Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis
Abstract
1. Introduction
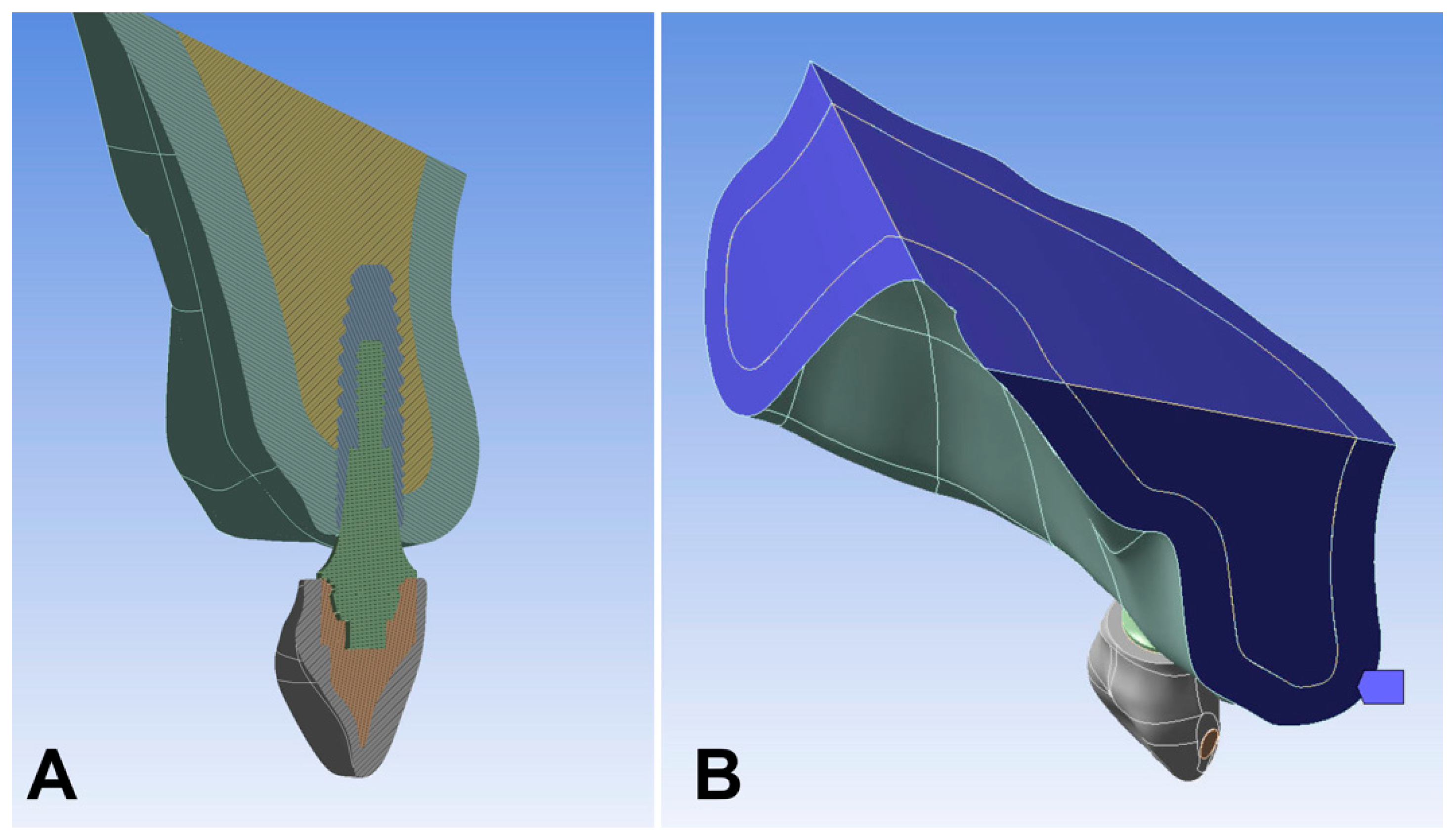
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wennstrom, J.L.; Ekestubbe, A.; Grondahl, K.; Karlsson, S.; Lindhe, J. Implant-supported single-tooth restorations: A 5-year prospective study. J. Clin. Periodontol. 2005, 32, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Buser, R.; Bragger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: A 20-year prospective case series study in partially edentulous patients. Clin. Implant Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef]
- Klein, M.O.; Schiegnitz, E.; Al-Nawas, B. Systematic review on success of narrow-diameter dental implants. Int. J. Oral Maxillofac. Implants 2014, 2, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental Implant Length and Diameter: A Proposed Classification Scheme. J. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 21–40. [Google Scholar] [CrossRef]
- Zinsli, B.; Sagesser, T.; Mericske, E.; Mericske-Stern, R. Clinical evaluation of small-diameter ITI implants: A prospective study. Int. J. Oral Maxillofac. Implants 2004, 19, 92–99. [Google Scholar]
- Cordeiro, J.M.; Barao, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Nicholson, J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E. Effect of dental implant diameter on fatigue performance. Part I: Mechanical behavior. Clin. Implant Dent. Relat. Res. 2014, 16, 172–177. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D.; Machtei, E.E.; Levin, L. Effect of dental implant diameter on fatigue performance. Part II: Failure analysis. Clin. Implant Dent. Relat. Res. 2014, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Bonfante, E.A.; Anchieta, R.B.; Machado, L.S.; Freitas, G.; Fardin, V.P.; Tovar, N.; Coelho, P.G. Reliability and failure modes of narrow implant systems. Clin. Oral Investig. 2016, 20, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Kopova, I.; Strasky, J.; Harcuba, P.; Landa, M.; Janecek, M.; Bacakova, L. Newly developed Ti-Nb-Zr-Ta-Si-Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Anitha, S.; Hegde, M.L.; Zecca, L.; Garruto, R.M.; Ravid, R.; Shankar, S.K.; Stein, R.; Shanmugavelu, P.; Jagannatha Rao, K.S. Aluminium in Alzheimer’s disease: Are we still at a crossroad? Cell. Mol. Life Sci. 2005, 62, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Bizot, P.; Mabilleau, G.; Hubert, L. Aluminum and bone: Review of new clinical circumstances associated with Al3+ deposition in the calcified matrix of bone. Morphologie 2016, 100, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Nie, J. Exposure to aluminum in daily life and alzheimer’s disease. In Neurotoxicity of Aluminum, Advances in Experimental Medicine and Biology, 1st ed.; Niu, Q., Ed.; Springer Nature: Singapore, 2018; pp. 99–111. [Google Scholar]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.L.; Cardoso, G.C.; Sousa, K.; Donato, T.A.G.; Pontes, F.M.L.; Grandini, C.R. Development of novel Ti-Mo-Mn alloys for biomedical applications. Sci. Rep. 2020, 10, 6298. [Google Scholar] [CrossRef]
- Martin-Camean, A.; Jos, A.; Puerto, M.; Calleja, A.; Iglesias-Linares, A.; Solano, E.; Camean, A.M. In vivo determination of aluminum, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by Inductively coupled plasma-mass spectrometry (ICP-MS). J. Trace Elem. Med. Biol. 2015, 32, 13–20. [Google Scholar] [CrossRef]
- Datte, C.E.; Tribst, J.P.; Dal Piva, A.O.; Nishioka, R.S.; Bottino, M.A.; Evangelhista, A.M.; Monteiro, F.M.M.; Borges, A.L. Influence of different restorative materials on the stress distribution in dental implants. J. Clin. Exp. Dent. 2018, 10, e439–e444. [Google Scholar] [CrossRef]
- Atalay, P.; Oztas, D.D. Fatigue resistance and fracture strength of narrow-diameter one-piece zirconia implants with angled abutments. J. Esthet. Restor. Dent. 2022, 34, 1060–1067. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Dias Corpa Tardelli, J.; Bolfarini, C.; Candido Dos Reis, A. Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review. J. Trace Elem. Med. Biol. 2020, 62, 126618. [Google Scholar] [CrossRef] [PubMed]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissure-Integrated Prostheses: Osseointegration in Clinical Dentistry; Brånemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Geng, J.P.; Tan, K.B.; Liu, G.R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, D.; Muftu, S.; Muftu, A. Evaluation of load transfer characteristics of five different implants in compact bone at different load levels by finite elements analysis. J. Prosthet. Dent. 2004, 92, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cho, I.H.; Kim, Y.S.; Heo, S.J.; Kwon, H.B.; Lim, Y.J. Bone-implant interface with simulated insertion stress around an immediately loaded dental implant in the anterior maxilla: A three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implants 2012, 27, 295–302. [Google Scholar]
- Baggi, L.; Pastore, S.; Di Girolamo, M.; Vairo, G. Implant-bone load transfer mechanisms in complete-arch prostheses supported by four implants: A three-dimensional finite element approach. J. Prosthet. Dent. 2013, 109, 9–21. [Google Scholar] [CrossRef]
- Alvarez-Arenal, A.; Segura-Mori, L.; Gonzalez-Gonzalez, I.; Gago, A. Stress distribution in the abutment and retention screw of a single implant supporting a prosthesis with platform switching. Int. J. Oral Maxillofac. Implants 2013, 28, e112–e121. [Google Scholar] [CrossRef][Green Version]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Beta titanium alloys: The lowest elastic modulus for biomedical applications: A review. Int. Schol. Sci. Res. Innov. 2014, 8, 822–827. [Google Scholar]
- Schneider, S.G.; Nunes, C.A.; Rogero, S.O.; Higa, O.Z.; Bressiani, J.C. Mechanical properties and cytotoxic evaluation of the Ti-3Nb-13Zr alloy. Biomecánica 2000, 8, 84–87. [Google Scholar] [CrossRef]
- Strasky, J.; Harcuba, P.; Vaclavova, K.; Horvath, K.; Landa, M.; Srba, O.; Janecek, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Barao, V.A.; Delben, J.A.; Faverani, L.P.; Hipolito, A.C.; Assuncao, W.G. Non-linear 3D finite element analysis of full-arch implant-supported fixed dentures. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ridzwan, M.; Shuib, S.; Hassan, A.; Shokri, A.; Mohamad, M. Problem of stress shielding and improvement to the hip implant designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Rizkalla, A.S.; Jones, D.W. Indentation fracture toughness and dynamic elastic moduli for commercial feldspathic dental porcelain materials. Dent. Mater. 2004, 20, 198–206. [Google Scholar] [CrossRef]
- Borie, E.; Orsi, I.A.; Noritomi, P.Y.; Kemmoku, D.T. Three-dimensional finite element analysis of the biomechanical behaviors of implants with different connections, lengths, and diameters placed in the maxillary anterior region. Int. J. Oral Maxillofac. Implants 2016, 31, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Leal, E.; Orsi, I.A.; Salamanca, C.; Dias, F.J.; Weber, B. Influence of transmucosal height in abutments of single and multiple implant-supported prostheses: A non-linear three-dimensional finite element analysis. Comput. Methods Biomech. Biomed. Engin. 2018, 21, 91–97. [Google Scholar] [CrossRef]
- Valera-Jimenez, J.F.; Burgueno-Barris, G.; Gomez-Gonzalez, S.; Lopez-Lopez, J.; Valmaseda-Castellon, E.; Fernandez-Aguado, E. Finite element analysis of narrow dental implants. Dent. Mater. 2020, 36, 927–935. [Google Scholar] [CrossRef]
- Froum, S.J.; Natour, M.; Cho, S.C.; Yu, P.Y.C.; Leung, M. Expanded Clinical Applications of Narrow-Diameter Implants for Permanent Use. Int. J. Periodontics Restor. Dent. 2020, 40, 529–537. [Google Scholar] [CrossRef]
- Reis, T.A.D.; Zancope, K.; Karam, F.K.; Neves, F.D.D. Biomechanical behavior of extra-narrow implants after fatigue and pull-out tests. J. Prosthet. Dent. 2019, 122, 54.e1–54.e6. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B.; et al. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. A. Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef] [PubMed]
- Melsen, B.; Lang, N.P. Biological reactions of alveolar bone to orthodontic loading of oral implants. Clin. Oral Implants Res. 2001, 12, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, Q.; Li, W.; Swain, M. Dental implant induced bone remodeling and associated algorithms. J. Mech. Behav. Biomed. Mater. 2009, 2, 410–432. [Google Scholar] [CrossRef] [PubMed]
- Sirandoni, D.; Leal, E.; Weber, B.; Noritomi, P.Y.; Fuentes, R.; Borie, E. Effect of Different Framework Materials in Implant-Supported Fixed Mandibular Prostheses: A Finite Element Analysis. Int. J. Oral Maxillofac. Implants 2019, 34, e107–e114. [Google Scholar] [CrossRef]
- Kayabaşi, O.; Yüzbasioğlu, E.; Erzincanli, F. Static, dynamic and fatigue behaviors of dental implant using finite element method. Adv. Eng. Soft. 2006, 37, 649–658. [Google Scholar] [CrossRef]
- Borie, E.; Orsi, I.A.; de Araujo, C.P. The influence of the connection, length and diameter of an implant on bone biomechanics. Acta. Odontol. Scand. 2015, 73, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Bordin, D.; Bergamo, E.T.P.; Fardin, V.P.; Coelho, P.G.; Bonfante, E.A. Fracture strength and probability of survival of narrow and extra-narrow dental implants after fatigue testing: In vitro and in silico analysis. J. Mech. Behav. Biomed. Mater. 2017, 71, 244–249. [Google Scholar] [CrossRef]

| Material | Young’s Modulus (GPa) | Poisson Ratio | Ultimate Strength (MPa) |
|---|---|---|---|
| Cortical bone | 13.7 [25] | 0.3 [25] | 100–130 [26] |
| Trabecular bone | 0.5 [27] | 0.3 [27] | 5 [28] |
| Ti–6Al–4V | 110 [29] | 0.35 [29] | 1000 [8] |
| Ti–35Nb–5Sn–6Mo–3Zr | 85 [8] | 0.34 [8] | 770 [8] |
| Ti–13Nb–13Zr | 77 [30] | 0.34 [30] | 1270 [31] |
| Ti–15Zr | 113 [8] | 0.34 [8] | N/A [8] |
| Ti–8Fe–5Ta | 120 [8] | 0.34 [8] | 1045 [8] |
| Ti–26.88Fe–4Ta | 175 [8] | 0.34 [8] | 2531 [8] |
| TNTZ–Fe–0.7O | 109 [32] | 0.35 [32] | N/A [32] |
| TNTZ–Fe–0.4O | 107 [32] | 0.35 [32] | 1130 [32] |
| Co–Cr | 205 [33] | 0.31 [33] | 655 [34] |
| Feldespatic ceramic | 68.9 [35] | 0.28 [35] | N/A [29] |
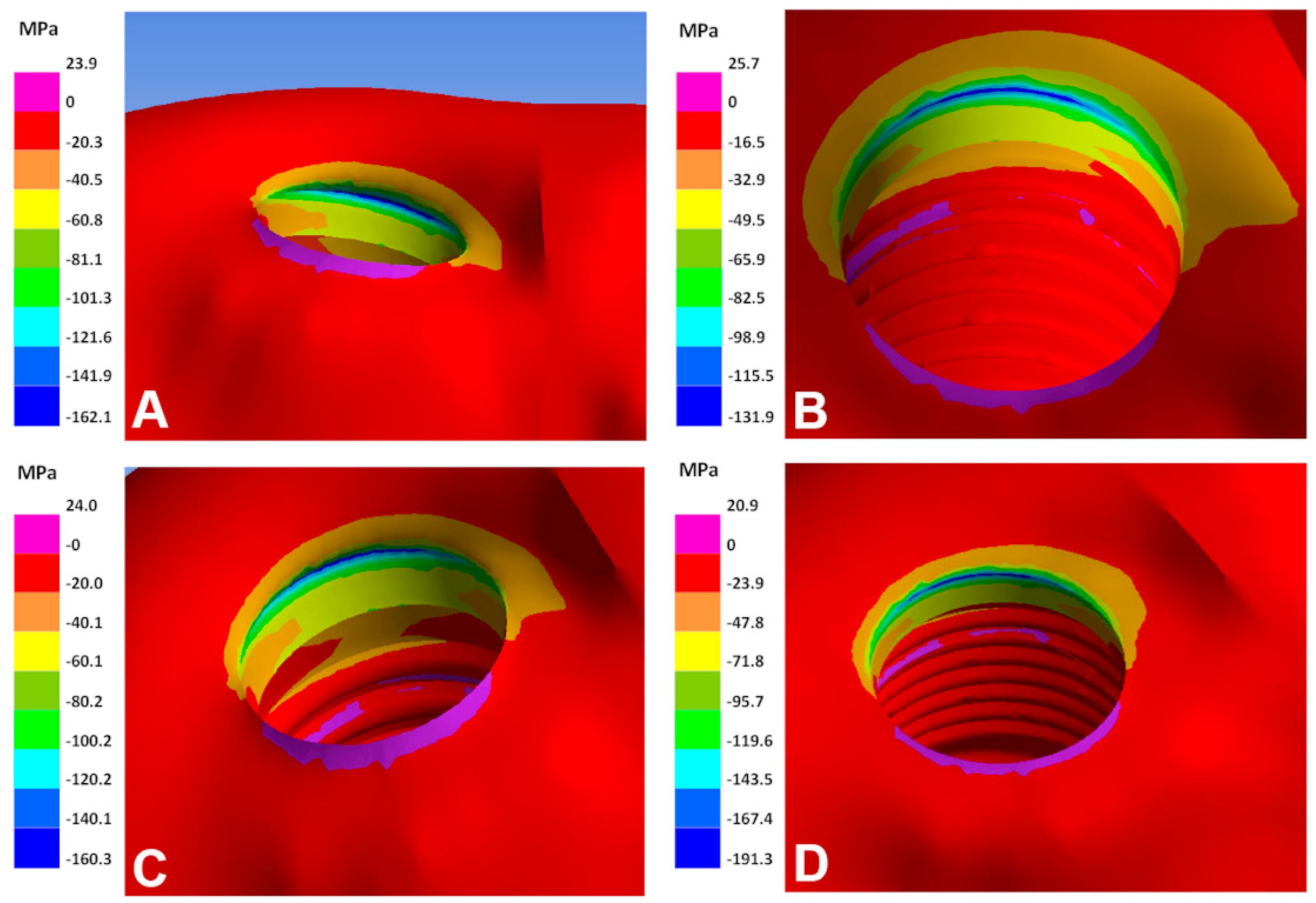
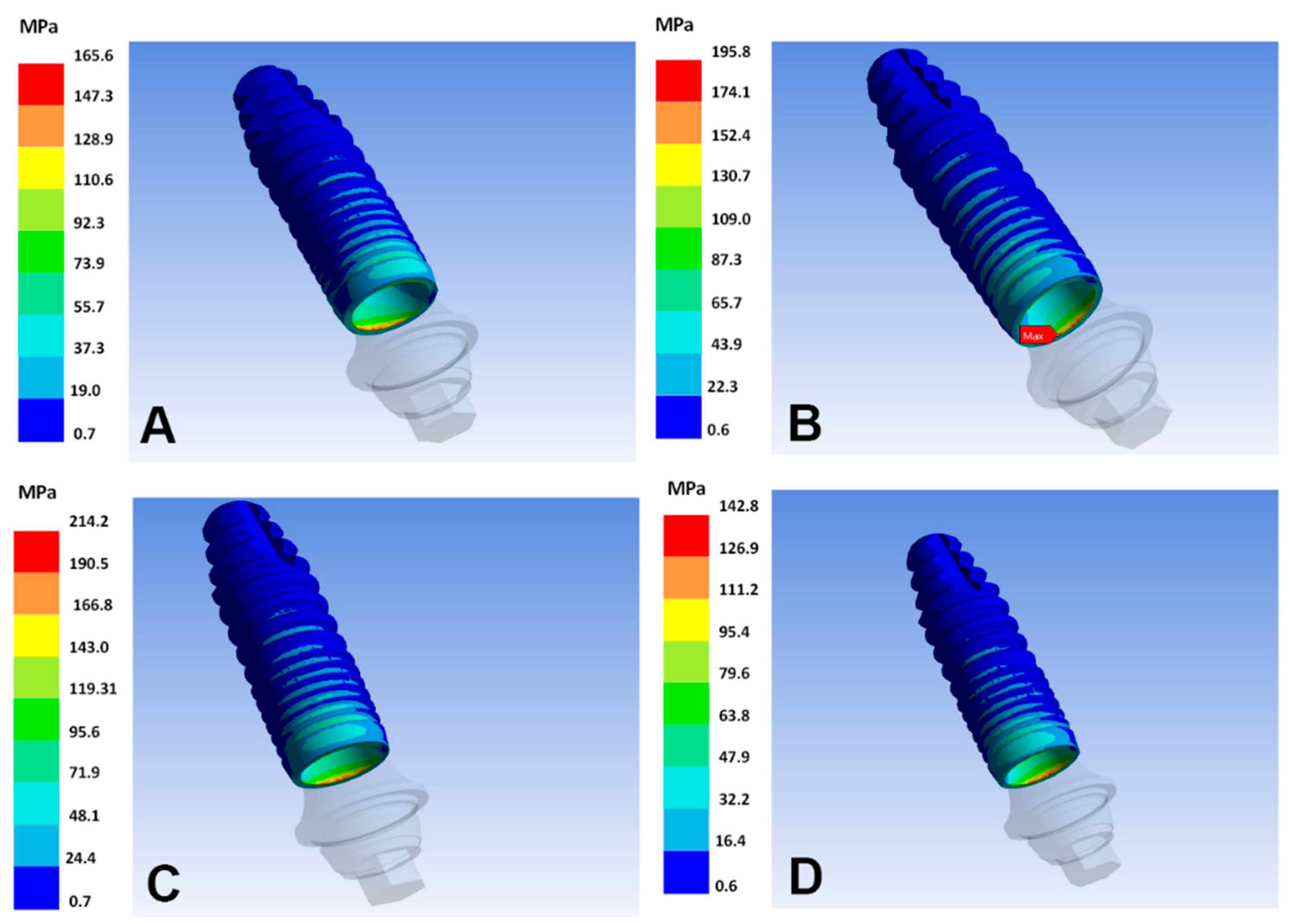
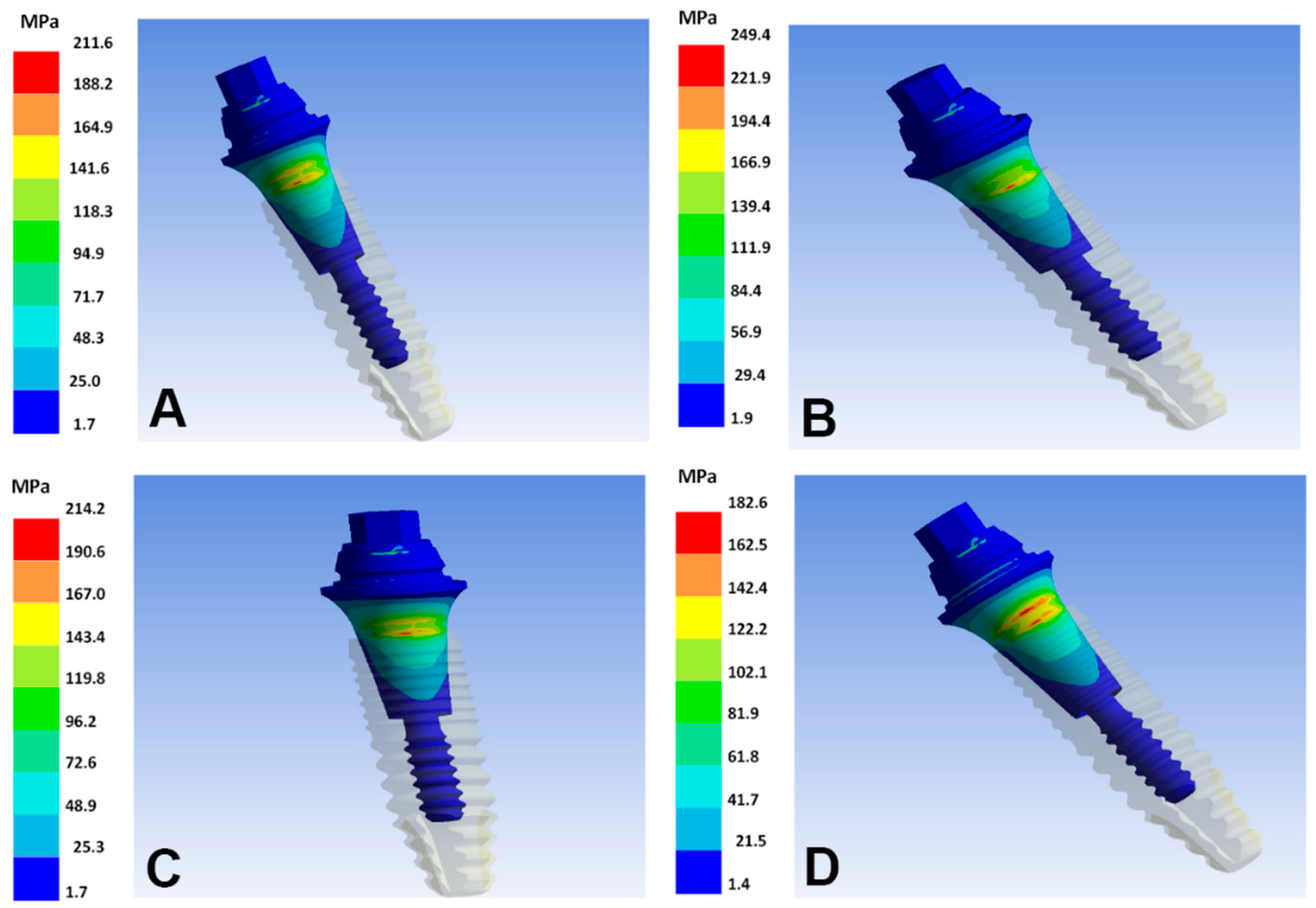
| Implant Alloy | Maximum Principal, Bone (MPa) | Minimum Principal, Bone (MPa) | VMES, Implants (MPa) | VMES, Abutments (MPa) |
|---|---|---|---|---|
| Ti–6Al–4V | 80 | −162 | 165 | 211 |
| Ti–35Nb–5Sn–6Mo–3Zr | 96 | −183 * | 149 | 190 |
| Ti–13Nb–13Zr | 103 | −191 * | 142 | 182 |
| Ti–15Zr | 79 | −160 | 168 | 214 |
| Ti–8Fe–5Ta | 75 | −155 | 171 | 219 |
| Ti–26.88Fe–4Ta | 58 | −131 | 195 | 249 |
| TNTZ–2Fe–0.4O | 82 | −164 | 163 | 209 |
| TNTZ–2Fe–0.7O | 81 | −163 | 165 | 210 |
| Implant Alloy | Maximum Principal, Bone (MPa) | Minimum Principal, Bone (MPa) | VMES, Implants (MPa) | VMES, Abutments (MPa) |
|---|---|---|---|---|
| Ti–6Al–4V | 81 | −149 | 143 | 176 |
| Ti–35Nb–5Sn–6Mo–3Zr | 93 | −164 | 117 | 183 |
| Ti–13Nb–13Zr | 101 | −170 * | 128 | 187 |
| Ti–15Zr | 79 | −147 | 178 | 145 |
| Ti–8Fe–5Ta | 75 | −144 | 148 | 182 |
| Ti–26.88Fe–4Ta | 57 | −125 | 161 | 203 |
| TNTZ–2Fe–0.4O | 80 | −150 | 122 | 180 |
| TNTZ–2Fe–0.7O | 79 | −164 | 123 | 181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, J.M.; Leal, E.; Hunter, R.; de Souza, R.F.; Borie, E. Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis. Materials 2022, 15, 8903. https://doi.org/10.3390/ma15248903
Zapata JM, Leal E, Hunter R, de Souza RF, Borie E. Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis. Materials. 2022; 15(24):8903. https://doi.org/10.3390/ma15248903
Chicago/Turabian StyleZapata, José Manuel, Eduardo Leal, Renato Hunter, Raphael Freitas de Souza, and Eduardo Borie. 2022. "Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis" Materials 15, no. 24: 8903. https://doi.org/10.3390/ma15248903
APA StyleZapata, J. M., Leal, E., Hunter, R., de Souza, R. F., & Borie, E. (2022). Biomechanical Behavior of Narrow Dental Implants Made with Aluminum- and Vanadium-Free Alloys: A Finite Element Analysis. Materials, 15(24), 8903. https://doi.org/10.3390/ma15248903








