Planar Perovskite Solar Cells Using Perovskite CsPbI3 Quantum Dots as Efficient Hole Transporting Layers
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of CsPbI3 QD Solution
2.3. Device Fabrication
2.4. Measuring Instruments
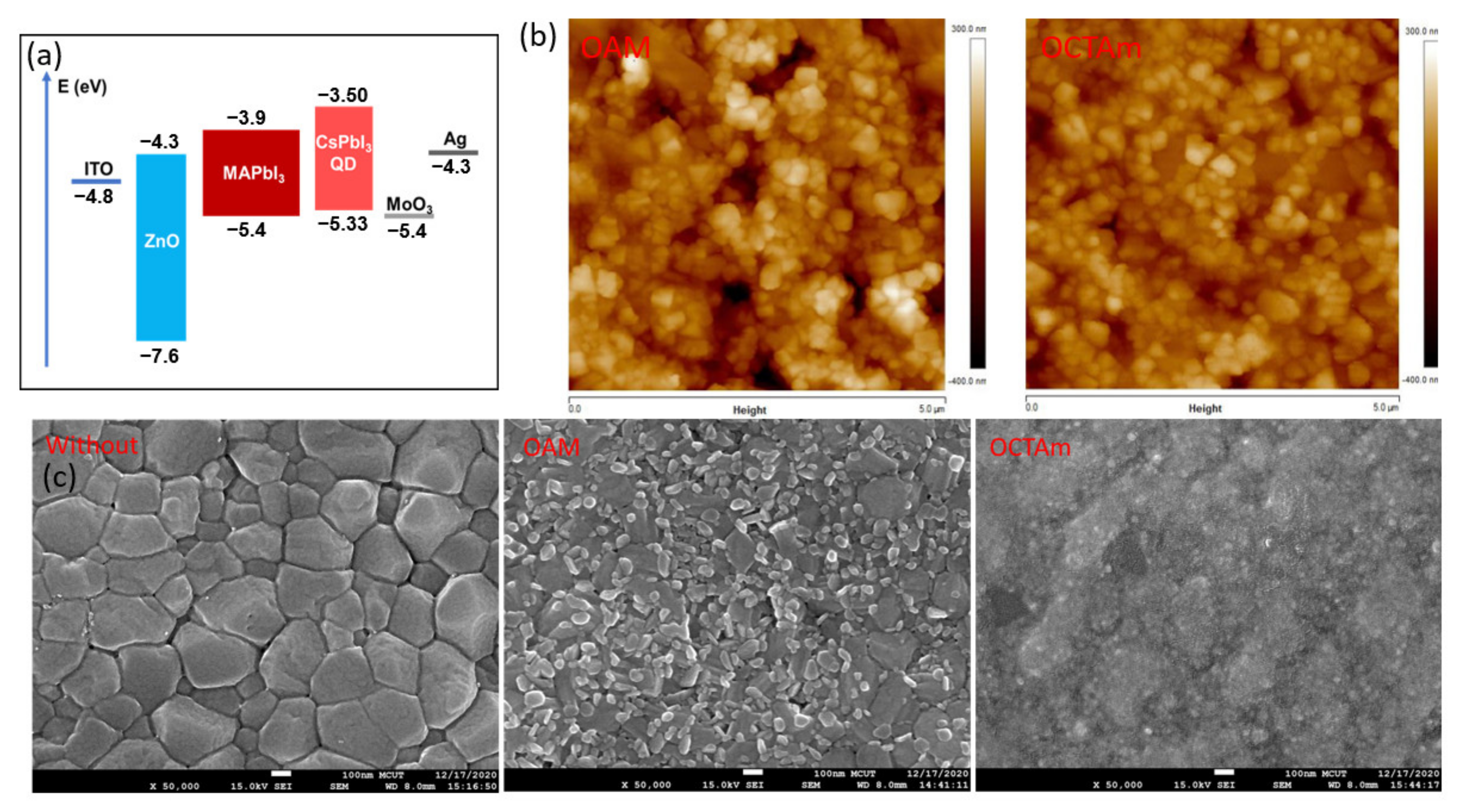
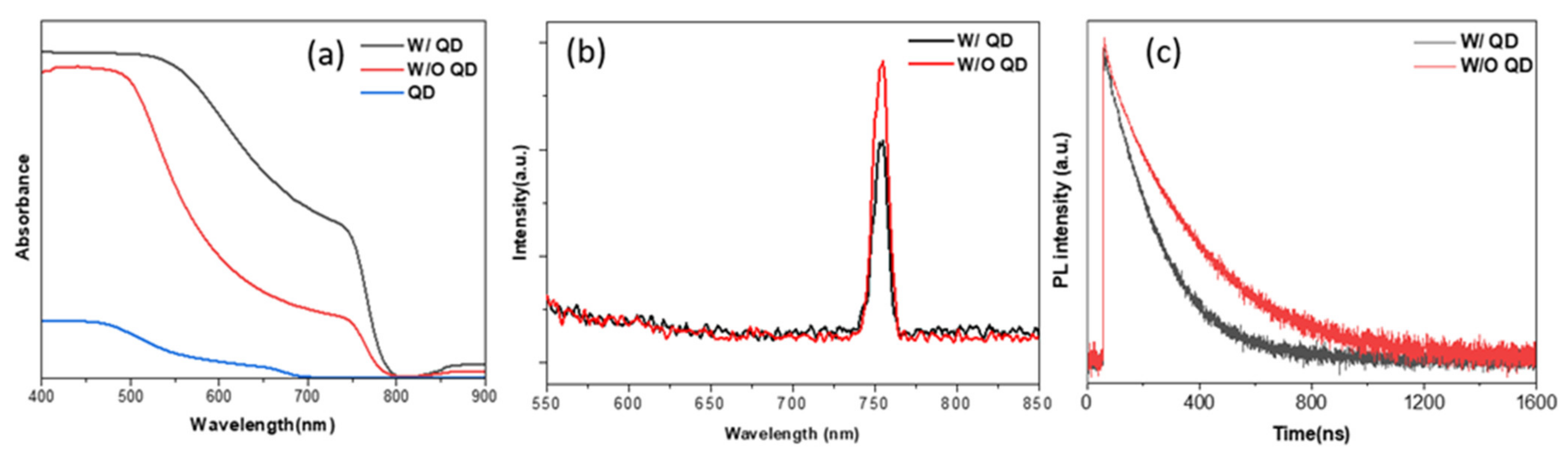
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paramati, S.R.; Shahzad, U.; Dogan, B. The role of environmental technology for energy demand and energy efficiency: Evidence from OECD countries. Renew. Sustain. Energy Rev. 2022, 153, 111735. [Google Scholar] [CrossRef]
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; D’Ambrosio, D.; Spencer, T. Net Zero by 2050, a Roadmap for the Global Energy Sector; IEA: Paris, France, 2021. [Google Scholar]
- Jones, D. Global Electricity Review 2022; Ember: London, UK, 2022. [Google Scholar]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Adhyaksa, G.W.P.; Veldhuizen, L.W.; Kuang, Y.; Brittman, S.; Schropp, R.E.I.; Garnett, E.C. Carrier diffusion lengths in hybrid perovskites: Processing, composition, aging, and surface passivation effects. Chem. Mater. 2016, 28, 5259–5263. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tien, C.-H.; Tseng, Z.-L.; Ruan, J.-H. Enhanced Efficiency of MAPbI3 Perovskite Solar Cells with FAPbX3 Perovskite Quantum Dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, M.; Zhou, X.; Wu, J.; Zhang, L.; Kong WLi, X.; Zhao, X.; Dai, S.; Xu, B.; Cheng, C. Efficiency and stability enhancement of perovskite solar cells by introducing CsPbI3 quantum dots as an interface engineering layer. NPG Asia Mater. 2018, 10, 552–561. [Google Scholar] [CrossRef]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, W. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kido, J. Lead halide perovskite quantum dots for light-emitting devices. J. Mater. Chem. C 2018, 6, 11868–11877. [Google Scholar] [CrossRef]
- Dai, J.; Xi, J.; Li, L.; Zhao, J.; Shi, Y.; Zhang, W.; Ran, C.; Jiao, B.; Hou, X.; Duan, X.; et al. Charge transport between coupling colloidal perovskite quantum dots assisted by functional conjugated ligands. Angew. Chem. Int. Ed. 2018, 57, 5754–5758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gibbs, M.; Puthussery, J.; Gaik, S.; Ihly, R.; Hillhouse, H.W.; Law, M. Dependence of carrier mobility on nanocrystal size and ligand length in PbSe nanocrystal solids. Nano Lett. 2010, 10, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Z.L.; Chiang, C.H.; Wu, C.G. Surface engineering of ZnO thin film for high efficiency planar perovskite solar cells. Sci. Rep. 2015, 5, 13211. [Google Scholar] [CrossRef]
- Chen, L.C.; Tseng, Z.L.; Lin, C.C. Low-temperature sol–gel derived ZnO films as electron transporting layers for perovskite-based solar cells. J. Nanoelectron. Optoelectron. 2019, 14, 723–728. [Google Scholar] [CrossRef]
- Chiang, C.H.; Tseng, Z.L.; Wu, C.G. Planar heterojunction perovskite/PC71BM solar cells with enhanced open-circuit voltage via a (2/1)-step spin-coating process. J. Mater. Chem. A 2014, 2, 15897–15903. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, G.H.; Kim, Y.T.; Wolf, C.; Yun, H.J.; Kwon, W.; Park, C.G.; Lee, T.W. High efficiency perovskite light-emitting diodes of ligand-engineered colloidal formamidinium lead bromide nanoparticles. Nano Energy 2017, 38, 51–58. [Google Scholar] [CrossRef]
- Lu, C.; Wright, M.; Ma, X.; Li, H.; Itanze, D.; Carter, J.A.; Hewitt, C.A.; Donati, G.L.; Carroll, D.L.; Lundin, P.M.; et al. Cs oleate precursor preparation for lead halide perovskite nanocrystal synthesis: The influence of excess oleic acid on achieving solubility, conversion, and reproducibility. Chem. Mater. 2018, 31, 62–67. [Google Scholar] [CrossRef]
- Xu, F.; Kong, X.; Wang, W.; Juan, F.; Wang, M.; Wei, H.; Li, J.; Cao, B. Quantum size effect and surface defect passivation in size-controlled CsPbBr3 quantum dots. J. Alloys Compd. 2020, 831, 154834. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy 2016, 1, 16148. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Roldán-Carmona, C.; Soriano, A.; Bandiello, E.; Camacho, L.; Nazeeruddin, M.K.; Bolink, H.J. Metal-oxide-free methylammonium lead iodide perovskite-based solar cells: The influence of organic charge transport layers. Adv. Energy Mater. 2014, 4, 1400345. [Google Scholar] [CrossRef]
- Girotto, C.; Voroshazi, E.; Cheyns, D.; Heremans, P.; Rand, B.P. Solution-Processed MoO3 Thin Films As a Hole-Injection Layer for Organic Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 3244–3247. [Google Scholar] [CrossRef]
- Schulz, P.; Edri, E.; Kirmayer, S.; Hodes, G.; Cahen, D.; Kahn, A. Interface energetics in organo-metal halide perovskite-based photovoltaic cells. Energy Environ. Sci. 2014, 7, 1377–1381. [Google Scholar] [CrossRef]
- Tseng, Z.L.; Huang, Y.S.; Liu, Y.L.; Wu, T.L.; Wei, Y.J. Tetraoctylammonium bromide-passivated CsPbI3−xBrx perovskite nanoparticles with improved stability for efficient red light-emitting diodes. J. Alloys Compd. 2022, 897, 163182. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Pan, J.; Ye, C. Alternating current electroluminescent devices with inorganic phosphors for deformable displays. Cell Rep. Phys. Sci. 2020, 1, 100213. [Google Scholar] [CrossRef]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Grätzel, M.; et al. Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.-C.; Su, H.-Y.; Chen, S.-A.; Chen, Y.-J.; Chiang, C.-Y.; Chiang, C.-H.; Kao, T.-T.; Chen, L.-C.; Lin, C.-C. Planar Perovskite Solar Cells Using Perovskite CsPbI3 Quantum Dots as Efficient Hole Transporting Layers. Materials 2022, 15, 8902. https://doi.org/10.3390/ma15248902
Liang T-C, Su H-Y, Chen S-A, Chen Y-J, Chiang C-Y, Chiang C-H, Kao T-T, Chen L-C, Lin C-C. Planar Perovskite Solar Cells Using Perovskite CsPbI3 Quantum Dots as Efficient Hole Transporting Layers. Materials. 2022; 15(24):8902. https://doi.org/10.3390/ma15248902
Chicago/Turabian StyleLiang, Tsair-Chun, Hsin-Yu Su, Sih-An Chen, Yen-Ju Chen, Chung-Yu Chiang, Chih-Hsun Chiang, Tzung-Ta Kao, Lung-Chien Chen, and Chun-Cheng Lin. 2022. "Planar Perovskite Solar Cells Using Perovskite CsPbI3 Quantum Dots as Efficient Hole Transporting Layers" Materials 15, no. 24: 8902. https://doi.org/10.3390/ma15248902
APA StyleLiang, T.-C., Su, H.-Y., Chen, S.-A., Chen, Y.-J., Chiang, C.-Y., Chiang, C.-H., Kao, T.-T., Chen, L.-C., & Lin, C.-C. (2022). Planar Perovskite Solar Cells Using Perovskite CsPbI3 Quantum Dots as Efficient Hole Transporting Layers. Materials, 15(24), 8902. https://doi.org/10.3390/ma15248902






