Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
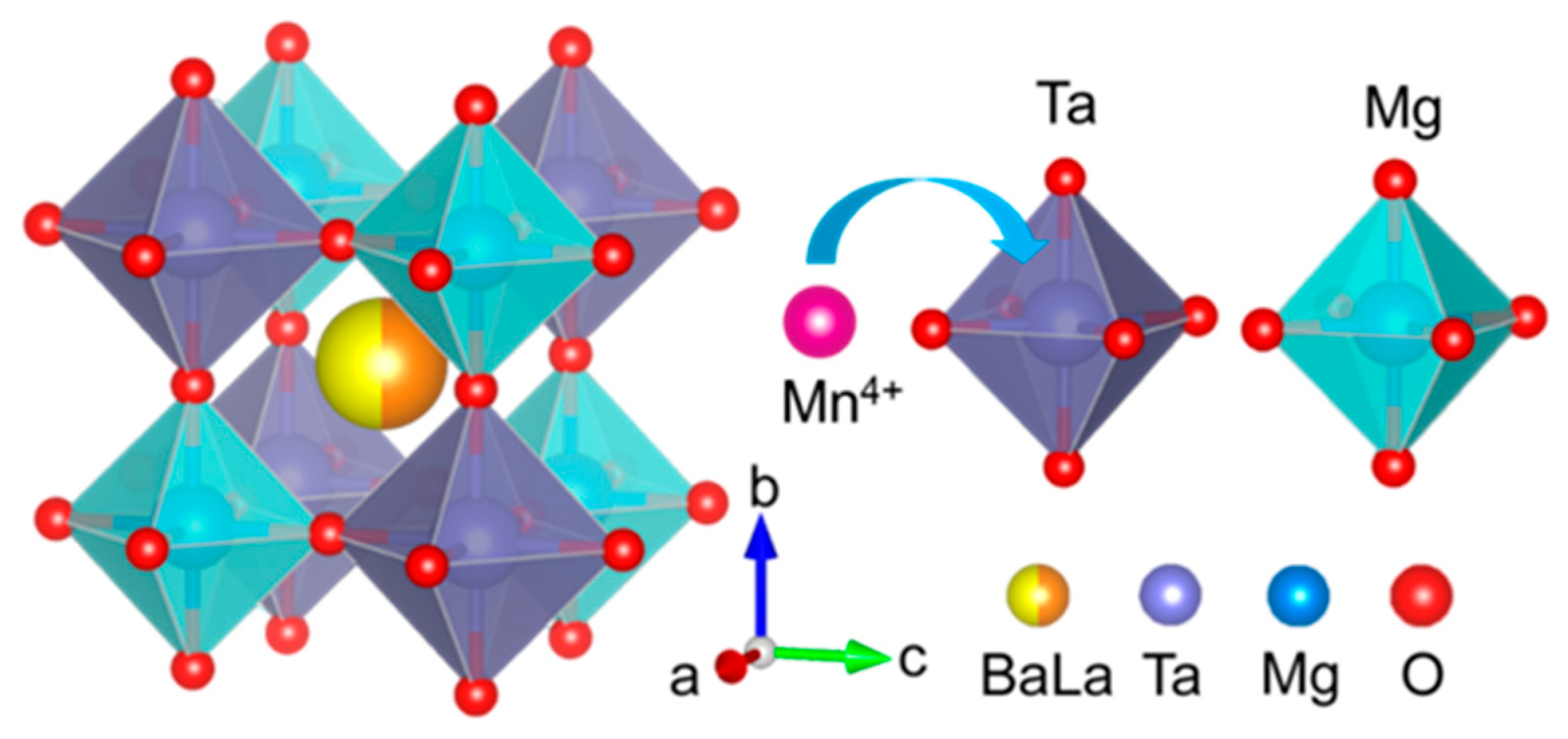
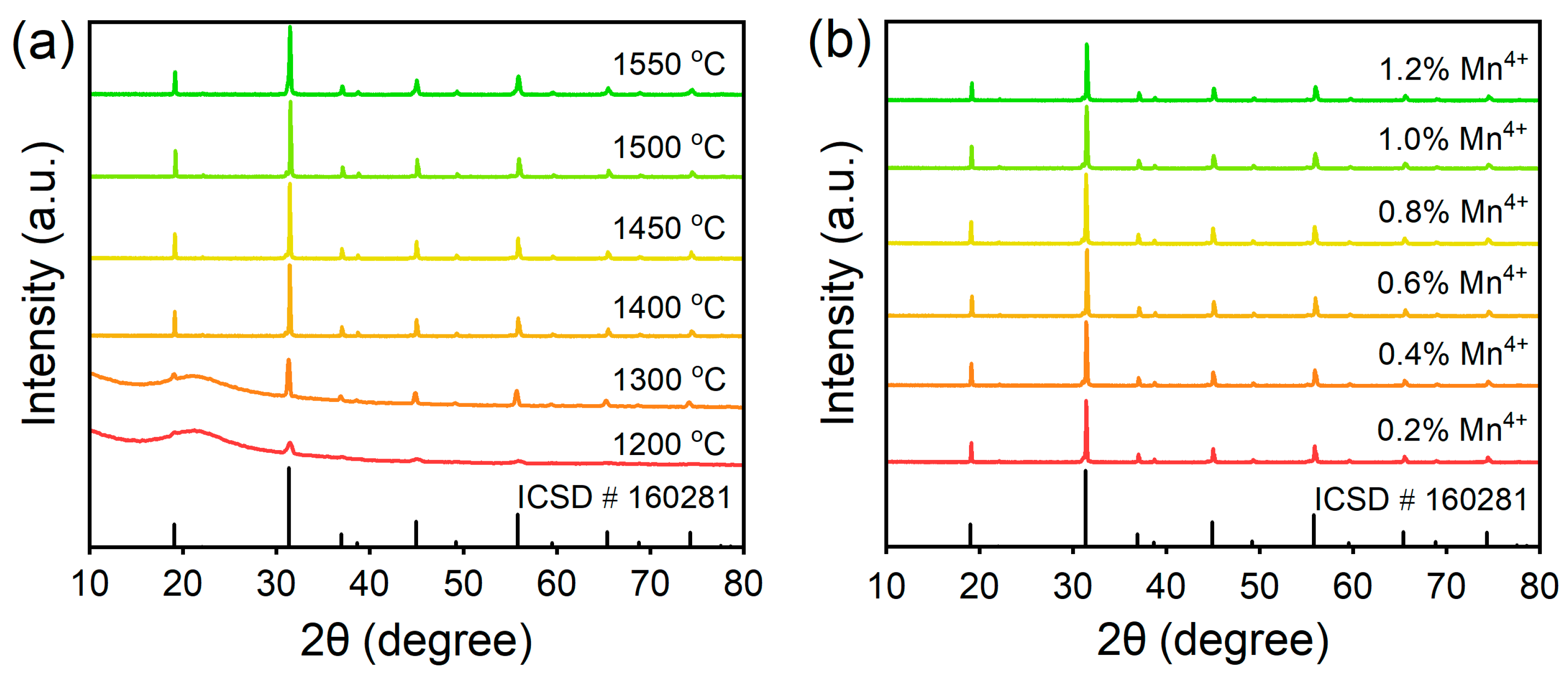
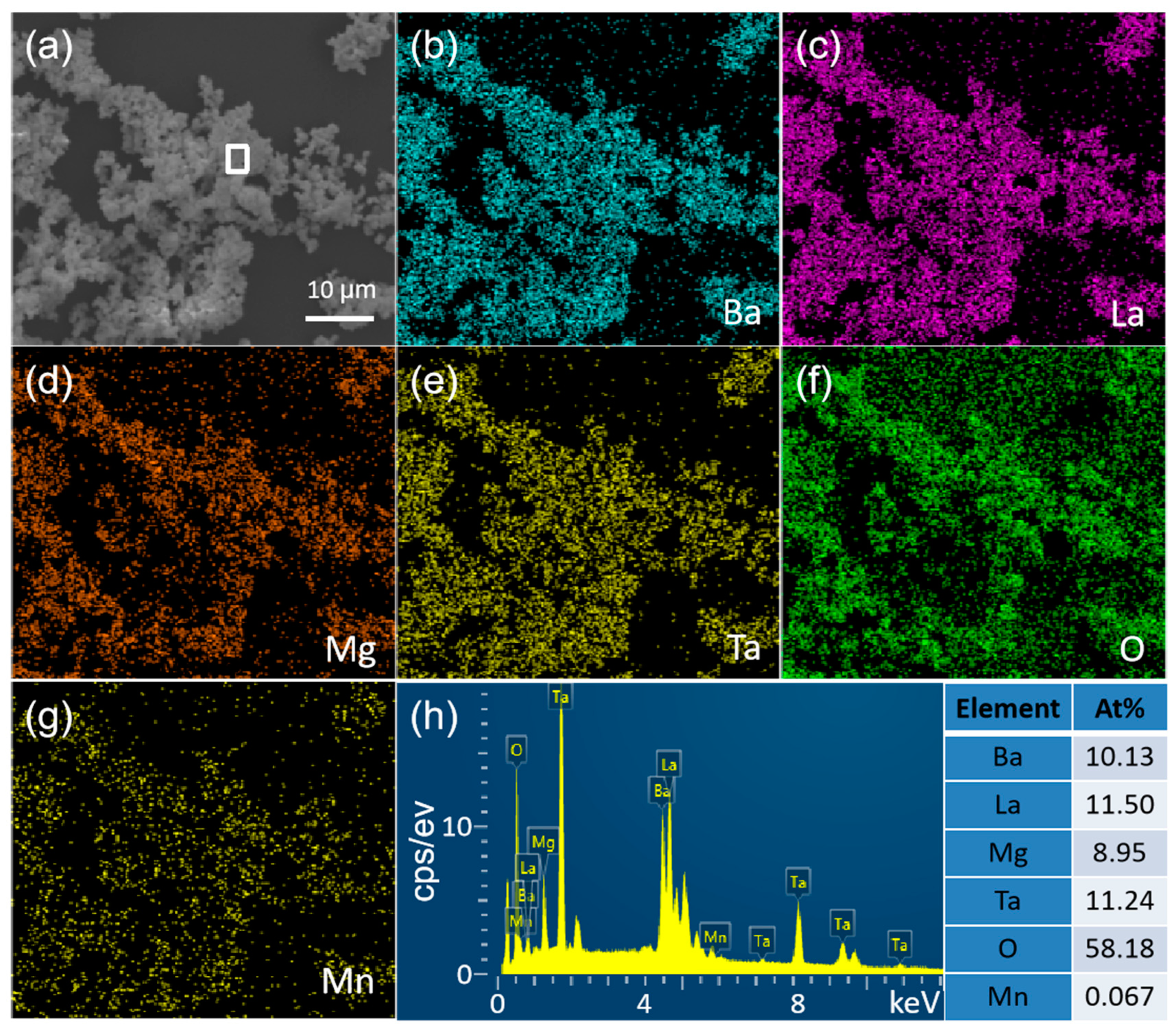
3.1. Structure and Morphology
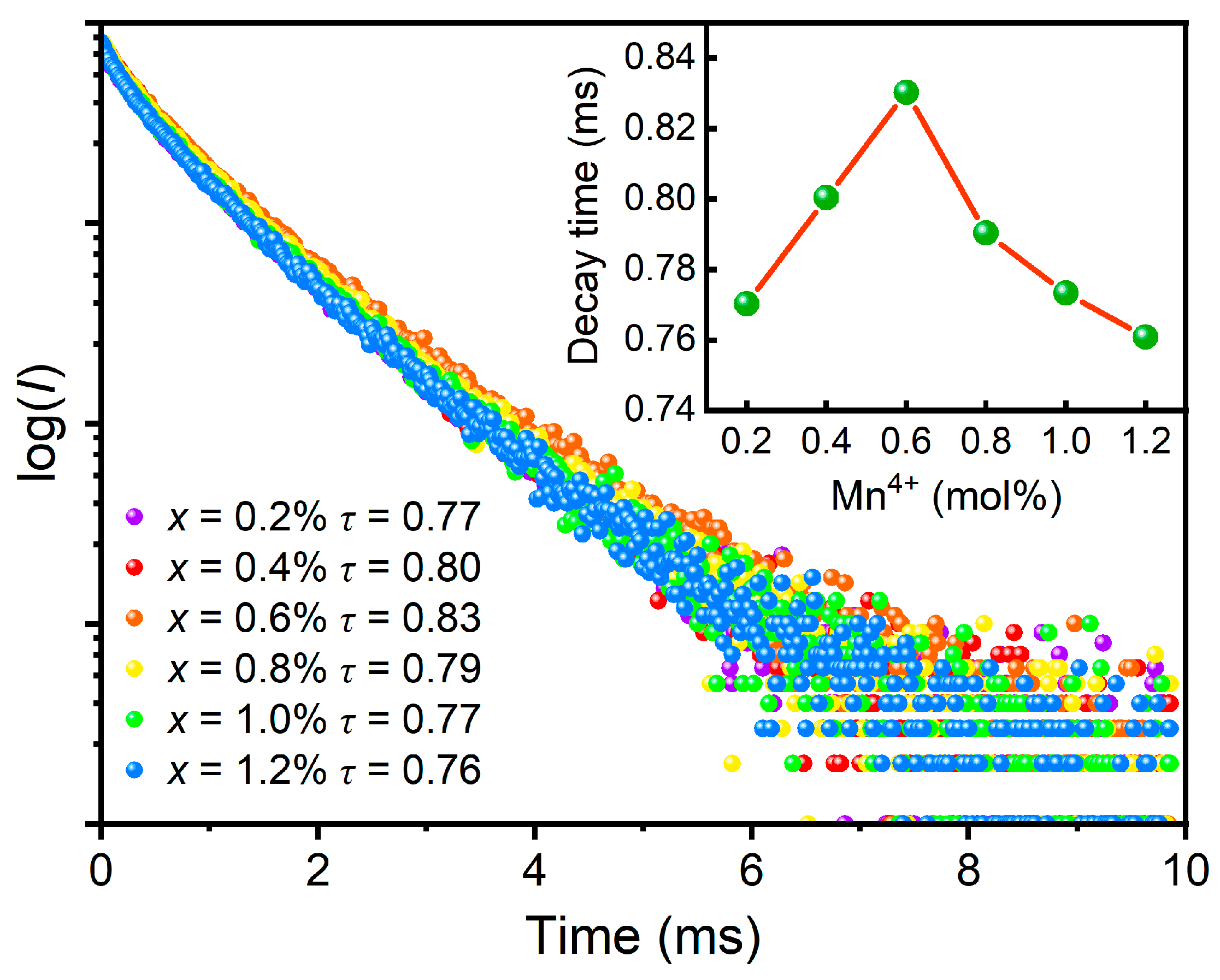
3.2. PL Properties
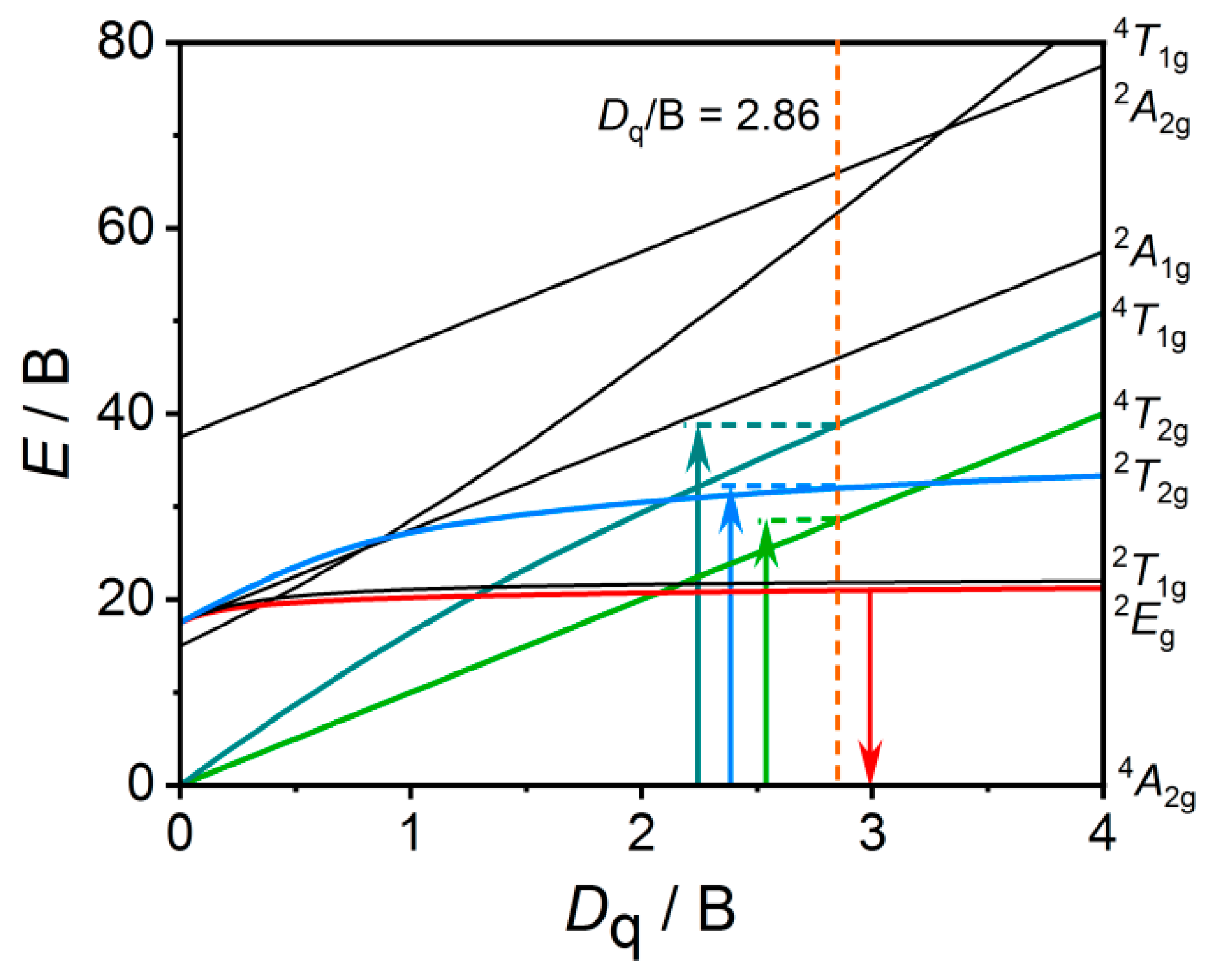
3.3. Crystal Field Analysis
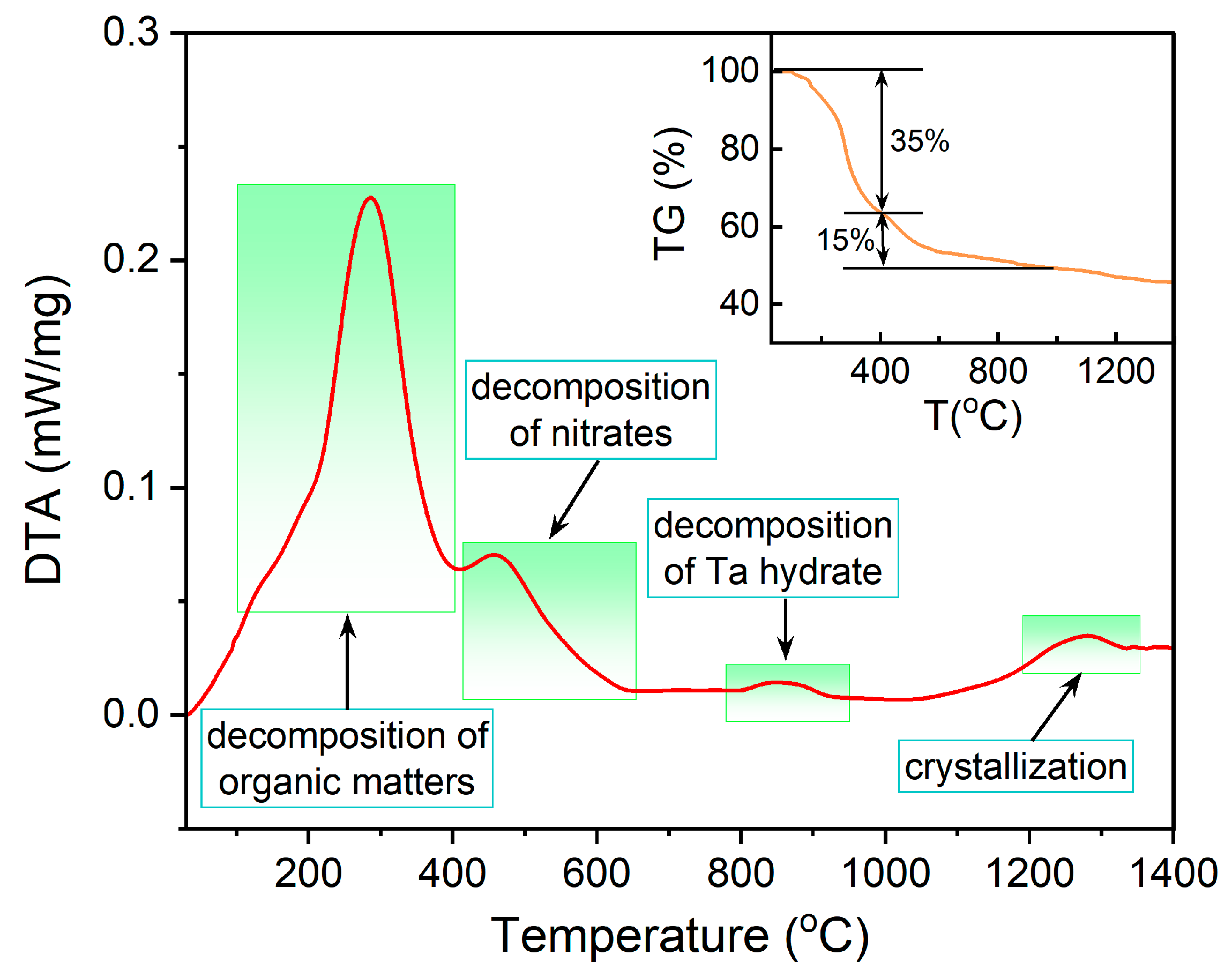
3.4. Thermal Stability
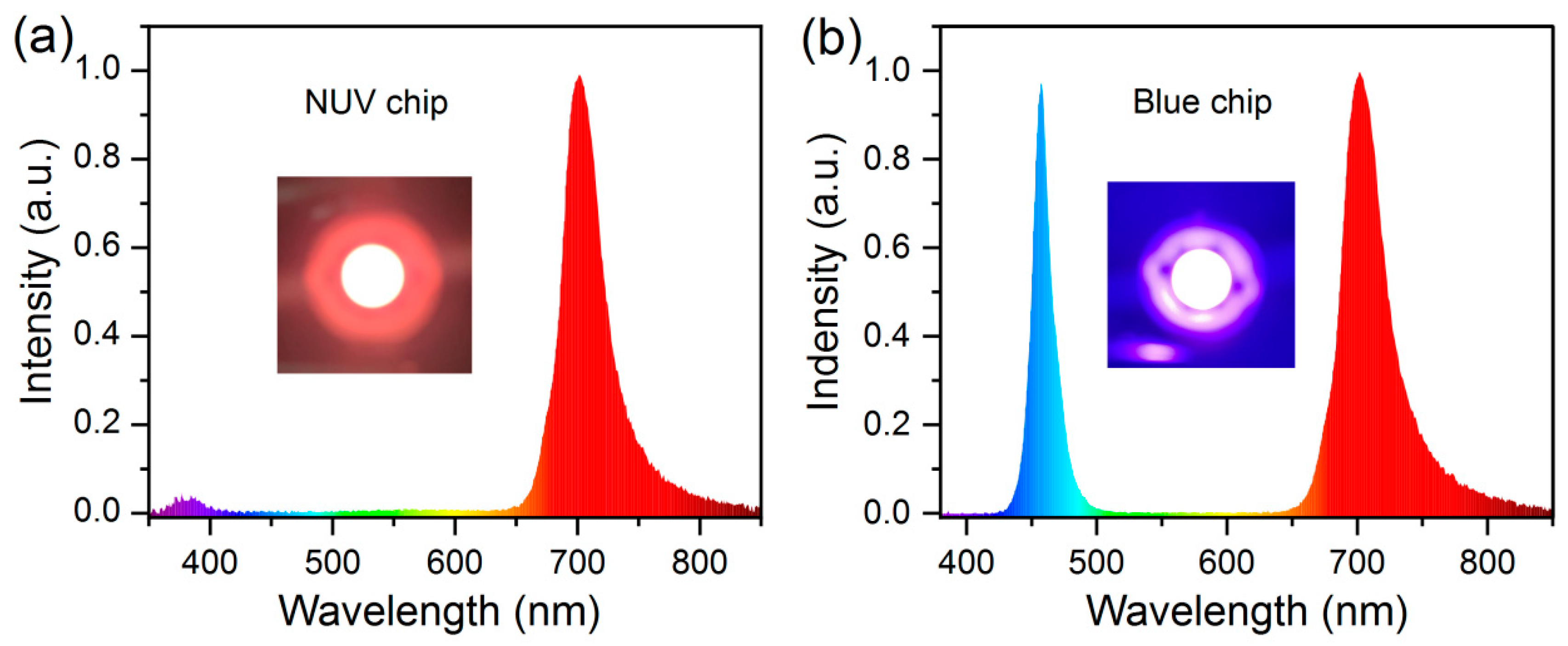
3.5. EL Properties of Fabricated LED Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Zheng, J.; Shi, R.; Zhang, N.; Chen, J.; Zhang, R.; Suo, H.; Goldys, E.M.; Guo, C. Ab initio site occupancy and far-red emission of Mn4+ in cubic-phase La(MgTi)1/2O3 for plant cultivation. ACS Appl. Mater. Interfaces 2017, 9, 6177–6185. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Ma, X.; Li, G.; Liu, S.; Li, H.; Yang, J.; Liang, Y.; Chen, Y. Tunable dual emission of Bi3+ and Mn4+ co-doped LaMg0.598Nb0.402O3 double perovskite via energy transfer for plant growth lighting. Mater. Res. Bull. 2020, 1126, 10814. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Yin, P.; Yang, Y.; Fu, L.; Wang, J.; Feng, X.; Rao, H.; Wang, Y. Luminescence-enhancement and tunable-excitation of far-red emitting La2LiSbO6:Mn4+, Bi3+ phosphors for plant growth lighting. J. Lumin. 2020, 224, 117268. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, Y.; Xia, M.; Zhou, N.; Lei, B.; Wang, J.; Wu, F. Tunable dual emission of Ca3Al4ZnO10:Bi3+, Mn4+ via energy transfer for indoor plant growth lighting. J. Mater. Chem. C 2018, 6, 8914–8922. [Google Scholar] [CrossRef]
- Cao, R.; Shi, Z.; Quan, G.; Chen, T.; Guo, S.; Hua, Z.; Liu, P. Preparation and luminescence properties of Li2MgZrO4:Mn4+ red phosphor for plant growth. J. Lumin. 2017, 188, 577–581. [Google Scholar] [CrossRef]
- Silva, V.O.; Freitas, A.A.; Maçanita, A.L.; Quina, F.H. Chemistry and photochemistry of natural plant pigments: The anthocyanins. J. Phys. Org. Chem. 2016, 29, 594–599. [Google Scholar] [CrossRef]
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Bazzaz, B.S.F. Biological activities of three natural plant pigments and their health benefits. J. Food Meas. Charact. 2018, 12, 356–361. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, Y.; Liu, Y.; Chen, S.; Luo, J.; Wang, J.; Wang, T.; Zhao, J.; Deng, B.; Yu, R. A new double perovskite CaY0.5Ta0.5O3:Mn4+ deep-red phosphor: Synthesis, optical properties, and potential applications in plant growth LEDs. J. Alloy. Compd. 2021, 851, 156875. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Wu, J.; Guo, R.; Luo, L.; Xiong, Y.; Wang, L.; Chen, W. A novel inequivalent double-site substituted red phosphor Li4AlSbO6:Mn4+ with high color purity: Structure, photoluminescence properties, and application in warm white LED. J. Mater. Chem. C 2021, 9, 13236–13246. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Luo, L.; Xiong, Y.; Jiang, L.; Guo, R.; Meng, L. A facile two-step synthesis of an efficient narrow-band red-emitting K2NbF7:Mn4+ phosphor for warm white LEDs and its thermal quenching behavior. J. Alloys Compd. 2021, 863, 158058. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, G.; Geng, W.; Wang, Q.; Wang, Y. Rare-earth-free high-efficiency narrow-band red-emitting Mg3Ga2GeO8:Mn4+ phosphor excited by near-UV light for white-light-emitting diodes. Inorg. Chem. 2016, 55, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lin, C.C.; Luo, W.; Shu, S.; Liu, Z.; Liu, Y.; Kong, J.; Ma, E.; Cao, Y.; Liu, R.-S.; et al. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef]
- Dong, X.; Pan, Y.; Jia, Y.; Liu, R.; Lian, H.; Lin, J. Improved luminescence properties of a novel red dodec-fluoride phosphor Ba3Sc2F12:Mn4+ with extraordinary thermal stability for WLED application. J. Mater. Chem. C 2020, 8, 6299–6305. [Google Scholar] [CrossRef]
- Fang, M.-H.; Wu, W.-L.; Jin, Y.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Brik, M.G.; Srivastava, A.M.; Chiang, C.-Y.; Zhou, W.; et al. Control of luminescence by tuning of crystal symmetry and local structure in Mn4+-activated narrow band fluoride phosphors. Angew. Chem. Int. Ed. 2018, 57, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Pan, Y.; Zhu, M.; Lian, H.; Lin, J. Room-temperature synthesis and optimized photoluminescence of a novel red phosphor NaKSnF6:Mn4+ for application in warm WLEDs. J. Mater. Chem. C 2017, 5, 9255–9263. [Google Scholar] [CrossRef]
- Bryknar, Z.; Trepakov, V.; Potůček, Z.; Jastrabík, L. Luminescence spectra of SrTiO3:Mn4+. J. Lumin. 2000, 87–89, 605–607. [Google Scholar] [CrossRef]
- Brik, M.G.; Camardello, S.J.; Srivastava, A.M. Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2015, 4, R39–R43. [Google Scholar] [CrossRef]
- Adachi, S. Photoluminescence properties of Mn4+-activated oxide phosphors for use in white-LED applications: A review. J. Lumin. 2018, 202, 263–281. [Google Scholar] [CrossRef]
- Adachi, S. Photoluminescence spectra and modeling analyses of Mn4+-activated fluoride phosphors: A review. J. Lumin. 2018, 197, 119–130. [Google Scholar] [CrossRef]
- Huang, X.; Liang, J.; Li, B.; Sun, L.; Lin, J. High-efficiency and thermally stable far-red-emitting NaLaMgWO6:Mn4+ phosphors for indoor plant growth light emitting diodes. Opt. Lett. 2018, 43, 3305–3308. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, H. Finding a novel highly efficient Mn4+-activated Ca3La2W2O12 far-red emitting phosphor with excellent responsiveness to phytochrome PFR: Towards indoor plant cultivation application. Dye. Pigment. 2018, 152, 36–42. [Google Scholar] [CrossRef]
- Li, L.; Tian, G.; Chang, W.; Yan, Y.; Ling, F.; Jiang, S.; Xiang, G.; Zhou, X. A novel double-perovskite LiLaMgTeO6:Mn4+ far-red phosphor for indoor plant cultivation white LEDs: Crystal and electronic structure, and photoluminescence properties. J. Alloys Compd. 2020, 832, 154905. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, L.; Ji, C.; Xu, D.; Zhang, C.; Bu, H.; Tan, X.; Yun, X.; Sun, J. Studies on luminescence properties of double perovskite deep red phosphor La2ZnTiO6:Mn4+ for indoor plant growth LED applications. J. Alloys Compd. 2019, 802, 628–635. [Google Scholar] [CrossRef]
- Shi, L.; Han, Y.; Zhang, Z.; Ji, Z.; Shi, D.; Geng, X.; Zhang, H.; Li, M.; Zhang, Z. Synthesis and photoluminescence properties of novel Ca2LaSbO6:Mn4+ double perovskite phosphor for plant growth LEDs. Ceram. Int. 2019, 45, 4739–4746. [Google Scholar] [CrossRef]
- Fu, A.; Zhou, C.; Chen, Q.; Lu, Z.; Huang, T.; Wang, H.; Zhou, L. Preparation and optical properties of a novel double-perovskite phosphor, Ba2GdNbO6:Mn4+, for light-emitting diodes. Ceram. Int. 2017, 43, 6353–6362. [Google Scholar] [CrossRef]
- Cao, R.; Chen, T.; Ren, Y.; Chen, T.; Ao, H.; Li, W.; Zheng, G. Synthesis and photoluminescence properties of Ca2LaTaO6:Mn4+ phosphor for plant growth LEDs. J. Alloys Compd. 2019, 780, 749–755. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Woodward, P.M. Crystal structures and dielectric properties of ordered double perovskites containing Mg2+ and Ta5+. J. Solid State Chem. 2007, 180, 2798–2807. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Zhu, J.; Ren, X.; Zhu, Y.; Yin, P.; Zhao, L.; Wang, J.; Feng, X. Enhanced luminescence performances of BaLaMgTaO6:Mn4+ red phosphor by Bi3+, Ca2+ doping for indoor plant lighting supplementary LED. Spectrochim. Acta A 2022, 268, 120655. [Google Scholar] [CrossRef]
- Todorovsky, D.S.; Getsova, M.M.; Vasileva, M.A. Thermal decomposition of lanthanum-titanium citric complexes prepared from ethylene glycol medium. J. Mater. Sci. 2002, 37, 4029–4039. [Google Scholar] [CrossRef]
- Popovici, C.I.; Ciupina, V.; Prodan, G.; Girtu, M.A. Structural characterisation of lanthanum aluminate synthetized by the Pechini method. J. Optoelectron. Adv. Mater. 2008, 10, 2942–2946. [Google Scholar]
- Zhong, Y.; Wang, M.; Wang, H.; Yuan, J. Thermodynamic description of the quaternary Mg(NO3)2-KNO3-NaNO3-LiNO3 system and investigation on the novel Mg(NO3)2 based nitrate salts with low temperature. Sol. Energy Mater. Sol. Cells 2021, 230, 111148. [Google Scholar] [CrossRef]
- Stern, K.H. High temperature properties and decomposition of inorganic salts part 3, nitrates and nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef]
- Gobichon, A.E.; Auffrédic, J.P.; Louër, D. Thermal decomposition of neutral and basic lanthanum nitrates studied with temperature-dependent powder diffraction and thermogravimetric analysis. Solid State Ionics 1996, 93, 51–64. [Google Scholar] [CrossRef]
- Drobot, D.; Nikishina, E.; Lebedeva, E.; Novoselov, A.; Yoshikawa, A. Synthesis of complexoxide phases by using of low hydrated niobium and tantalum hydroxides. Mater. Res. Bull. 2008, 43, 1232–1238. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of Concentration Quenching in Inorganic Phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Ming, H.; Liu, S.; Liu, L.; Peng, J.; Fu, J.; Du, F.; Ye, X. Highly regular, uniform K3ScF6:Mn4+ phosphors: Facile synthesis, microstructures, photoluminescence properties, and application in light-emitting diode devices. ACS Appl. Mater. Interfaces 2018, 10, 19783–19795. [Google Scholar] [CrossRef]
- Han, Y.; Wang, S.; Liu, H.; Shi, L.; Zhang, J.; Zhang, Z.; Mao, Z.; Wang, D.; Mu, Z.; Zhang, Z.; et al. Synthesis and luminescent properties of a novel deep-red phosphor Sr2GdNbO6:Mn4+ for indoor plant growth lighting. J. Lumin. 2020, 220, 116968. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Huang, Q.; Huang, F.; Xu, J.; Wang, B.; Lin, Z.; Zhou, J.; Wang, Y. A novel double-perovskite Gd2ZnTiO6:Mn4+ red phosphor for UV-based w-LEDs: Structure and luminescence properties. J. Mater. Chem. C 2016, 4, 2374–2381. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, B.; Zhao, G.; Liu, Y.; Zou, J.; Sun, H.; Ou, H.; Fang, Y.; Hou, J. High quantum efficiency far red emission from double perovskite structured CaLaMgMO6:Mn4+ (M = Nb, Ta) phosphor for UV-based light emitting diodes application. Opt. Mater. 2018, 83, 93–98. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Y.; Duan, H.; Chen, L.; Fu, Y.; Ju, G.; Wang, T.; He, M. Novel La3GaGe5O16:Mn4+ based deep red phosphor: A potential color converter for warm white light. RSC Adv. 2015, 5, 90499–90507. [Google Scholar] [CrossRef]
- Reisfeld, M.J.; Matwiyoff, N.A.; Asprey, L.B. The electronic spectrum of cesium hexafluoromanganese(IV). J. Mol. Spectrosc. 1971, 39, 8–20. [Google Scholar] [CrossRef]
- Sijbom, H.F.; Verstraete, R.; Joos, J.J.; Poelman, D.; Smet, P.F. K2SiF6:Mn4+ as a red phosphor for displays and warm-white LEDs: A review of properties and perspectives. Opt. Mater. Express 2017, 7, 3332. [Google Scholar] [CrossRef]
- Adachi, S. Review—Temperature dependence of luminescence intensity and decay time in Mn4+-activated oxide phosphors. ECS J. Solid State Sci. Technol. 2022, 11, 056003. [Google Scholar] [CrossRef]
- Adachi, S. Review—Mn 4+-Activated Red and Deep Red-Emitting Phosphors. ECS J. Solid State Sci. Technol. 2020, 9, 016001. [Google Scholar] [CrossRef]
- Bhushan, S.; Chukichev, M. Temperature dependent studies of cathodoluminescence of green band of ZnO crystals. J. Mater. Sci. Lett. 1988, 7, 319–321. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Q.; Devakumar, B. Novel efficient deep-red-emitting Ca2LuTaO6:Mn4+ double-perovskite phosphors for plant growth LEDs. J. Lumin. 2020, 222, 117177. [Google Scholar] [CrossRef]
- Huang, D.; Dang, P.; Xiao, X.; Bai, B.; Lian, H.; Zeng, Q.; Lin, J. (Ba,Sr)LaZnTaO6:Mn4+ far red emitting phosphors for plant growth LEDs: Structure and photoluminescence properties. New J. Chem. 2020, 44, 6163–6172. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, N.; Du, Q.; Guo, R.; Luo, L.; Wang, L. Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs. Materials 2023, 16, 4029. https://doi.org/10.3390/ma16114029
Fan N, Du Q, Guo R, Luo L, Wang L. Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs. Materials. 2023; 16(11):4029. https://doi.org/10.3390/ma16114029
Chicago/Turabian StyleFan, Niansi, Quan Du, Rui Guo, Lan Luo, and Li Wang. 2023. "Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs" Materials 16, no. 11: 4029. https://doi.org/10.3390/ma16114029
APA StyleFan, N., Du, Q., Guo, R., Luo, L., & Wang, L. (2023). Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs. Materials, 16(11), 4029. https://doi.org/10.3390/ma16114029






