A Self-Rechargeable Electrochromic Battery Based on Electrodeposited Polypyrrole Film. Sol. Energy Mater. Sol. Cells 2019, 192, 1–7. [Google Scholar] [CrossRef]
- Meng, H. Organic Electronics for Electrochromic Materials and Devices; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 531. [Google Scholar]
- Jarosz, T.; Gebka, K.; Stolarczyk, A.; Domagala, W. Transparent to Black Electrochromism—The “Holy Grail” of Organic Optoelectronics. Polymers 2019, 11, 273. [Google Scholar] [CrossRef]
- Wagner, J.S.; Smith, E.; Bacsa, J.; Tomlinson, A.L.; Reynolds, J.R. Color Control in Bis-ethylenedioxythiophene Phenylene Anodically Coloring Electrochromes. Chem. Mater. 2023. [Google Scholar] [CrossRef]
- Christiansen, D.T.; Ohtani, S.; Chujo, Y.; Tomlinson, A.L.; Reynolds, J.R. All Donor Electrochromic Polymers Tunable across the Visible Spectrum via Random Copolymerization. Chem. Mater. 2019, 31, 6841–6849. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-Processable Triarylamine-Based Electroactive High Performance Polymers for Anodically Electrochromic Applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Yu, G.; Hou, Y.; Niu, H. Electrochromism of Novel Triphenylamine-Containing Polyamide Polymers. J. Appl. Polym. Sci. 2018, 136, 47264. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Solodukhin, A.N.; Chuyko, I.A.; Peregudova, S.M.; Ponomarenko, S.A. Highly Electrochemically and Thermally Stable Donor–π–Acceptor Triphenylamine-based Hole-transporting Homopolymersviaoxidative Polymerization. New J. Chem. 2022, 46, 12311–12317. [Google Scholar] [CrossRef]
- Yu, T.; Theato, P.; Yao, H.; Liu, H.; Di, Y.; Sun, Z.; Guan, S. Colorless Electrochromic/Electrofluorochromic Dual-Functional Triphenylamine-Based Polyimides: Effect of a Tetraphenylethylene-Based π-Bridge on optoelectronic properties. Chem. Eng. J. 2023, 451, 138441. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Hsiao, S.-H.; Su, T.-H.; Liou, G.-S. Novel Electrochromic Aromatic Poly(Amine–Amide–Imide)s with Pendent Triphenylamine Structures. Polymer 2005, 46, 5939–5948. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Fang, Y.-K. Electrochromic Properties of Novel Strictly Alternating Poly(Amine–Amide–Imide)s with Electroactive Triphenylamine Moieties. Eur. Polym. J. 2006, 42, 1533–1540. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Multicolor Electrochromic Poly(Amide-Imide)s with N,N-Diphenyl-N′,N′-Di-4-Tert-Butylphenyl-1,4-Phenylenediamine Moieties. Polym. Chem. 2010, 1, 1013–1023. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Guo, W.; Kung, Y.-C.; Lee, Y.-J. Redox-Active and Electrochromic Aromatic Poly(Amide-Imide)S with 2,4-Dimethoxytriphenylamine Chromophores. J. Polym. Res. 2010, 18, 1353–1364. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liu, N.-E.; Kung, Y.-R. Synthesis of Electroactive and Electrochromic Poly(Amide-Imide)s Containing Diphenylpyrenylamine Moieties. J. Polym. Res. 2015, 22, 9. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, C.-Y.; Li, L.-J.; Liaw, D.-J. Colorless-to-Colorful Switching Electrochromic Polyimides with Very High Contrast Ratio. Nat. Commun. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-S.; Hsiao, S.-H.; Chen, H.-W. Novel High-Tg Poly(Amine-Imide)s Bearing Pendent N-Phenylcarbazole Units: Synthesis and Photophysical, Electrochemical and Electrochromic Properties. J. Mater. Chem. 2006, 16, 1831–1842. [Google Scholar] [CrossRef]
- Chang, C.-W.; Yen, H.-J.; Huang, K.-Y.; Yeh, J.-M.; Liou, G.-S. Novel Organosoluble Aromatic Polyimides Bearing Pendant Methoxy-Substituted Triphenylamine Moieties: Synthesis, Electrochromic, and Gas Separation Properties. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7937–7949. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Electrochemically and Electrochromically Stable Polyimides Bearing Tert-Butyl-Blocked N,N,N′,N′-Tetraphenyl-1,4-Phenylenediamine Units. Polymer 2009, 50, 1692–1699. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.-C.; Pan, H.-Y.; Kuo, C.-H. Electroactive Aromatic Polyamides and Polyimides with Adamantylphenoxy-Substituted Triphenylamine Units. Eur. Polym. J. 2009, 45, 2234–2248. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Substituent Effects on Electrochemical and Electrochromic Properties of Aromatic Polyimides with 4-(Carbazol-9-yl)Triphenylamine Moieties. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1172–1184. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R. Synthesis and Characterization of New Redox-Active and Electrochromic Polyimides with (4-Morpholinyl)Triphenylamine Units. J. Electroanal. Chem. 2016, 764, 31–37. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, Y.-Z. Electrochemical Synthesis of Stable Ambipolar Electrochromic Polyimide Film from a Bis(Triphenylamine) Perylene Diimide. J. Electroanal. Chem. 2017, 799, 417–423. [Google Scholar] [CrossRef]
- Yen, H.J.; Tsai, C.L.; Chen, S.H.; Liou, G.S. Electrochromism and Nonvolatile Memory Device Derived from Triphenylamine-Based Polyimides with Pendant Viologen Units. Macromol. Rapid Commun. 2017, 38, 1600715. [Google Scholar] [CrossRef]
- Yen, H.-J.; Chen, C.-J.; Liou, G.-S. Flexible Multi-Colored Electrochromic and Volatile Polymer Memory Devices Derived from Starburst Triarylamine-Based Electroactive Polyimide. Adv. Funct. Mater. 2013, 23, 5307–5316. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yen, H.-J.; Hu, Y.-C.; Liou, G.-S. Novel Programmable Functional Polyimides: Preparation, Mechanism of CT Induced Memory, and Ambipolar Electrochromic Behavior. J. Mater. Chem. C 2013, 1, 7623–7634. [Google Scholar] [CrossRef]
- Sun, N.; Meng, S.; Zhou, Z.; Chao, D.; Yu, Y.; Su, K.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. Electroactive (A3+B2)-Type Hyperbranched Polyimides with Highly Stable and Multistage Electrochromic Behaviors. Electrochim. Acta 2017, 256, 119–128. [Google Scholar] [CrossRef]
- Su, K.; Sun, N.; Tian, X.; Guo, S.; Yan, Z.; Wang, D.; Zhou, H.; Zhao, X.; Chen, C. Highly Soluble Polyimide Bearing Bulky Pendant Diphenylamine-Pyrene for Fast-Response Electrochromic and Electrofluorochromic Applications. Dye. Pigment. 2019, 171, 107668. [Google Scholar] [CrossRef]
- Gao, Y.; Zhai, M.; Sui, Y.; Li, D.; Lin, X.; Pan, S.; Pan, Q.; Niu, H.; Wang, W. Multifunctional Polyimides Containing Triarylamine for Electrochromic Flexible Device, Photodetector and Resistance Memory Device. Multifunct. Mater. 2021, 4, 034002. [Google Scholar] [CrossRef]
- Zheng, R.-R.; Huang, T.; Niu, H.-J.; Wang, C.; Chang, H.-Y.; Sun, Z.-Y.; Wang, L.-Y.; Guo, L.-Y.; Zhang, Z.-P.; Zhang, S. Multifunctional Flexible Polyimides for Electroactive Devices with Electrochromic, Electrofluorochromic, and Photodetection Properties. ACS Appl. Polym. Mater. 2021, 3, 1338–1348. [Google Scholar] [CrossRef]
- Huang, L.-T.; Yen, H.-J.; Liou, G.-S. Substituent Effect on Electrochemical and Electrochromic Behaviors of Ambipolar Aromatic Polyimides Based on Aniline Derivatives. Macromolecules 2011, 44, 9595–9610. [Google Scholar] [CrossRef]
- Yen, H.-J.; Guo, S.-M.; Yeh, J.-M.; Liou, G.-S. Triphenylamine-Based Polyimides with Trimethyl Substituents for Gas Separation Membrane and Electrochromic Applications. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3637–3646. [Google Scholar] [CrossRef]
- Constantin, C.-P.; Damaceanu, M.-D. A Refreshing Perspective on Electrochromic Materials: Phenoxazine as an Opportune Moiety Towards Stable and Efficient Electrochromic Polyimides. Chem. Eng. J. 2023, 465, 142883. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Zhu, J.; Bai, X.; Jiang, X.; Zhang, Q.; Niu, H. High Coloration Efficiency and Fast Switching Speed of Poly(Amic Acid-Imide)S Containing Triphenylamine in Acidic Electrolyte. RSC Adv. 2015, 5, 11071–11076. [Google Scholar] [CrossRef]
- Cai, S.; Niu, H. Synthesis and Characterization of Triarylamine-Based Polyimides for Electrochromic and Optoelectronic Conversion Behaviors. J. Appl. Polym. Sci. 2019, 137, 48808. [Google Scholar] [CrossRef]
- Zheng, R.; Huang, T.; Zhang, Z.; Sun, Z.; Niu, H.; Wang, C.; Wang, W. Novel Polyimides Containing Flexible Carbazole Blocks with Electrochromic and Electrofluorescencechromic Properties. RSC Adv. 2020, 10, 6992–7003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, X.; Sun, B.; Huang, R.; Wang, C.; Chao, D. A Piezoelectric-Driven Electrochromic/Electrofluorochromic Dual-Modal Display Device. Small 2023, 19, e2301886. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xia, S.; Zhai, M.; Zhang, C.; Hong, T.; Zhang, B.; Cai, W.A.; Niu, H.; Wang, W. Non-Planar Polyimides with Monomer Containing Four Triphenylamines for Near-Infrared Electrochromism and TNP Detection. J. Appl. Polym. Sci. 2023, 140, e54377. [Google Scholar] [CrossRef]
- Bejan, A.E.; Constantin, C.P.; Damaceanu, M.D. Evidence of Diimide Structure Variation on Overall Performance of Electro(fluoro)chromic Devices Integrating Versatile Triphenylamine-Based Polyimides. Mater. Today Chem. 2022, 26, 101100. [Google Scholar] [CrossRef]
- Kung, Y.C.; Liou, G.S.; Hsiao, S.H. Synthesis and Characterization of Novel Electroactive Polyamides and Polyimides with Bulky 4-(1-Adamantoxy)Triphenylamine Moieties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1740–1755. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Triphenylamine-Based Redox-Active Aramids with 1-Piperidinyl Substituent as an Auxiliary Donor: Enhanced Electrochemical Stability and Electrochromic Performance. React. Funct. Polym. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsiao, Y.H.; Kung, Y.R. Highly Redox-Stable and Electrochromic Aramids with Morpholinyl-Substituted Triphenylamine Units. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 1289–1298. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Flexible Electrofluorochromic Devices with the Highest Contrast Ratio Based on Aggregation-Enhanced Emission (AEE)-Active Cyanotriphenylamine-Based Polymers. Chem. Commun. 2013, 49, 9797–9799. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, K.-H. A Comparative Study on the Properties of Aromatic Polyamides with Methyl- or Trifluoromethyl-Substituted Triphenylamine Groups. J. Fluor. Chem. 2016, 188, 33–42. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, Z.; Meng, S.; Chao, D.; Chu, X.; Zhao, X.; Wang, D.; Zhou, H.; Chen, C. Aggregation-Enhanced Emission (AEE)-Active Polyamides with Methylsulfonyltriphenylamine Units for Electrofluorochromic Applications. Dye. Pigment. 2017, 141, 356–362. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent Advances in Triphenylamine-Based Electrochromic Derivatives and Polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Yen, H.-J.; Lin, H.-Y.; Liou, G.-S. Novel Starburst Triarylamine-Containing Electroactive Aramids with Highly Stable Electrochromism in Near-Infrared and Visible Light Regions. Chem. Mater. 2011, 23, 1874–1882. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Elunger, S.; Reynolds, J.R. The Donor-Acceptor Approach Allows a Black-to-Transmissive Switching Polymeric Electrochrome. Nat. Mater. 2008, 7, 795. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, L.; Yan, S.; Kuai, Y.; Fu, H.; Li, W.; Ouyang, M.; Zhang, C. Black-to-Transmissive Polymer Films via Electrochemical Copolymerization with High-Performance Electrochromic and Supercapacitor Bifunction. Sol. Energy Mater. Sol. Cells 2022, 245, 111857. [Google Scholar] [CrossRef]
- Beverina, L.; Pagani, G.A.; Sassi, M. Multichromophoric Electrochromic Polymers: Colour Tuning of Conjugated Polymers Through the Side Chain Functionalization Approach. Chem. Commun. 2014, 50, 5413–5430. [Google Scholar] [CrossRef]
- Sassi, M.; Salamone, M.M.; Ruffo, R.; Mari, C.M.; Pagani, G.A.; Beverina, L. Gray to Colorless Switching, Crosslinked Electrochromic Polymers with Outstanding Stability and Transmissivity From Naphthalenediimmide-Functionalized EDOT. Adv. Mater. 2012, 24, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.H.R.; Schmidt, A.; Zarbin, A.J.G. A Tunable Color Palette of Electrochromic Materials Achieved through an Ingenious Stacking of Ordinary Conducting Polymers. J. Mater. Chem. A 2023, 11, 18853–18861. [Google Scholar] [CrossRef]
- Liu, H.-S.; Pan, B.-C.; Huang, D.-C.; Kung, Y.-R.; Leu, C.-M.; Liou, G.-S. Highly Transparent to Truly Black Electrochromic Devices Based on an Ambipolar System of Polyamides and Viologen. NPG Asia Mater. 2017, 9, e388. [Google Scholar] [CrossRef]
- Yu, T.; Han, Y.; Yao, H.; Chen, Z.; Guan, S. Polymeric Optoelectronic Materials with Low-Voltage Colorless-to-Black Electrochromic and AIE-Activity Electrofluorochromic Dual-Switching Properties. Dye. Pigment. 2020, 181, 108499. [Google Scholar] [CrossRef]
- Shao, Y.-J.; Yen, T.-C.; Hu, C.-C.; Liou, G.-S. Non-Conjugated Triarylamine-Based Intrinsic Microporous Polyamides for Electrochromic Supercapacitor: Diffusion Dynamics and Charge-Discharge Studies. J. Mater. Chem. A 2023, 11, 1877–1885. [Google Scholar] [CrossRef]
- Pai, M.H.; Hu, C.C.; Liou, G.S. Enhancement of Electrochromic Switching Properties with Troger’s Base-Derived Intrinsic Microporous Polyamide Films. Macromol. Rapid Commun. 2021, 42, e2100492. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.W.; Pai, M.H.; Liou, G.S. Facile Approach of Porous Electrochromic Polyamide/ZrO(2) Films for Enhancing Redox Switching Behavior. ACS Appl. Mater. Interfaces 2020, 12, 35273–35281. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.-C.; Chen, W.-H.; Lee, T.-M.; Liou, G.-S. Synthesis and Characterization of Novel Electrochromic Devices Derived from Redox-Active Polyamide–TiO2 Hybrids. J. Mater. Chem. C 2018, 6, 12422–12428. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, H.; Chen, C.; Yu, Y. Preparation of Porous Polyamide Films with Enhanced Electrochromic Performance by Electrostatic Spray Deposition. J. Electroanal. Chem. 2021, 887, 115038. [Google Scholar] [CrossRef]
- Liou, G.-S.; Chang, C.-W. Highly Stable Anodic Electrochromic Aromatic Polyamides Containing N,N,N‘,N‘-Tetraphenyl-p-Phenylenediamine Moieties: Synthesis, Electrochemical, and Electrochromic Properties. Macromolecules 2008, 41, 1667–1674. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chung, C.-H.; Liou, G.-S. Novel Anodic Polyelectrochromic Aromatic Polyamides Containing Pendent Dimethyltriphenylamine Moieties. Macromolecules 2008, 41, 8441–8451. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Pyrenylamine-Functionalized Aromatic Polyamides as Efficient Blue-Emitters and Multicolored Electrochromic Materials. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3475–3490. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Han, J.-S. Solution-Processable Transmissive-to-Green Switching Electrochromic Polyamides Bearing 2,7-Bis(Diphenylamino)Naphthalene Units. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1409–1421. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. Synthesis and Electrochromism of Highly Organosoluble Polyamides and Polyimides with Bulky Trityl-Substituted Triphenylamine Units. Polymers 2017, 9, 511. [Google Scholar] [CrossRef]
- Sun, N.; Meng, S.; Chao, D.; Zhou, Z.; Du, Y.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. Highly Stable Electrochromic and Electrofluorescent Dual-Switching Polyamide Containing Bis(Diphenylamino)-Fluorene Moieties. Polym. Chem. 2016, 7, 6055–6063. [Google Scholar] [CrossRef]
- Meng, S.; Sun, N.; Su, K.; Feng, F.; Wang, S.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. Optically Transparent Polyamides Bearing Phenoxyl, Diphenylamine And Fluorene Units with High-Contrast of Electrochromic and Electrofluorescent Behaviors. Polymer 2017, 116, 89–98. [Google Scholar] [CrossRef]
- Su, K.; Sun, N.; Tian, X.; Guo, S.; Yan, Z.; Wang, D.; Zhou, H.; Zhao, X.; Chen, C. High-Performance Blue Fluorescent/Electroactive Polyamide Bearing P-Phenylenediamine and Asymmetrical SBF/TPA-Based Units for Electrochromic and Electrofluorochromic Multifunctional Applications. J. Mater. Chem. C 2019, 7, 4644–4652. [Google Scholar] [CrossRef]
- Perez, I. Ab Initio Methods for the Computation of Physical Properties and Performance Parameters of Electrochemical Energy Storage Devices. Phys. Chem. Chem. Phys. 2023, 25, 1476–1503. [Google Scholar] [CrossRef]
- Sun, N.; Su, K.; Zhou, Z.; Wang, D.; Fery, A.; Lissel, F.; Zhao, X.; Chen, C. “Colorless-to-Black” Electrochromic and AIE-Active Polyamides: An Effective Strategy for the Highest-Contrast Electrofluorochromism. Macromolecules 2020, 53, 10117–10127. [Google Scholar] [CrossRef]
- Su, K.; Sun, N.; Yan, Z.; Jin, S.; Li, X.; Wang, D.; Zhou, H.; Yao, J.; Chen, C. Dual-Switching Electrochromism and Electrofluorochromism Derived from Diphenylamine-Based Polyamides with Spirobifluorene/Pyrene as Bridged Fluorescence Units. ACS Appl. Mater. Interfaces 2020, 12, 22099–22107. [Google Scholar] [CrossRef]
- Su, K.; Sun, N.; Tian, X.; Li, X.; Chao, D.; Wang, D.; Zhou, H.; Chen, C. Novel Polyamides with Pendant P-Phenylenediamine and α-/β-Substituted Naphthalene: Synthesis, Characteristics, and Effects of Substitution Sites on Electro-Switchable Optical Behaviors. Mater. Today Chem. 2021, 22, 100536. [Google Scholar] [CrossRef]
- Li, X.; Su, K.; Zeng, Q.; Wang, D.; Zhao, X.; Chen, C. Highly Stable Electrochromism and Electrofluorochromism Derived from a Bi-Functional Polyamide Containing Conjugated Bis(Diphenylamine-Spirodifluorene) Moieties. Dye. Pigment. 2022, 199, 110072. [Google Scholar] [CrossRef]
- Lv, X.; Li, D.; Ma, Y.; Li, J.; Liu, Y.; Guo, J.; Niu, H.; Zhou, T.; Wang, W. From Gas Separation to Ion Transport in The Cavity of Hyperbranched Polyamides Based on Triptycene Aimed for Electrochromic and Memory Devices. Polym. Chem. 2022, 13, 808–818. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, M.; Xia, S.; Fu, X.; Hong, T.; Zhang, B.; Liu, H.; Cai, W.; Niu, H.; Wang, W. Boosting Electrochromic Properties of Polyamides Through Altering the Structure Bonded to Triarylamine Groups by Xanthene. Sol. Energy Mater. Sol. Cells 2023, 260, 112497. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Pang, L.; Guo, J.; Wang, J.; Qi, D.; Li, W.; Shen, K. Novel Triphenylamine Polyamides Bearing Carbazole and Aniline Substituents for Multi-Colored Electrochromic Applications. Dye. Pigment. 2020, 173, 107995. [Google Scholar] [CrossRef]
- Yang, C.; Shi, W.; Chen, X.; Zhang, K.; Zhou, X.; Sun, X.; Ding, S.; Liu, S.; Xie, Z. Novel Triphenylamine-Based Polyamides: Efficient Preparation via Benzoxazine-Isocyanide-Chemistry at Room Temperature and Electrochromic Properties Investigation. Dye. Pigment. 2020, 176, 108206. [Google Scholar] [CrossRef]
- Rusu, R.-D.; Damaceanu, M.-D.; Ursache, S.; Constantin, C.-P. Tuning the Main Electrochromic Features by Polymer Backbone Variation of Triphenylamine-Based Polyamides. J. Photochem. Photobiol. A Chem. 2023, 435, 114272. [Google Scholar] [CrossRef]
- Zhu, T.; Xiong, J.; Chen, J.; Zhou, X.; Cai, G.; Lai, Y.; Lee, P.S. Flexible Electrochromic Fiber with Rapid Color Switching and High Optical Modulation. Nano Res. 2021, 16, 5473–5479. [Google Scholar] [CrossRef]
- Minami, Y.; Matsuyama, N.; Takeichi, Y.; Watanabe, R.; Mathew, S.; Nakajima, Y. Depolymerization of Robust Polyetheretherketone to Regenerate Monomer Units Using Sulfur Reagents. Commun. Chem. 2023, 6, 14. [Google Scholar] [CrossRef]
- Jia, X.; Chao, D.; Berda, E.B.; Wang, S.; Yang, R.; Yao, L.; Wang, C. Synthesis and Properties of a Novel Electroactive Poly(aryl ether ketone) Bearing Pendant Aniline Tetramer. Macromol. Chem. Phys. 2012, 213, 1475–1481. [Google Scholar] [CrossRef]
- Chen, Z.; Ono, R.J.; Wiggins, K.M.; Cui, H.; Rong, C.; Bielawski, C.W.; Jiang, Z. Synthesis and Characterization of Polyketones Containing Pendant Carbazoles. Polymer 2011, 52, 1731–1737. [Google Scholar] [CrossRef]
- Han, Y.; Lin, Y.; Sun, D.; Xing, Z.; Jiang, Z.; Chen, Z. Poly(Aryl Amino Ketone)-Based Materials with Excellent Electrochromic and Electrofluorochromic Behaviors. Dye. Pigment. 2019, 163, 40–47. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Zheng, Z.; Chen, Z.; Jiang, Z. Rapid and Effective Electrochemical Strategy for the Synthesis of PAASs/PAAKs Electrochromic High-Performance Polymers. Sol. Energy Mater. Sol. Cells 2023, 254, 112256. [Google Scholar] [CrossRef]
- Djafri, D.E.; Henni, A.; Zerrouki, D. Electrochemical Synthesis of Highly Stable and Rapid Switching Electrochromic Ni(OH)2 Nanoflake Array Films as Low-Cost Method. Mater. Chem. Phys. 2022, 279, 125704. [Google Scholar] [CrossRef]
- Jia, S.; Xing, Z.; Wang, Q.; Wang, S.; Chen, Z. Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis. Molecules 2023, 28, 5297. [Google Scholar] [CrossRef]
- Ziemann, E.; Das, A.K.; Manna, P.; Sharon-Gojman, R.; Sela-Adler, M.; Linder, C.; Kasher, R.; Bernstein, R. Crosslinked Polyethersulfone Membranes for Organic Solvent Nanofiltration in Polar Aprotic and Halogenated solvents. J. Membr. Sci. 2022, 663, 120963. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Bornhorst, K. Syntheses of Multicyclic Poly(Ether Sulfone)s from 5,5′,6,6′-Tetrahydroxy-3,3,3′,3′-Tetramethyl Spirobisindane and 4,4′-Bis(4-Chlorophenyl) Sulfones. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3732–3739. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Zhou, M.; Liu, X.; Wang, C.; Chao, D. Synthesis and Electrochemical Properties of a Novel Poly(ether sulfone) with Oligoaniline Pendants. Chem. Res. Chin. Univ. 2015, 31, 1066–1071. [Google Scholar] [CrossRef]
- Cheng, S.-W.; Chang Chien, Y.-H.; Huang, T.-Y.; Liu, C.-L.; Liou, G.-S. Linkage Effects of Triphenylamine-Based Aromatic Polymer Electrets on Electrical Memory Performance. Polymer 2018, 148, 382–389. [Google Scholar] [CrossRef]
- Wu, J.-T.; Fan, Y.-Z.; Liou, G.-S. Synthesis, Characterization and Electrochromic Properties of Novel Redox Triarylamine-Based Aromatic Polyethers with Methoxy Protecting Groups. Polym. Chem. 2019, 10, 345–350. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, Y.; Han, Y.; Zhai, Y.; Tian, Y.; Qi, S.; Zhu, X.; Jiang, Z.; Chen, Z. The Effect of Constructing Discontinuous Side Chain D-A Structure on High-Performance Poly (Ether Sulfone)s Optoelectronic Materials. Dye. Pigment. 2021, 189, 109259. [Google Scholar] [CrossRef]
- Xing, Z.; Jia, S.; Li, S.; Wang, Q.; Zhong, J.; Qi, H.; Sun, W.; Jiang, Z.; Chen, Z. Preparation and Characterization of Novel High-Performance N, N, N′, N′-tetraphenyl-p-phenylenediamine-Based Poly (ether sulfone)s. Electrochim. Acta 2023, 452, 142316. [Google Scholar] [CrossRef]
- Huang, C.-L.; Kung, Y.-R.; Shao, Y.-J.; Liou, G.-S. Synthesis and Characteristics of Novel TPA-Containing Electrochromic Poly(Ether Sulfone)s with Dimethylamino Substituents. Electrochim. Acta 2021, 368, 137552. [Google Scholar] [CrossRef]
- Han, Y.; Xing, Z.; Ma, P.; Li, S.; Wang, C.; Jiang, Z.; Chen, Z. Design Rules for Improving the Cycling Stability of High-Performance Donor-Acceptor-Type Electrochromic Polymers. ACS Appl. Mater. Interfaces 2020, 12, 7529–7538. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Chen, C.-J.; Yen, H.-J.; Lin, K.-Y.; Yeh, J.-M.; Chen, W.-C.; Liou, G.-S. Novel Triphenylamine-Containing Ambipolar Polyimides with Pendant Anthraquinone Moiety for Polymeric Memory Device, Electrochromic and Gas Separation Applications. J. Mater. Chem. 2012, 22, 20394–20402. [Google Scholar] [CrossRef]
- Zheng, R.-R.; Zhang, X.; Zhang, Z.-P.; Niu, H.-J.; Wang, C.; Wang, W. Preparation and Multifunction of Electrochromic Polyamides Containing Flexible Backbone Chains with Electrochemical, Fluorescence and Memory Properties. Appl. Surf. Sci. 2019, 478, 906–915. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Wang, M.; Li, B.; Wang, K.; Fan, X.; Li, N.; Wang, H.; Hu, S.; Diao, X. Low Self-Discharge All-Solid-State Electrochromic Asymmetric Supercapacitors at Wide Temperature toward Efficient Energy Storage. Chem. Eng. J. 2023, 456, 141022. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Zhou, M.; Chao, D. High-Performance Electrochromic Supercapacitor Based on an Electroactive Polyimide Containing Pentaaniline. Dye. Pigment. 2023, 212. [Google Scholar] [CrossRef]
- Ho, P.Y.; Dmitrieva, E.; Sun, N.; Guskova, O.; Lissel, F.S.C. Synthesis of Novel Ruthenium-Polymetallaynes and Their Application in Multistate Electrochromic Memory. Adv. Mater. Technol. 2022, 7, 2200316. [Google Scholar] [CrossRef]
- Sun, N.; Su, K.; Zhou, Z.; Tian, X.; Jianhua, Z.; Chao, D.; Wang, D.; Lissel, F.; Zhao, X.; Chen, C. High-Performance Emission/Color Dual-Switchable Polymer-Bearing Pendant Tetraphenylethylene (TPE) and Triphenylamine (TPA) Moieties. Macromolecules 2019, 52, 5131–5139. [Google Scholar] [CrossRef]
- Sun, N.; Su, K.; Zhou, Z.; Yu, Y.; Tian, X.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. AIE-Active Polyamide Containing Diphenylamine-TPE Moiety with Superior Electrofluorochromic Performance. ACS Appl. Mater. Interfaces 2018, 10, 16105–16112. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, N.; Li, F.; Jia, X.; Wang, C.; Chao, D. Multiple Stimuli-Responsive Fluorescence Behavior of Novel Polyamic Acid Bearing Oligoaniline, Triphenylamine, and Fluorene Groups. ACS Appl. Mater. Interfaces 2017, 9, 6497–6503. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, Z.; Chao, D.; Chu, X.; Du, Y.; Zhao, X.; Wang, D.; Chen, C. Novel Aromatic Polyamides Containing 2-Diphenylamino-(9,9-Dimethylamine) Units as Multicolored Electrochromic and High-Contrast Electrofluorescent Materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 213–222. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Y.; Ye, Y.; Shen, R. Unique Applications of Carbon Materials in Infrared Stealth: A Review. Chem. Eng. J. 2023, 452, 139147. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Zhao, P.; Ji, Y.; Ma, L.; Wang, C.; Bai, X.; Wang, W. Multicolored Near-Infrared Electrochromic Polyimides: Synthesis, Electrochemical, and Electrochromic Properties. Dye. Pigment. 2013, 99, 1124–1131. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, X.; Cai, W.; Wang, Y.; Yang, C.; Chen, Y.; Zhang, W.; Zhang, Z.; Niu, H.; Wang, W. Donor−Acceptor Conjugated Polymers Containing Isoindigo Block for Novel Multifunctional Materials for Electrochromic, Resistance Memory, and Detector Devices. Sol. Energy Mater. Sol. Cells 2019, 200, 109979. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, W.; Zhang, X.; Yang, C.; Ge, H.; Chen, Y.; Niu, H.; Wang, W. Multifunctional Polymers for Electrochromic, Memory Device, Explosive Detection and Photodetector: Donor-Acceptor Conjugated Isoindigo Derivatives with Strong Fluorescence. Eur. Polym. J. 2018, 108, 124–137. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, C.; Qiao, X.; Zhang, X.; Cai, W.; Chen, Y.; Wang, Y.; Zhang, W.; Lin, X.; Niu, H.; et al. Multifunctional AIE-Active Polymers Containing TPA-TPE Moiety for Electrochromic, Electrofluorochromic and Photodetector. Dye. Pigment. 2019, 166, 340–349. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Q.; Yang, C.; Zhao, S.; Chen, Y.; Niu, H.; Zhao, P.; Wang, W. Multi-Stimuli Responsive Novel Polyimide Smart Materials Bearing Triarylamine and Naphthalimide Groups. Eur. Polym. J. 2019, 112, 291–300. [Google Scholar] [CrossRef]
- Zhai, M.; Lv, X.; Zhang, C.; Fu, X.; Zhang, Q.; Huang, R.; Cai, W.; Niu, H.; Wang, W. Multi-Stimuli-Responsive Polyamides Containing Benzophenothiazine Being Sensitive to TNP, Acid, Voltage, and Light. ACS Appl. Polym. Mater. 2023, 5, 9466–9476. [Google Scholar] [CrossRef]
- Grigoras, M.; Catargiu, A.M.; Ivan, T.; Vacareanu, L.; Minaev, B.; Stromylo, E. Tuning Optical and Electronic Properties of Poly(4,4’-triphenylamine vinylene)s by Post-Modification Reactions. Dye. Pigment. 2015, 113, 227–238. [Google Scholar] [CrossRef]














| S/N | PAI– ECPs | Tg (°C) | Td at 5% Weight Loss (°C) | Original Color | Oxidation Potential and Color (V) a | Reduction Potential (V) | Eonset Ox (V) b | Eg/eV c | ΔT% | Tc/Tb (s) d | Cycle | CE (cm2/C) e | REF | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N2 | Air | 1st E1/2 | 2nd E1/2 | 1st E1/2 | 2nd E1/2 | |||||||||||||
| 1 | PAI–2M | 279 | 495 | 483 | brownish | 0.67 (green) | 1.08 (deep blue) | - | - | 0.52 | 3.13 | 0.202 | 3/2 | 100 cycles | 305 | [28] | ||
| 2 | 2a | - | - | - | pale brownish | 0.65 (green) | 1.02 (blue) | - | - | - | 2.95 | - | 4/3 | 10 cycles | - | [29] | ||
| 3 | 4c | - | - | - | colorless | 0.69 (yellow) | 1.04 (blue) | - | - | 0.42 | 3.09 | - | - | - | - | [30] | ||
| 4f | - | - | - | pale yellow | 0.69 (yellowish green) | 0.89 (green) | 1.05 (blue) | - | - | 0.47 | 2.89 | - | - | - | - | |||
| 4g | - | - | - | pale yellow | 0.63 (deeper yellow) | 0.69 (yellowish green) | - | - | 0.42 | 2.70 | - | - | - | - | ||||
| 4 | 5c | 278 | 533 (10%) | 540 (10%) | nearly colorless | 0.93 (pale blue) | - | −1.10 | - | 0.82 | 3.06 | 39% | 8.5/4.2 | 100 cycles | - | [31] | ||
| 5 | 1 | 340 | 635 (10%) | 632 (10%) | pale yellow | 1.08 (purplish blue) | - | −1.18 (pink) | −2.01 (red-brown) | 0.90 | 2.68 | - | 3.1/1.3 | 10 cycles | - | [32] | ||
| 2 | 352 | 650 (10%) | 621 (10%) | yellow | 0.81 (deep blue) | 1.00 | −1.19 | −2.11 | 0.65 | 2.15 | - | - | 30 cycles | - | ||||
| 3 | 335 | 623 (10%) | 626 (10%) | yellow | 0.86 (bluish gray) | - | −1.15 (orange) | −2.04 (red-brown) | 0.66 | 2.29 | - | 3.5/1.3 | 10 cycles | - | ||||
| 6 | 1a | - | - | - | colorless | 1.3 (black) | 1.0 | 3.1 | 91.4% | 1.3/1.1 | 5000 cycles | 99 | [33] | |||||
| 2a | - | - | - | colorless | 1.2 (blue) | 0.9 | 3.2 | 81.2% | 2.2/3.7 | 5000 cycles | 78 | |||||||
| S/N | PA–ECPs | Tg (°C) a | Td at 5% Weight Loss (°C) b | Original Color | Oxidation (V) and Color a | Reduction Potential (V) | Eonset Ox (V) b | Eg/eV c | ΔT% | Tc/Tb (s) d | Cycle | CE (cm2/C) e | REF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N2 | Air | 1st E1/2 | 2nd E1/2 | 1st E1/2 | 2nd E1/2 | ||||||||||||||||
| 1 | DH–PI | - | - | - | colorless | 0.36 (black) | - | - | - | 0.31 | 3.14 | - | 2.1/1.7 | 500 cycles | 212 | [27] | |||||
| TH–PI | - | - | - | pale yellow | 0.54 (light green) | 0.15 (black) | - | - | 0.40 | 2.88 | - | 2.5/1.5 | 230 cycles | 177 | |||||||
| TOH–PI | - | - | - | colorless | 0.42 (blue) | 0.93 (dark purple) | - | - | 0.25 | 3.23 | - | 10.7/6.8 | 500 cycles | 113 | |||||||
| 2 | F | 310 | 604 | 553 | pale yellow | 1.10 (green) | 1.44 (dark blue) | - | - | - | 3.29 | - | 3/1.5 | 10 cycles | [34] | ||||||
| 3 | PI–VIa | 292 | 475 | 490 | colorless | 0.65 (green) | 1.00 (blue) | - | - | 0.56 | 2.12 | 60% (1st) 84% (2nd) | 3.26/1.47 (1st) 4.22/1.42 (2nd) | 30 cycles | 118 (1st) 123 (2nd) | [35] | |||||
| 4 | 6e | 305 | 538 (10%) | 519 (10%) | colorless | 0.73 (green) | 1.06 (blue) | - | - | 0.60 | 2.90 | 44% (1st) 98% (2nd) | 2.4/2.4 (1st) 3.0/2.4 (2nd) | 50 cycles | 214 | [36] | |||||
| 5 | 6d | 290 | 497 | 514 | pale yellowish | 0.63 (cyan) | 0.95 (bluish purple) | - | - | 0.53 | 2.99 | - | 16/23 | 100 cycles | 180 | [39] | |||||
| 6 | TPA–PTPI | - | - | - | Indian red | 0.99 (deep purple) | - | −0.67 (bright violet) | - | - | - | 73% (Ox) 60% (Red) | 6.1/4.2 (Ox) 2.9/1.3 (Red) | 100 cycles | 371 (Ox) 271 (Red) | [40] | |||||
| 7 | Vio–PM | 170 | 250 | 245 | pale yellow | 1.02 (dark cyan) | - | −0.55 (light purple) | −0.76 (dark purple) | −0.95 (light green) | −1.31 (magenta) | 0.85 | 2.99 | 64% | - | - | - | [41] | |||
| Vio–BP | 175 | 255 | 255 | pale yellow | 0.96 (dark cyan) | - | −0.32 (purple) | −0.84 (dark yellow) | −1.21 (dark olive green) | −1.38 (navy) | 0.79 | 3.12 | 50% | - | - | - | |||||
| 8 | 9Ph–PMPI | 265 | 460 | 460 | colorless | 0.60 (yellow) | 0.90 (purple) | 1.25 (blue) | 1.50 (bluish-green) | −0.9 | −1.5 | 0.35 | 2.13 | 82% (2nd) 80% (4th) | 3.99/0.90 (Ox 1st) 3.12/1.74 (Ox 2nd) | 1000 cycles | 226 (1st) 280 (2nd) | [43] | |||
| 9 | 6FDA–HBPI | 286 | - | - | near colorless | 0.46 (yellow) | 0.72 (red) | 0.99 (indigo) | 1.24 (black) | - | - | 0.33 | 2.86 | 63% (1st) 42% (2nd) 44% (3rd) 49% (4th) | 2.6/1.9 (1st) 1.8/4.3 (2nd) 2.3/1.8 (3rd) 4.9/3.1 (4th) | 10,000 s | 196 (1st) 92 (2nd) 86 (3rd) 89 (4th) | [44] | |||
| 10 | PI | - | - | - | yellow | 0.79 (blueviolet) | - | - | - | 0.36 | - | - | 1.7/0.9 | 155 | [45] | ||||||
| 11 | PI–4 | 221 | 434 | - | colorless | 0.99 (light orange) | 1.45 (blue) | - | - | 0.80 | 1.84 | 28% | 1.6/1.2 | 2000 cycles | 186 | [46] | |||||
| 12 | 1a | 339 | 425 | 390 | pale green | 0.42 (light green) | 0.70 (light bluish green) | −0.98 | - | 0.28 | 1.69 | 57%(3rd) | - | - | - | [48] | |||||
| 2a | 322 | 520 | 510 | pale yellow | 0.48 (green) | 0.78 (dark blue) | −1.05 | - | 0.34 | 2.20 | 81% (1st) 76% (2nd) | - | - | - | |||||||
| 3a | 270 | 440 | 430 | pale yellowish | 0.52 (yellowish-green) | 0.74 (purple) | - | - | 0.40 | - | 66% (1st) 77% (2nd) | - | 100 cycles | - | |||||||
| 13 | I–DS | 340 | 470 | 460 | pale yellow | 1.24 (green) | - | −0.82 | - | 1.07 | 2.35 | 77% | - | 10 cycles | 136 | [49] | |||||
| 14 | P3 | 312 | - | - | colorless | 0.75 (deep purple) | - | - | - | - | - | 62% | 6.5/5.1 | 500 cycles | 193 | [50] | |||||
| 15 | PAA–IM–1 | - | - | - | light yellow | 1.26 (blue) | - | - | - | - | 3.22 | - | 1.7/0.8 | 50 cycles | 834 | [51] | |||||
| PAA–IM–2 | - | - | - | light yellow | 1.35 (green) | - | - | - | - | 3.16 | - | 3.8/1.6 | 50 cycles | 523 | |||||||
| 16 | PyTPMPI | - | 317 | - | colorless | 0.90 (light blue) | - | - | - | 0.89 | 3.08 | - | 3.69/7.08 | 1000 s | 72.73 | [52] | |||||
| PyTPTPI | - | 299 | - | yellow | 0.90 (dark blue) | - | - | - | 0.91 | 3.12 | - | 3.25/5.78 | 1000 s | 190.91 | |||||||
| PyHPMPI | - | 348 | - | colorless | 0.98 (light blue) | - | - | - | 082 | 3.21 | - | 3.13/3.00 | 1000 s | 72.42 | |||||||
| PyHPTPI | - | 296 | - | yellow | 0.95 (dark blue) | - | - | - | 0.85 | 3.10 | - | 1.19/3.30 | 1000 s | 42.30 | |||||||
| 17 | PI–6A | - | 354 | - | light yellow | 0.82 (brown yellow) | 1.07 (deep yellow) | - | - | 0.59 | 3.33 | 55% | 10.0/7.7 | 600 cycles | 191 | [53] | |||||
| 18 | CzpP–SPP | - | 422 | - | colorless | 0.7 (green) | 1.2 (black) | - | - | - | - | 50% | 2.8/2.9 | 2000 cycles | 108 | [54] | |||||
| CzpP–PREP | - | 410 | - | colorless | 0.93 (yellow) | 1.19 (yellow−green) | 1.38 (green−black) | - | - | - | - | 30% | 3.3/2.5 | 2000 cycles | 90 | ||||||
| CzpP–FRNP | - | 454 | - | colorless | 0.91 (yellow) | 1.33 | - | - | - | - | 34% | 3.6/2.7 | 2000 cycles | 187 | |||||||
| 19 | P1 | - | 411 | - | yellow | 0.69 (reddish brown) | 0.82 (inky blue) | - | - | 0.41 | 3.74 | 26% | 3.2/8.7 | - | 239 | [55] | |||||
| P2 | - | 405 | - | yellow | 0.95 (reddish brown) | 1.15 (inky blue) | - | - | 0.51 | 3.43 | 52% | 3.1/2.4 | 200 cycles | 310 | |||||||
| P3 | - | 378 | - | yellow | 0.67 (reddish brown) | 0.84 (inky blue) | - | - | 0.31 | 3.32 | 34% | 2.3/2.8 | 300 cycles | 170 | |||||||
| 20 | P1 | 307 | - | - | yellow-orange | 0.91 (blue) | - | −0.94 | −1.56 | 0.72 | 1.46 | 75% | 1.98/5.14 | 100 cycles | 316 | [56] | |||||
| P2 | 280 | - | - | pale yellow | 0.90 (light blue) | - | −1.41 | - | 0.71 | 1.82 | 24% | 17.92/18.14 | 100 cycles | 158 | |||||||
| P3 | 325 | - | - | pale yellow | 0.92 (light blue) | - | −1.43 | - | 0.71 | 1.77 | 19% | 17.22/18.39 | 100 cycles | 108 | |||||||
| S/N | PA–ECPs | Tg (°C) | Td at 5% Weight Loss (°C) | Original Color | Oxidation (V) and Color a | Reduction Potential (V) | Eonset Ox (V) b | Eg/eV c | ΔT% | Tc/Tb (s) d | Cycle | CE (cm2/C) e | REF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N2 | Air | 1st E1/2 | 2nd E1/2 | 1st E1/2 | 2nd E1/2 | |||||||||||
| 1 | BCT–1 | - | - | 303 | pale yellow | 0.67 (green) | - | - | - | - | - | 4.25% | 3.71/3.89 | - | 266 | [25] |
| 2 | PA–AgNWs-ES | - | - | - | - | - | - | - | - | 86.9% | 0.8/1.2 | - | 697.1 | [77] | ||
| 3 | 6g | 248 | 495 | 490 | colorless | 0.48 (green) | 0,84 (blue) | - | - | 0.36 | 2.95 | 86% (2nd) | 3.04/1.87 (1st) 4.02/2.02 (2nd) | 1000 cycles | 285 (1st) 272 (2nd) | [78] |
| 4 | Ib | 262 | 505 | 490 | colorless | 0.58 (green) | 0.96 (blue) | - | - | 0.48 | 2.94 | 87% (2nd) | 4.21/1.17 (1st) 3.96/2.04 (2nd) | 1600 cycles | 276 (1st) 174 (2nd) | [79] |
| 5 | 4g | 312 | 574 (10%) | 576 (10%) | pale yellow | 1.0 (greenish-gray) | 1.5 (dark purplish-gray) | −1.90 (bright yellow) | −2.4 (reddish orange) | - | 2.74 | - | 3.8/1.6 | 100 cycles | 112 | [80] |
| 6 | 5b | 248 | 465 | 442 | colorless | 0.75 (pale green) | 0.92 (deeper green) | - | - | 0.63 | 2.94 | 82% | 8.0/1.8 | 20 cycles | 140 | [81] |
| 7 | 5b | 288 | 430 (10%) | 430 (10%) | colorless | 0.85 (greenish blue) | - | - | - | 0.74 | 3.13 | 84% | - | 100 cycles | 186 | [82] |
| 8 | 5a | 251 | 440 (10%) | 439 (10%) | colorless | 0.54 (orange-red) | 0.75 (brilliant blue) | - | - | 0.41 | 2.97 | - | 4.8/2.7 (1st) 4.6/2.0 (2nd) | 1000 cycles | 257 (1st) 278 (2nd) | [83] |
| 9 | 6a | 244 | 440 | 407 | colorless | 0.82 (green) | - | - | - | 0.72 | 3.20 | 86% | 2.5/0.6 | 100 cycles | 268 | [84] |
| 10 | SBF–HPA | - | >420 | - | colorless | 0.53 (green) | 0.90 (blue) | - | - | 0.37 | - | 65% | 2.3/1.6 (1st) 1.9/3.2 (2nd) | 200 cycles | 246 (1st) 127 (2nd) | [85] |
| 11 | 11–TPE–PA | 247 | - | - | colorless | 0.77 (gray) | - | - | - | 0.62 | - | 86% | 2.2/0.8 | 300 cycles | - | [86] |
| Z–12–TPE– PA | 245 | - | - | colorless | 0.66 (black) | - | - | - | 0.57 | - | 92% | 0.6 s/0.3 | 300 cycles | - | ||
| 12 | S–HPA | 284 | 448 | - | colorless | 0.61 (orange red) | 0.81 (blue) | - | - | - | - | 64% | 2.6/1.7 | 500 cycles | - | [87] |
| P–HPA | 317 | - | - | yellow | 0.77 (green) | (blue green) | - | - | - | - | 48% | 2.8/2.4 | 500 cycles | - | ||
| 13 | a–HPA | 251 | - | - | colorless | 0.62 (green) | 1.03 (blue) | - | - | - | - | - | 1.4/1.1 (1st) 1.8/1.2 (2nd) | 500 cycles | 296 (1st) 248 (2nd) | [88] |
| b–HPA | 241 | - | - | colorless | 0.65 (green) | 1.03 (blue) | - | - | - | - | - | 1.8/1.4 (1st) 2.2/1.1 (2nd) | 500 cycles | 197 (1st) 187 (2nd) | ||
| 14 | PA–4 | 266 | 403 | - | colorless | 0.70 (green) | 1.07 (blue) | - | - | - | - | 69.2% | 2.9/1.8 | 400 cycles | 280 | [89] |
| 15 | T3TPA–OA | - | 321 | - | yellow | dark green | - | - | - | −3.0 | 3.38 | 65% | 4.3/3.7 | 12,000 s | 206 | [90] |
| 16 | SFXMOPb–OA | - | - | - | colorless | 0.63 (red) | 0.80 (blue) | - | - | 62% | 1.0/2.2 | 2500 cycles | 463 | [91] | ||
| 17 | PA– VIIID | 315 | 448 | - | light yellow | 0.63 (green) | 1.58 (dark blue) | - | - | 0.3 | 2.97 | 58.7% | 3.4/3.9 (1st) 3.9/3.9 (2nd) | 200 cycles | 203 | [92] |
| 18 | P1 | - | 308 | - | colorless | 0.84 (blue-green) | - | - | - | 0.66 | 3.25 | 22% | 6.2/5.8 | 300 cycles | 225 | [93] |
| 19 | P2 | - | - | - | colorless | 1.00 (dark blue) | - | −1.17 | - | 0.78 | 2.20 | 6.69/2.29 | 500 cycles | 219 | [94] | |
| 20 | TPABTA | - | - | 295 | reddish brown | 1.6 (blue) | - | - | - | 0.24 | 3.30 | 14% | 3.2/4.4 | - | 141 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xing, Z.; Jia, S.; Shi, X.; Chen, Z.; Jiang, Z. Research Progress in Special Engineering Plastic-Based Electrochromic Polymers. Materials 2024, 17, 73. https://doi.org/10.3390/ma17010073
Liu Y, Xing Z, Jia S, Shi X, Chen Z, Jiang Z. Research Progress in Special Engineering Plastic-Based Electrochromic Polymers. Materials. 2024; 17(1):73. https://doi.org/10.3390/ma17010073
Chicago/Turabian StyleLiu, Yixuan, Zhen Xing, Songrui Jia, Xiangfu Shi, Zheng Chen, and Zhenhua Jiang. 2024. "Research Progress in Special Engineering Plastic-Based Electrochromic Polymers" Materials 17, no. 1: 73. https://doi.org/10.3390/ma17010073
APA StyleLiu, Y., Xing, Z., Jia, S., Shi, X., Chen, Z., & Jiang, Z. (2024). Research Progress in Special Engineering Plastic-Based Electrochromic Polymers. Materials, 17(1), 73. https://doi.org/10.3390/ma17010073






