Abstract
An aqueous sol-gel preparation technique was applied for the synthesis of calcium-substituted lanthanum molybdate with the initial composition of La2–xCaxMo2O9–x/2. The influence of the substitution effect, which plays a crucial role in the formation of final ceramics, was investigated. The thermal behavior tendencies of phase transition at elevated temperatures from the monoclinic crystal phase to cubic as well as reversible transformation were identified and discussed in detail. It was proved that the phase transformation in the obtained mixture significantly depends only on the impurities’ amount, while the partial substitution by calcium atoms above the value of x = 0.05 does not create a homogeneous multicomponent system for La2–xCaxMo2O9–x/2 composition.
1. Introduction
Since the discovery of enhanced ionic conductivity for the La2Mo2O9 compound by Lacorre in 2000 [1], the efforts of application [2] for this system in different electrochemical devices have continuously increased [3]. Oxygen pumps, sensors, and solid oxide fuel cells (SOFCs) [4,5,6,7] are only a few types of equipment where lanthanum molybdenum oxide can be successfully applied. Despite a reversible phase transformation [8,9] above 540 °C from a low-temperature form α-La2Mo2O9 [10] to a high-temperature form β-La2Mo2O9 [11], its chemical stability [12] under air atmosphere in the range of temperature from 600 °C to 1000 °C creates the conditions for using this compound as a solid electrolyte of oxygen ions [13]. Moreover, the densification [14] of the corresponding ceramic could be successfully applied below the temperature of 1200 °C while creating desirable surface and crystalline properties [15,16]. The synthesis technique [17,18,19] that allows the preparation of the initial mixture of lanthanum and molybdenum oxides also plays an important role during the formation of the final ceramic at high temperatures. However, the molar ratio of initial metals remains the main factor that determines the formation of the La2Mo2O9 composition. This is the reason why the partial substitution [20,21,22] of either lanthanum [23,24,25] or molybdenum [26,27,28,29] leads to the crystallization of side phases [30,31], which significantly affects the physical properties [32,33] of the corresponding compound. This effect is directly related to both the amount of the La2Mo2O9 phase in the final ceramic mixture and the increased stabilization of the cubic phase at room temperature. Therefore, the main aim of this work was to study the dependence of the phase transition of La2Mo2O9 ceramics on the degree of calcium substitution in the corresponding system.
2. Materials and Methods
La–Ca–Mo–O tartrate gel precursor for La2–xCaxMo2O9–x/2 ceramic was prepared by an aqueous sol-gel synthesis using tartaric acid as a chelating agent that interacts as a ligand at the molecular level with the reaction mixture during both the dissolution in water and either sol or gel formation. The general scheme of this experiment is illustrated and presented in Figure 1.
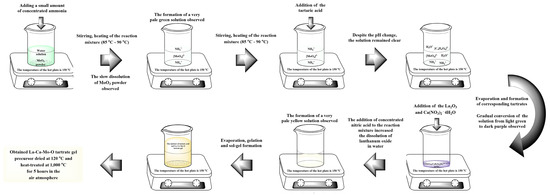
Figure 1.
Synthesis scheme of the La–Ca–Mo–O tartrate precursor for La2–xCaxMo2O9–x/2 ceramic.
Lanthanum (III) oxide (La2O3, 99.99% Alfa Aesar), molybdenum (VI) oxide (MoO3, 99.95% Alfa Aesar), and calcium (II) nitrate tetrahydrate (Ca(NO3)2·4H2O 99.98% Alfa Aesar) were used as starting materials and weighed before the dissolution procedure according to the desired stoichiometric ratio. It should be noted that, despite the high purity of the lanthanum (III) oxide, it was additionally heat-treated at 1000 °C for 5 h because of its tendency of the reaction with humidity and carbon dioxide from the air. In this case, even a slight deviation in the lanthanum amount from the ideal composition for La2Mo2O9 ceramic creates conditions for the formation of impurity phases such as La2Mo3O12 or La2MoO6 [34]. Nitric acid (HNO3 66% Reachem (Mississauga, Canada)), distilled water, and concentrated ammonia solution (NH3 · H2O 25% Penta (Prague, Czech Republic)) were used as solvents and reagents to regulate the pH of the solution. Tartaric acid (L–(+)–Tartaric acid (C4H6O6) (TA) ≥ 99.5% Sigma-Aldrich (Darmstadt, Germany)) was applied for escalation of solubility via coordination of starting compounds in the reaction mixture, especially during the pH changes and evaporation before sol-gel formation. The mechanism of the corresponding chemical process in the frame of the aqueous tartaric acid-assisted synthesis for the preparation of the La–Mo–O gel precursor was discussed in our previous work [35]. Finally, the obtained La−Ca−Mo−O tartrate gel precursor for La2−xCaxMo2O9−x/2 ceramics was heat-treated for 5 h at 1000 °C in the air atmosphere.
The thermal analysis of heat-treated powders was performed with TG–DSC, with a STA 6000 PerkinElmer instrument using a sample mass of about 20 mg and a heating rate of 40 °C min–1 under an airstream of 20 cm3·min–1 at ambient pressure. The heating and cooling cycle was fulfilled twice from 300 °C to 800 °C and from 800 °C to 300 °C. The sample mass, heating rate, atmosphere, and its flow rate were selected empirically during numerous tests to ensure the best signal peak efficiency and to minimize the noises and background signals, which occur because of the influence of the corundum crucible and equipment limits. The characteristics of the phase transition peak were evaluated in the ranges of temperature from 530 °C to 600 °C for heating and from 560 °C to 490 °C for the cooling regime. X-ray diffraction (XRD) patterns were recorded in air at room temperature by employing a powder X-ray diffractometer Rigaku MiniFlex II using CuKα1 radiation. XRD patterns were recorded at the standard rate of 1.5 2θ min–1. The sample was spread on the glass holder to obtain the maximum intensity of the characteristic peaks in the XRD diffractograms. The Rietveld refinements of the obtained XRD patterns were performed using X’Pert HighScore Plus version 2.0a software.
3. Results and Discussion
3.1. Thermal Analysis
In this work, thermal analysis as a powerful investigation technique was used for a detailed investigation of the crystal phase transition from the monoclinic α-phase to cubic β-phase and from the cubic β-phase to monoclinic α-phase in the La2–xCaxMo2O9–x/2 ceramic system. An example of a differential scanning calorimetry (DSC) curve for the La1.95Ca0.05Mo2O8.975 compound is presented in Figure 2. The corresponding results for other samples are presented in the Appendix A. Meanwhile, the data of the phase transition during the repeated heat treatments are collected in Table 1.
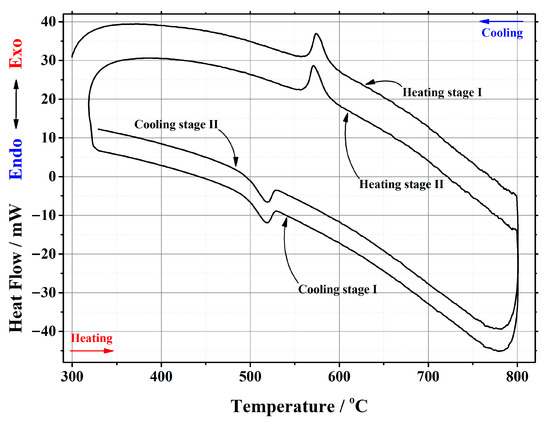
Figure 2.
DSC curve of the phase transition cycles for La1.95Ca0.05Mo2O8.975 ceramic heat-treated at 1000 °C.

Table 1.
Thermoanalytical data and α↔β phase transition peak properties for La2–xCaxMo2O9–x/2 ceramic.
It is seen from Table 1 that the enthalpy values of the first heating cycle are slightly lower, especially in the cases with a smaller amount of calcium ions, compared with the second one. The reversible stabilization of the cubic phase at room temperature after partial transformation from the monoclinic α-phase determines the main reason for such behavior. According to the measurement conditions, the second heating cycle corresponds to phase transition energy more precisely. Therefore, the representation of the tendency of enthalpy change of only the second heating and cooling cycles according to the substitution degree of calcium ions is shown in Figure 3 and Figure 4. The decrease in the tendency of phase transition enthalpy by increasing the calcium amount in the corresponding system is directly related to the amount of the monoclinic crystal phase of the La2Mo2O9 compound. Nevertheless, during the cooling stage, the increased enthalpy of the phase transition in the La1.9Ca0.1Mo2O8.95 sample shows that the reduction of the La2Mo2O9 phase is not the only factor that determines the energetics of the phase transition.
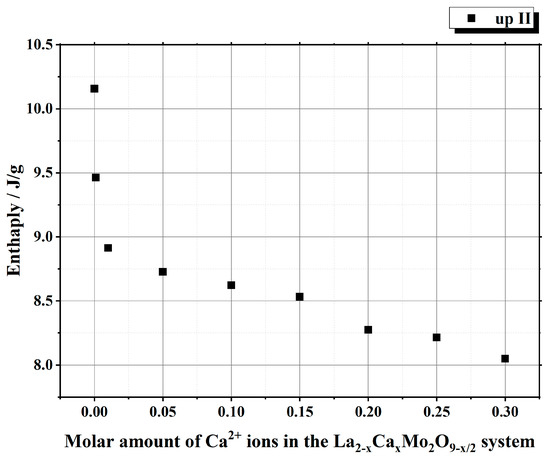
Figure 3.
Dependency of the phase transition enthalpy values from the substitution degree by calcium in the La2–xCaxMo2O9–x/2 system under the second heating stage.
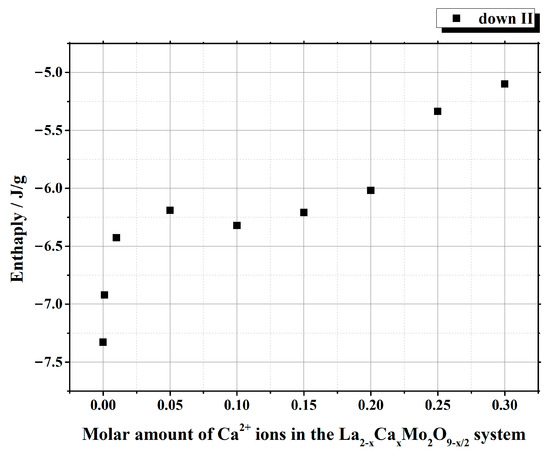
Figure 4.
Dependency of the phase transition enthalpy values from the substitution degree by calcium in the La2–xCaxMo2O9–x/2 system under the second cooling stage.
This phenomenon could be explained either by the increase in the amount of the monoclinic phase or by the influence of calcium ions on the formation of side phases in the final ceramic mixture. By further increasing the concentration of calcium ions in the La2–xCaxMo2O9–x/2 system, the enthalpy of the phase transition starts to decrease, and this result is directly related to the decrease in the amount of the crystalline phase for La2Mo2O9 in the final ceramic.
Summarizing the phase transition results obtained from cooling cycles, it can be concluded that homogeneous substitution by Ca2+ ions in the La2–xCaxMo2O9–x/2 system takes place up to the value of x = 0.05. In this case, the phase transition mainly depends only on the amount of the monoclinic crystal phase in the La2Mo2O9 ceramic homogeneously substituted by Ca2+ ions. The increase in enthalpy values of the phase transition for La2–xCaxMo2O9–x/2 (x = 0.10 and 0.15) samples during the cooling stages could be explained by the side phase effect, which increases the amount of pure La2Mo2O9 compound and its monoclinic phase in the final ceramic mixture.
3.2. X-ray Diffraction
In order to prove the crystalline composition in the obtained La2–xCaxMo2O9–x/2 system, the XRD analysis of the corresponding ceramic was also performed. The XRD patterns of all samples that correspond to the data collected in Table 2 are presented in the Appendix B.

Table 2.
Crystal system, mass fraction, crystallite size, lattice parameters, and agreement indices for the La2–xCaxMo2O9–x/2 ceramic.
Meanwhile, Figure 5 is consistent with XRD data, which show the formation process and trends of La1–xCaxMo2O9–x/2 and CaMoO4 crystalline phases. As it seen, the enthalpy of the phase transition for La2Mo2O9 mostly depends on the amount of the monoclinic phase in the ceramic mixture. This assumption is confirmed by the increased stabilization of the cubic phase up to 48.0% even after insignificant substitution of lanthanum by calcium ions in the La1.999Ca0.001Mo2O8.9995 system.
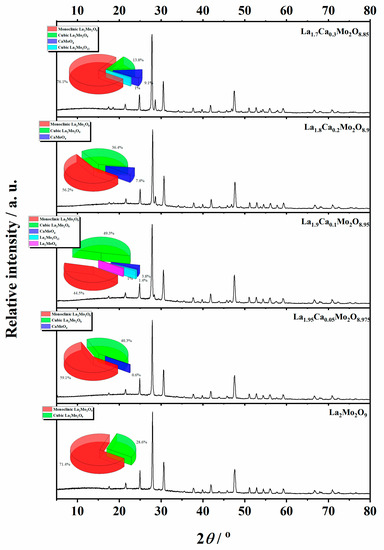
Figure 5.
XRD patterns of the La1–xCaxMo2O9–x/2 ceramic heat-treated at a 1000 °C temperature.
Nevertheless, by a further increase in the substitution degree of lanthanum by calcium (x = 0.01 and 0.05), the amount of the monoclinic phase for the La2Mo2O9 compound slightly increases; however, the trend of phase transition enthalpy change remains in a decreasing manner as concluded from Figure 3. Considering the fact that the amount of impurity phases in the obtained ceramics is really small, this decrease in the enthalpy of phase transition is basically determined by the increase in the concentration of the mixed-phase La2–xCaxMo2O9–x/2. This statement is partially confirmed by the XRD diffractogram of the Ca1.9Ca0.1Mo2O8.95 compound, in which quite a significant amount of the crystalline side phase for the CaMoO4 was identified. It seems that this impurity phase effect reduces the amount of the La2–xCaxMo2O9–x/2 homogeneous phase in the mixture and creates conditions for the formation of pure La2Mo2O9 compound. This explains the increase in the phase transition enthalpy in La1.9Ca0.1Mo2O8.95 and La1.85Ca0.15Mo2O8.925 samples during both cooling stages (Figure 4). Meanwhile, by the further increase in the calcium substitution degree in the La2–xCaxMo2O9–x/2 system, the decrease in the phase transition enthalpy is already determined by a significant lack of the La2Mo2O9 crystalline phase. This conclusion is confirmed by the constant increase in the concentration of the crystalline phase of calcium molybdate in the final mixture of the obtained ceramics.
4. Conclusions
This study showed that the homogeneous substitution of lanthanum by calcium ions takes place up to the compound of initial composition for La1.95Ca0.05Mo2O8.975. In this case, the decrease in the phase transition enthalpy is determined by the increase in the concentration of the formation of the mixed compound for the initial composition of La2–xCaxMo2O9–x/2. Meanwhile, the influence of the monoclinic phase amount on the phase transition enthalpy remained important only in the case of the formation of a pure La2Mo2O9 compound, the amount of which significantly increases with the appearance of the CaMoO4 impurity phase in the ceramic mixture. In summary, it can be concluded that the formation of the impurity of the calcium molybdate crystal phase, which compensates for the lack of lanthanum and the excess of molybdenum in the multicomponent oxide La2–xCaxMo2O9–x/2 system, has a significant influence on the decrease in the phase transition enthalpy in the La2Mo2O9 compound. The influence of the monoclinic phase amount on the phase transition enthalpy remains an important factor only in the case of the pure lanthanum molybdate.
Author Contributions
A.Ž.: Conceptualization, Methodology, Software, Validation, Resources, Data curation, Writing—original draft, Writing—review and editing, Visualization, Supervision. G.G.: Methodology, Investigation, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A
The DSC curves for the La2–xCaxMo2O9–x/2 ceramic contain details and data supporting the results presented in Table 1. For comparison, the phase transition DSC curve for the La2Mo2O9 compound is also presented in this section.
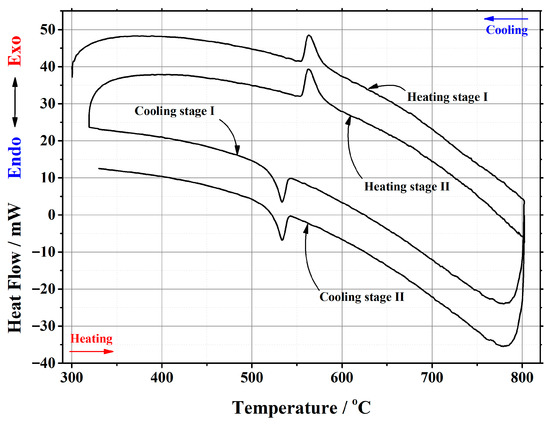
Figure A1.
DSC curve of the phase transition cycles for La2Mo2O9 ceramic heat-treated at 1000 °C.
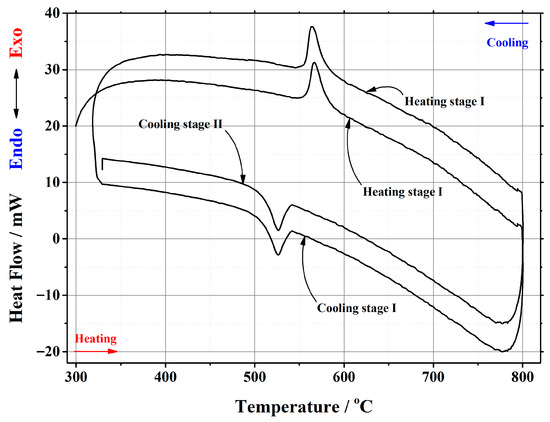
Figure A2.
DSC curve of the phase transition cycles for La1.999Ca0.001Mo2O8.9995 ceramic heat-treated at 1000 °C.
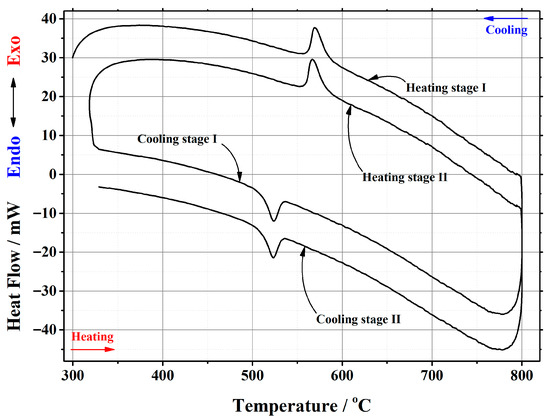
Figure A3.
DSC curve of the phase transition cycles for La1.99Ca0.01Mo2O8.995 ceramic heat-treated at 1000 °C.
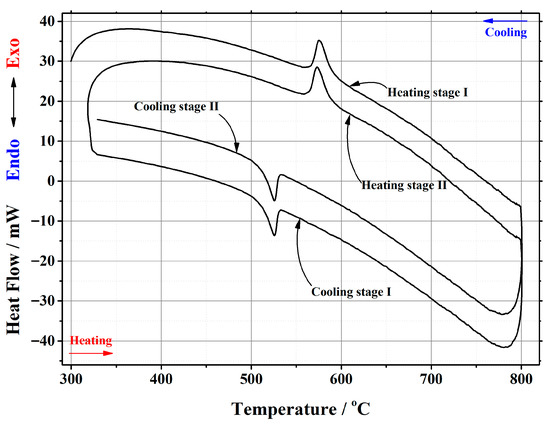
Figure A4.
DSC curve of the phase transition cycles for La1.9Ca0.1Mo2O8.95 ceramic heat-treated at 1000 °C.
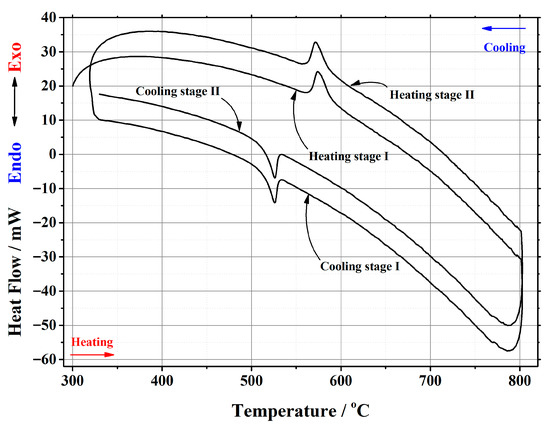
Figure A5.
DSC curve of the phase transition cycles for La1.85Ca0.15Mo2O8.925 ceramic heat-treated at 1000 °C.
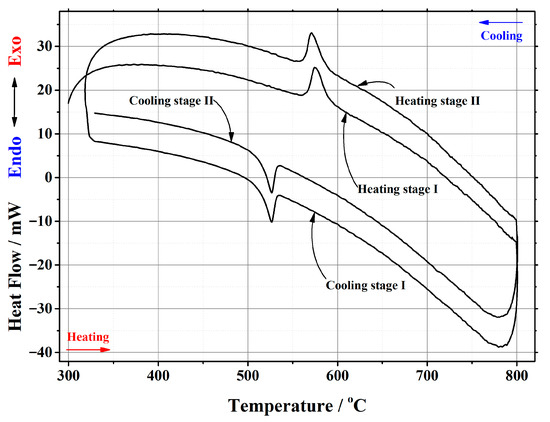
Figure A6.
DSC curve of the phase transition cycles for La1.8Ca0.2Mo2O8.9 ceramic heat-treated at 1000 °C.
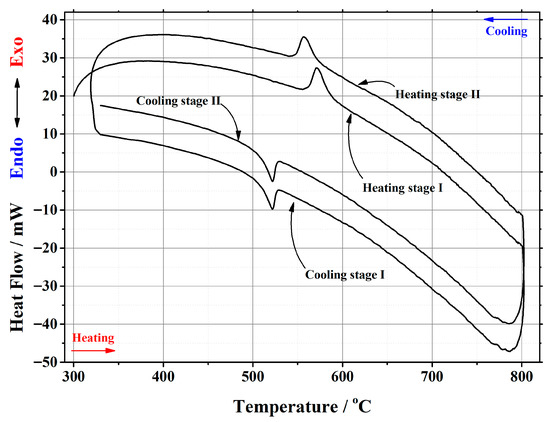
Figure A7.
DSC curve of the phase transition cycles for La1.75Ca0.25Mo2O8.875 ceramic heat-treated at 1000 °C.
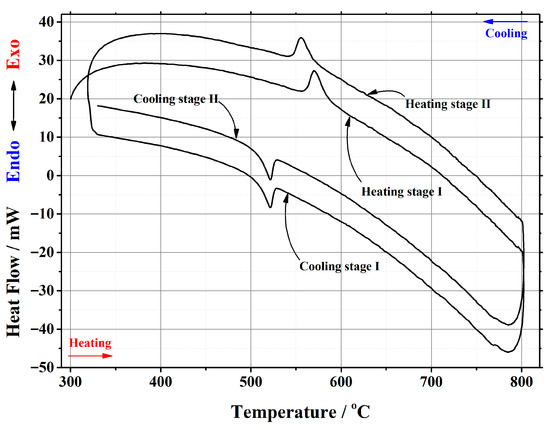
Figure A8.
DSC curve of the phase transition cycles for La1.7Ca0.3Mo2O8.85 ceramic heat-treated at 1000 °C.
Appendix B
Rietveld refinement analysis results of the corresponding XRD patterns for the La2–xCaxMo2O9–x/2 ceramic supporting the data presented in Table 2.
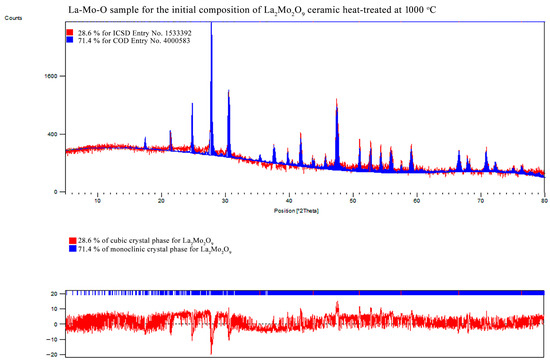
Figure A9.
XRD pattern of the La2Mo2O9 ceramic heat-treated at 1000 °C.
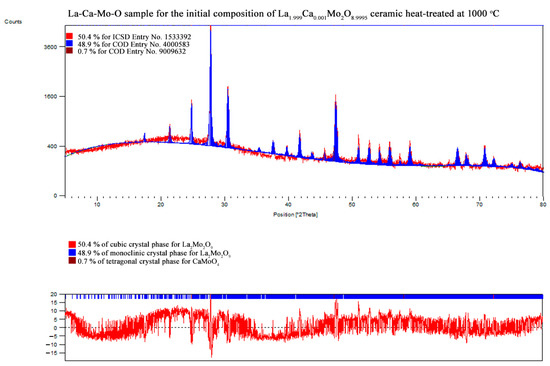
Figure A10.
XRD pattern of the La1.999Ca0.001Mo2O8.9995 ceramic heat-treated at 1000 °C.
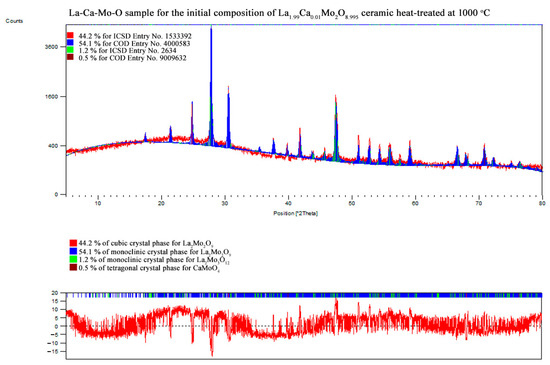
Figure A11.
XRD pattern of the La1.99Ca0.01Mo2O8.995 ceramic heat-treated at 1000 °C.
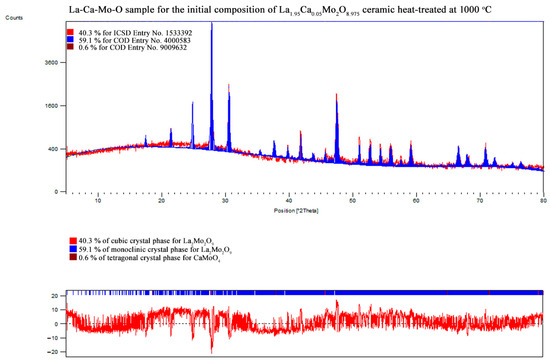
Figure A12.
XRD pattern of the La1.95Ca0.05Mo2O8.975 ceramic heat-treated at 1000 °C.
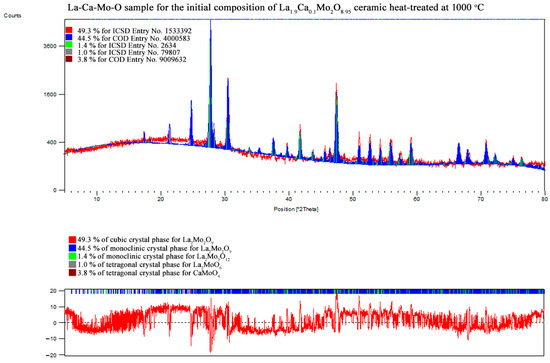
Figure A13.
XRD pattern of the La1.9Ca0.1Mo2O8.95 ceramic heat-treated at 1000 °C.
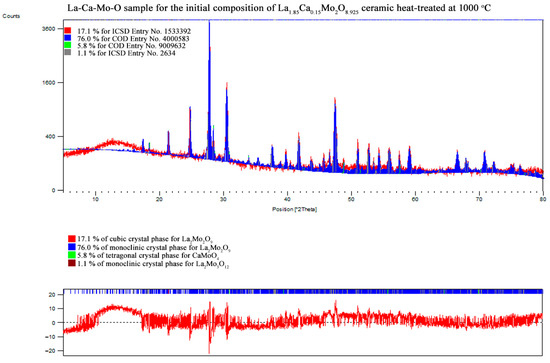
Figure A14.
XRD pattern of the La1.85Ca0.15Mo2O8.925 ceramic heat-treated at 1000 °C.
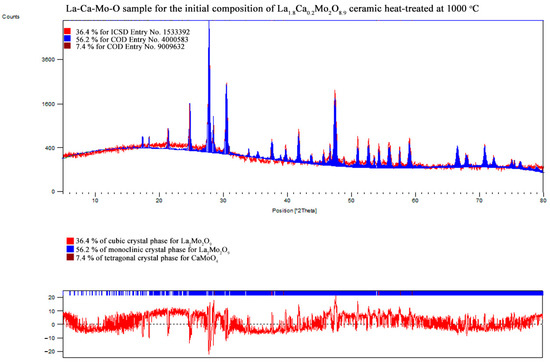
Figure A15.
XRD pattern of the La1.8Ca0.2Mo2O8 ceramic heat-treated at 1000 °C.
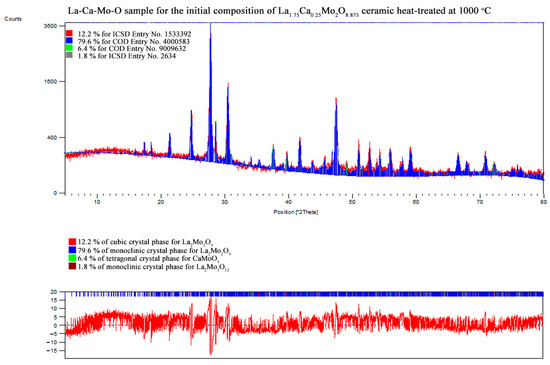
Figure A16.
XRD pattern of the La1.75Ca0.25Mo2O8.875 ceramic heat-treated at 1000 °C.
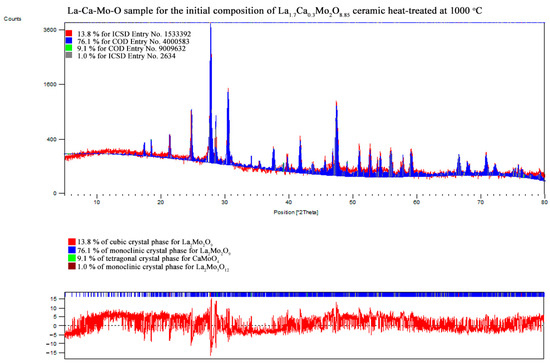
Figure A17.
XRD pattern of the La1.7Ca0.3Mo2O8.85 ceramic heat-treated at 1000 °C.
References
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Yang, B.; Tian, C.; Liu, Y.; Yang, L. Research progress of La2Mo2O9-based oxide-ion conductor electrolyte materials. Nanomater. Nanotechnol. 2022, 11, 1–7. [Google Scholar] [CrossRef]
- Jacquens, J.; Farrusseng, D.; Georges, S.; Viricelle, J.P.; Gaudillere, C.; Corbel, G.; Lacorre, P. Tests for the Use of La2Mo2O9-based Oxides as Multipurpose SOFC Core Materials. Fuel Cells 2010, 10, 433–439. [Google Scholar] [CrossRef]
- Lo, J.C.; Tsai, D.S.; Chen, Y.C.; Le, M.V.; Chung, W.H.; Liu, F.J. La2Mo2O9-Based Electrolyte: Ion Conductivity and Anode-Supported Cell under Single Chamber Conditions. J. Am. Ceram. Soc. 2011, 94, 806–811. [Google Scholar] [CrossRef]
- Kilner, J.; Druce, J.; Ishihara, T. Electrolytes. In High-Temperature Solid Oxide Fuel Cells for the 21st Century: Fundamentals, Design and Applications: Second Edition, 2nd ed.; Kendall, K., Kendall, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 85–132. [Google Scholar] [CrossRef]
- Siddharth Sil, A.; Bysakh, S. Effect of K doping on Mo6+ stability and ionic conductivity study in La2Mo2O9 as oxide-ion conductor. Mater. Res. Express 2019, 6, 056203. [Google Scholar] [CrossRef]
- Ali, M.; Wani, B.N.; Bharadwaj, S.R. Phase transition in LAMOX type compounds. J. Therm. Anal. Calorim. 2009, 96, 463–468. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, H.O.; Huo, D.X.; Tan, W.S. Room-temperature magnetoresistive and magnetocaloric effect in La1-xBaxMnO3 compounds: Role of Griffiths phase with ferromagnetic metal cluster above Curie temperature. J. Appl. Phys. 2022, 131, 043901. [Google Scholar] [CrossRef]
- Evans, I.R.; Howard, J.A.K.; Evans, J.S.O. The crystal structure of alpha-La2Mo2O9 and the structural origin of the oxide ion migration pathway. Chem. Mater. 2005, 17, 4074–4077. [Google Scholar] [CrossRef]
- Goutenoire, F.; Isnard, O.; Retoux, R.; Lacorre, P. Crystal structure of La2Mo2O9, a new fast oxide-ion conductor. Chem. Mater. 2000, 12, 2575–2580. [Google Scholar] [CrossRef]
- Selmi, A.; Galven, C.; Corbel, G.; Lacorre, P. Thermal stability of alkali and alkaline-earth substituted LAMOX oxide-ion conductors. Dalton Trans. 2010, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tealdi, C.; Chiodelli, G.; Flor, G.; Leonardi, S. Electrode stability and electrochemical performance of Lamox electrolytes under fuel cell conditions. Solid State Ionics 2010, 181, 1456–1461. [Google Scholar] [CrossRef]
- El Khal, H.; Cordier, A.; Batis, N.; Siebert, E.; Georges, S.; Steil, M.C. Effect of porosity on the electrical conductivity of LAMOX materials. Solid State Ionics 2017, 304, 75–84. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Zhuang, Z.; Gao, Y.X.; Wang, X.P.; Fang, Q.F. Electrical properties and microstructure of nanocrystalline La2-xAxMo2O9-delta (A = Ca, Sr, Ba, K) films. Solid State Ionics 2010, 181, 1510–1515. [Google Scholar] [CrossRef]
- Corbel, G.; Durand, P.; Lacorre, P. Comprehensive survey of Nd3+ substitution In La2Mo2O9 oxide-ion conductor. J. Solid State Chem. 2009, 182, 1009–1016. [Google Scholar] [CrossRef]
- Khaled, A.; Pireaux, J.J.; Khelili, S. Synthesis and Characterization of Ca and Ba Doped LAMOX Materials and Surface Study by X-ray Photoelectron Spectroscopy. Acta Chim. Slov. 2012, 59, 766–778. [Google Scholar]
- Das, A.; Lakhanlal, L.; Shajahan, I.; Dasari, H.P.; Saidutta, M.B.; Dasari, H. Dilatometer studies on LAMOX based electrolyte materials for solid oxide fuel cells. Mater. Chem. Phys. 2021, 258, 123958. [Google Scholar] [CrossRef]
- Corbel, G.; Laligant, Y.; Goutenoire, F.; Suard, E.; Lacorre, P. Effects of partial substitution of Mo6+ by Cr6+ and W6+ on the crystal structure of the fast oxide-ion conductor structural effects of W6+. Chem. Mater. 2005, 17, 4678–4684. [Google Scholar] [CrossRef]
- Georges, S.; Bohnke, O.; Goutenoire, F.; Laligant, Y.; Fouletier, J.; Lacorre, P. Effects of tungsten substitution on the transport properties and mechanism of fast oxide-ion conduction in La2Mo2O9. Solid State Ionics 2006, 177, 1715–1720. [Google Scholar] [CrossRef]
- Pinet, P.; Fouletier, J.; Georges, S. Conductivity of reduced La2Mo2O9 based oxides: The effect of tungsten substitution. Mater. Res. Bull. 2007, 42, 935–942. [Google Scholar] [CrossRef]
- Kazakevicius, E.; Kezionis, A.; Kazlauskas, S.; Zalga, A.; Barre, M.; Juskenas, R. Phase transformations in La2-xYxMo2O9 (x=0.05, x=0.075): Temperature cycling and DRT analysis. Solid State Ionics 2019, 339, 114989. [Google Scholar] [CrossRef]
- Liao, Y.W.; Kawabata, S.; Yabutsuka, T.; Chen, W.J.; Okumura, H.; Takai, S. Low temperature phase transition phenomena in Ba- and Pb-substituted La2Mo2O9 oxide ion conductors. Solid State Ionics 2020, 354, 115405. [Google Scholar] [CrossRef]
- Acharya, S.; Naz, R. Nd-Nb co-dopant effect on suppression of phase transition, ionic conductivity and dielectrics relaxation phenomenon of La2Mo2O9 system. Ferroelectrics 2022, 589, 243–251. [Google Scholar] [CrossRef]
- Borah, L.N.; Sanjay; Pandey, A. Effect of Sn-doping at Mo-site on the conductivity of La2Mo2O9 series of compounds. Indian J. Phys. 2010, 84, 699–704. [Google Scholar] [CrossRef]
- Pahari, B.; Mhadhbi, N.; Corbel, G.; Lacorre, P.; Dittmer, J. Analysis of the local structure of phosphorus-substituted LAMOX oxide ion conductors. Dalton Trans. 2012, 41, 5696–5703. [Google Scholar] [CrossRef]
- Dammak, K.; Mhadhbi, N.; Tozri, A.; Naili, H. Influence of Isovalent Partial Sulfur Substitution on the Structural, Thermal, Electrical and Spectroscopic Properties of La2Mo2O9 Oxide Ion Conductors. Eur. J. Inorg. Chem. 2022, e202200165. [Google Scholar] [CrossRef]
- Paul, T.; Tsur, Y. Influence of Isovalent ‘W’ Substitutions on the Structure and Electrical Properties of La2Mo2O9 Electrolyte for Intermediate-Temperature Solid Oxide Fuel Cells. Ceramics 2021, 4, 502–515. [Google Scholar] [CrossRef]
- Selmi, A.; Corbel, G.; Kojikian, S.; Voronkova, V.; Kharitonova, E.; Lacorre, P. Complex effect of partial substitution of La3+ by Ca2+ on the stability of fast oxide-ion conductor La2Mo2O9. Eur. J. Inorg. Chem. 2008, 1813–1821. [Google Scholar] [CrossRef]
- Porotnikova, N.; Khrustov, A.; Farlenkov, A.; Khodimchuk, A.; Partin, G.; Animitsa, I.; Kochetova, N.; Pavlov, D.; Ananyev, M. Promising La2Mo2O9-La2Mo3O12 Composite Oxygen-Ionic Electrolytes: Interphase Phenomena. ACS Appl. Mater. Interfaces 2022, 14, 6180–6193. [Google Scholar] [CrossRef]
- Borah, L.N.; Pandey, A. Impedance Studies of La2Mo2-xSnxO9-delta Oxide Ion Conductors. Acta Metall. Sin. (Engl. Lett.) 2013, 26, 425–434. [Google Scholar] [CrossRef]
- Le, M.V.; Tsai, D.S.; Yao, C.C.; Lo, J.C.; Vo, T.P.G. Properties of 10% Dy-doped La2Mo2O9 and its electrolyte performance in single chamber solid oxide fuel cell. J. Alloys. Compd. 2014, 582, 780–785. [Google Scholar] [CrossRef]
- Stankevičiūtė, R.; Žalga, A. Sol-gel synthesis, crystal structure, surface morphology, and optical properties of Eu2O3-doped La2Mo3O12 ceramic. J. Therm. Anal. Calorim. 2014, 118, 925–935. [Google Scholar] [CrossRef]
- Žalga, A.; Gaidamavičienė, G.; Gricius, Ž.; Užpurvytė, E.; Gadeikis, J.; Diktanaitė, A.; Barré, M.; Šalkus, T.; Kežionis, A.; Kazakevičius, E. Aqueous sol–gel synthesis, thermoanalytical study and electrical properties of La2Mo2O9. J. Therm. Anal. Calorim. 2018, 132, 1499–1511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).