Quantitative Strengthening Evaluation of Powder Metallurgy Titanium Alloys with Substitutional Zr and Interstitial O Solutes via Homogenization Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Mixing of Raw Powder
2.2. Sintering, Homogenization, and Tempering Process Conditions
2.3. Hot Extrusion
2.4. Materials Characterization
3. Results and Discussions
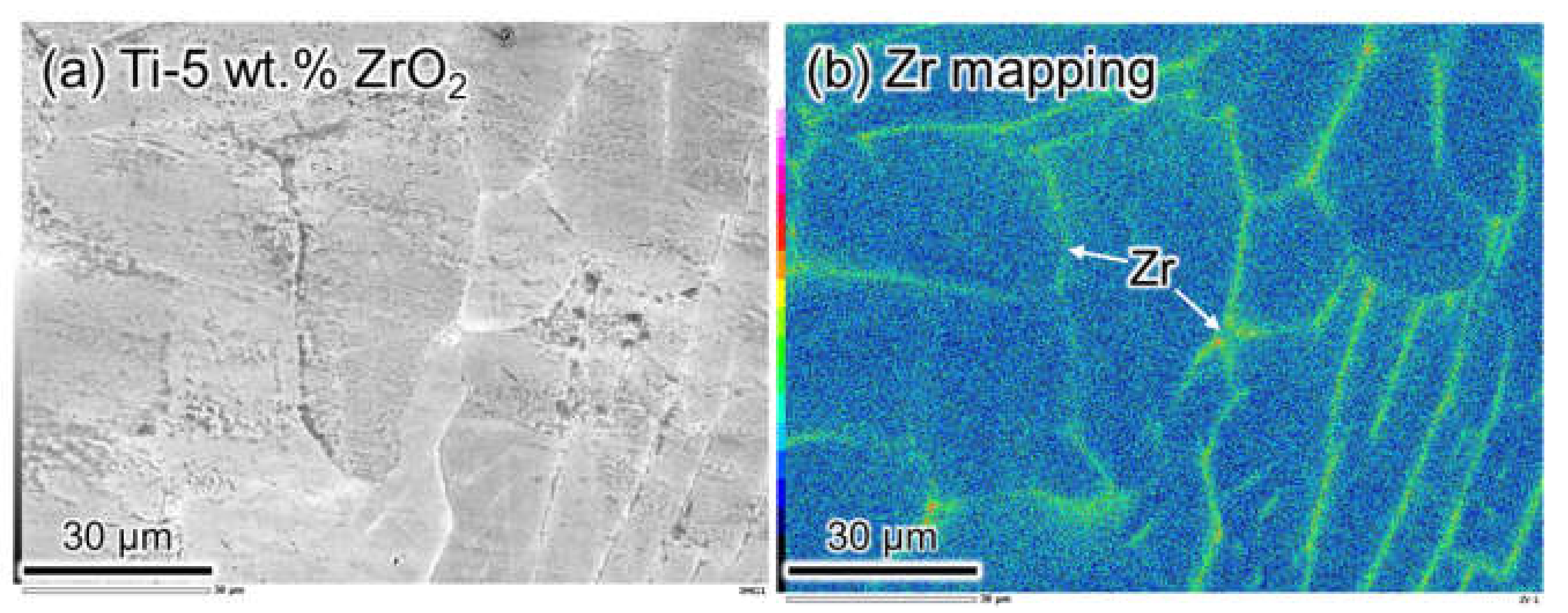
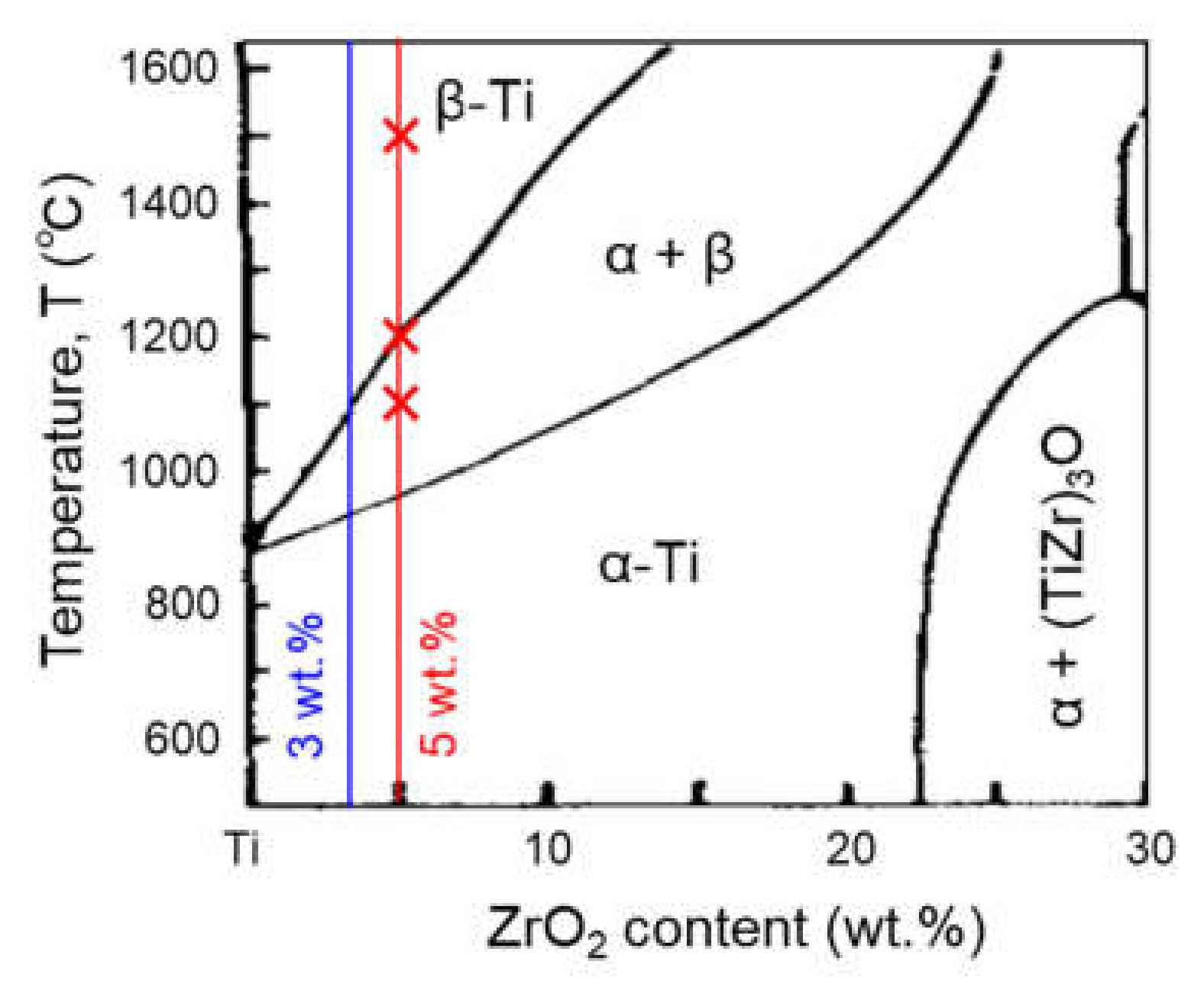
3.1. ZrO2 Decomposition and Zr Homogenization Behavior
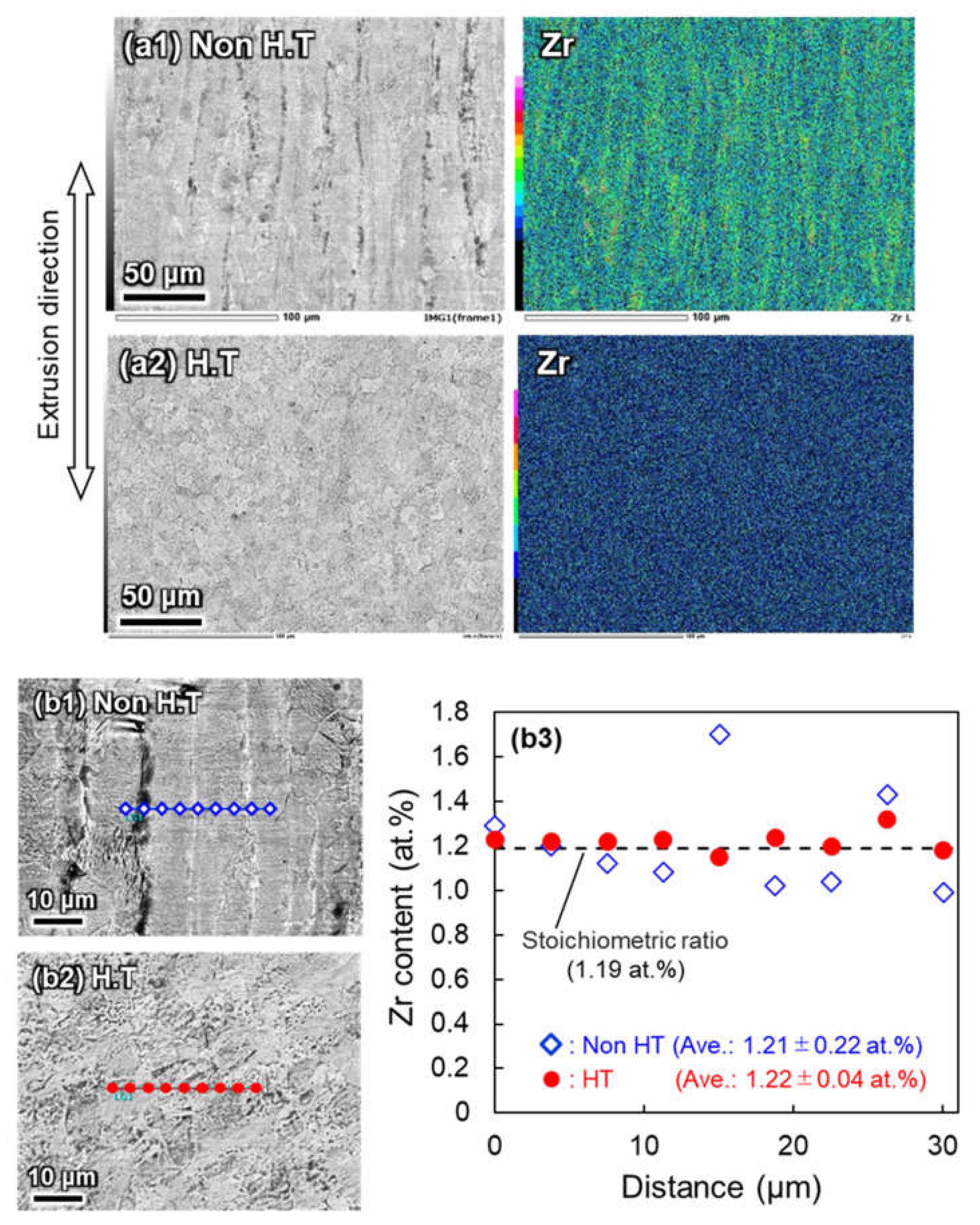
3.2. Zr Agglomeration Behavior during Cooling after Homogenization
3.3. Role of Homogenization on Microstructural and Mechanical Properties of Extruded Ti-Zr-O Alloys
3.4. Quantitative Evaluation of Strengthening Mechanism of Ti-Zr-O Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurul Amin, A.K.M. (Ed.) Titanium Alloys-Towards Achieving Enhanced Properties for Diversified Applications; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Williams, J.C. (Ed.) Titanium and Titanium Alloys: Scientific and Technological Aspects; Springer: New York, NY, USA; Volume 3, 1982. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Amigó, A.; Vicente, A.; Afonso, C.R.M.; Amigó, V. Mechanical properties and the microstructure of β Ti-35Nb-10Ta-xFe alloys obtained by powder metallurgy for biomedical applications. Metals 2019, 9, 158–161. [Google Scholar] [CrossRef] [Green Version]
- Phuong, D.D.; Duong, L.V.; Luan, N.V.; Anh, N.N.; Trinh, P.V. Microstructure and mechanical properties of Ti6Al4V alloy consolidated by different sintering techniques. Metals 2019, 9, 1033. [Google Scholar] [CrossRef] [Green Version]
- Bahador, A.; Umeda, J.; Ghandvar, H.; Bakar, T.A.A.; Yamanoglu, R.; Issariyapat, A.; Kondoh, K. Microstructure globularization of high oxygen concentration dual-phase extruded Ti alloys via powder metallurgy route. Mater. Charact. 2021, 172, 110855. [Google Scholar] [CrossRef]
- Kamiyama, K.; Kariya, S.; Fukuo, M.; Umeda, J.; Kondoh, K. Ductility improvement mechanism of Ti-6Al-4V+O sintered material. Mater. Trans. 2020, 61, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Chen, B.; Umeda, J.; Zhang, J.; Li, Y.; Kondoh, K. An in-situ study on deformation and cracking initiation in oxygen-doped commercial purity titanium. Mech. Mater. 2020, 148, 103519. [Google Scholar] [CrossRef]
- Issariyapat, A.; Visuttipitukul, P.; Song, T.; Bahador, A.; Umeda, J.; Qian, M.; Kondoh, K. Tensile properties improvement by homogenized nitrogen solid solution strengthening of commercially pure titanium through powder metallurgy process. Mater. Charac. 2020, 170, 110700. [Google Scholar] [CrossRef]
- Umeda, J.; Ishizaka, H.; Li, S.; Alhazaa, A.; Kondoh, K. Comparison study on mechanical properties of powder metallurgy titanium materials with nitrogen solutes and TiN dispersoids. J. Alloys Compd. 2020, 846, 156455. [Google Scholar] [CrossRef]
- Issariyapat, A.; Visuttipitukul, P.; Song, T.; Umeda, J.; Qian, M.; Kondoh, K. Strength-ductility improvement of extruded Ti-(N) materials using pure Ti powder with high nitrogen solution. Mater. Sci. Eng. A 2020, 779, 139136. [Google Scholar] [CrossRef]
- Umeda, J.; Tanaka, T.; Teramae, T.; Kariya, S.; Fujita, J.; Nishikawa, H.; Shibutani, Y.; Shen, J.; Kondoh, K. Microstructures analysis and quantitative strengthening evaluation of powder metallurgy Ti-Fe binary extruded alloys with (α+β)-dual-phase. Mater. Sci. Eng. A 2021, 803, 140708. [Google Scholar] [CrossRef]
- Bahador, A.; Issariyapat, A.; Umeda, J.; Yamanoglu, R.; Pruncu, C.; Amrin, A.; Kondoh, K. Strength-ductility balance of powder metallurgy Ti-2Fe-2W alloy extruded at high-temperature. J. Mater. Res. Tech. 2021, 14, 677–691. [Google Scholar] [CrossRef]
- Bahador, A.; Umeda, J.; Yamanoglu, R.; Bakar, T.A.A.; Kondoh, K. Strengthening evaluation and high-temperature behavior of Ti-Fe-O-Cu-Si alloy. Mater. Sci. Eng. A 2021, 800, 140324. [Google Scholar] [CrossRef]
- Conrad, H. Effect of interstitial solutes on the strength and ductility of titanium. Prog. Mater. Sci. 1981, 26, 123–403. [Google Scholar] [CrossRef]
- Kondoh, K.; Ichikawa, E.; Issariyapat, A.; Shitara, K.; Umeda, J.; Chen, B.; Li, S. Tensile property enhancement by oxygen solutes in selectively laser melted titanium materials fabricated from pre-mixed pure Ti and TiO2 powder. Mater. Sci. Eng. A 2020, 795, 139983. [Google Scholar] [CrossRef]
- Kondoh, K.; Issariyapat, A.; Umeda, J.; Visuttipitukul, P. Selective laser-melted titanium materials with nitrogen solid solutions for balanced strength and ductility. Mater. Sci. Eng. A 2020, 790, 139641. [Google Scholar] [CrossRef]
- Gerber, H.; Perren, S.M. Evaluation of tissue compatibility of in vitro cultures of embryonic bone. In Evaluation of Biomaterials; Winter, G.D., Leray, J.L., de Groot, K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1980; pp. 307–314. [Google Scholar]
- Steinemann, S.G. Corrosion of Surgical Implants-in vivo and in vitro tests. In Evaluation of Biomaterials; Winter, G.D., Leray, J.L., de Groot, K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1980; pp. 1–34. [Google Scholar]
- Murray, J.L. (Ed.) Phase Diagrams of Binary Titanium Alloys: Monograph Series on Alloy Phase Diagrams; AMS International: Ann Arbor, MI, USA, 1987. [Google Scholar]
- Williams, D.F.; Williams, R.L. Degradative effects of the biological environment on metals and ceramics. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2004; pp. 430–439. [Google Scholar]
- Nakasuji, K.; Okada, M. New high strength titanium alloy Ti-10%Zr for spectacle frames. Mater. Sci. Eng. A 1996, 213, 162–165. [Google Scholar] [CrossRef]
- Homma, T.; Matayoshi, Y.; Voskoboinikov, R. Application of the Bons-Azuma method and determination of grain growth mechanism in rolled Ti-Zr alloys. Philos. Mag. Lett. 2015, 95, 564–573. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuo, M.; Kariya, S.; Shitara, K.; Li, S.; Alhazaa, A.; Umeda, J. Quantitative strengthening evaluation of powder metallurgy Ti–Zr binary alloys with high strength and ductility. J. Alloys Compd. 2021, 852, 156954. [Google Scholar] [CrossRef]
- Teramae, T.; Tanaka, T.; Fukuo, M.; Shitara, K.; Umeda, J.; Li, S.; Alhazaa, A.; Kondoh, K. Acicular microstructure formation and strengthening behavior of Ti-4%Fe alloys by Zr addition. J. Alloys Compd. 2021, 858, 158292. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Molotnikov, A.; Lapovok, R.; Zeller, R.; Berner, S.; Habersetzer, P.; Torre, F.D. Microstructure and mechanical properties of Ti-15Zr alloy used as dental implant material. J. Mech. Behav. Biomed. Mater. 2016, 62, 384–398. [Google Scholar] [CrossRef]
- Labusch, R. A statistical theory of solid solution hardening. Phys. Status Solidi B 1970, 41, 659–669. [Google Scholar] [CrossRef]
- Kariya, S.; Fukuo, M.; Umeda, J.; Kondoh, K. Quantitative analysis on light elements solution strengthening in pure titanium sintered materials by Labusch model using experimental data. Mater. Trans. 2019, 60, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Fukuo, M.; Kariya, S.; Umeda, J.; Kondoh, K.; Yoshiya, M. Strengthening mechanisms of powder metallurgy extruded CP titanium materials with zirconium and oxygen solid solution via decomposition of ZrO2 additives in sintering. J. Jpn. Soc. Powder Powder Metall. 2018, 65, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Bragg, W.L. The diffraction of short electromagnetic waves by a crystal. Scientia 1929, 23, 153. [Google Scholar]
- Domagala, R.F.; Lyon, S.R.; Ruh, R. The pseudobinary Ti-ZrO2. J. Am. Ceram. Soc. 1973, 56, 584–587. [Google Scholar] [CrossRef]
- Hall, E.O. Variation of hardness of metals with grain size. Nature 1954, 173, 948–949. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 173, 25–28. [Google Scholar]
- Kato, M. Introduction to the Theory of Dislocations; Shokabo Co. Ltd.: Tokyo, Japan, 1999. [Google Scholar]











| ZrO2 (wt.%) | Chemical Compositions (at.%) | Microstructural and Mechanical Properties | Calculated Stress Increment/MPa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr | O | N | H | Grain Size (µm) | Schmid Factor, SF | YS, σYS (MPa) | UTS, σ(MPa) | Elongation (%) | YS increment, ΔσYS[E] (MPa) | Δσ[GR] | Δσ[Zr-SS] | Δσ[O-SS] | Δσ[N-SS] | YS Increment, ΔσYS[C] | |
| 0 | 0 | 1.08 | 0.07 | 0.79 | 13.51 | 0.43 | 471.4 | 614.0 | 30.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.0 | 0.39 | 1.82 | 0.06 | 0.88 | 8.14 | 0.44 | 753.0 | 870.5 | 27.2 | 281.6 | 44.6 | 31.6 | 181.7 | −7.1 | 250.8 |
| 2.0 | 0.79 | 2.65 | 0.06 | 0.93 | 8.89 | 0.42 | 964.1 | 1042.8 | 26.4 | 492.7 | 36.0 | 52.7 | 408.3 | −4.6 | 492.4 |
| 3.0 | 1.19 | 3.34 | 0.06 | 0.97 | 7.65 | 0.41 | 1144.5 | 1173.5 | 7.8 | 673.1 | 50.9 | 71.0 | 580.4 | −3.2 | 699.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondoh, K.; Kariya, S.; Khantachawana, A.; Alhazaa, A.; Umeda, J. Quantitative Strengthening Evaluation of Powder Metallurgy Titanium Alloys with Substitutional Zr and Interstitial O Solutes via Homogenization Heat Treatment. Materials 2021, 14, 6561. https://doi.org/10.3390/ma14216561
Kondoh K, Kariya S, Khantachawana A, Alhazaa A, Umeda J. Quantitative Strengthening Evaluation of Powder Metallurgy Titanium Alloys with Substitutional Zr and Interstitial O Solutes via Homogenization Heat Treatment. Materials. 2021; 14(21):6561. https://doi.org/10.3390/ma14216561
Chicago/Turabian StyleKondoh, Katsuyoshi, Shota Kariya, Anak Khantachawana, Abdulaziz Alhazaa, and Junko Umeda. 2021. "Quantitative Strengthening Evaluation of Powder Metallurgy Titanium Alloys with Substitutional Zr and Interstitial O Solutes via Homogenization Heat Treatment" Materials 14, no. 21: 6561. https://doi.org/10.3390/ma14216561
APA StyleKondoh, K., Kariya, S., Khantachawana, A., Alhazaa, A., & Umeda, J. (2021). Quantitative Strengthening Evaluation of Powder Metallurgy Titanium Alloys with Substitutional Zr and Interstitial O Solutes via Homogenization Heat Treatment. Materials, 14(21), 6561. https://doi.org/10.3390/ma14216561









