LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Transport Experiments
2.3. Mathematic Model
2.4. Breakthrough Sorption Capacity
3. Results and Discussion
3.1. Characterization of PE MPs
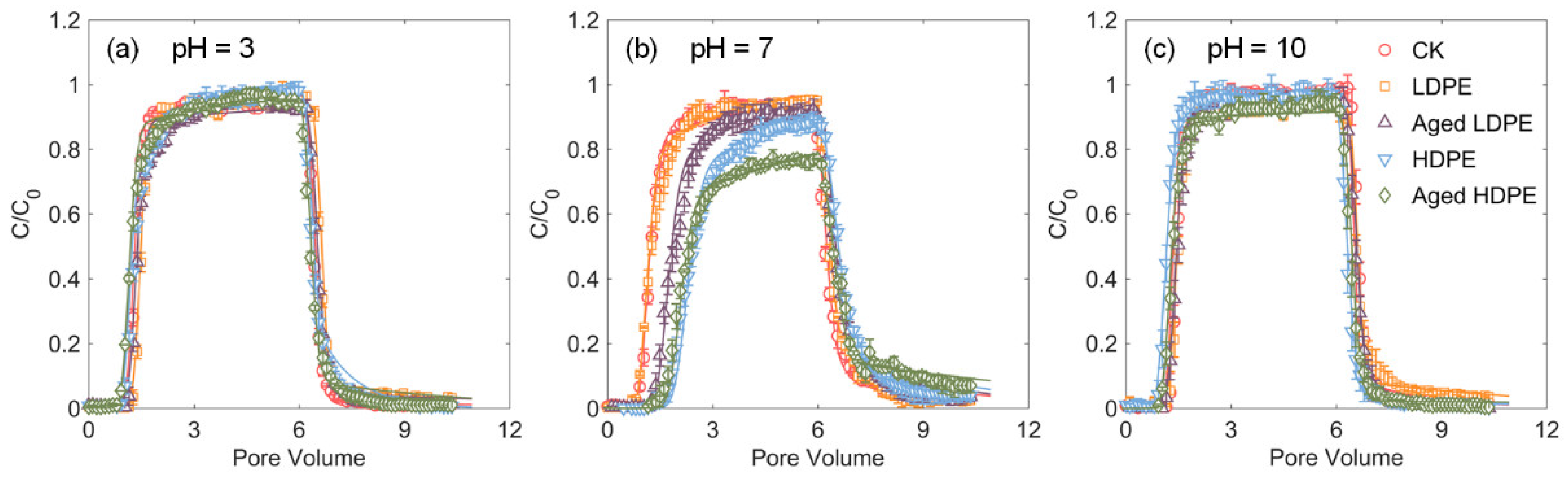
3.2. Effect of pH on Tetracycline Transport
3.3. Effect of PE Type on Tetracycline Transport
3.4. Effect of PE Weathering on Tetracycline Transport
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Graham, J.; Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. Projecting the sorption capacity of heavy metal ions onto microplastics in global aquatic environments using artificial neural networks. J. Hazard. Mater. 2021, 402, 123709. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Milic, N.; Milanovic, M.; Letic, N.G.; Sekulic, M.T.; Radonic, J.; Mihajlovic, I.; Miloradov, M.V. Occurrence of antibiotics as emerging contaminant substances in aquatic environment. Int. J. Environ. Health Res. 2013, 23, 296–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, L.; Zhu, S.; Lu, H.; Liu, W. Occurrence, abundance, and distribution of sulfonamide and tetracycline resistance genes in agricultural soils across China. Sci. Total Environ. 2017, 599–600, 1977–1983. [Google Scholar] [CrossRef]
- Ma, J.; Sheng, G.D.; O’Connor, P. Microplastics combined with tetracycline in soils facilitate the formation of antibiotic resistance in the Enchytraeus crypticus microbiome. Environ. Pollut. 2020, 264, 114689. [Google Scholar] [CrossRef]
- Wang, S.; Xue, N.; Li, W.; Zhang, D.; Pan, X.; Luo, Y. Selectively enrichment of antibiotics and ARGs by microplastics in river, estuary and marine waters. Sci. Total Environ. 2020, 708, 134594. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Wu, J.; Luo, Y. Effects of microplastics on distribution of antibiotic resistance genes in recirculating aquaculture system. Ecotoxicol. Environ. Saf. 2019, 184, 109631. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, D.; Li, Z.; Song, K.; He, Y. Evaluation of microplastic polyvinylchloride and antibiotics tetracycline co-effect on the partial nitrification process. Mar. Pollut. Bull. 2020, 160, 111671. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Koci, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Lu, T.; Li, Y.; Song, Y.; Shang, Z.; Liu, S.; Li, D.; Qi, Z. Effects of divalent metal cations and inorganic anions on the transport of tetracycline in saturated porous media: Column experiments and numerical simulations. Environ. Sci. Process. Impacts 2019, 21, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Huang, G.; Zhou, T.; Zhao, Y.; Ma, J. Interfacial interaction between diverse microplastics and tetracycline by adsorption in an aqueous solution. Sci. Total Environ. 2020, 721, 137729. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef]
- Sassman, S.A.; Lee, L.S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef]
- Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. A methodological approach of the current literature on microplastic contamination in terrestrial environments: Current knowledge and baseline considerations. Sci. Total Environ. 2020, 730, 139164. [Google Scholar] [CrossRef]
- Zhang, X.M.; Elkoun, S.; Ajji, A.; Huneault, M.A. Oriented structure and anisotropy properties of polymer blown films: HDPE, LLDPE and LDPE. Polymer 2004, 45, 217–229. [Google Scholar] [CrossRef]
- Herbert, S.; Shinozaki, D.M.; Collacott, R.J. Fine-scale morphology of ultraviolet-ozone etched polyethylene. J. Mater. Sci. 1996, 31, 4655–4661. [Google Scholar] [CrossRef]
- Hu, E.; He, Z.; Nan, X.; Yuan, Z.; Li, X. Removal of phenanthrene and pyrene from contaminated sandy soil using hydrogen peroxide oxidation catalyzed by basic oxygen furnace slag. Environ. Sci. Pollut. Res. 2019, 26, 9281–9292. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liao, P.; Yuan, S. Effects of ionic strength and cationic type on humic acid facilitated transport of tetracycline in porous media. Chem. Eng. J. 2016, 284, 389–394. [Google Scholar] [CrossRef]
- Huffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Zakari, S.; Liu, H.; Tong, L.; Wang, Y.; Liu, J. Transport of bisphenol-A in sandy aquifer sediment: Column experiment. Chemosphere 2016, 144, 1807–1814. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, J.; Hu, B.X.; Wei, W. Adsorption and desorption for dynamics transport of hexavalent chromium (Cr(VI)) in soil column. Environ. Sci. Pollut. Res. 2018, 25, 459–468. [Google Scholar] [CrossRef]
- Chabira, S.F.; Sebaa, M.; G’Sell, C. Oxidation and crosslinking processes during thermal aging of low-density polyethylene films. J. Appl. Polym. Sci. 2011, 124, 5200–5208. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Zhao, Y.; Xiang, Y.; He, D.; Pan, X. Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environ. Pollut. 2020, 257, 113475. [Google Scholar] [CrossRef]
- Wool, R.P.; Bretzlaff, R.S.; Li, B.Y.; Wang, C.H.; Boyd, R.H. Infrared and raman spectroscopy of stressed polyethylene. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 1039–1066. [Google Scholar] [CrossRef]
- Snyder, R.G.; Poore, M.W. Conformational structure of polyethylene chains from the infrared spectrum of the partially deuterated polymer. Macromolecules 1973, 6, 708–715. [Google Scholar] [CrossRef]
- Pagès, P.; Carrasco, F.; Surina, J.; Colom, X. FTIR and DSC study of HDPE structural changes and mechanical properties variation when exposed to weathering aging during Canadian winter. J. Appl. Polym. Sci. 1996, 60, 153–159. [Google Scholar] [CrossRef]
- Ye, S.; Cheng, M.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Shen, M.; Song, B.; Liu, J.; Yang, H.; et al. Insights into catalytic removal and separation of attached metals from natural-aged microplastics by magnetic biochar activating oxidation process. Water Res. 2020, 179, 115876. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, D.; Fu, J.; Chen, Y.; Ou, H. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020, 186, 116360. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Gomez-Pacheco, C.V.; Sanchez-Polo, M.; Lopez-Penalver, J.J.; Ocampo-Perez, R. Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. J. Environ. Manag. 2013, 131, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L. Nonequilibrium transport of organic chemicals: The impact of pore-water velocity. J. Contam. Hydrol. 1992, 9, 353–368. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wang, F.; Yang, H.; Liu, L. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation. Chemosphere 2021, 265, 129133. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Zarfl, C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE). Environ. Sci. Pollut. Res. 2012, 19, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
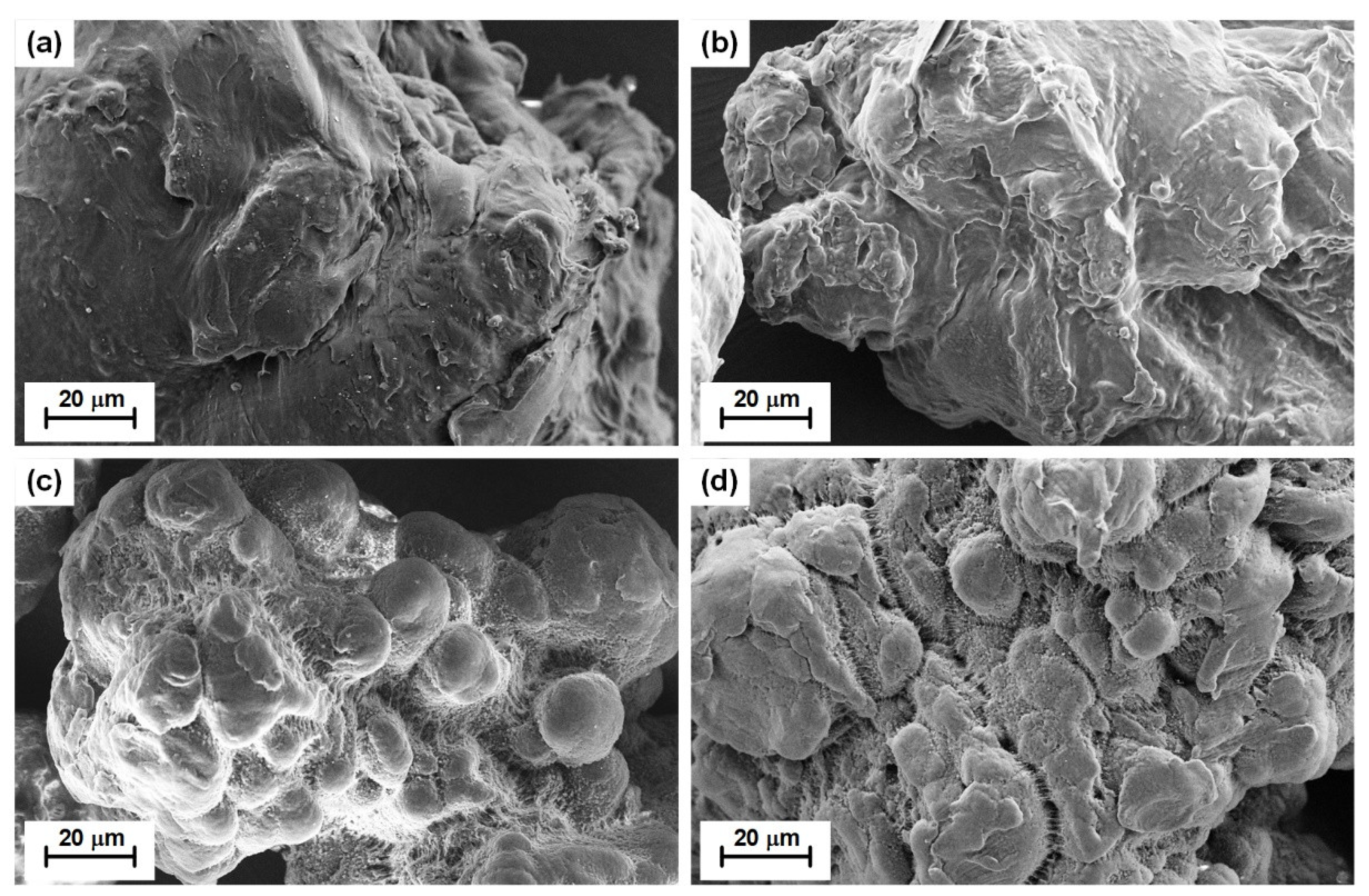

| Property | Pristine LDPE | UV-Weathered LDPE | Pristine HDPE | UV-Weathered HDPE |
|---|---|---|---|---|
| Dv50 (µm) | 46.8 ± 0.1 | 47.9 ± 0.5 | 29.7 ± 0.1 | 167.0 ± 1.2 |
| BET surface area (m2·g−1) | 0.31 ± 0.02 | 0.38 ± 0.02 | 3.06 ± 0.04 | 7.32 ± 0.10 |
| O/C ratio | 0.017 ± 0.000 | 0.020 ± 0.000 | 0.023 ± 0.000 | 0.030 ± 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, E.; Yuan, H.; Du, Y.; Chen, X. LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media. Materials 2021, 14, 1757. https://doi.org/10.3390/ma14071757
Hu E, Yuan H, Du Y, Chen X. LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media. Materials. 2021; 14(7):1757. https://doi.org/10.3390/ma14071757
Chicago/Turabian StyleHu, Enzhu, Hongbo Yuan, Yichun Du, and Xijuan Chen. 2021. "LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media" Materials 14, no. 7: 1757. https://doi.org/10.3390/ma14071757
APA StyleHu, E., Yuan, H., Du, Y., & Chen, X. (2021). LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media. Materials, 14(7), 1757. https://doi.org/10.3390/ma14071757







