Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zirconia Samples and Laser Structuring
2.2. Wettability of Surfaces
2.3. Cold Argon (Ar-) Plasma Activation
2.4. Gingival Cell Culture, Morphology, and Spreading
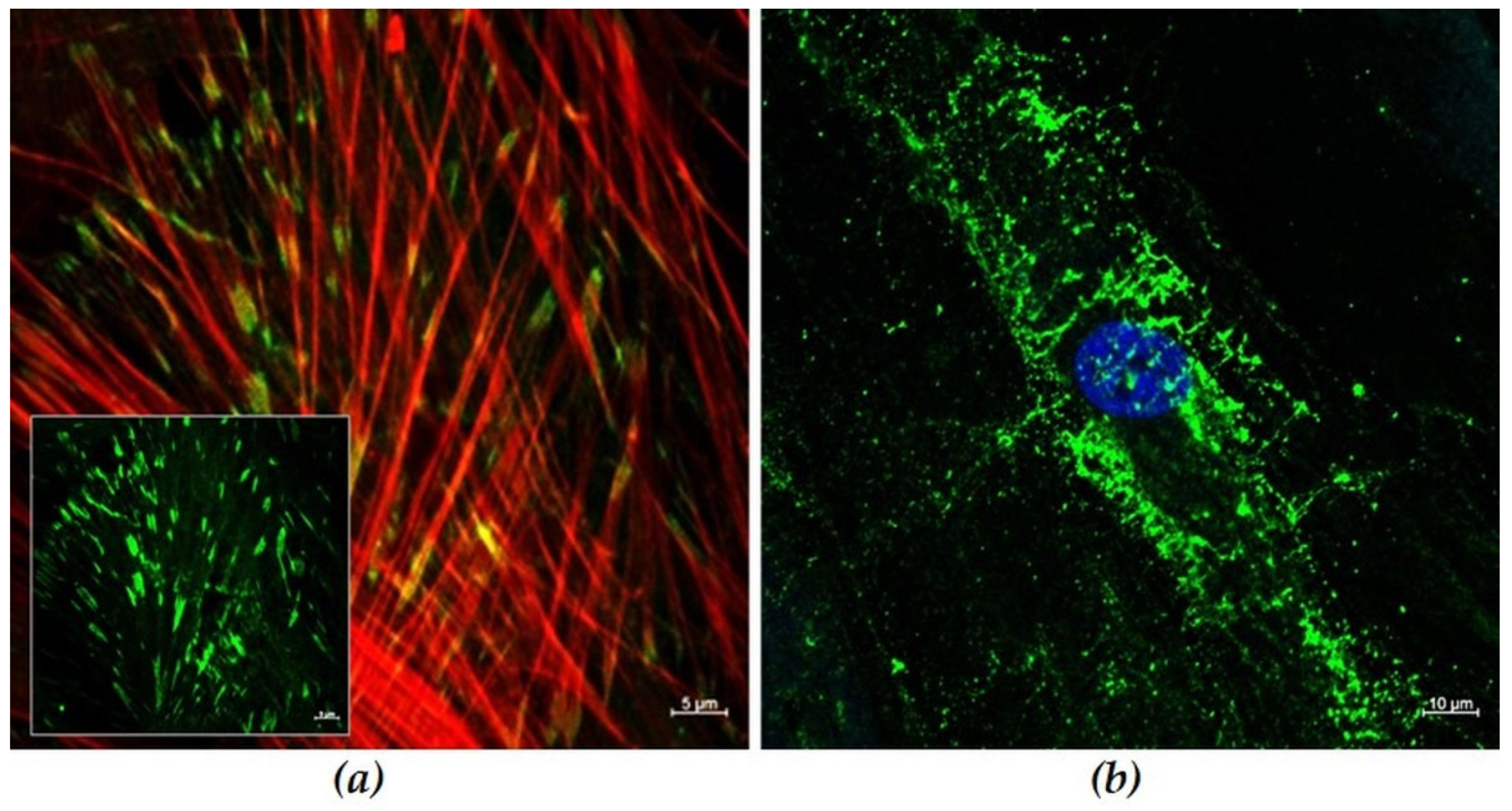
2.5. Confocal Laser Scanning Microscopy
2.6. Statistic
3. Results
3.1. Characterization of Human Gingival Fibroblasts
3.2. Material Surface Characterization
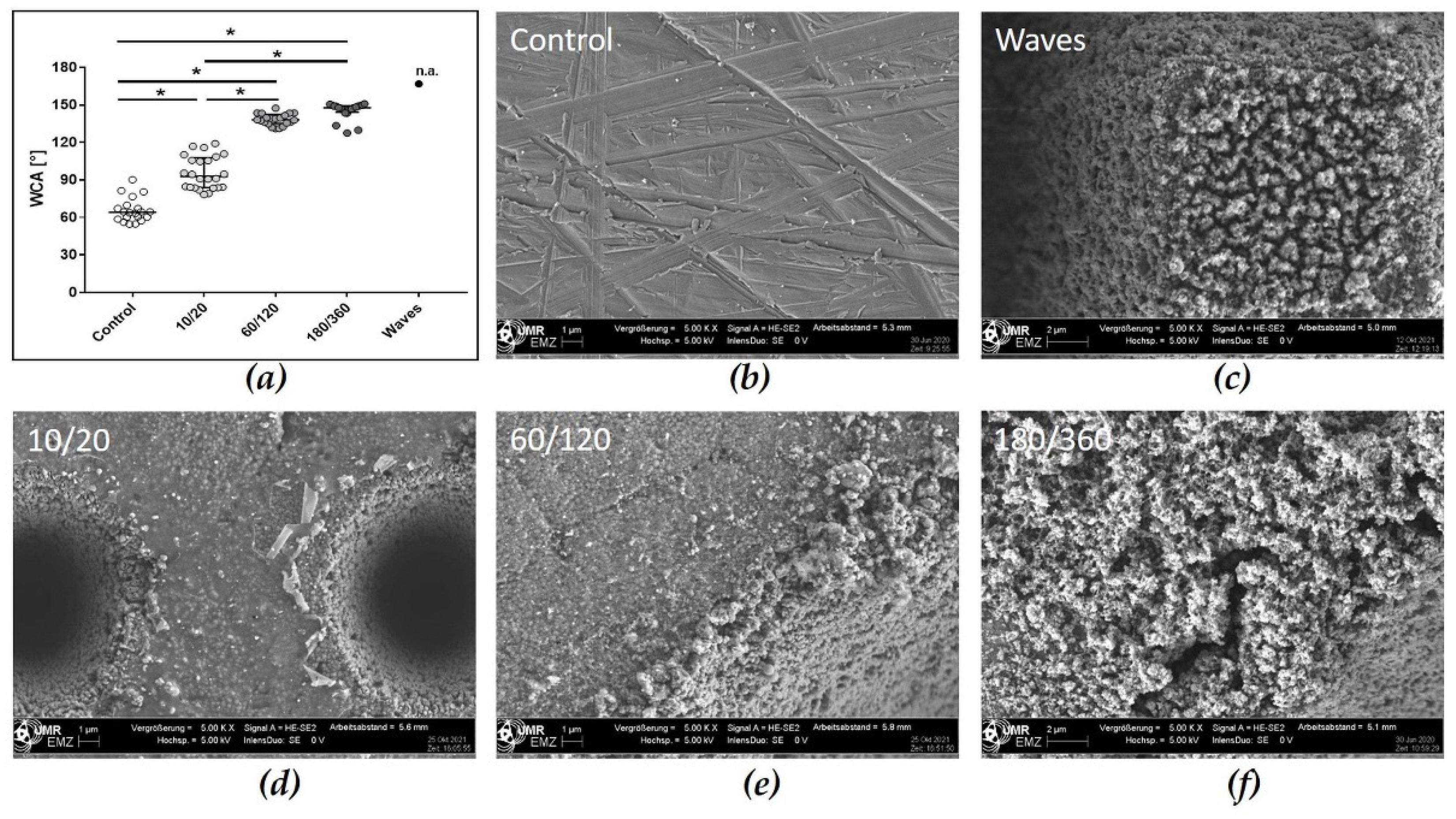
3.2.1. Profiles of Zirconia Samples
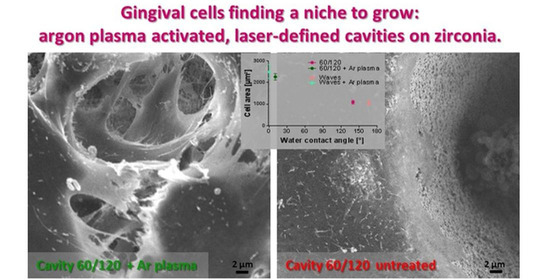
3.2.2. Wetting Properties of Laser Structured Zirconia Samples
3.3. Cellular Behavior
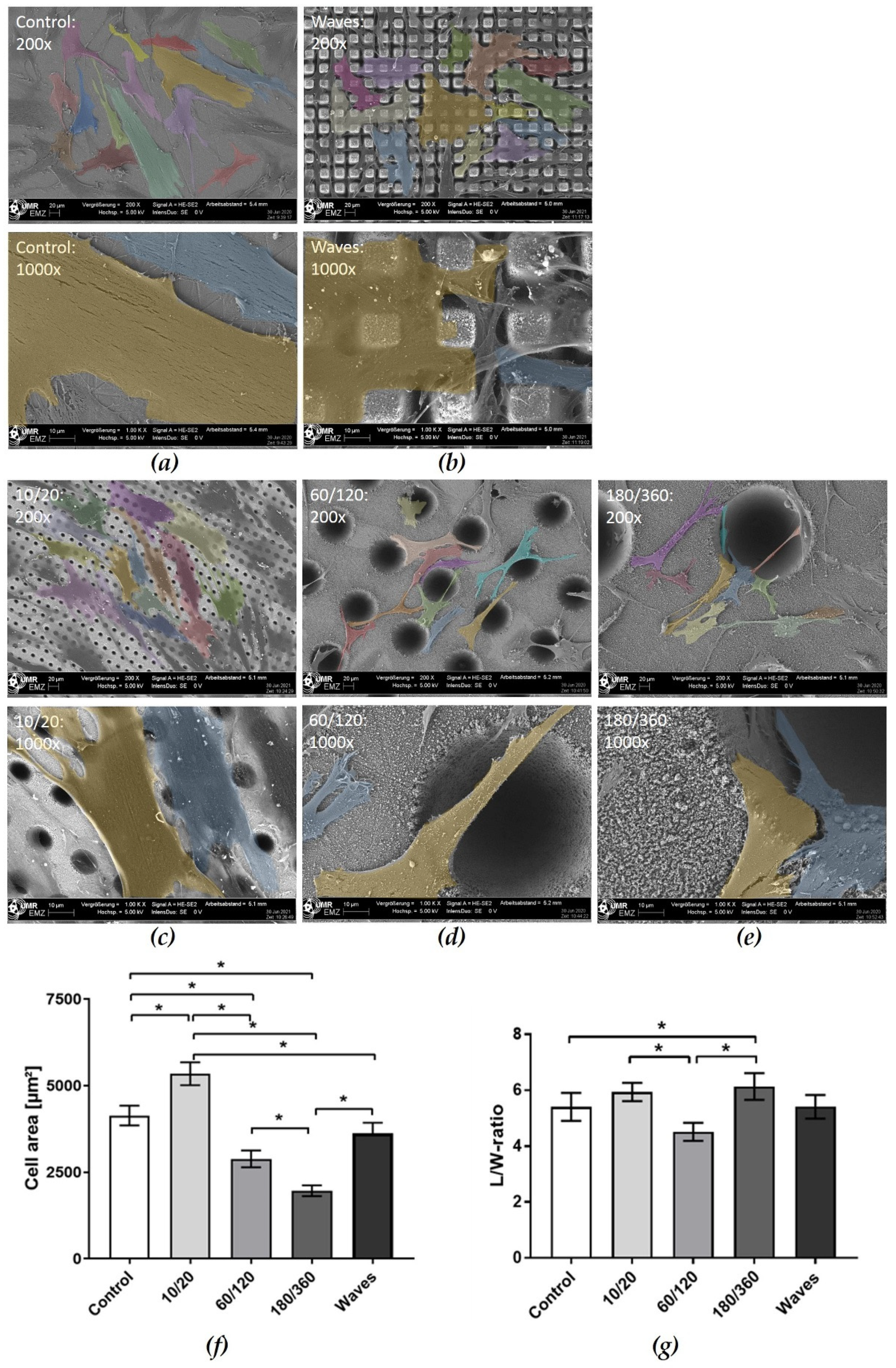
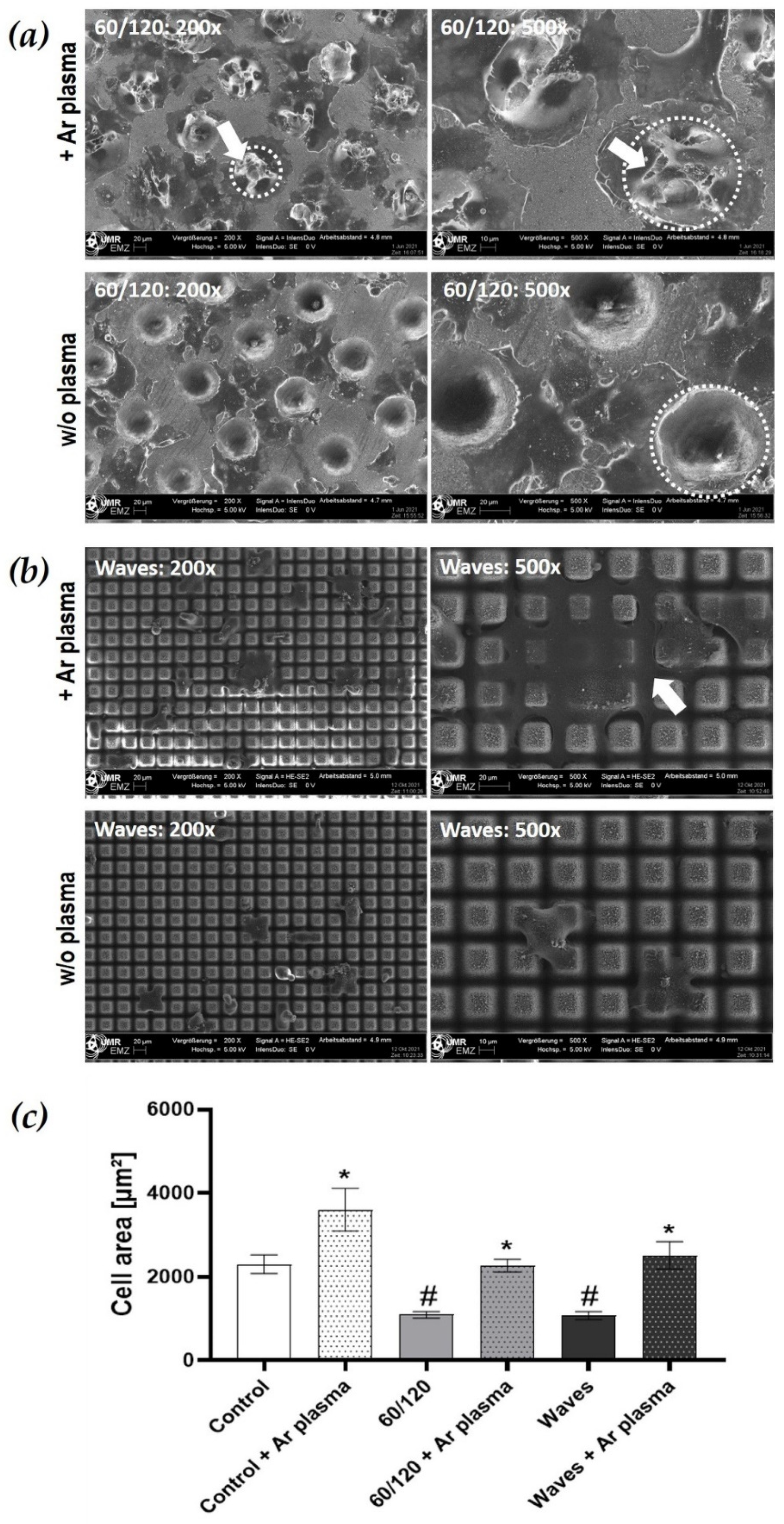
3.3.1. Morphology and Spreading on Laser Structured Zirconia
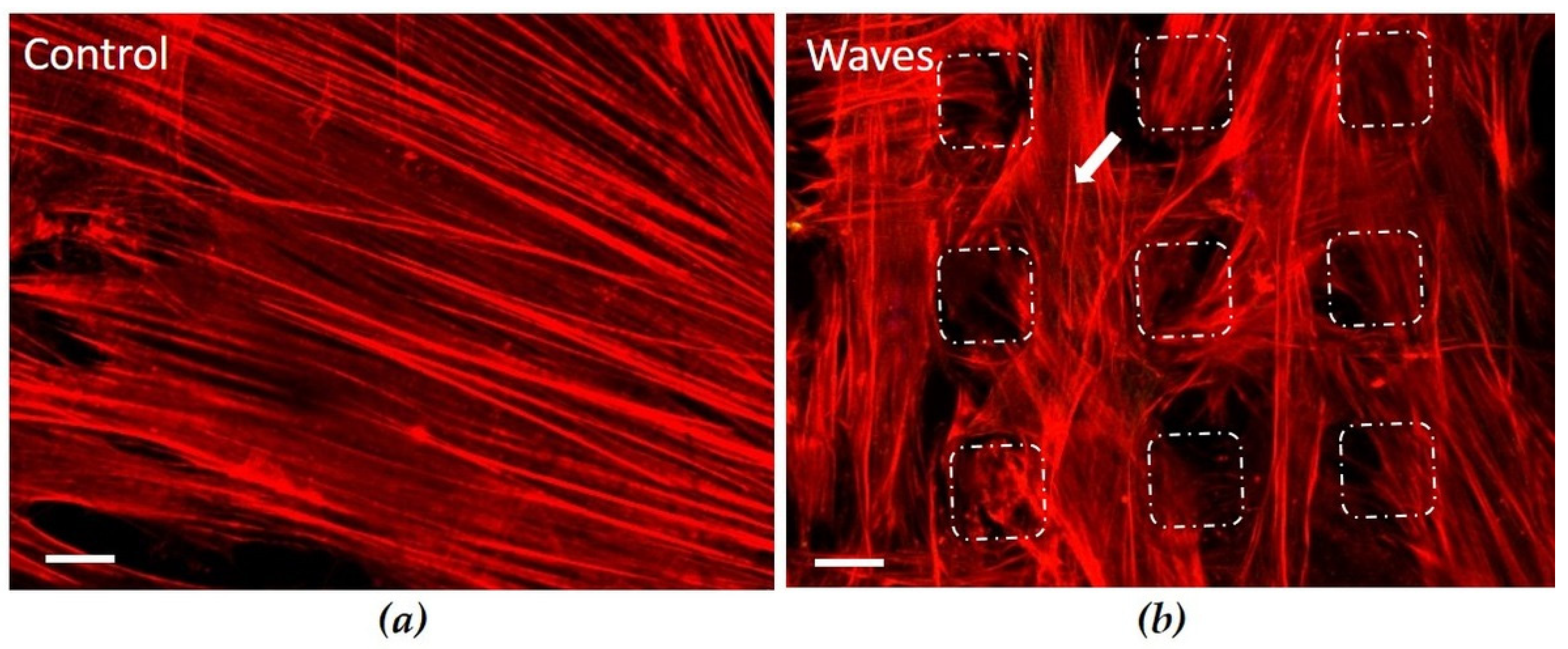
3.3.2. Actin Cytoskeleton on Convex Waves
3.3.3. Cell Spreading after Wettability Modulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29, 135–153. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 55–67. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Brägger, U.; Bürgin, W.; Lang, N.P. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin. Oral Implant. Res. 1996, 7, 111–119. [Google Scholar] [CrossRef]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Discoloration of the peri-implant mucosa caused by zirconia and titanium implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Zirconia compared to titanium dental implants in preclinical studies—A systematic review and meta-analysis. Clin. Oral Implant. Res. 2019, 30, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P. Biologic width around implants. An evidence-based review. Stomatologija 2008, 10, 27–35. [Google Scholar] [PubMed]
- Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J. Periodontol. 1997, 68, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Fischer, J.; Schott, A.; Märtin, S. Surface micro-structuring of zirconia dental implants. Clin. Oral Implant. Res. 2016, 27, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Han, J.; Zhang, F.; Van Meerbeek, B.; Vleugels, J.; Braem, A.; Castagne, S. Laser surface texturing of zirconia-based ceramics for dental applications: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112034. [Google Scholar] [CrossRef]
- Schnell, G.; Staehlke, S.; Duenow, U.; Nebe, J.B.; Seitz, H. Femtosecond laser nano/micro textured Ti6Al4V surfaces–effect on wetting and MG-63 cell adhesion. Materials 2019, 12, 2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Pacha-Olivenza, M.A.; Tejero, R.; Fernández-Calderón, M.C.; Anitua, E.; Troya, M.; González-Martín, M.L. Relevance of Topographic Parameters on the Adhesion and Proliferation of Human Gingival Fibroblasts and Oral Bacterial Strains. Biomed. Res. Int. 2019, 2019, 8456342. [Google Scholar] [CrossRef] [Green Version]
- Gristina, A.G.; Naylor, P.T.; Myrvik, Q. The Race for the Surface: Microbes, Tissue Cells, and Biomaterials. In Molecular Mechanisms of Microbial Adhesion; Switalski, L., Höök, M., Beachey, E., Eds.; Springer: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Piest, C.; Wille, S.; Strunskus, T.; Polonskyi, O.; Kern, M. Efficacy of Plasma Treatment for Decontaminating Zirconia. J. Adhes Dent. 2018, 20, 289–297. [Google Scholar] [CrossRef]
- Bergemann, C.; Quade, A.; Kunz, F.; Ofe, S.; Klinkenberg, E.-D.; Laue, M.; Schroeder, K.; Weissmann, V.; Hansmann, H.; Weltmann, K.-D.; et al. Ammonia Plasma Functionalized Polycarbonate Surfaces Improve Cell Migration Inside an Artificial 3D Cell Culture Module. Plasma Process. Polym. 2012, 9, 261–272. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.-D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Rolauffs, B.; Adolfsson, E.; Altmann, B. Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma. Sci. Rep. 2021, 11, 17302. [Google Scholar] [CrossRef] [PubMed]
- Hoentsch, M.; von Woedtke, T.; Weltmann, K.-D.; Nebe, J.B. Time-dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J. Phys. D Appl. Phys. 2012, 45, 025206. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric pressure plasma jet for medical therapy: Plasma parameters and risk estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000, 24, 28–55. [Google Scholar] [CrossRef]
- Oksala, O.; Salo, T.; Tammi, R.; Hakkinen, L.; Jalkanen, M.; Inki, P.; Larjava, H. Expression of proteoglycans and hyalu-ronan during wound healing. J. Histochem. Cytochem. 1995, 43, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Bergemann, C.; Waldner, A.-C.; Emmert, S.; Nebe, J.B. The Hyaluronan pericellular coat and cold atmospheric plasma treatment of cells. Appl. Sci. 2020, 10, 5024. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. H 2010, 224, 1487–1507. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2012, 28, 843–863. [Google Scholar] [CrossRef]
- Bergemann, C.; Duske, K.; Nebe, J.B.; Schöne, A.; Bulnheim, U.; Seitz, H.; Fischer, J. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J. Mater. Sci. Mater. Med. 2015, 26, 26. [Google Scholar] [CrossRef] [Green Version]
- Rohr, N.; Zeller, B.; Matthisson, L.; Fischer, J. Surface structuring of zirconia to increase fibroblast viability. Dent. Mater. 2020, 36, 779–786. [Google Scholar] [CrossRef]
- Staehlke, S.; Springer, A.; Freitag, T.; Brief, J.; Nebe, J.B. The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles. Dent. J. 2020, 8, 127. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandoleon, P.; Bakopoulou, A.; Papadopoulou, L.; Koidis, P. Evaluation of the biological behaviour of various dental implant abutment materials on attachment and viability of human gingival fibroblasts. Dent. Mater. 2019, 35, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, M.; Liao, Y.; Zhou, J.; Li, H.; Tan, J. Different behavior of human gingival fibroblasts on surface modified zirconia: A comparison between ultraviolet (UV) light and plasma. Dent. Mater. J. 2019, 38, 756–763. [Google Scholar] [CrossRef]
- Zheng, M.; Yang, Y.; Liu, X.-Q.; Liu, M.-Y.; Zhang, X.-F.; Wang, X.; Li, H.-P.; Tan, J.-G. Enhanced biological behavior of in vitro human gingivalfibroblasts on cold plasma-treated zirconia. PLoS ONE 2015, 10, e0140278. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Cha, S.; Park, Y.-S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of argon increases cell attachment and bacterial decontamination on different implant surfaces. Int. J. Oral Maxillofac. Surg. 2017, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Contr. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Hiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, P.; Lopez-Suarez, C.; Pelaez, J.; Tobar, C.; Rodriguez-Alonso, V.; Suarez, M.J. Influence of Low-Pressure Plasma on the Surface Properties of CAD-CAM Leucite-Reinforced Feldspar and Resin Matrix Ceramics. Appl. Sci. 2020, 10, 8856. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of Ultrathin Nano-Hydroxyapatite Films on Laser Micro-Textured Titanium Surfaces to Prepare a Multiscale Surface Topography for Improved Surface Wettability/Energy. Materials 2016, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Belaud, V.; Petithory, T.; Ponche, A.; Mauclair, C.; Donnet, C.; Pieuchot, L.; Benayoun, S.; Anselme, K. Influence of multiscale and curved structures on the migration of stem cells. Biointerphases 2018, 13, 06D408. [Google Scholar] [CrossRef]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics in dependence on topographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Canullo, L.; Cassinelli, C.; Götz, W.; Tarnow, D. Plasma of argon accelerates murine fibroblast adhesion in early stages of titanium disk colonization. Int. J. Oral Maxillofac. Implant. 2013, 28, 957–962. [Google Scholar] [CrossRef] [PubMed]
| Surface | Method | Control | 10/20 | 60/120 | 180/360 | Waves |
|---|---|---|---|---|---|---|
| w/o plasma |  |  |  |  |  | |
| WCA [°] | 65.88 ± 2.15 | 95.84 ± 2.67 | 138.4 ± 0.99 | 145.4 ± 1.53 | ≥150 | |
| SFE [mN/m] dispersive polar | 46.82 ± 4.29 31.21 ± 1.62 15.61 ± 2.67 | 48.12 ± 1.45 47.34 ± 1.45 0.78 ± 0.89 | 54.84 ± 3.09 45.51 ± 1.53 9.32 ± 1.56 | 43.13 ± 3.60 33.89 ± 2.52 9.24 ± 1.08 | ||
| +Ar-plasma |  |  |  |  |  | |
| WCA [°] | 17.27 ± 1.35 | 7.34 ± 2.41 | 13.67 ± 1.13 | 10.25 ± 1.12 | ≥0 | |
| SFE [mN/m] dispersive polar | 72.46 ± 6.21 35.26 ± 3.35 37.20 ± 2.86 | 80.61 ± 0.4 50.56 ± 0.26 30.05 ± 0.13 | 77.65 ± 1.75 46.39 ± 0.71 31.25 ± 1.06 | 79.96 ± 1.05 49.68 ± 0.34 30.28 ± 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials 2022, 15, 732. https://doi.org/10.3390/ma15030732
Staehlke S, Oster P, Seemann S, Kruse F, Brief J, Nebe B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials. 2022; 15(3):732. https://doi.org/10.3390/ma15030732
Chicago/Turabian StyleStaehlke, Susanne, Philip Oster, Susanne Seemann, Fabian Kruse, Jakob Brief, and Barbara Nebe. 2022. "Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation" Materials 15, no. 3: 732. https://doi.org/10.3390/ma15030732
APA StyleStaehlke, S., Oster, P., Seemann, S., Kruse, F., Brief, J., & Nebe, B. (2022). Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials, 15(3), 732. https://doi.org/10.3390/ma15030732