Abstract
Solar energy is an inexhaustible clean energy providing a key solution to the dual challenges of energy and environmental crises. Graphite-like layered molybdenum disulfide (MoS2) is a promising photocatalytic material with three different crystal structures, 1T, 2H and 3R, each with distinct photoelectric properties. In this paper, 1T-MoS2 and 2H-MoS2, which are widely used in photocatalytic hydrogen evolution, were combined with MoO2 to form composite catalysts using a bottom-up one-step hydrothermal method. The microstructure and morphology of the composite catalysts were studied by XRD, SEM, BET, XPS and EIS. The prepared catalysts were used in the photocatalytic hydrogen evolution of formic acid. The results show that MoS2/MoO2 composite catalysts have an excellent catalytic effect on hydrogen evolution from formic acid. By analyzing the photocatalytic hydrogen production performance of composite catalysts, it suggests that the properties of MoS2 composite catalysts with different polymorphs are distinct, and different content of MoO2 also bring differences. Among the composite catalysts, 2H-MoS2/MoO2 composite catalysts with 48% MoO2 content show the best performance. The hydrogen yield is 960 µmol/h, which is 1.2 times pure 2H-MoS2 and two times pure MoO2. The hydrogen selectivity reaches 75%, which is 22% times higher than that of pure 2H-MoS2 and 30% higher than that of MoO2. The excellent performance of the 2H-MoS2/MoO2 composite catalyst is mainly due to the formation of the heterogeneous structure between MoS2 and MoO2, which improves the migration of photogenerated carriers and reduces the possibilities of recombination through the internal electric field. MoS2/MoO2 composite catalyst provides a cheap and efficient solution for photocatalytic hydrogen production from formic acid.
1. Introduction
With the progress of science and technology, while productivity has developed rapidly, problems such as environmental damage and energy shortage have also deteriorated. In order to relieve the dependence on fossil energy, cheap and clean energy is desirable. Many new energy technologies have emerged, but some of them will be severely limited by factors such as geography and policy. Among them, solar photocatalysis is a new promising sustainable technology [1,2]. Solar energy is an inexhaustible source of energy in nature. However, it has fluctuations in supply time and is not convenient to use. Therefore, we can convert solar energy into other forms that are easy to use and store—hydrogen energy. Small-molecule hydrogen storage materials, such as hydrazine hydrate [3,4], sodium borohydride [5,6], formic acid and other materials, have been extensively studied as hydrogen storage materials with high hydrogen content and stable properties. Formic acid, as a by-product of the organic chemical industry, can be prepared as a product in the catalytic reaction of carbon dioxide hydrogenation [7]. Besides having the advantages common to small molecule hydrogen storage materials, formic acid also has many distinct properties.
The commonly used catalysts for hydrogen production from formic acid are precious metals and composite catalysts with precious metal components [8,9]. Shaybanizadeh’s team [10] stabled Pd–Au bimetallic alloy nanoparticles stabilized on boron nitride nanosheets via the deposition–precipitation method. When the catalyst contains 3% Pd and 5% Au, respectively, a hydrogen selectivity of 100% was reached at 50 °C. However, high costs and complex preparation processes limit the use of catalysts containing precious metals.
The development of photocatalytic hydrogen production technology depends on the development of high-performance materials. Many semiconductor materials have been found to have the ability of water decomposition, such as TiO2 [11], ZnO [12,13], WO3 [14,15,16] and CdS [17]. MoS2 is a layered material consisting of alternating layers of Mo and S atoms [18,19]. The layers are connected by van der Waals forces, and the atoms within the layers are bonded by covalent bonds. The common MoS2 has three crystal structure types, namely the 1T phase of the trigonal system, the 3R phase of the rhombic system, and the 2H phase of the hexagonal system [20]. 1T-MoS2 exhibits strong metallic properties and has good electron transfer ability. However, 2H-MoS2 is a material with semiconductor properties, and its high carrier recombination rate leads to poor catalytic efficiency [21]. In addition, the 2H-MoS2 conduction band potential is greater than the reduction potential of H+/H2, which does not meet the requirements of photocatalytic HER [22].
Among Mo compounds, MoO2 with various valence states and HER activity stands out [23,24]. Oxygen vacancies with sufficient concentration generate a large number of free electrons, and the Fermi energy level of MoO2 is located in the Mo4d orbital, resulting in a metallic property. Its excellent conductivity provides efficient carrier transfer [25]. If a material can be prepared to retain the advantages of MoS2 and MoO2, it will be a progressive step in the field of photocatalytic hydrogen evolution.
MoS2/MoO2 is considered a promising non-noble metal catalyst for hydrogen evolution reaction. By calcination, Zhang formed a Schottky heterojunction between MoS2 and MoO2, and the hydrogen yield of the composite product was 3.4 times that of pure MoS2 [26]. To further explore the role of MoO2 in the HER of MoS2, Wang used MoO3 with different morphologies as a molybdenum source to produce MoO2 and studied the effects of pH value, temperature and molybdenum-sulfur mole ratio on the morphology of the produced MoO2. The relevant electrochemical properties were tested in subsequent experiments. They believed that there were many active sites in S [27], which is the reason that HER catalytic performance is better. Kang et al. modified the MoS2/MoO2 ratio by soft annealing, which caused no change in the structure, yet an improvement in the hydrogen evolution efficiency [28].
In past research, researchers often prepared MoS2 and MoO2 separately and then composite the two. Such multi-step reaction often requires high temperature, which not only has high requirements of experimental equipment, but also complicated the operation steps, which is not conducive to repetition, and limits its further application in practice. The one-step hydrothermal synthesis has the advantages of simple operation, high repeatability, and low preparation cost.
In order to solve the above problems, the authors used ammonium molybdate tetrahydrate as molybdenum source and thiourea as sulfur source, and prepared 1T-MoS2 by bottom-up hydrothermal method. By increasing synthesis temperature and time, the phase transformation process of molybdenum sulfide from the 1T phase to the 2H phase was realized. On this basis, a certain amount of 5% diluted hydrochloric acid is introduced to adjust the environment suitable for MoO2 formation. The morphology, structure, surface area and valence state of the composite catalyst were analyzed by XRD, SEM, XPS and BET. Using formic acid as the hydrogen storage material, the photocatalytic performance of the composite catalysts was evaluated, and then the mechanism was explored. When 1T-MoS2 is combined with MoO2, the hydrogen selectivity improves to 72.5%, with the cost of a 10% decrease in hydrogen production. However, when 2H-MoS2 was combined with MoO2, hydrogen production and hydrogen selectivity were significantly improved. Though the hydrogen production rate and selectivity of the catalysts used in this work are much higher than those reported in the literature, the energy to irradiate the reaction was from the Unfiltered Hg lamp which contains a much higher amount of ultraviolet light. The catalysts studied in this work are applicable in high-concentration pollutant control in water source and hydrogen production under high irradiation.
2. Materials and Methods
2.1. Materials
Thiourea (CH4N2S) was purchased from Tianjin Zhiyuan Chemical Reagent Co. (Tianjin, China). Ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, 99%) was purchased from Sinophosphate Chemical Reagent Co. (Tianjin, China). Hydrochloric acid (HCl) was supplied by Nanjing Chemical Specimen Co. (Nanjing, China). Formic acid (HCOOH) is supplied by Shanghai Lingfeng Chemical Reagent Co. (Shanghai, China). All chemicals are analytical grade and used without further purification.
2.2. Synthesis of Photocatalysts
MoS2 particles: 1T-MoS2 was synthesized by hydrothermal method. A total of 1.059 g of (NH4)6Mo7O24·4H2O and 2.284 g of CH4N2S(thiourea) were added in 60 mL deionized water at room temperature and was stirred until a clear solution was obtained. The solution was then transferred to a 100 mL Teflon-lined autoclave and held at 200 °C for 3 h. The resulting black precipitate was then dried at 40 °C for 8 h. For the preparation of 2H-MoS2, a similar procedure was applied except the hydrothermal temperature was increased to 240 °C and the duration was increased to 8 h.
MoO2 Flakes: 0.288 g of molybdenum powders and 0.384 g of molybdenum trioxide were added into a quartz boat and then calcined in a tubular furnace with an N2 atmosphere. The powder was heated to 700 °C at a rate of 5 °C/min and kept at 700 °C for 10 h. A black powder was obtained after cooling down to room temperature naturally.
MoS2/MoO2 composite catalyst: Since hydrothermal temperature is a crucial parameter to make the desired polymorph of MoS2, the same strategy was applied to prepare the MoS2/MoO2 composite catalyst with different polymorphs of MoS2. For the preparation of 1T-MoS2/MoO2, 1.0593 g of (NH4)6Mo7O24·4H2O and 0.4568 g of CH4N2S were dissolved in 40 mL deionized water at room temperature. After continuous stirring, an appropriate amount of 5% HCl was added to the solution. Then, the solution was moved into a 100 mL Teflon-lined autoclave and kept at 220 °C for 20 h. The HCl solution was used to control the amount of MoO2 produced and thus the MoS2/MoO2 ratio can be changed accordingly. Three 1T-MoS2/MoO2 samples with different ratios of MoS2/MoO2 were obtained using 18 mL, 20 mL and 22 mL of 5% HCl solution and the resulting products were denoted as 1T-18HCl, 1T-20HCl and 1T-22HCl, respectively. For the preparation of 2H-MoS2/MoO2 with different MoS2/MoO2 ratios 18 mL, 20 mL, 22 mL and 30 mL of 5% HCl solution was used, and the hydrothermal synthesis was carried out at 240 °C for 20 h. The resulting 2H-MoS2/MoO2 composites were named 2H-18HCl, 2H-20HCl, 2H-22HCl and 2H-30HCl.
2.3. Characterization
Powder X-ray diffraction patterns were obtained by Rigaku SmartLab diffractometer (Tokyo, Japan) using Cu Kα (λ = 0.154178 nm) as a radiation source. Rietveld refinement of XRD data was performed using GSAS-II (version 5511). The morphology of the samples was measured by scanning electron microscope (FESEM, Ultra-55, Zeiss, Oberkochen, Germany). Nitrogen adsorption-desorption isotherms were measured at −196 °C using a specific surface area and pore analyzer V-Sorb 1800 (NETZSCH-Gerätebau GmbH, Selb, Germany). The samples were pretreated at 105 °C for 12 h prior to measurement. The electrochemical impedance was measured at the electrochemical workstation CH1660E. X-ray photoelectron spectroscopy was recorded using Kratos AXIS Supra (Shimadzu, Kyoto, Japan).
2.4. Photocatalytic Degradation and Photoelectrochemical Test
The photocatalytic performance of the composite catalyst was evaluated by catalytic hydrogen evolution from formic acid under ultraviolet light using a 500 UV Hg lamp as the light source. For each test, 50 mg of the catalyst was dispersed in 500 mL of 10% formic acid solution. Prior to the test, the suspension was stirred in the dark for 30 min and the photocatalyst reactor was vacuumed to eliminate the air in the reactor. The produced gas was extracted every 5 min using a 500 µL gas sampler and the composition was analyzed using a gas chromatograph (SP-6890,Shandong Lunan Ruihong Chemical Instrument Co., Ltd., Tengzhou City, China). The amount of gas produced by photocatalysis was evaluated using the drainage method.
Electrochemical impedance measurement was conducted to study the electrochemical characteristics. A total of 20 mg of the catalyst was dispersed into 40 µL of a mixture of 5 wt% Nafion and 0.5 mL of anhydrous ethanol under ultrasound, and then 200 µL of the suspension was spread onto the surface of the conductive glass to prepare the working electrode. A total of 0.1 M Na2SO4 solution is used as the electrolyte, platinum electrode as the counter electrode and saturated glycerol electrode as the reference electrode Electrochemical impedance measurement was carried out using CH1660E electrochemical workstation.
3. Results and Discussion
3.1. Characterization and Properties of 1T-MoS2/MoO2 Composite Catalysts
3.1.1. XRD Characterization
Figure 1a shows the XRD patterns of 1T-MoS2/MoO2 composite catalyst with different MoO2 contents. It can be seen from Figure 1a that the characteristic peaks of the MoS2 component at 2θ equal to 9.6°, 33.1°, 35.4°, and 57.4° correspond to (001), (100), (102), and (110) crystallographic planes, respectively [29]. The characteristic peaks at 26.1°, 37.3°, 53.7°, and 61° correspond to (011), (211), (311), and (013) crystallographic planes of MoO2, respectively. In the composite sample of 1T-MoS2/MoO2, with the increase in the dosage of 5% HCl solution, the characteristic peak intensity of 1T-MoS2 at 2θ equal to 9.6° gradually decreases. On the contrary, the intensity of the characteristic peaks of MoO2 at 2θ equal to 26.1° and 37.7° gradually increases.
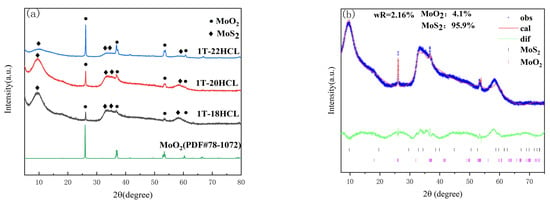
Figure 1.
Powder X-ray diffraction pattern of 1T-MoS2/MoO2 composite catalyst, and the figure of fitting calculation; (a) Overlay of powder X-ray diffraction patterns of 1T composite catalyst (b) 1T-18; (c) 1T-20HCl; (d) 1T-22HCl.
Rietveld quantitative analysis was carried out using the GSAS-II software package (version 5511) [30,31]. Figure 1b–d are the Rietveld plots of 1T-MoS2/MoO2 with different MoO2 contents. The blue line represents the raw XRD data obtained from the actual test, the red line is the data calculated by the software, the green line is the difference between the actual data and the fitted data, and the different colored tick marks represent the Bragg peak positions of different substances. The Rwp after refinement are 2.16%, 1.97%, and 1.93% for 1T-18HCl, 1T-20HCl and 1T-22HCl, respectively, indicating a good fit was obtained. The contents for each substance in 1T-MoS2/MoO2 are listed in Table 1. Rietveld quantitative analysis suggests that the content of MoO2 is positively correlated with the amount of dilute hydrochloric acid used.

Table 1.
The content of different substances in 1T-MoS2/MoO2 in different amounts of hydrochloric acid.
3.1.2. Morphology Analysis
Different additions of 5% HCl solution will cause changes in the composition of the composite catalyst as described in the preceding section. Further characterization of morphology is carried out and Figure 2 shows scanning electron microscope images of three 1T-MoS2/MoO2 composite catalysts together with the images of 1T-MoS2 and MoO2 for comparison. As shown in Figure 2a, MoO2 exhibits a regular shape with a size of about 1μm, while 1T-MoS2 presents a nano-flower-like morphology with abundant petals shown in Figure 2b. Figure 2c shows the SEM image of 1T-18HCl, which is basically dominated by 1T-MoS2 nanoflowers. In Figure 2d, more MoO2 can be seen, while nano-flower-like MoS2 grows on block MoO2. In Figure 2e, the flower-like 1T-MoS2 still grows on the block MoO2, but more block MoO2 can be seen. It can be seen from Figure 2 that HCl promotes the growth of MoO2 in the composite catalyst. The SEM study is consistent with the results of the Rietveld quantitative analysis using GSAS-II.
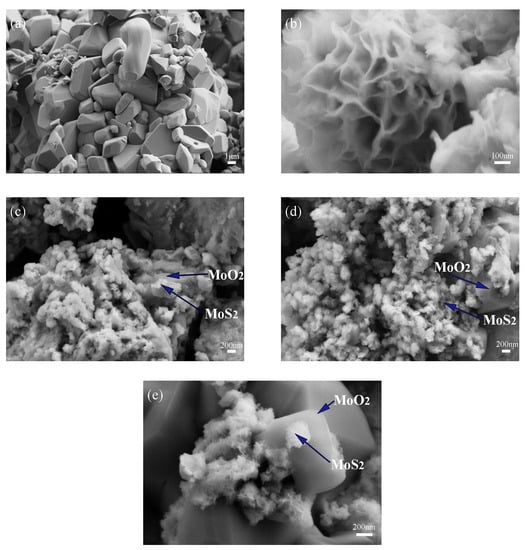
Figure 2.
SEM images of (a) MoO2; (b) 1T-MoS2; (c) 1T-18HCl; (d) 1T-20HCl; (e) 1T-22HCl.
3.1.3. Electrochemical Impedance Measurement
Figure 3 shows the electrochemical impedance measurements of the 1T-18HCl, 1T-20HCl, and 1T-22HCl composite catalysts. The arc radius of the high-frequency region of the measured electrochemical impedance represents the photoexcited carrier transfer efficiency of the material. The smaller the curvature radius, the lower the electrical impedance of the catalyst and the higher the transfer efficiency of the electron–hole pair. The results show that the impedance radius of 1T-18HCl is large, indicating that the transfer efficiency of electron–hole pairs is low. The radius of 1T-20HCl is smaller than that of 1T-18HCl, while the radius of 1T-22HCl is significantly smaller than that of 1T-20HCl and 1T-18HCl. The electrochemical impedance measurement showed that the electron transfer efficiency of the composite catalyst increased with the increase in MoO2 content.
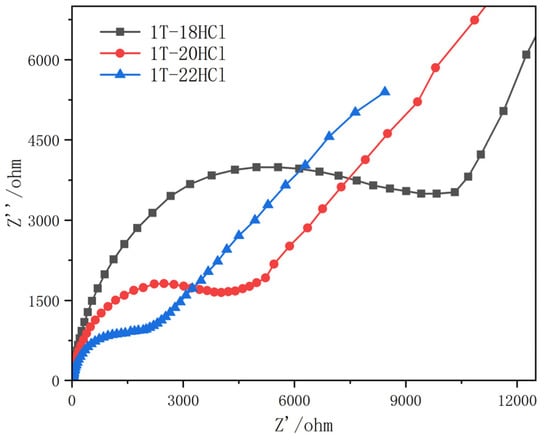
Figure 3.
Electrochemical impedance of 1TMoS2/MoO2 composite catalyst with different MoO2 contents.
3.1.4. Photocatalytic Performance
The photocatalytic performance of hydrogen evolution from formic acid using 1T-MoS2/MoO2 composite catalysts is summarized in Figure 4. It can be seen from Figure 4 that in the composite catalyst, the hydrogen production and hydrogen selectivity will increase with the increase in MoO2 content. 1T-22HCl shows the best hydrogen selectivity (73%) which is increased by 7% and 27% compared with that of pure 1T-MoS2 and MoO2, respectively. However, in terms of hydrogen production per unit time, the composite catalyst is inferior to pure 1T-MoS2.
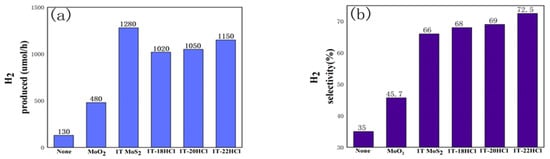
Figure 4.
Histogram of photocatalytic performance comparison of different catalysts, (a) hydrogen production comparison chart, (b) hydrogen selectivity comparison chart.
3.2. Characterization and Properties of 2H-MoS2/MoO2
3.2.1. XRD Characterization
Figure 5a shows the XRD patterns of the 2H-MoS2/MoO2 composite catalysts prepared at 240 °C for 20 h, and the addition of 5% dilute HCl was 18, 20, 24, and 30 mL. It can be seen from Figure 5a shows the XRD patterns of 2H-MoS2/MoO2 composite catalysts with different MoO2 contents. It can be seen from Figure 5a that the characteristic peaks of the MoS2 component at 2θ equal to14°, 32.3°, 35.4°, and 57.4° correspond to (002), (100), (103), and (110) crystallographic planes, respectively. The characteristic peaks at 26.1°, 37.3°, 53.7°, and 61° correspond to (011), (211), (311), and (013) crystallographic planes of MoO2, respectively.
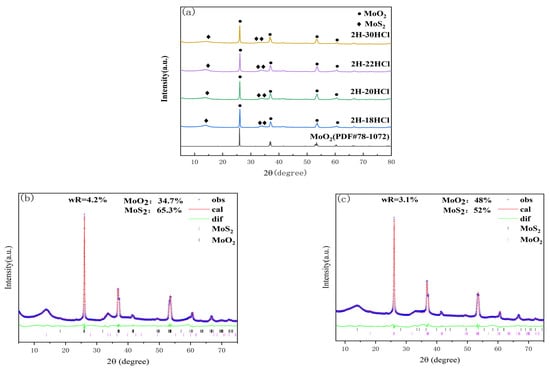
Figure 5.
Powder X-ray diffraction pattern of 2H-MoS2/MoO2 composite catalyst, and the figure of fitting calculation; (a) Overlay of powder X-ray diffraction patterns of 2H composite catalyst (b) 2H-20HCl; (c) 2H-30HCl.
Rietveld quantitative analysis was carried out between the differentiated 2H-30HCl and the intermediate value of 2H-20HCl. The blue line represents the raw XRD data obtained from the actual test, the red line is the data calculated by the software, the green line is the difference between the actual data and the fitted data, and the different colored tick marks represent the Bragg peak positions of different substances. The smaller Rwp after refining were 4.2% and 3.1%, respectively, indicating a good fit. Table 2 lists the content of each substance in 2H-MoS2/MoO2. Rietveld quantitative analysis showed that the content of MoO2 was positively correlated with the amount of dilute chloric acid.

Table 2.
The content of different substances in 2H-MoS2/MoO2 in different amounts of hydrochloric acid.
The Rwp of Rietveld quantitative analysis for 2H-20HCl and 2H-30HCl are 4.2% and 3.1%, respectively, indicating that the fittings are reliable. The results of the analysis illustrate that the content of MoO2 in 2H-30HCl is significantly more than that in 2H-20HCl. This agrees well with the fact that HCl can promote the formation of MoO2 as indicated by the preparation for 1T-MoS2/MoO2.
3.2.2. Morphology Analysis of 2H-MoS2/MoO2
The scanning electron microscopy images of 2H-20HCl and 2H-30HCl are shown in Figure 6. Due to a longer period of high temperature in hydrothermal treatment, it is difficult for MoS2 to maintain the flower-like morphology, and the agglomeration of crystal grains is intensified. Instead, MoS2 nanospheres are observed. Figure 6b shows that MoO2 transforms into a rod-like morphology when the temperature is 240 °C. Figure 6c shows a similar rod-like shape for MoO2 in 2H-30HCl compared with that of 2H-20HCl, indicating excess HCl have little effect on the morphology of MoO2. 2H-20HCl and 2H-30HCl exhibit similar morphology for MoS2.
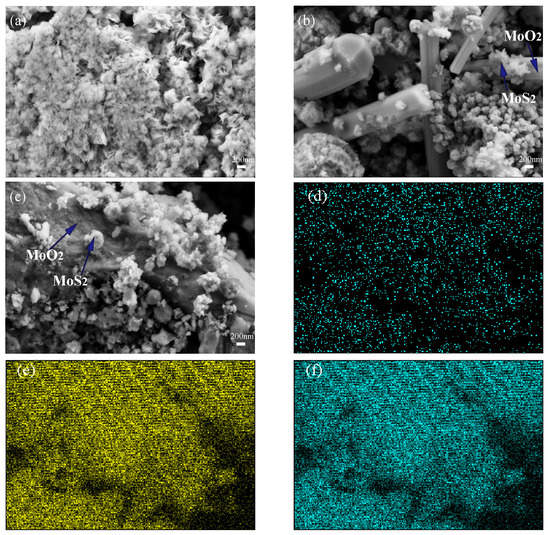
Figure 6.
Scanning electron microscopy of 2H-MoS2, composite catalyst, and energy spectrum of 2H-30HCl (a) 2H-MoS2 (b) 2H-20HCl (c) 2H-30HCl (d) oxygen element (e) sulfur element (f) molybdenum element.
Figure 6d,e is the EDS elemental mapping of 2H-30HCl, it can be seen that S, Mo, and O elements are evenly distributed on the surface of the catalyst material. The elemental analysis using EDS is listed in Table 3, which is approximately consistent with the quantitative analysis using the Rietveld method.

Table 3.
Elemental content for EDS analysis of 2H-30HCl.
3.2.3. BET Measurements
Table 4 shows the specific surface area results obtained by the catalyst after nitrogen adsorption-desorption, and it can be seen from the data in Table 4 that the specific surface area of 2H-20HCl is 10% larger than that of 2H-30HCl. According to the calculated average pore size, both 2H-20HCl and 2H-30HCl belong to mesoporous materials.

Table 4.
BET analysis of composite catalysts with different dilute hydrochloric acid additions.
3.2.4. Electrochemical Impedance Measurement
The electrochemical impedance measured results of the 2H-MoS2/MoO2 composite catalyst are shown in Figure 7. Compared with 2H-30HCl, the curve of 2H-20HCl is closer to a straight line, indicating that its radius of curvature is greater than that of 2H-30HCl, that is, the charge transfer resistance is greater than 2H-30HCl, and the charge transfer resistance and the photocatalytic performance of the material often show a negative correlation. It is speculated that the photocatalytic performance of 2H-20HCl is not as good as that of 2H-30HCl. In summary, the decrease in pH caused by the addition of dilute hydrochloric acid can increase the MoO2 content in the 2H composite catalyst, and with the increase in MoO2 content, the charge transfer resistance of the material decreases.

Figure 7.
Electrochemical impedance diagrams of 2H-MoS2/MoO2 composite catalysts with different MoO2 contents.
3.2.5. X-ray Photoelectronic Spectrum Analysis of 2H-MoS2/MoO2
XPS analysis was performed for 2H-20HCl and 2H-30HCl. The spectrum for each sample shows similar characteristics and 2H-30HCl is discussed as an example. Figure 8 shows a narrow spectrum. (Please view the Figure S1 for the full spectrum) The peak fitting of Mo 3d and S 2p are shown in Figure 8a,b. As to the S 2p spec, the characteristic doublets at 162.4 eV, 163.6 eV,163.8 eV and 166.0 eV correspond to S2− [32], while the band at 169.6 eV indicates that S is partially oxidized [33]. Figure 8a, the band corresponding to 233.3 eV is formed by Mo6+ spin orbit, which can be attributed to the slight oxidation of the sample surface [34,35]. The band at other positions such as 229.6 eV and 233.2 eV conform to the characteristic peak regularity of Mo4+3d5/2 and Mo4+3d3/2, and a characteristic band belonging to Mo6+3d3/2 appears near 236.5 eV. The small band at 226.7 eV is the interference peak of S 2 s, which indicates that MoS2 exists on the surface of the detected substance. Calculated by the XPS data, the molar ratio of Mo: S is 1:1.50, larger than the stoichiometric ratio of pure MoS2 (1:2), providing direct evidence for the incomplete sulfurization of MoO2.

Figure 8.
XPS diagram of catalyst 2H−30HCl (a) Peak fitting for Mo 3d; (b) Peak fitting for S 2P.
3.2.6. Photocatalytic Performance
Figure 9 is a performance of the photocatalytic hydrogen production from formic acid using a 2H-MoS2/MoO2 composite catalyst. When the ratio of MoO2 to 2H-MoS2 in the sample changes, the selectivity of hydrogen increases. For 2H-30HCl, hydrogen selectivity reaches 75%, which is 2.14 times that of catalyst-free conditions and 1.42 times that of pure 2H-MoS2. The hydrogen production per unit time of the 2H composite catalyst is also higher than that of pure 2H-MoS2. Taking 2H-30HCl as an example, its hydrogen production has reached 960µmol/h, which is not only twice that of pure MoO2, but also higher than that of pure 2H-MoS2.
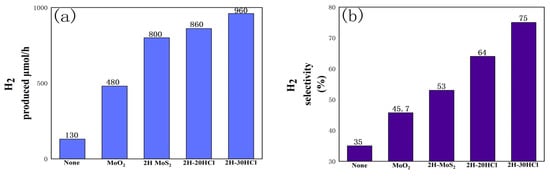
Figure 9.
Histogram of photocatalytic performance comparison of 2H-MoS2/MoO2 catalysts with different MoO2 content, (a) comparison of hydrogen production;(b) comparison of hydrogen selectivity.
4. Conclusions
MoS2 of different polymorphs can be synthesized under appropriate hydrothermal conditions. 1T-MoS2 can be obtained at 200 °C for 3 h while 2H-MoS2 is formed at 240 °C for 8 h. MoS2/MoO2 composite catalysts can be synthesized by a one-step hydrothermal method. The polymorph of MoS2 in the composite can be adjusted using a similar strategy for the synthesis of pure 1T-MoS2 and 2H-MoS2.
Hydrochloric acid promotes the formation of MoO2 in the process of preparing MoS2/MoO2 composite catalysts. In the process of composite catalyst generation, an acidic environment promotes the formation of MoO3 from ammonium molybdate in aqueous solution. MoO3 is then reduced by thiourea to form MoO2, and then MoO2 is further vulcanized to produce MoS2. When the amount of hydrochloric acid is low, the MoOx produced will be completely vulcanized to MoS2. With the gradual increase in the dosage of hydrochloric acid, the thiourea involved in sulfide MoOx was gradually reduced, and the content of MoO2 phase in the product was gradually increased. Therefore, the ratio of MoS2 to MoO2 in the composite catalysts can be modified using different amounts of hydrochloric acid.
Comparing 1T-MoS2/MoO2 and 2H-MoS2/MoO2, it can be found that each has its own advantages. Based on the relatively high hydrogen production of 1T-MoS2, the overall hydrogen production per unit time of 1T-MoS2/MoO2 is higher than that of 2H-MoS2/MoO2, and hydrogen production on 1T-22HCl reached 1150 µmol/h. The advantage of 2H-MoS2/MoO2 is presented in its hydrogen selectivity, with a hydrogen selectivity of 75% for 2H-30HCl.
Due to the synergistic effect, the hydrogen selectivity of composite catalyst 1T-MoS2/MoO2 is improved compared to pure 1T-MoS2 and MoO2. The hydrogen selectivity also increases with the increase in MoO2 content, though the hydrogen yield on 1T-MoS2/MoO2 is lower than that of pure 1T-MoS2, which may be caused by the reduction in active sites from 1T-MoS2 with the addition of MoO2 [27]. However, with the increase in the amount of composite MoO2, the synergistic effect increases, and the hydrogen yield of the composite catalyst increases gradually.
P-type semiconductor 2H-MoS2 has a similar crystal structure matching lattice constant and crossed band gaps to N-type semiconductor MoO2. A heterostructure of 2H-MoS2 and MoO2 is formed [36,37]. Due to the formation of heterojunctions, oxidation and reduction occur in different components of the composite catalyst, which greatly reduced the recombination rate of charge carriers. Moreover, due to the presence of a junction electric field, the migration efficiency of charge carriers has also been significantly improved. Therefore, the photocatalytic performance of 2H-MoS2/MoO2 is better than that of pure 2H-MoS2 and MoO2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16114030/s1, Figure S1: Full-spectrum scanning of the XPS.
Author Contributions
Conceptualization, Z.P.; software, W.Y., Y.L. (Yunfei Liu) and Y.L. (Yinong Lu); data curation, D.D.; writing—original draft preparation, D.D.; writing—review and editing, Y.T. and Z.P. project administration, Y.L. (Yinong Lu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the priority academic program development of Jiangsu Higher Education Institution (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the assistance from Zhigang Pan and Yaqiu Tao from NJTECH, and the staff from the State Key Laboratory of Materials-Oriented Chemical Engineering.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, D.D.; Wang, L.; Yu, P.; Zhao, L.; Tian, C.U.; Zhou, W.; Zhang, L.; Fu, H.G. From graphite to porous graphene-like nanosheets for high rate lithium-ion batteries. Nano Res. 2015, 8, 2998–3010. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. Spent rechargeable lithium batteries in e-waste: Composition and its implications. Front. Environ. Sci. Eng. 2014, 8, 792–796. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, D.X.; Yin, H.; Qu, Y.P.; Zhou, L.L.; Chen, C.; Wu, H.; Wang, P. Noble-Metal-Free Ni-W-O-Derived Catalysts for High-Capacity Hydrogen Production from Hydrazine Monohydrate. ACS Sustain. Chem. Eng. 2020, 8, 5595–5603. [Google Scholar] [CrossRef]
- Qiu, Y.P.; Shi, Q.; Zhou, L.L.; Chen, M.H.; Chen, C.; Tang, P.P.; Walker, G.S.; Wang, P. Ni Pt Nanoparticles Anchored onto Hierarchical Nanoporous N-Doped Carbon as an Efficient Catalyst for Hydrogen Generation from Hydrazine Monohydrate. ACS Appl. Mater. Interfaces 2020, 12, 18617–18624. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Lup, W.J.; Kuan, Y.D. Preparation of Catalyst for Hydrogen Production Reaction of Sodium Borohydride and Its Effectiveness. Sens. Mater. 2020, 32, 3659–3668. [Google Scholar] [CrossRef]
- Ghodke, N.P.; Rayaprol, S.; Bhoraskar, S.V.; Mathe, V.L. Catalytic hydrolysis of sodium borohydride solution for hydrogen production using thermal plasma synthesized nickel nanoparticles. Int. J. Hydrog. Energy 2020, 45, 16591–16605. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.B. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.M.; He, H.Y.; Huang, F.Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew Chem. Int. Ed. Engl. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Shaybanizadeh, S.; Chermahini, A.N.; Luque, R. Boron nitride nanosheets supported highly homogeneous bimetallic AuPd alloy nanoparticles catalyst for hydrogen production from formic acid. Nanotechnology 2022, 33, 27. [Google Scholar] [CrossRef]
- Wang, G.Z.; Chang, J.L.; Tang, W.Y.; Xie, W.J.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D-Appl. Phys. 2022, 55, 29. [Google Scholar] [CrossRef]
- He, Y.B.; Li, G.R.; Wang, Z.L.; Su, C.Y.; Tong, Y.X. Single-crystal ZnO nanorod/amorphous and nanoporous metal oxide shell composites: Controllable electrochemical synthesis and enhanced supercapacitor performances. Energy Environ. Sci. 2011, 4, 1288–1292. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.S.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.Y.; Ye, H.C.; Chen, S.M.; Ju, H.X.; Liu, D.B.; Lin, Y.; Ye, W.; Wang, C.M.; Xu, Q.; et al. Oxide Defect Engineering Enables to Couple Solar Energy into Oxygen Activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Paik, T.; Cargnello, M.; Gordon, T.R.; Zhang, S.; Yun, H.; Lee, J.D.; Woo, H.Y.; Oh, S.J.; Kagan, C.R.; Fornasiero, P.; et al. Photocatalytic Hydrogen Evolution from Substoichiometric Colloidal WO3-x Nanowires. ACS Energy Lett. 2018, 3, 1904–1910. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.L.; Guan, N.J.; Li, L.D.; Gong, J.L. Tungsten Oxide Single Crystal Nanosheets for Enhanced Multichannel Solar Light Harvesting. Adv. Mater. 2015, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Liu, L.; Xi, X.X.; Li, Y.M.; Geng, Z.L.; Jiang, G.Y.; Zhao, Z. Novel metal doped carbon quantum dots/CdS composites for efficient photocatalytic hydrogen evolution. Nanoscale 2019, 11, 1618–1625. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.Y.; Hong, G.S.; Dai, H.J. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Jia, F.; Jia, F.F.; Song, S.X. Adsorption of heavy metals on molybdenum disulfide in water: A critical review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide TS2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, Y.X.; Yang, Z.Y.; Yan, Y.M.; Sun, K.N. Synthesis of MoS2 and MoO2 for their applications in H2 generation and lithium ion batteries: A review. Sci. Technol. Adv. Mater. 2013, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Y.; Zhang, H.Y.; Li, Z.H. Synthesis of Double-Layer Nitrogen-Doped Microporous Hollow Carbon@MoS2/MoO2 Nanospheres for Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 29511–29520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Shao, J.; Li, J.; Zhang, L.; Qu, Q.T.; Zheng, H.H. Ordered mesoporous MoO2 as a high-performance anode material for aqueous supercapacitors. J. Power Sour. 2013, 237, 80–83. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.M.; Yang, X.F.; Jiao, M.G.; Zhou, Z.; Zhang, M.H.; Wang, D.H.; Bu, X.H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xin, X.; Song, Y.R.; Yang, L.; Wang, B.L.; Tan, L.L.; Li, X.H. Plasmonic MoO2 as co-catalyst of MoS2 for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 504, 144291. [Google Scholar] [CrossRef]
- Wang, W.; Yao, Q.; Ma, J.; Xu, Y.; Jiang, J.Q.; Liu, X.E.; Li, Z.C. Engineering MoS2 nanostructures from various MoO3 precursors towards hydrogen evolution reaction. Crystengcomm 2020, 22, 2258–2267. [Google Scholar] [CrossRef]
- Kang, H.; Youn, J.-S.; Oh, I.; Manavalan, K.; Jeon, K.J. Controllable atomic-ratio of CVD-grown MoS2-MoO2 hybrid catalyst by soft annealing for enhancing hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 1399–1408. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, Y.Y.; Luo, X.N.; Wu, Z.Z.; Wang, Y.J.; Fang, B.Z. Swollen Ammoniated MoS2 with 1T/2H Hybrid Phases for High -Rate Electrochemical Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 2509–2515. [Google Scholar] [CrossRef]
- Toby, B.H.; Von dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Von Dreele, R.B. Small-angle scattering data analysis in GSAS-II. J. Appl. Crystallogr. 2014, 47, 1784–1789. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Z.J.; Luo, X.N.; Sun, A.K.; Wu, Z.Z.; Wang, D.Z. Template-free fabrication of hierarchical MoS2/MoO2 nanostructures as efficient catalysts for hydrogen production. Appl. Surf. Sci. 2018, 433, 723–729. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Sharma, R.K.; Reddy, G.S. A study on role of partial pressure in controlled synthesis of core-shell MoO2/MoS2 nanoflakes. Mater. Chem. Phys. 2016, 178, 6–11. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, B.C.; Zhou, J.F.; Fang, Z.T.; Li, K.Z.; Ma, H.Y.; Dai, J.M.; Zhu, X.B.; Sun, Y.P. Improved Electrochemical Performance of Ultrathin MoS2 Nanosheet/Co Composites for Lithium-Ion Battery Anodes. Chemelectrochem 2019, 6, 1930–1938. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.M.; Chen, Y.F.; Chen, Y.F.; Li, M.X.; Liu, P.Y.; Wang, C.C.; Wang, P.; Lu, H.B. Designing vertical channels with expanded interlayers for Li-ion batteries. Chem. Commun. 2019, 55, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Modak, A.; Pant, K.; Sinhamahapatra, A.; Biswas, P. MoS2-Nanosheets-Based Catalysts for Photocatalytic CO2 Reduction: A Review. ACS Appl. Nano Mater. 2021, 4, 8644–8667. [Google Scholar] [CrossRef]
- Tian, J.; Yang, C.; Liu, Z.; Li, F.N.; He, X.; Chen, W.; Xia, N.; Lin, C. Construction of MoO2@MoS2 heterostructures in situ on carbon cloth for the hydrogen evolution reaction. New J. Chem. 2021, 45, 19826–19830. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).