SBA-16 Cage-Like Porous Material Modified with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Pure SBA-16
2.2. Functionalization of the SBA-16 with APTES
2.3. Characterization Techniques
2.4. Adsorption Experiments
3. Results and Discussion
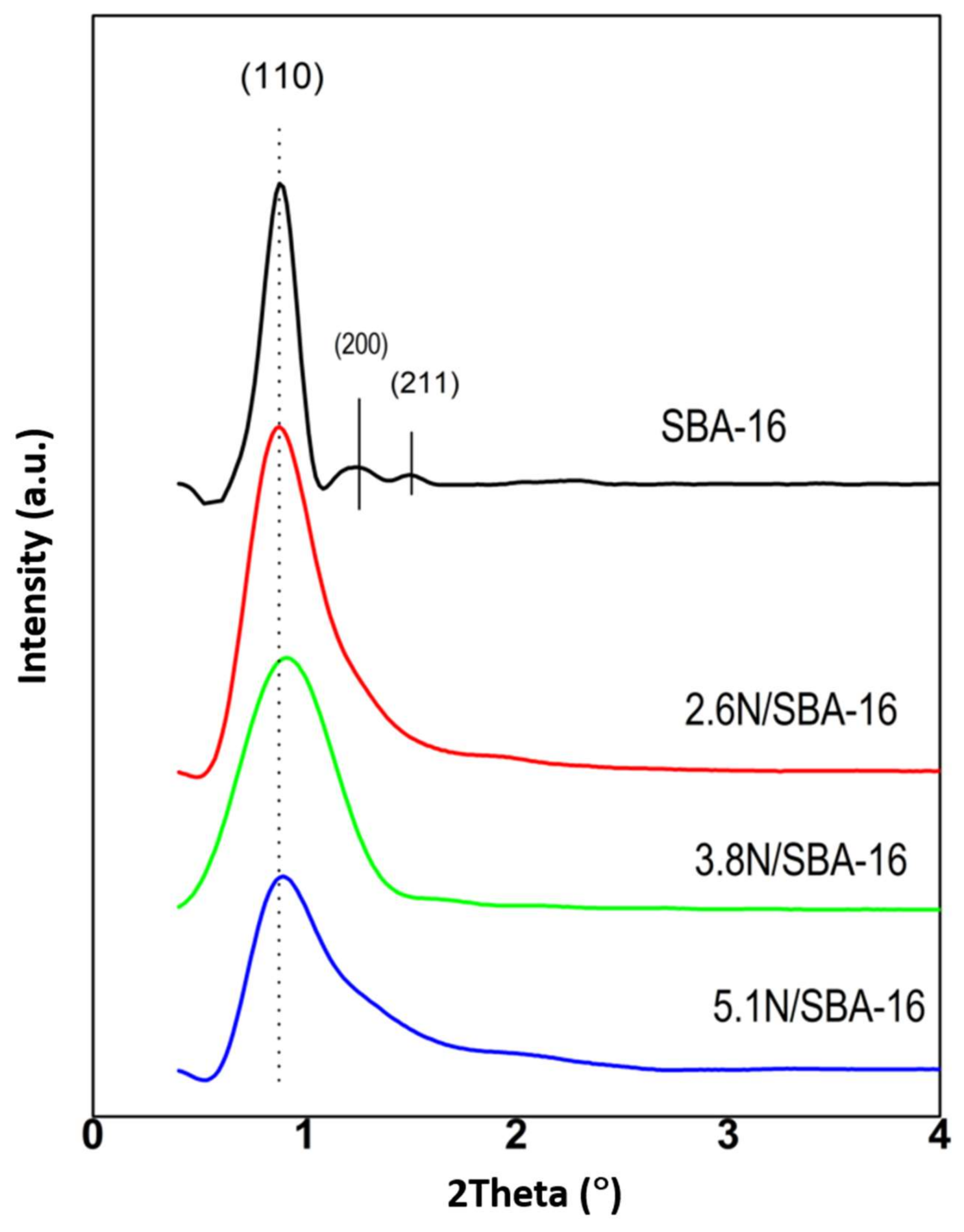
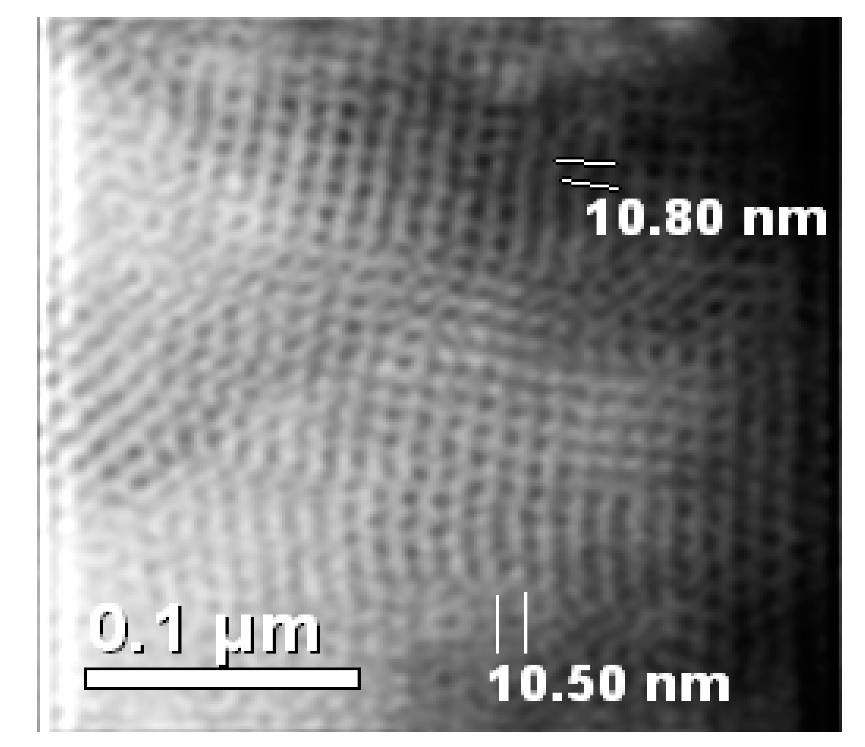
3.1. Chemical Analysis and Low-Angle X-ray Diffractions
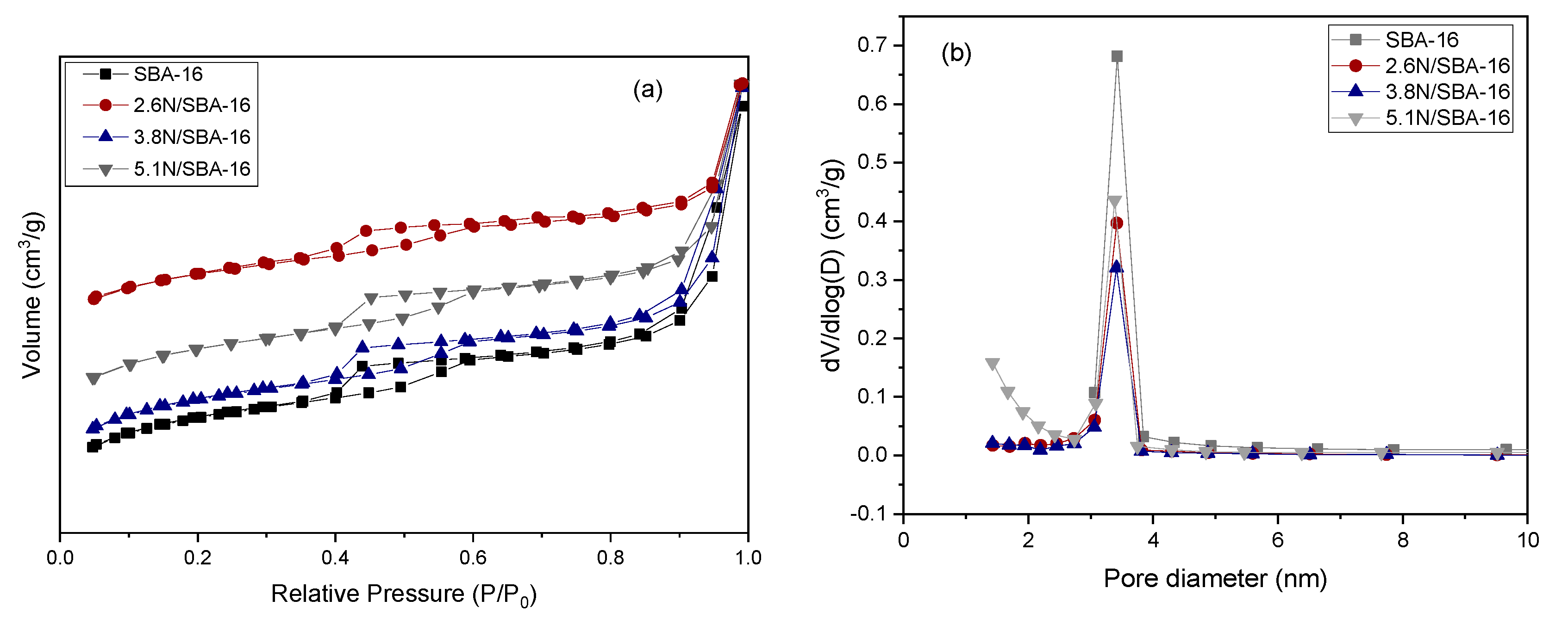
3.2. Textural Properties
3.3. FTIR of Framework Vibration
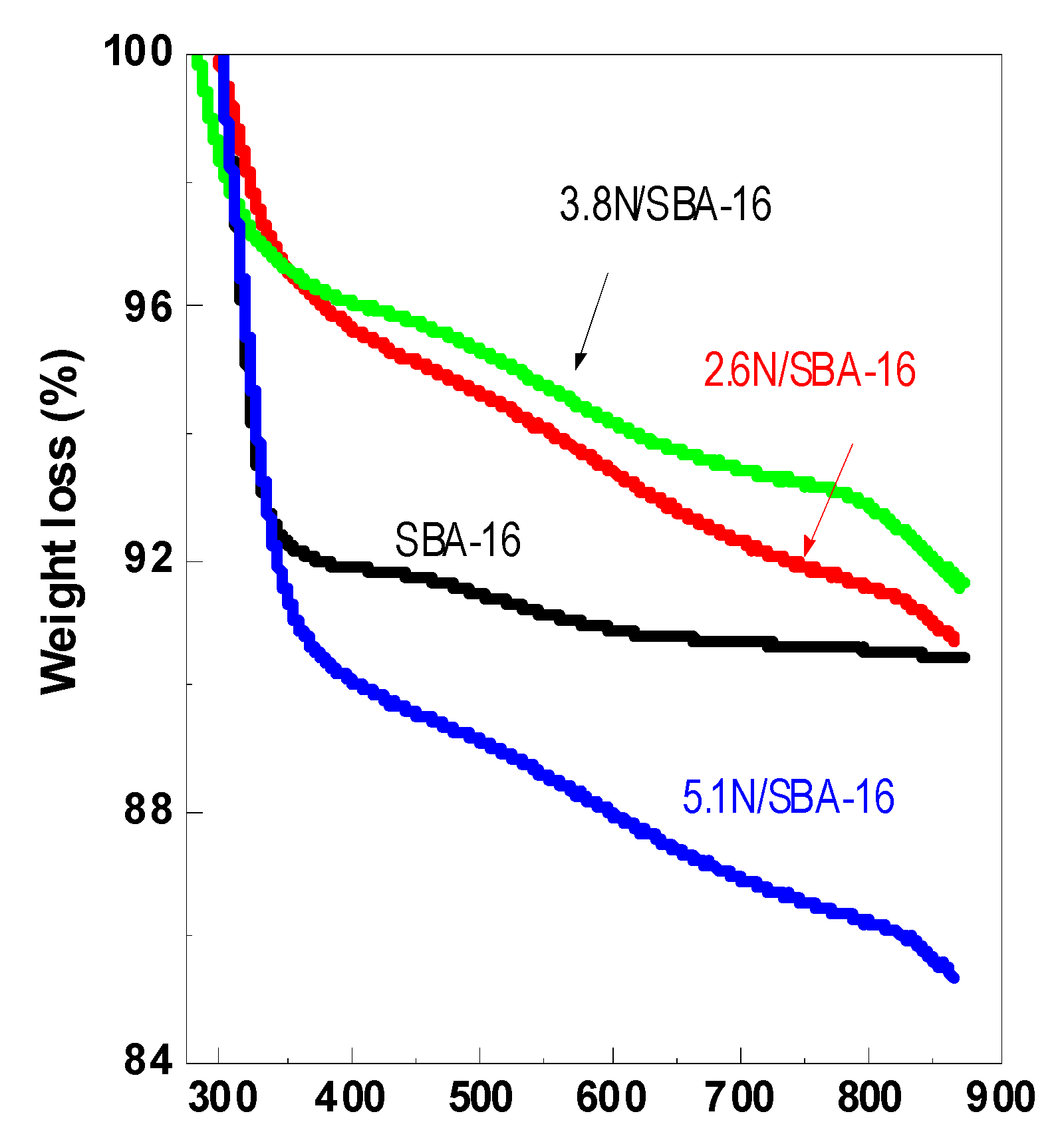
3.4. Thermogravimetry (TG)
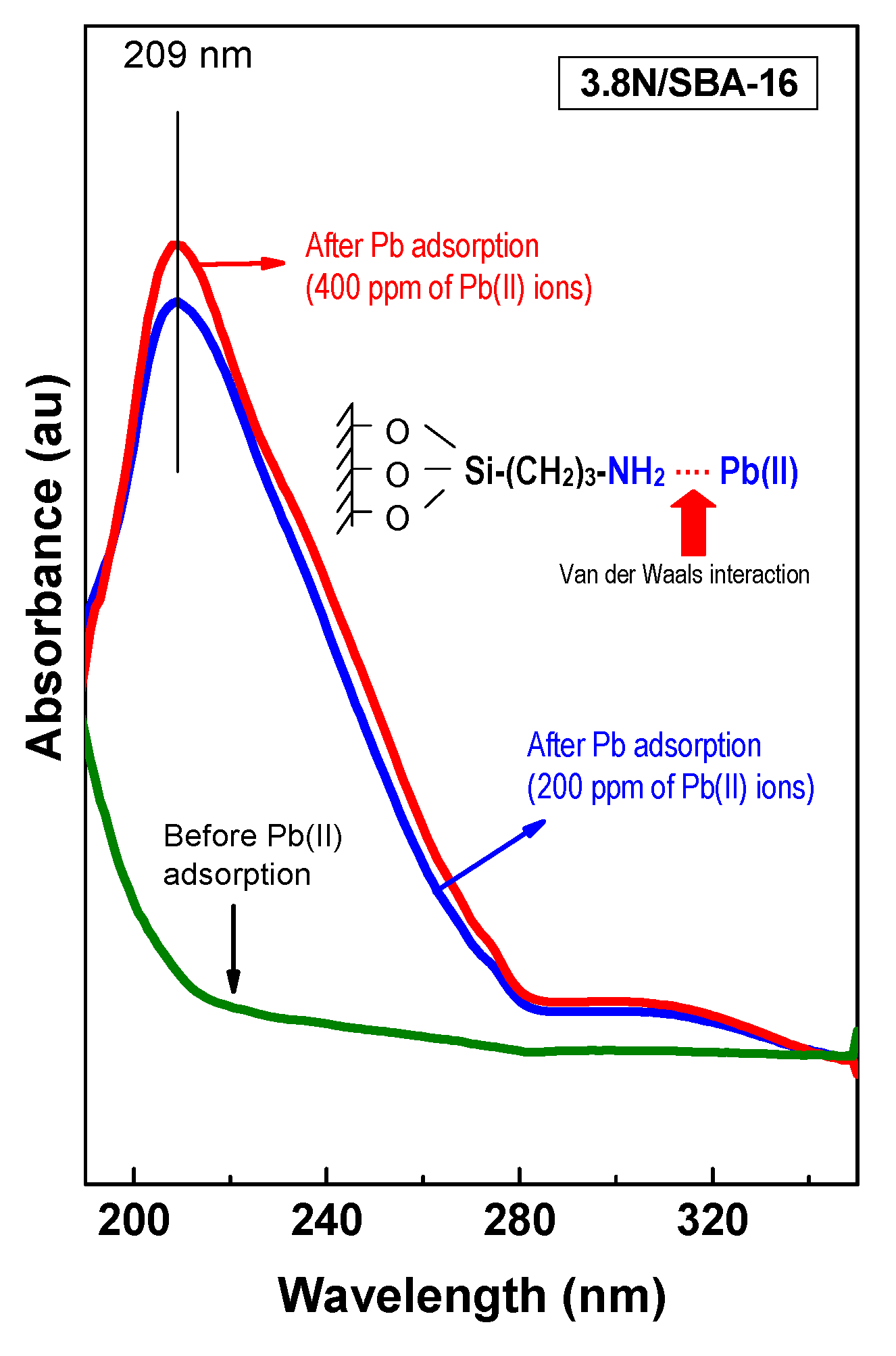
3.5. DRS UV-Vis
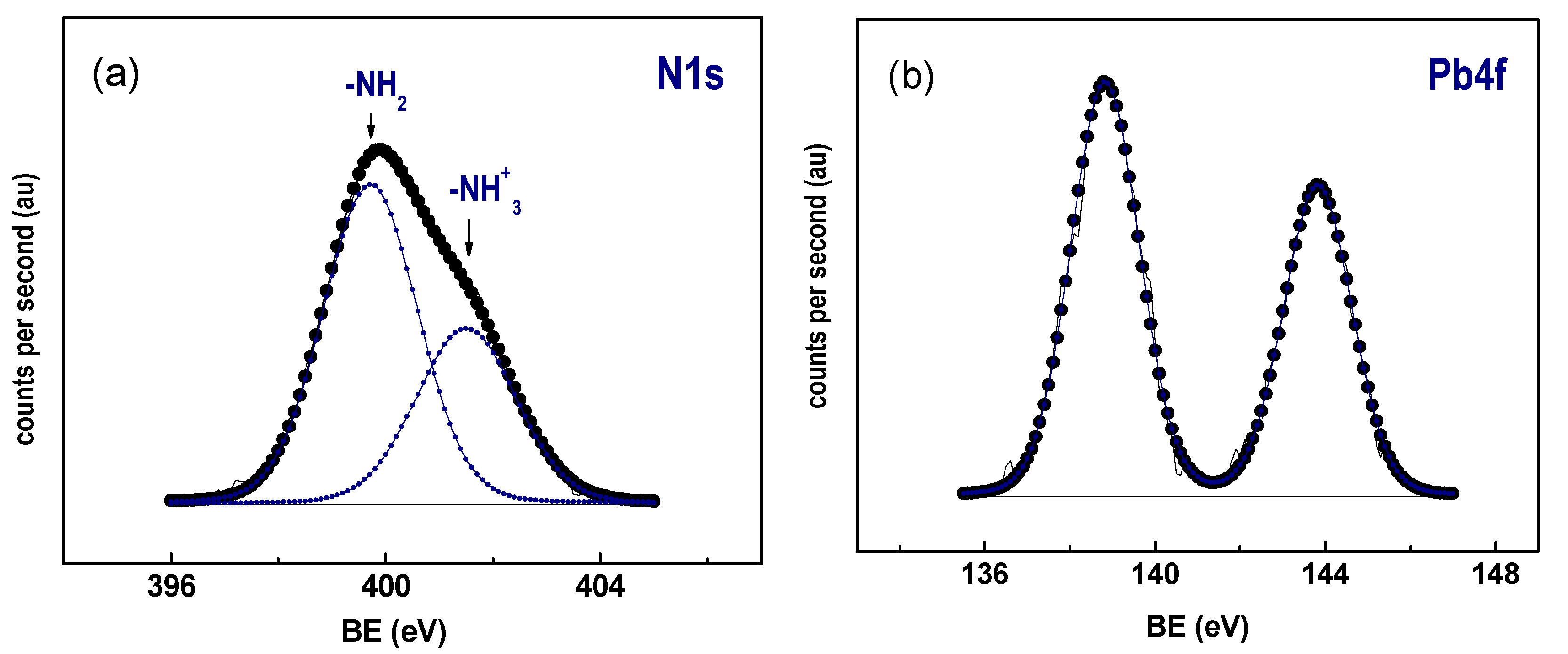
3.6. X-ray Photoelectron Spectroscopy
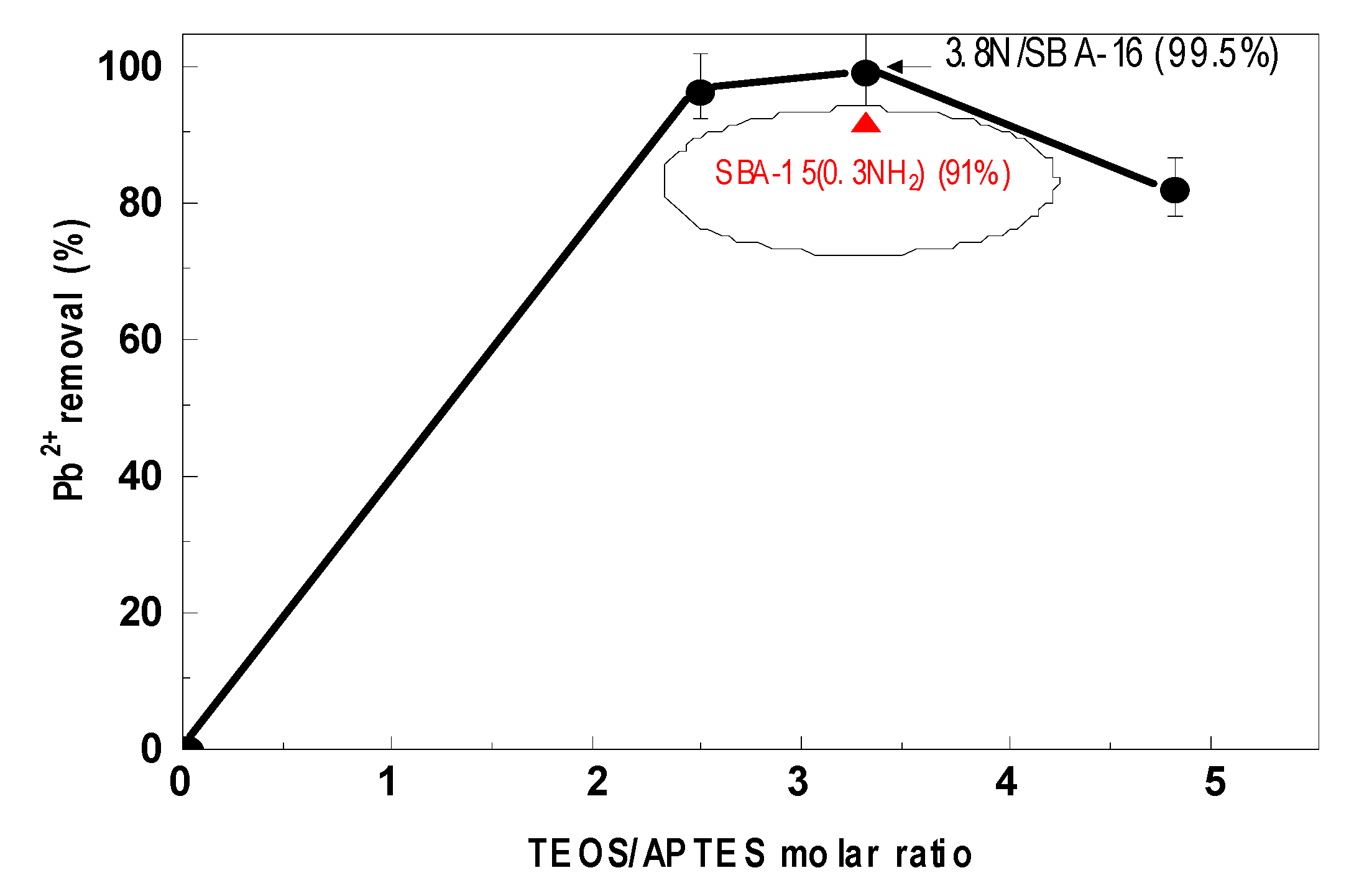
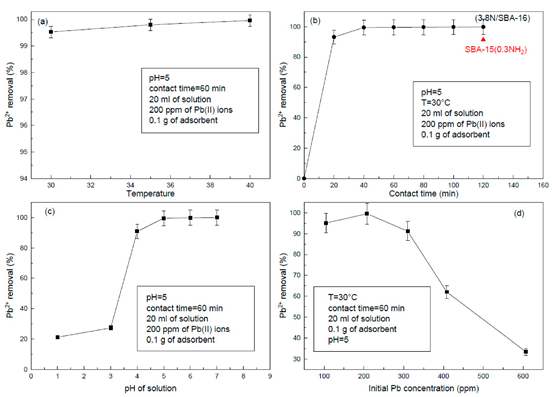
3.7. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morales, V.H.; Nava, R.; Silva, Y.J.A.; Sánchez, S.A.M.; Bueno, J.L.P.; Pawelec, B. Adsorption of Lead (II) on SBA 15 Mesoporous Molecular Sieve Functionalized with -NH2 Groups. Microporoporous Mesoporous Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Vu, D.-H.; Bui, H.-B.; Bui, X.-N.; An-Nguyen, D.; Le, Q.-T.; Do, N.-H.; Nguyen, H. A novel Approach in Adsorption of Heavy Metal Ions from Aqueous Solution Using Synthesized MCM-41 from Coal Bottom Ash. Int. J. Environ. Anal. Chem. 2019, 1–19. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Anirudhan, T.; Divya, L.; Ramachandran, M. Mercury (II) Removal from Aqueous Solutions and Wastewaters Using a Novel Cation Exchanger Derived from Coconut Coir Pith and Its Recovery. J. Hazard. Mater. 2008, 157, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Kumar, A.; Rout, L.; Rath, J.; Dash, P.; Sahoo, H. An Investigation of Heavy Metal Adsorption by Hexa Dentate Ligand Modified Magnetic Nanocomposites. Sep. Sci. Technol. 2018, 53, 863–876. [Google Scholar] [CrossRef]
- Mahmood-Ul-Hassan, M.; Suthar, V.; Ahmad, R.; Yousra, M. Biosorption of Metal Ions on Lignocellulosic Materials: Batch and Continuous-Flow Process Studies. Environ. Monit. Assess. 2018, 190, 287. [Google Scholar] [CrossRef]
- Swarnalatha, K.; Ayoob, S. Adsorption Studies on Coir Pith for Heavy Metal Removal. Int. J. Sustain. Eng. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Todorciuc, T.; Bulgariu, L.; Popa, V. Adsorption of Cu (II) from Aqueous Solution on Wheat Straw Lignin: Equilibrium and Kinetic Studies. Cell Chem. Technol. 2015, 49, 439–447. [Google Scholar]
- Šćiban, M.; Klasnja, M.; Skrbic, B. Modified Softwood Sawdust As Adsorbent of Heavy Metal Ions from Water. J. Hazard. Mater. 2006, 136, 266–271. [Google Scholar] [CrossRef]
- Bernard, E.; Jimoh, A. Adsorption of Pb, Fe, Cu, and Zn from Industrial Electroplating Wastewater by Orange Peel Activated Carbon. Int. J. Eng. Appl. Sci. 2013, 4, 95–103. [Google Scholar]
- Abdallah, B.; Baudu, M.; Derriche, Z.; Basly, J. Aqueous Heavy Metals Removal on Amine-Functionalized Si-MCM-41 and Si-MCM-48. J. Hazard. Mater. 2009, 171, 1001–1008. [Google Scholar]
- Liu, A.M.; Hidajat, K.; Kawi, S.; Zhao, D.Y. A New Class of Hybrid Mesoporous Materials with Functionalized Organic Monolayers for Selective Adsorption of Heavy Metal Ions. Chem. Commun. 2000, 13, 1145–1146. [Google Scholar] [CrossRef]
- Kang, T.; Park, Y.; Yi, J. Highly Selective Adsorption of Pt2+ and Pd2+ Using Thiol Functionalized Mesoporous Silica. Ind. Eng. Chem. Res. 2004, 43, 1478–1484. [Google Scholar] [CrossRef]
- Nooney, R.I.; Kalyanaraman, M.; Kennedy, G.; Maginn, E.J. Heavy Metal Remediation Using Functionalized Mesoporous Silicas with Controlled Macrostructure. Langmuir 2001, 17, 528–533. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Prelle, A.; Sarzanini, C.; Onida, B.; Fiorilli, S.; Garrone, E. Retention of Heavy Metals Ion on SBA 15 Mesoporous Silica Functionalized with Carboxylic Groups. J. Sep. Sci. 2007, 30, 2414–2420. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous Heavy Metals Removal by Adsorption on Amine-Functionalized Mesoporous Silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Xue, X.; Li, F.; Li, G. Hybrid Surfactant-Templated Mesoporous Silica Formed in Ethanol and Its Application for Heavy Metal Removal. J. Hazard. Mater. 2008, 152, 690–698. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Study on the Adsorption of Heavy Metal Ions from Aqueous Solution on Modified SBA-15. Mater. Res. 2013, 16, 745–754. [Google Scholar] [CrossRef][Green Version]
- Da’Na, E.; Sayari, A. Adsorption of Heavy Metals on Amine-Functionalized SBA-15 Prepared by Co-Condensation: Applications to Real Water Samples. Desalination 2012, 285, 62–67. [Google Scholar] [CrossRef]
- Cheraghali, R.; Tavakoli, H.; Sepehrian, H. Preparation, Characterization and Lead Soption Performance of Aliginate SBA 15 Composite as a Novel Adsorbent. Scientia Iranica. F 2013, 20, 1028–1034. [Google Scholar]
- Yang, Y.; Wang, D. The Synthesis of Novel thiol/Amino Bifunctionalized SBA-15 and Application on the Cr (VI) Absorption. IOP Conf. Series: Earth Environ. Sci. 2017, 82, 12074. [Google Scholar] [CrossRef]
- Flodström, K.; Alfredsson, V. Influence of the Block Length of Triblock Copolymers on the Formation of Mesoporous Silica. Microporous Mesoporous Mater. 2003, 59, 167–176. [Google Scholar] [CrossRef]
- Huirache Acuña, R.; Pawelec, B.; Muñoz, E.; Nava, R.; Espino, J.; Fierro, J.L.G. Comparison of the Morphology and HDS Activity of Ternary Co Mo W Catalysts Supported on P Modified SBA 15 and SBA 16 Substrates. Appl. Catal. B Environ. 2009, 92, 168–184. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.L.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications, 2nd ed.; Academic Press: London, UK,, 2013. [Google Scholar]
- Grudzien, R.M.; Grabicka, B.E.; Jaroniec, M. Adsorption and Structural Properties of Channel-Like and Cage-Like Organosilicas. Adsorption 2006, 12, 293–308. [Google Scholar] [CrossRef]
- Boss, C.B.; Fredeen, K.J. Inductively Coupled Plasma Atomic Emission Spectrometry; Methods for geochemical analysis; The Perkin-Elmer Corporation: Norwalk, CT, USA, 1989. [Google Scholar]
- Gallo, J.M.R.; Bisio, C.; Marchese, L.; Pastore, H.O. Surface Acidity of Novel Mesostructurated Silicas with Framework Aluminium Obtained by SBA 16 Related Synthesis. Micropor. Mesopor. Mater. 2008, 111, 632–635. [Google Scholar] [CrossRef]
- Sauer, J.; Marlow, F.; Schuth, F. Simulation of Powder Diffraction Patterns of Modified Ordered Mesoporous Materials. Phys. Chem. Chem. Phys. 2001, 3, 5579–5584. [Google Scholar] [CrossRef]
- Bottazzi, G.S.B.; Nartínez, M.L.; Costa, M.B.G.; Anunziata, O.A.; Beltramone, A.R. Inhibition of the Hydrogenation of Tetralin by Nitrogen and Sulfur Compounds over Ir/SBA 16. Appl. Catal. A: Gen. 2011, 404, 30–38. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Lin, Y.-C.; Cheng, H.-H.; Chen, Y.-C. The Effect and Model of Silica Concentrations on Physical Properties and Particle Sizes of Three-Dimensional SBA-16 Nanoporous Materials. Chem. Phys. Lett. 2003, 382, 496–501. [Google Scholar] [CrossRef]
- Walcarius, A.; Etienne, M.; Bessiere, J. Rate of Acess to the Binding Sites in Organically Modified Silicates. Amorphous Silica Gels Grafted with Amine or Thiol Groups. Chem. Mater. 2002, 14, 2757–2766. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Experimental Confirmation of Different Mechanisms of Evaporation from Ink-Bottle Type Pores: Equilibrium, Pore Blocking, and Cavitation. Langmuir 2002, 18, 9830–9837. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Boutros, M.; Onfroy, T.; Da Costa, P. Mesostructured or Alumina-Mesostructured Silica SBA-16 As Potential Support for NOx Reduction and Ethanol Oxidation. Catal. Lett. 2010, 139, 50–55. [Google Scholar] [CrossRef]
- Stevens, W.J.J.; Lebeau, K.; Mertens, M.; Van Tendeloo, G.; Cool, P.; VanSant, E.F. Investigation of the Morphology of the Mesoporous SBA-16 and SBA-15 Materials. J. Phys. Chem. B 2006, 110, 9183–9187. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, B. HDS of Dibenzothiophene over Polyphosphates Supported on Mesoporous Silica. J. Catal. 2004, 223, 86–97. [Google Scholar] [CrossRef]
- Decottiguies, M.; Phalippou, J.; Zarzycki, J. Synthesis of Glasses by Hot Pressing of Gels. J. Mater. Sci. 1978, 13, 2605–2618. [Google Scholar] [CrossRef]
- White, L.; Tripp, C. Reaction of (3-Aminopropyl) dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef]
- Scott, R.P.W. Silica Gel and Bonded Phases: Their Production, Properties & Use in LC; Wiley Science: New York, NY, USA, 1993. [Google Scholar]
- Chong, A.S.M.; Zhao, X.S. Functionalization of SBA-15 With APTES and Characterization of Functionalized Materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Luan, Z.; Fournier, J.A.; Wooten, J.B.; Miser, D.E. Preparation and Characterization of (3 aminopropyl) triethoxysilane Modified Mesoporous SBA 15 Silica Molecular Sieves. Micropor. Mesopor. Mater. 2005, 83, 150–158. [Google Scholar] [CrossRef]
- Payne, J.C.; Horst, M.A.; Godwin, H.A. Lead Fingers: Pb2+ Binding to Structural Zinc Binding Domians Determined Directly by Monitoring Lead Thiolate Charge Transfer Bands. J. Am. Chem. Soc. 1999, 121, 6850–6855. [Google Scholar] [CrossRef]
- Vögler, A.; Nikol, H. Photochemistry and Photophysics of Coordination Compounds of the Main Group Metals. Pure Appl. Chem. 1992, 64, 1311–1317. [Google Scholar] [CrossRef]
- Mariscal, R.; Soria, J.; Peña, M.A.; Fierro, J.L.G. Structure and Reactivity of Undoped and Sodium Doped PbO/α Al2O3 Catalysts for Oxidative Coupling of Methane. Appl. Catal. A: Gen. 1994, 111, 79–97. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Vass, A.K.; Alisson, J.W.; Powell, C.J.; Rumble, J.R.J. NIST X-Ray Photoelectron Spectroscopy Database 20 Version 3.4; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003. [Google Scholar]
- García, N.; Benito, E.; Guzmán, J.; Tiemblo, P.; Morales, V.; Garcia, R. Functionalization of SBA-15 by an Acid-Catalyzed Approach: A Surface Characterization Study. Microporous Mesoporous Mater. 2007, 106, 129–139. [Google Scholar] [CrossRef]

| Sample | N (mmol/g) | Pb/N (Atomic Ratio) | SBET (m2/g) | Loss of SBET (%) | Vtotal (cm3/g) | dp (nm) |
|---|---|---|---|---|---|---|
| SBA-16 | - | - | 650 | - | 0.64 | 3.4 |
| 2.6N/SBA-16 | 2.6 | 0.07 | 507 | 22.0 | 0.45 | 3.1 |
| 3.8N/SBA-16 | 3.8 | 0.05 | 500 | 23.1 | 0.42 | 3.2 |
| 5.1N/SBA-16 | 5.1 | 0.03 | 494 | 24.0 | 0.43 | 3.1 |
| Sample | aob (nm) | wtc (nm) | wt/dp Ratio |
|---|---|---|---|
| SBA-16 | 14.13 | 8.84 | 2.6 |
| 2.6N/SBA-16 | 13.91 | 8.95 | 2.9 |
| 3.8N/SBA-16 | 13.91 | 8.85 | 2.8 |
| 5.1N/SBA-16 | 14.19 | 9.19 | 3.0 |
| XPS Data | Before Pb2+ Adsorption | After Pb2+ Adsorption a |
|---|---|---|
| Si 2p (eV) | 103.5 | 103.5 |
| O 1s (eV) | 532.7 | 532.7 |
| N 1s (eV) | 399.7 (60) 401.5 (40) | 399.7 (63) 401.5 (37) |
| Pb4f7/2 | - | 138.8 |
| N/Si | 0.070 | 0.071 (0.109) b |
| Pb/N | - | 0.133 (0.139) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palos-Barba, V.; Moreno-Martell, A.; Hernández-Morales, V.; Peza-Ledesma, C.L.; Rivera-Muñoz, E.M.; Nava, R.; Pawelec, B. SBA-16 Cage-Like Porous Material Modified with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution. Materials 2020, 13, 927. https://doi.org/10.3390/ma13040927
Palos-Barba V, Moreno-Martell A, Hernández-Morales V, Peza-Ledesma CL, Rivera-Muñoz EM, Nava R, Pawelec B. SBA-16 Cage-Like Porous Material Modified with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution. Materials. 2020; 13(4):927. https://doi.org/10.3390/ma13040927
Chicago/Turabian StylePalos-Barba, Viviana, Abigail Moreno-Martell, Verónica Hernández-Morales, Carmen L. Peza-Ledesma, Eric M. Rivera-Muñoz, Rufino Nava, and Barbara Pawelec. 2020. "SBA-16 Cage-Like Porous Material Modified with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution" Materials 13, no. 4: 927. https://doi.org/10.3390/ma13040927
APA StylePalos-Barba, V., Moreno-Martell, A., Hernández-Morales, V., Peza-Ledesma, C. L., Rivera-Muñoz, E. M., Nava, R., & Pawelec, B. (2020). SBA-16 Cage-Like Porous Material Modified with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution. Materials, 13(4), 927. https://doi.org/10.3390/ma13040927






