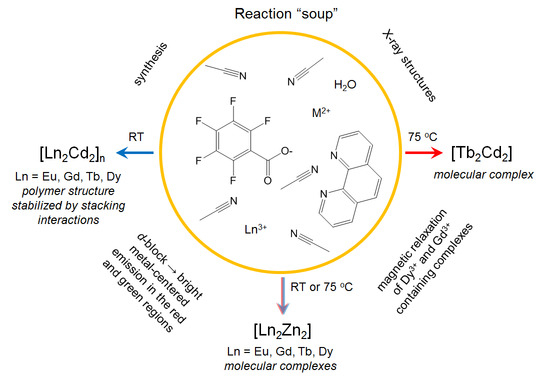
Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties
Abstract
1. Introduction
2. Results
2.1. Synthesis of Complexes
2.2. Photoluminescence
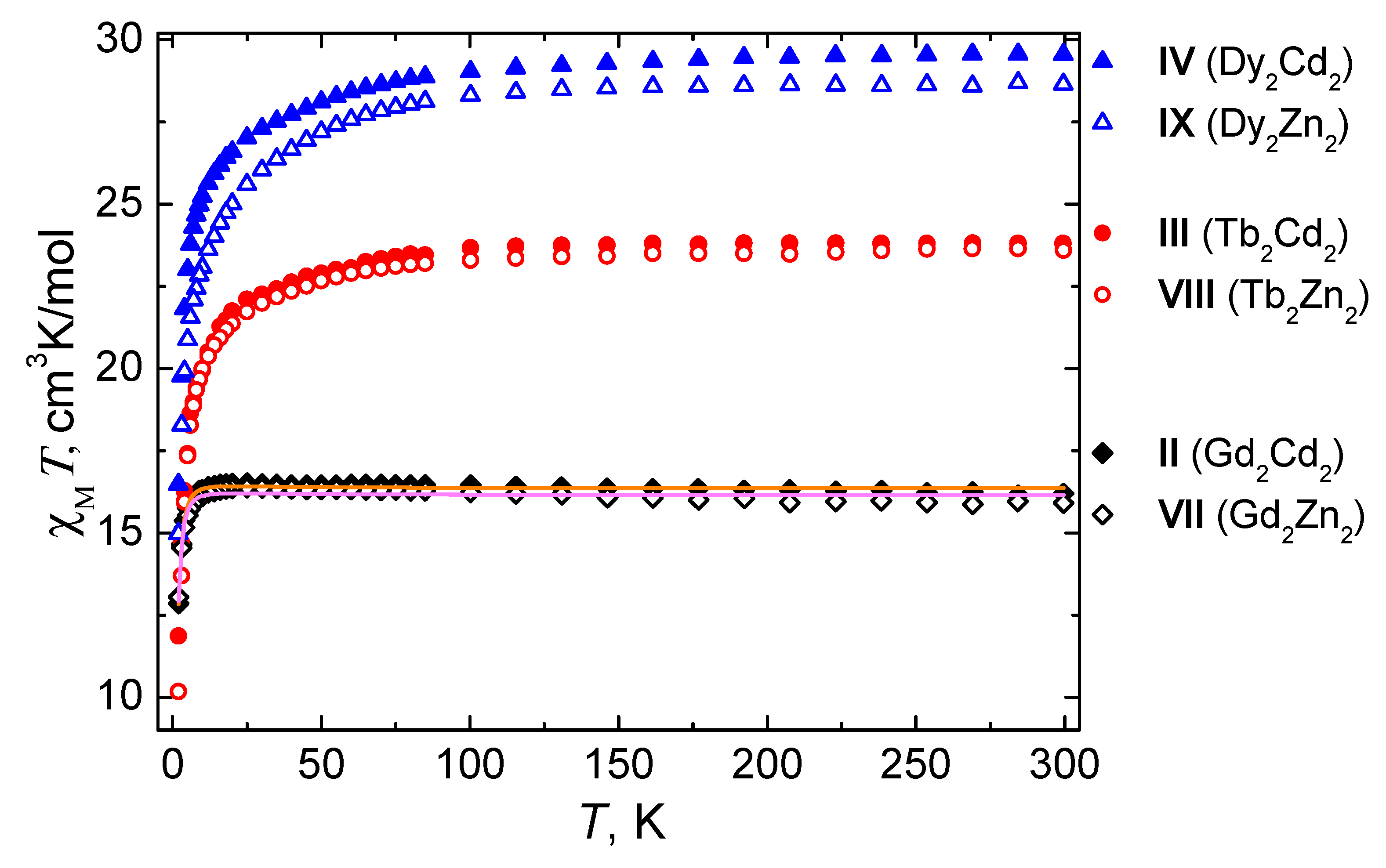
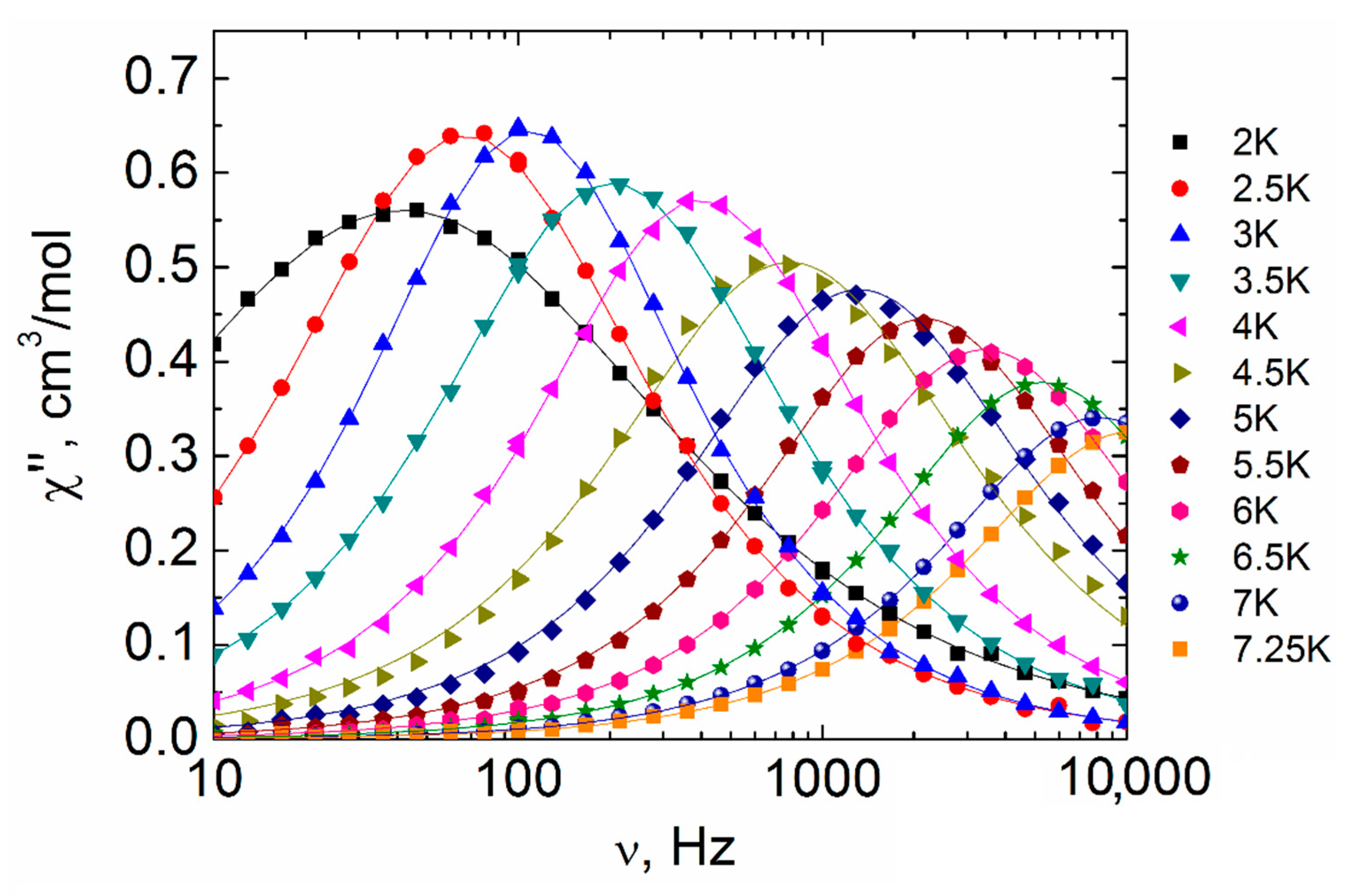
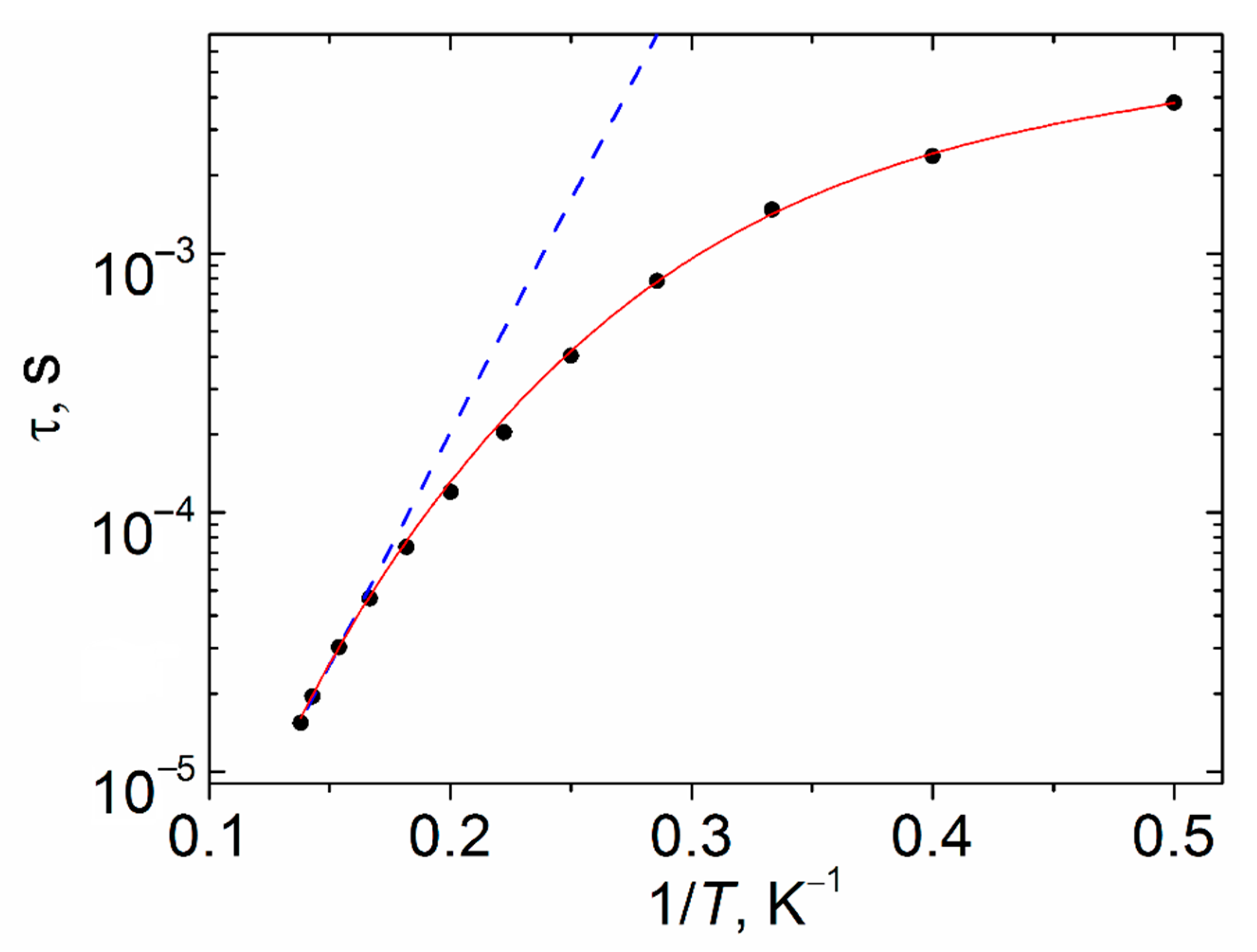
2.3. Magnetic Properties
3. Discussion
4. Experimental
4.1. Materials and Methods
4.2. Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, J.; Wang, L.; Mao, D.; Wang, W.; Zhang, L.; Wu, S.; Xie, Y. Ytterbium pentafluorobenzoate as a novel fluorous Lewis acid catalyst in the synthesis of 2,4-disubstituted quinolines. Tetrahedron 2011, 67, 8465–8469. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Kondinski, A. Exploring High-Symmetry Lanthanide-Functionalized Polyoxopalladates as Building Blocks for Quantum Computing. Inorganics 2018, 6, 101. [Google Scholar] [CrossRef]
- Efimov, N.N.; Koroteev, P.S.; Gavrikov, A.; Ilyukhin, A.B.; Dobrokhotova, Z.V.; Novotortsev, V.M. Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand. Magnetochemistry 2016, 2, 38. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Fedin, V.P. Main Approaches to the Synthesis of Heterometallic Metal-Organic Frameworks. Russ. J. Coord. Chem. 2020, 46, 443–457. [Google Scholar] [CrossRef]
- Han, L.-J.; Kong, Y.-J.; Sheng, N.; Jiang, X.-L. A new europium fluorous metal–organic framework with pentafluorobenzoate and 1,10-phenanthroline ligands: Synthesis, structure and luminescent properties. J. Fluor. Chem. 2014, 166, 122–126. [Google Scholar] [CrossRef]
- Utochnikova, V.; Kuzmina, N.P. Photoluminescence of lanthanide aromatic carboxylates. Russ. J. Coord. Chem. 2016, 42, 679–694. [Google Scholar] [CrossRef]
- Feng, X.; Guo, N.; Li, R.; Chen, H.; Ma, L.; Li, Z.; Wang, L. A facile route for tuning emission and magnetic properties by controlling lanthanide ions in coordination polymers incorporating mixed aromatic carboxylate ligands. J. Solid State Chem. 2018, 268, 22–29. [Google Scholar] [CrossRef]
- Raj, D.B.A.; Biju, S.; Reddy, M.L.P. One-, Two-, and Three-Dimensional Arrays of Eu3+-4,4,5,5,5-pentafluoro-1-(naphthalen-2-yl)pentane-1,3-dione complexes: Synthesis, Crystal Structure and Photophysical Properties. Inorg. Chem. 2008, 47, 8091–8100. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bìnzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of EuIII-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 11095–11107. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, P.; Sun, J.; An, G.; Yao, X.; Li, Y.-X.; Li, G. Luminescence and white-light emitting luminescent sensor of tetrafluoroterephthalate-lanthanide metal–organic frameworks. Dalton Trans. 2017, 46, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Kalyakina, A.S.; Utochnikova, V.; Bushmarinov, I.S.; Ananyev, I.V.; Eremenko, I.L.; Volz, D.; Rönicke, F.; Schepers, U.; Van Deun, R.; Trigub, A.L.; et al. Highly Luminescent, Water-Soluble Lanthanide Fluorobenzoates: Syntheses, Structures and Photophysics, Part I: Lanthanide Pentafluorobenzoates. Chem. Eur. J. 2015, 21, 17921–17932. [Google Scholar] [CrossRef] [PubMed]
- Kalyakina, A.S.; Utochnikova, V.; Bushmarinov, I.S.; Le-Deygen, I.M.; Volz, D.; Weis, P.; Schepers, U.; Kuzmina, N.P.; Bräse, S. Lanthanide Fluorobenzoates as Bio-Probes: A Quest for the Optimal Ligand Fluorination Degree. Chem. Eur. J. 2017, 23, 14944–14953. [Google Scholar] [CrossRef]
- Utochnikova, V.; Solodukhin, N.N.; Aslandukov, A.N.; Marciniak, L.; Bushmarinov, I.S.; Vashchenko, A.A.; Kuzmina, N.P. Lanthanide tetrafluorobenzoates as emitters for OLEDs: New approach for host selection. Org. Electron. 2017, 44, 85–93. [Google Scholar] [CrossRef]
- Kalyakina, A.S.; Utochnikova, V.; Zimmer, M.; Dietrich, F.; Van Deun, R.; Nieger, M.; Gerhards, M.; Schepers, U.; Bräse, S.; Kaczmarek, A.M.; et al. Remarkable high efficiency of red emitters using Eu(iii) ternary complexes. Chem. Commun. 2018, 54, 5221–5224. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, H.; Li, R.; Wang, W.; Zhang, D.; Wang, L.; Li, Z. Enhanced luminescence and tunable magnetic properties of lanthanide coordination polymers based on fluorine substitution and phenanthroline ligand. RSC Adv. 2018, 9, 16328–16338. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Han, Y.; Yan, P.; Li, Y.-X.; Li, G. Structure, color-tunable luminescence, and UV-vis/NIR benzaldehyde detection of lanthanide coordination polymers based on two fluorinated ligands. CrystEngComm 2018, 20, 3335–3343. [Google Scholar] [CrossRef]
- Casanovas, B.; Font-Bardía, M.; Speed, S.; El Fallah, M.S.; Vicente, R. Field-Induced SMM and Visible/NIR-Luminescence Behaviour of Dinuclear LnIII Complexes with 2-Fluorobenzoate. Eur. J. Inorg. Chem. 2018, 2018, 1928–1937. [Google Scholar] [CrossRef]
- Casanovas, B.; Speed, S.; Vicente, R.; Font-Bardía, M. Sensitization of visible and NIR emitting lanthanide(III) ions in a series of dinuclear complexes of formula [Ln2(μ-2-FBz)2(2-FBz)4(terpy)2]·2(2-HFBz)·2(H2O). Polyhedron 2019, 173, 114113. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Dolgushin, F.M.; Lyssenko, K.A.; Kiskin, M.A.; Varaksina, E.A.; Taidakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Influence of Substituents in the Aromatic Fragment of the Benzoate Anion on the Structures and Compositions of the Formed {Cd–Ln} Complexes. Russ. J. Coord. Chem. 2020, 46, 493–504. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.; Sidorov, A.A.; Kiskin, M.A.; Voronina, J.K.; Nelyubina, Y.V.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Eremenko, I.L. Coordination polymers based on 3,5-di-tert-butylbenzoate {Cd2Eu} moieties. Inorg. Chim. Acta 2020, 515, 120050. [Google Scholar] [CrossRef]
- Li, S.-S.; Ye, Z.-N.; Xu, S.-S.; Zhang, Y.-J.; Tao, A.-R.; Liu, M.; Zeng, C.-H.; Zhong, S. Highly luminescent lanthanide CPs based on dinuclear cluster: Crystal structure and sensitive Trp sensor. RSC Adv. 2015, 5, 71961–71967. [Google Scholar] [CrossRef]
- Yu, H.-H.; Chi, J.-Q.; Su, Z.-M.; Li, X.; Sun, J.; Zhou, C.; Hu, X.-L.; Liu, Q. A water-stable terbium metal–organic framework with functionalized ligands for the detection of Fe3+ and Cr2O72− ions in water and picric acid in seawater. CrystEngComm 2020, 22, 3638–3643. [Google Scholar] [CrossRef]
- Sobieray, M.; Gode, J.; Seidel, C.; Poß, M.; Feldmann, C.; Ruschewitz, U. Bright luminescence in lanthanide coordination polymers with tetrafluoroterephthalate as a bridging ligand. Dalton Trans. 2015, 44, 6249–6259. [Google Scholar] [CrossRef]
- Feng, X.; Sun, Y.-L.; Li, R.-F.; Zhang, T.; Guo, N.; Wang, L.-Y. Two novel europium coordination polymers based on fluorine substituted and similar carboxylate ligands: Syntheses, structures and luminescence. Inorg. Chem. Commun. 2016, 73, 190–195. [Google Scholar] [CrossRef]
- Li, J.-J.; Fan, T.-T.; Qu, X.-L.; Han, H.-L.; Li, X. Temperature-induced 1D lanthanide polymeric frameworks based on Lnn (n = 2, 2, 4, 6) cores: Synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 2924–2935. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Kuznetsova, G.N.; Kiskin, M.A.; Voronina, Y.K.; Yakushev, I.A.; Ivanova, T.M.; Nelyubina, Y.V.; Sidorov, A.A.; Eremenko, I.L. Cd(II) and Cd(II)–Eu(III) Complexes with Pentafluorobenzoic Acid Anions and N-Donor Ligands: Synthesis and Structures. Russ. J. Coord. Chem. 2020, 46, 557–572. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Makarov, D.A.; Kiskin, M.A.; Yakushev, I.A.; Dolgushin, F.M.; Aleksandrov, G.G.; Varaksina, E.A.; Taidakov, I.V.; Aleksandrov, E.V.; et al. Synthesis of Coordination Polymers from the Heterometallic Carboxylate Complexes with Chelating N-Donor Ligands. Russ. J. Coord. Chem. 2020, 46, 1–14. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Sharma, R.P.; Aree, T.; Venugopalan, P. Second sphere coordination in fluoroanion binding: Synthesis, spectroscopic and X-ray structural study of [Co(phen)2CO3](Pfbz)·6H2O. J. Fluor. Chem. 2009, 130, 650–655. [Google Scholar] [CrossRef]
- Sharma, R.P.; Saini, A.; Singh, S.; Venugopalan, P.; Harrison, W.T.A. Segregated aromatic π–π stacking interactions involving fluorinated and non-fluorinated benzene rings: Cu(py)2(pfb)2 and Cu(py)2(pfb)2(H2O) (py=pyridine and pfb=pentafluorobenzoate). J. Fluor. Chem. 2010, 131, 456–460. [Google Scholar] [CrossRef]
- Kong, Y.-J.; Li, P.; Han, L.-J.; Fan, L.-T.; Yin, S. Two cadmium(II) fluorous coordination compounds tuned by different bipyridines. Acta Crystallogr. Sect. C 2017, 73, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.-T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.-T.; Koshevoy, I.O. Halogen Bonding to Amplify Luminescence: A Case Study Using a Platinum Cyclometalated Complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Wang, W.; Jin, W.J. Interactions between haloperfluorobenzenes and fluoranthene in luminescent cocrystals from π-hole⋯π to σ-hole⋯π bonds. CrystEngComm 2017, 19, 5058–5067. [Google Scholar] [CrossRef]
- Mikhalyova, E.A.; Yakovenko, A.V.; Zeller, M.; Kiskin, M.A.; Kolomzarov, Y.V.; Eremenko, I.L.; Addison, A.W.; Pavlishchuk, V.V. Manifestation of π–π Stacking Interactions in Luminescence Properties and Energy Transfer in Aromatically-Derived Tb, Eu and Gd Tris(pyrazolyl)borate Complexes. Inorg. Chem. 2015, 54, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Larionov, S.V.; Glinskaya, L.A.; Leonova, T.G.; Klevtsova, R.F.; Uskov, E.M.; Platonov, V.E.; Karpov, V.M.; Fadeeva, V.P. Luminescence properties of complexes Ln(Phen)(C6F5COO)3 (Ln = Tb, Eu) and Ln(C6F5COO)3 · nH2O (Ln = Tb, n = 2; Ln = Eu, n = 1). Structures of the [Tb2(H2O)8(C6F5COO)6] complex and its isomer in the supramolecular compound [Tb2(H2O)8(C6F5COO)6] · 2C6F5COOH. Russ. J. Coord. Chem. 2009, 35, 798–806. [Google Scholar] [CrossRef]
- Crosby, G.; Highland, R.; Truesdell, K. Spectroscopic properties of (nd)10 transition metal complexes. Coord. Chem. Rev. 1985, 64, 41–52. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Sidorov, A.A.; Nelyubina, Y.A.; Dolgushin, F.M.; Voronina, Y.K.; Kiskin, M.A.; Aleksandrov, G.G.; Varaksina, E.A.; Taydakov, I.V.; et al. Chemical assembling of heterometallic {Cd–M} (M=Li, Mg, Eu, Tb) molecules with 3,5-Di-tert-butylbenzoate bridges and N-donor ligands. ChemistrySelect 2020, 5, 8475–8482. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Voronina, Y.K.; Gogoleva, N.V.; Sidorov, A.A.; Kiskin, M.A.; Dolgushin, F.M.; Nelyubina, Y.V.; Aleksandrov, G.G.; Varaksina, E.A.; Taydakov, I.V.; et al. Influence of the steric properties of pyridine ligands on the structure of complexes containing the {LnCd2(bzo)7} fragment. Russ. Chem. Bull. 2020, 69, 1544–1560. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; Van Der Tol, E.B.; Verhoeven, J.W. New Sensitizer-Modified Calix [4] arenes Enabling Near-UV Excitation of Complexed Luminescent Lanthanide Ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Solodukhin, N.N.; Aslandukov, A.A.; Zaitsev, K.V.; Kalyakina, A.S.; Averin, A.A.; Ananyev, I.A.; Churakov, A.V.; Kuzmina, N.P. Luminescence enhancement by p-substituent variation. Eur. J. Inorg. Chem. 2017, 1, 107–114. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers Inc.: New York, NY, USA, 1993; p. 380. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- John, D.; Urland, W. Crystal structure and magnetic behaviour of the new gadolinium carboxylates Gd2(ClF2CCOO)6(Hypy)2, Gd2(F3CCOO)6(Hypy)2, Gd2(F2HCCOO)6(Hypy)2 and Gd2(Cl2HCCOO)6(H2O)2(Hypy)2. Eur. J. Inorg. Chem. 2006, 2006, 3503–3509. [Google Scholar] [CrossRef]
- John, D.; Urland, W. Crystal Structure and Magnetic Behaviour of the New Gadolinium Complex Compound Gd2(ClH2CCOO)6(bipy)2. Eur. J. Inorg. Chem. 2005, 2005, 4486–4489. [Google Scholar] [CrossRef]
- Gavrikov, A.; Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Kostopoulos, A.; Ariciu, A.-M.; Novotortsev, V.M. Novel mononuclear and 1D-polymeric derivatives of lanthanides and (η6-benzoic acid)tricarbonylchromium: Synthesis, structure and magnetism. Dalton Trans. 2017, 46, 3369–3380. [Google Scholar] [CrossRef]
- Mamontova, E.; Long, J.; Ferreira, R.A.S.; Botas, A.M.P.; Luneau, D.; Guari, Y.; Carlos, L.A.D.; Larionova, J. Magneto-Luminescence Correlation in the Textbook Dysprosium(III) Nitrate Single-Ion Magnet. Magnetochemistry 2016, 2, 41. [Google Scholar] [CrossRef]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution to Elucidate the Slow Magnetic Relaxation Effects of a Dy2Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.; Novotortsev, V.M. Self-assembly and SMM properties of lanthanide cyanocobaltate chain complexes with terpyridine as blocking ligand. Inorg. Chim. Acta 2018, 482, 813–820. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Zubavichus, Y.V.; Trigub, A.L.; Shubina, E.S.; Eremenko, I.L.; et al. Heterometallic Na6 Co3 Phenylsilsesquioxane Exhibiting Slow Dynamic Behavior in its Magnetization. Chem. Eur. J. 2015, 21, 18563–18565. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Babeshkin, K.A.; Gavrikov, A.; Ilyukhin, A.B.; Belova, E.V.; Efimov, N.N. Towards comparative investigation of Er- and Yb-based SMMs: The effect of the coordination environment configuration on the magnetic relaxation in the series of heteroleptic thiocyanate complexes. Dalton Trans. 2019, 48, 12644–12655. [Google Scholar] [CrossRef] [PubMed]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clérac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(iii)–dysprosium(iii) 2-pyridyloximate complexes. Dalton Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Shmelev, M.A.; Kuznetsova, G.N.; Dolgushin, F.M.; Voronina, J.K.; Gogoleva, N.V.; Kiskin, M.A.; Ivanov, V.K.; Sidorov, A.A.; Eremenko, I.L. Influence of fluorinated aromatic fragments on the structure of cadmium and zinc carboxylate complexes by the example of pentafluorobenzoates and 2,3,4,5-tetrafluorobenzoates. Russ. J. Coord. Chem. 2021, 47. in press. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Ariño, A.; Sesé, J.; et al. Gd-Based Single-Ion Magnets with Tunable Magnetic Anisotropy: Molecular Design of Spin Qubits. Phys. Rev. Lett. 2012, 108, 247213. [Google Scholar] [CrossRef]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolomé, E.; Cases, R.; Luzón, J.; Turta, C. Structural and magnetic properties of some lanthanide (Ln = Eu(iii), Gd(iii) and Nd(iii)) cyanoacetate polymers: Field-induced slow magnetic relaxation in the Gd and Nd substitutions. Dalton Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Ho, L.T.A.; Ungur, L.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Observation of unusual slow-relaxation of the magnetisation in a Gd-EDTA chelate. Dalton Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef]
- Yoshida, T.; Cosquer, G.; Izuogu, D.C.; Ohtsu, H.; Kawano, M.; Lan, Y.; Wernsdorfer, W.; Nojiri, H.; Breedlove, B.K.; Yamashita, M. Field-Induced Slow Magnetic Relaxation of GdIII Complex with a Pt−Gd Heterometallic Bond. Chem. Eur. J. 2017, 23, 4551–4556. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Arczyński, M.; Sheveleva, A.M.; Tuna, F.; Sarewicz, M.; Osyczka, A.; Rams, M.; Vieru, V.; Chibotaru, L.F.; et al. Magnetization Dynamics and Coherent Spin Manipulation of a Propeller Gd(III) Complex with the Smallest Helicene Ligand. J. Phys. Chem. Lett. 2020, 11, 1508–1515. [Google Scholar] [CrossRef]
- Khalfaoui, O.; Beghidja, A.; Long, J.; Beghidja, C.; Guari, Y.; Larionova, J. Field-Induced Slow Relaxation in a Dinuclear Dysprosium(III) Complex Based on 3-Methoxycinnamic Acid. Inorganics 2018, 6, 35. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, A.S.-Y.; Kaizu, Y. Mononuclear Lanthanide Complexes with a Long Magnetization Relaxation Time at High Temperatures: A New Category of Magnets at the Single-Molecular Level. J. Phys. Chem. B 2004, 108, 11265–11271. [Google Scholar] [CrossRef]
- Mandal, L.; Biswas, S.; Yamashita, M. Magnetic Behavior of Luminescent Dinuclear Dysprosium and Terbium Complexes Derived from Phenoxyacetic Acid and 2,2′-Bipyridine. Magnetochemistry 2019, 5, 56. [Google Scholar] [CrossRef]
- SMART (control) and SAINT (integration) Software; Version 5.0; Bruker AXS Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. SADABS-2004/1, Program for Scaling and Correction of Area Detector Data; Göttingen University: Göttinngen, Germany, 1997. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.A. Short history of ShelX. Acta Cryst. Sect. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Program. for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools, version 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
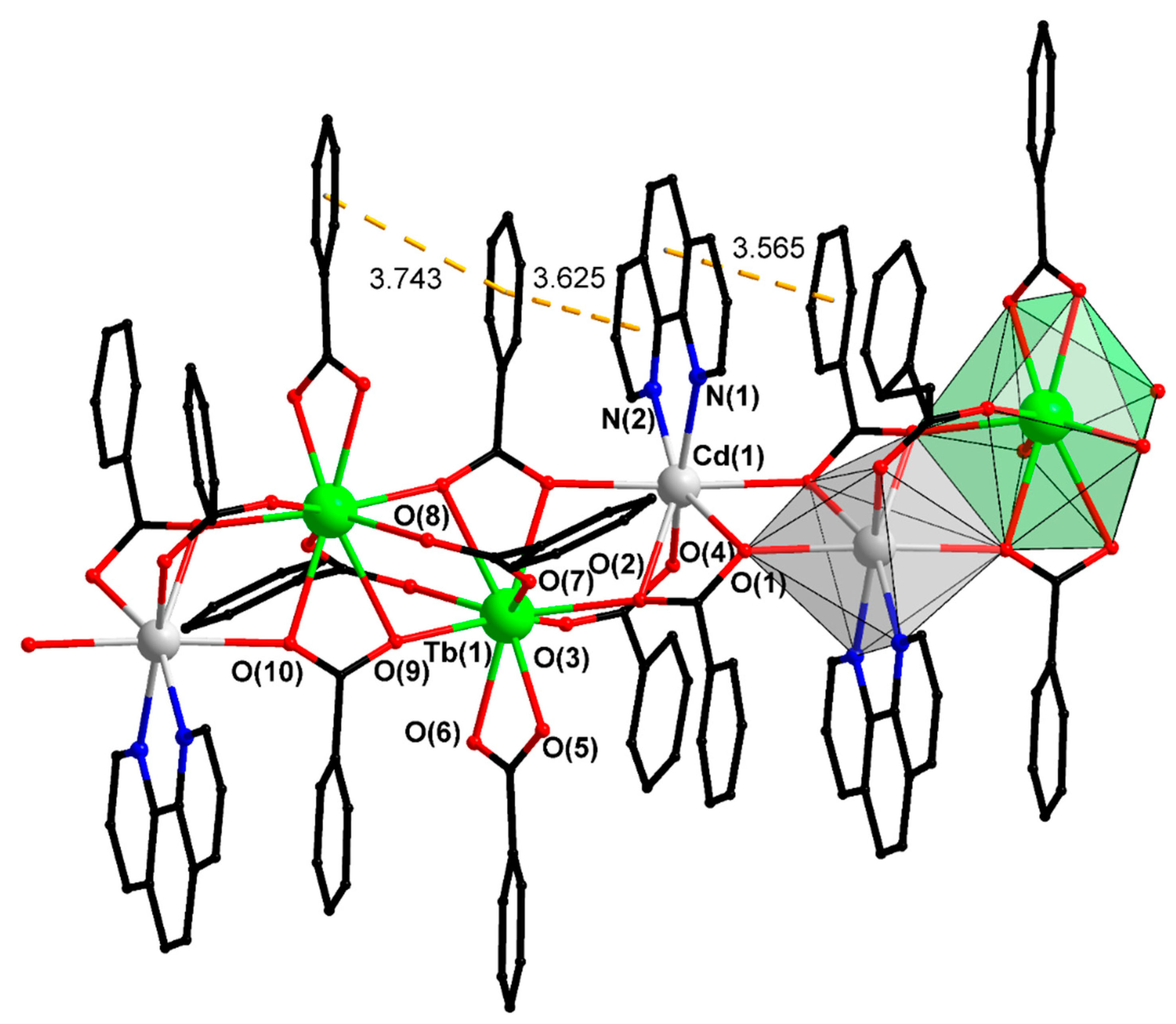
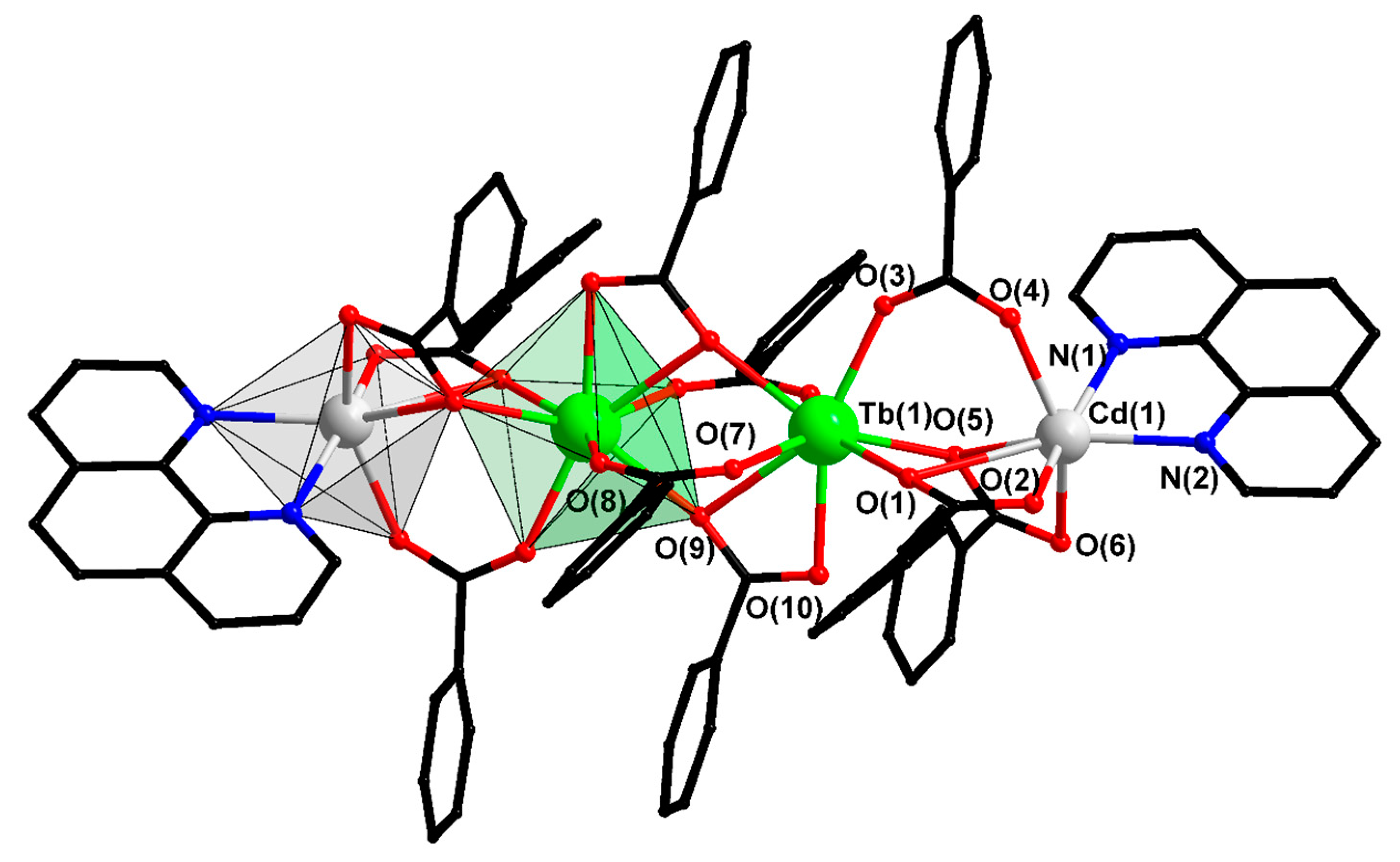

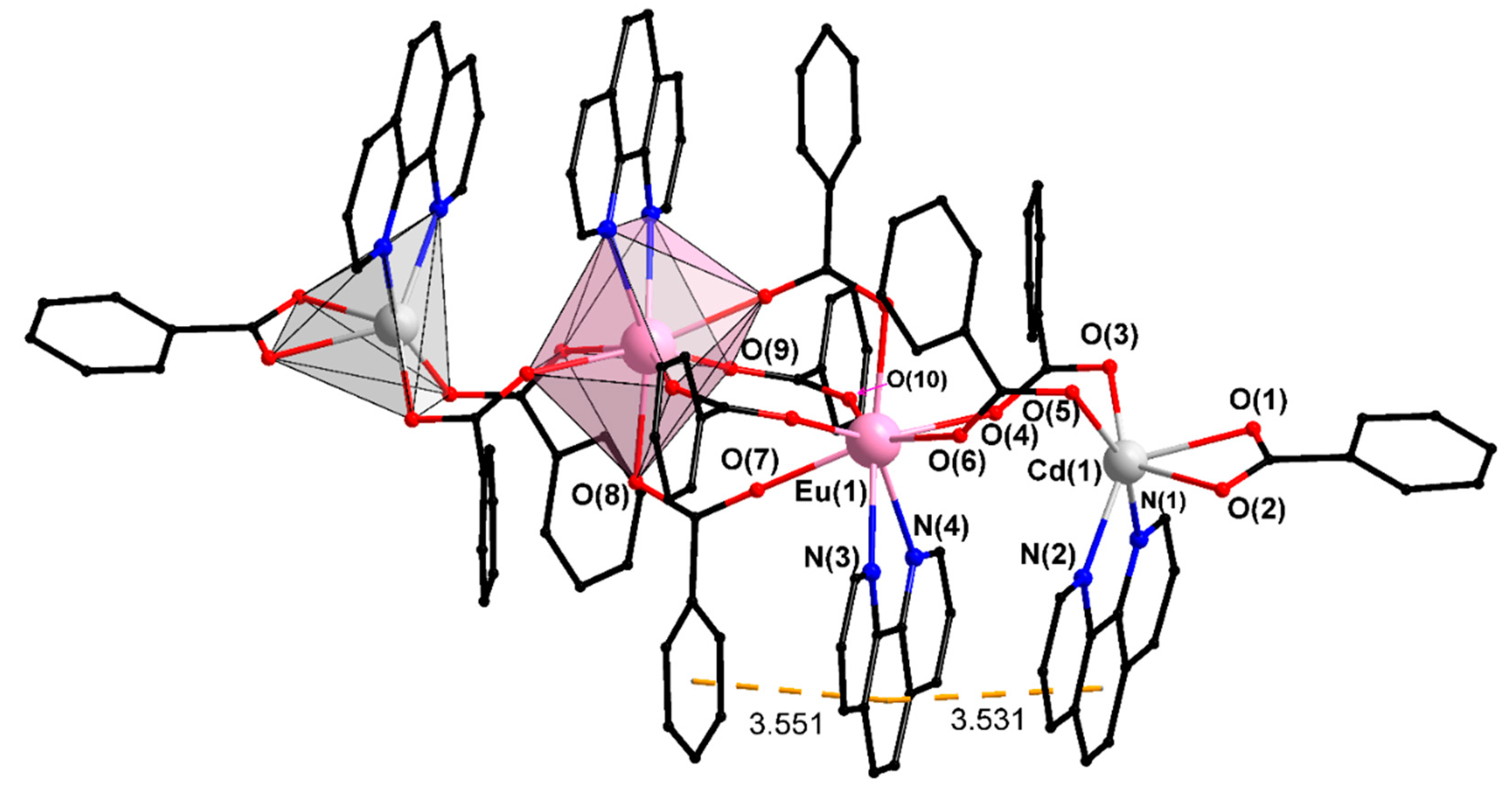
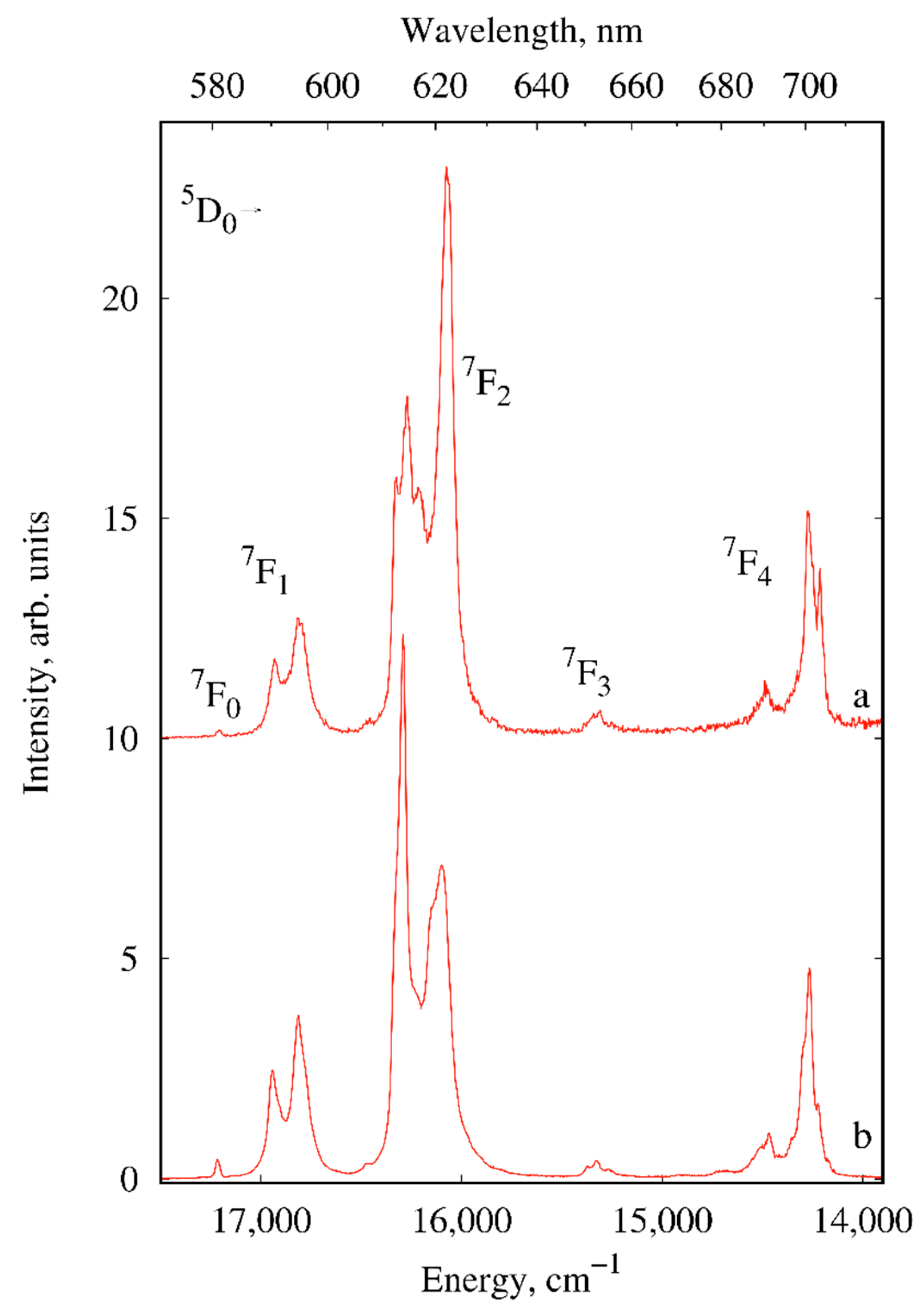
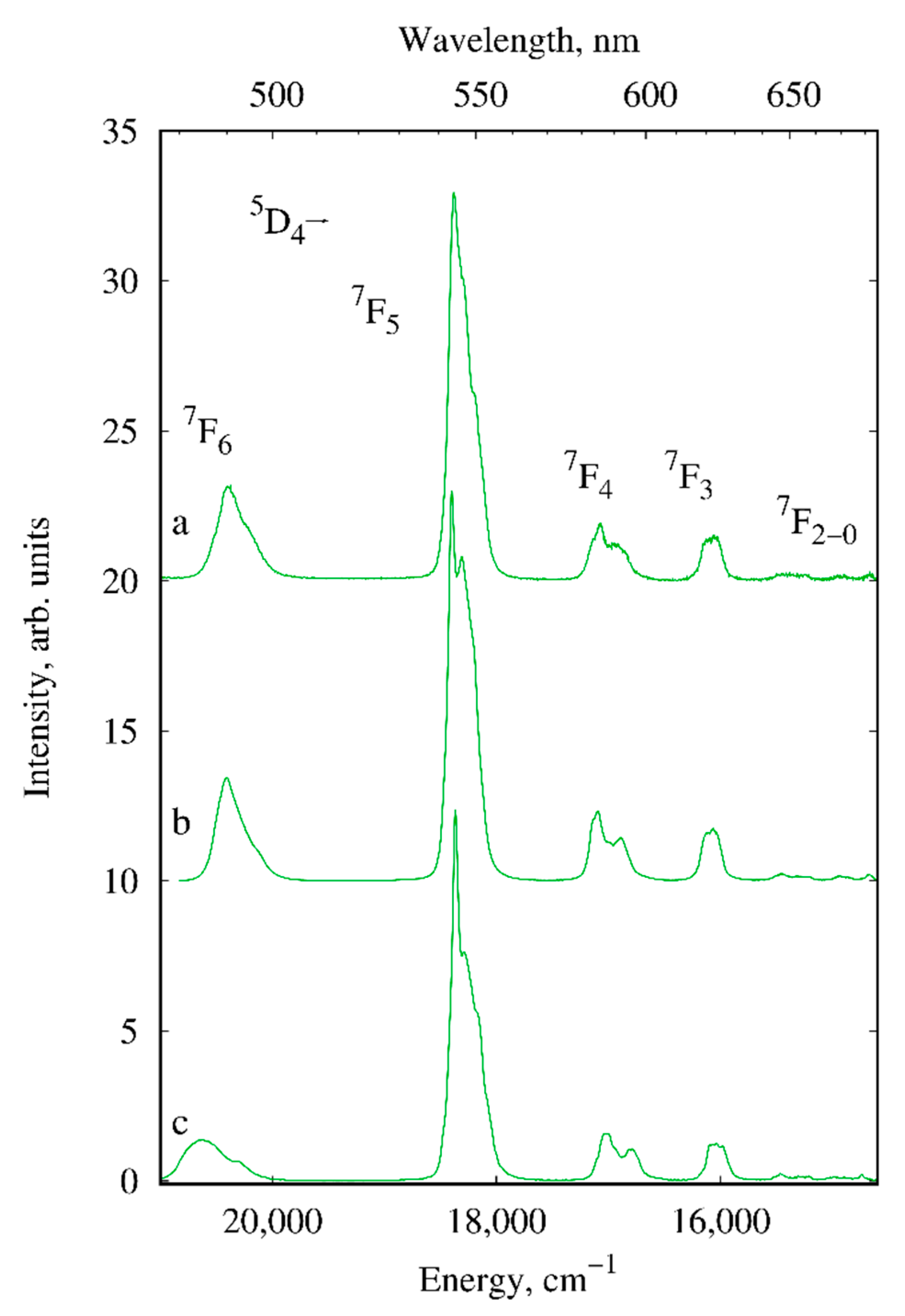
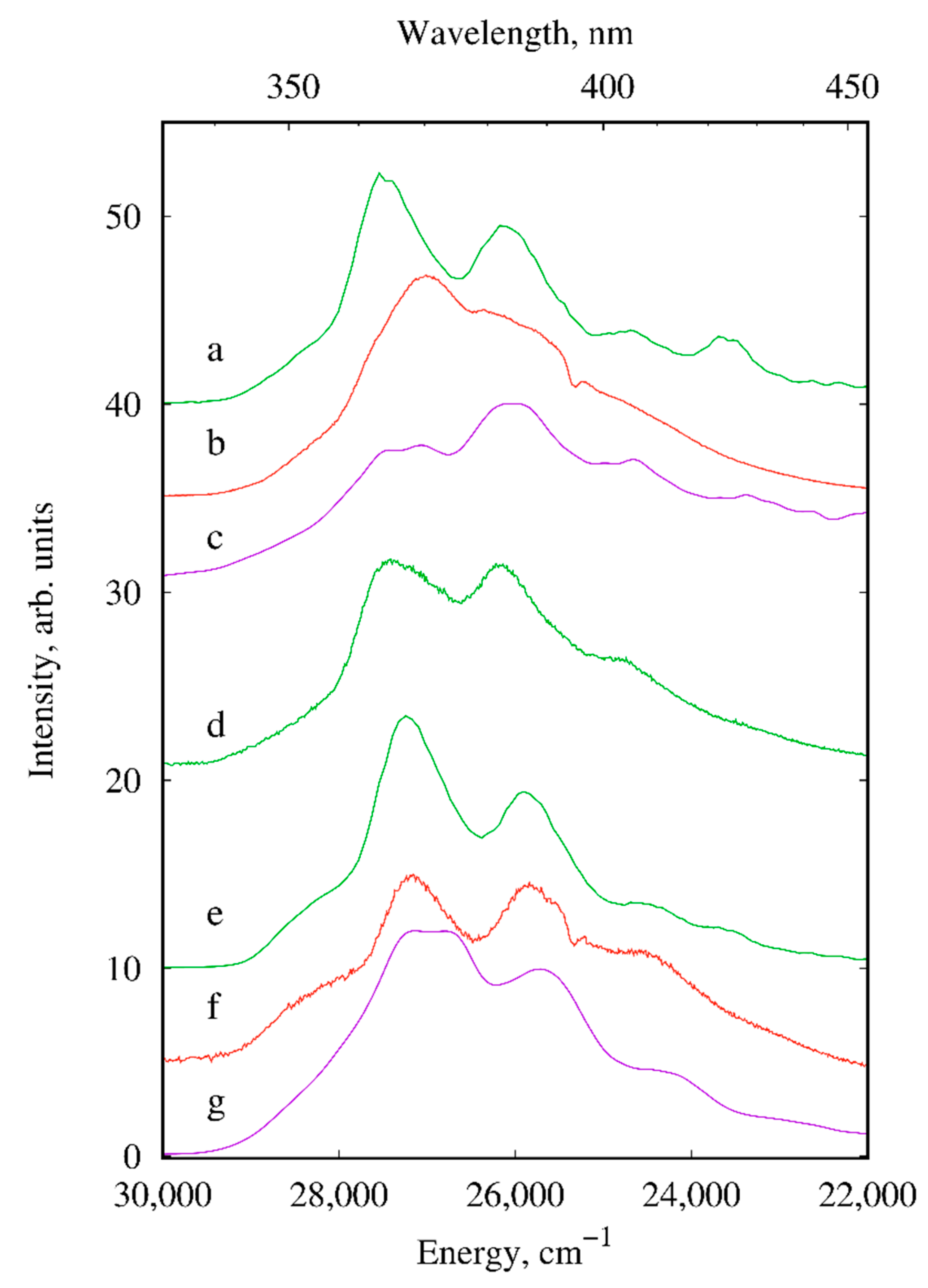
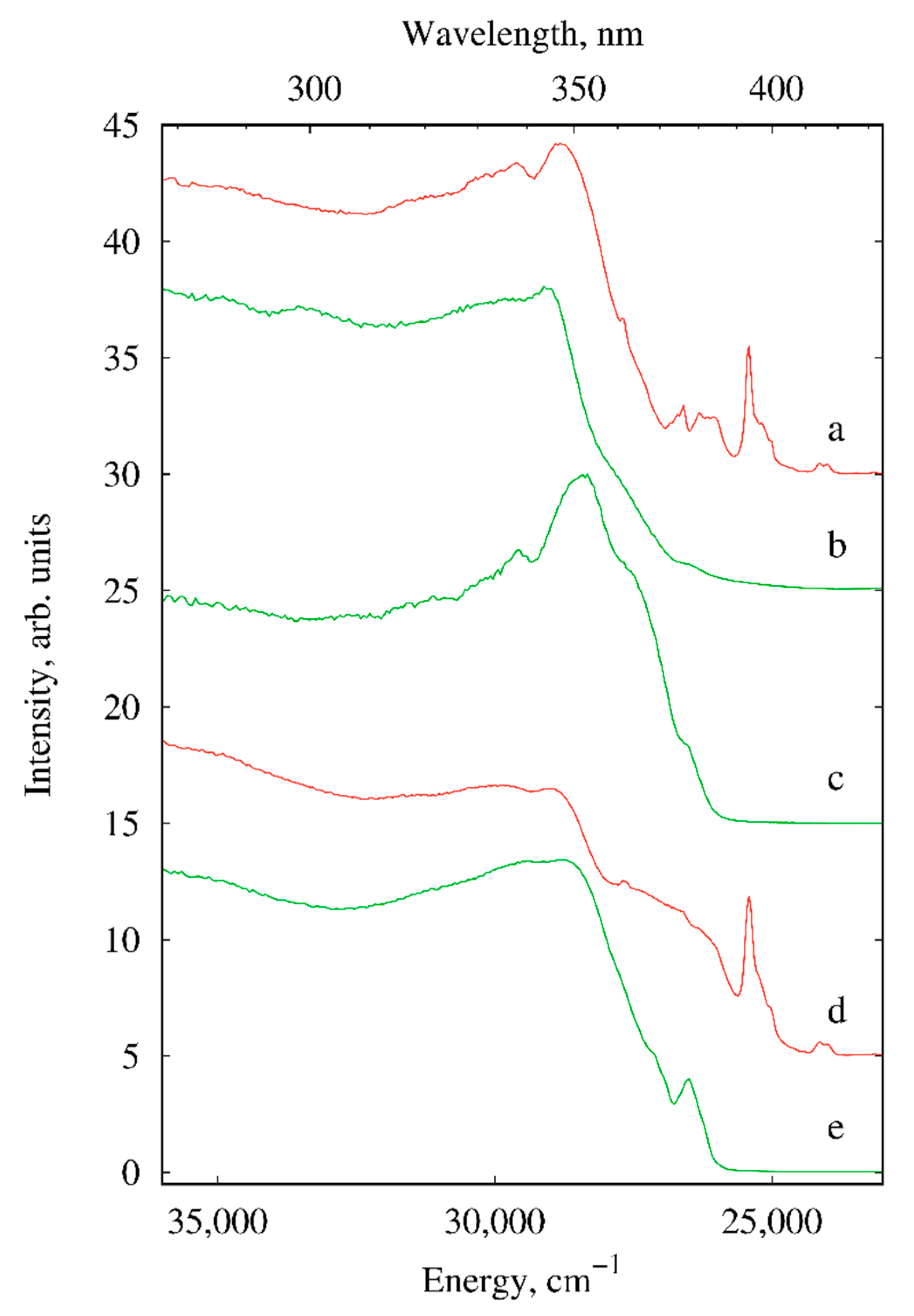
| Compound/Parameter | I | II | III | IV | V | X |
|---|---|---|---|---|---|---|
| Bond Lengths | d (Å) | |||||
| Cd–O (pfb) | 2.207(4)–2.468(5) | 2.206(5)–2.480(5) | 2.215(4)–2.469(4) | 2.212(5)–2.485(6) | 2.252(3)–2.405(3) | 2.210(8)–2.457(10) |
| Cd–N (phen) | 2.314(5), 2.320(5) | 2.304(5), 2.317(5) | 2.319(5), 2.324(5) | 2.262(15)–2.44(2) | 2.290(3), 2.345(3) | 2.264(10), 2.320(11) |
| Ln–O (pfb) | 2.370(3)–2.728(4) | 2.364(4)–2.719(4) | 2.350(4)–2.729(4) | 2.329(4)–2.734(4) | 2.314(3)–2.655(3) | 2.325(7)–2.442(7) |
| Ln–N (phen) | - | - | - | - | - | 2.602(9), 2.636(9) |
| Interatomic distances | d (Å) | |||||
| Cd…Ln | 3.987(1) | 3.971(2) | 3.974(1) | 3.964(1) | 3.917(1) | 4.453(1) |
| Ln…Ln | 4.178(1) | 4.173(2) | 4.164(1) | 4.153(1) | 4.015(1) | 4.408(1) |
| Cd…Cd | 3.923(1) | 3.948(2) | 3.926(4) | 3.940(1) | 7.793(1) | 6.782(19) |
| Bond angles | ω (°) | |||||
| Cd–Ln–Ln | 115.25(3) | 115.57(5) | 115.23(3) | 115.39(2) | 171.62(1) | 162.57(3) |
| Compound/Parameter | VI | VII | VIII | IX |
|---|---|---|---|---|
| Bond Lengths | d (Å) | |||
| Zn–O (pfb) | 2.037(4)–2.454(5) | 2.037(2)–2.357(2) | 2.037(3)–2.436(3) | 2.028(3)–2.452(3) |
| Zn–N (phen) | 2.088(5), 2.137(5) | 2.108(3), 2.135(2) | 2.091(3), 2.137(4) | 2.088(3), 2.135(3) |
| Ln–O (pfb) | 2.340(4)–2.608(4) | 2.357(2)–2.572(2) | 2.317(3)–2.607(3) | 2.291(2)–2.576(2) |
| Ln–O (H2O) | - | 2.426(2) | - | - |
| Interatomic distances | d (Å) | |||
| Zn…Ln | 3.857(1) | 3.392(1) | 3.864(1) | 3.819(1) |
| Ln…Ln | 3.986(1) | 3.991(1) | 3.965(1) | 3.917(1) |
| Zn…Zn | 7.783(1) | 7.936(1) | 7.828(1) | 7.946(1) |
| Bond angles | ω (°) | |||
| Zn–Ln–Ln | 161.77 | 160.30 | 162.12 | 162.17 |
| Compound | Arad, s−1a | Anrad, s−1 | τ, ms | 𝜂sens, % | ||
|---|---|---|---|---|---|---|
| I (EuCd) | 325 | 195 | 1.92 ± 0.05 | 62 | 36 | 58 |
| VI (Eu2Zn2) | 425 | 100 | 1.90 ± 0.05 | 81 | 41 | 51 |
| [Eu(pfb)3(H2O)n] [13] | - | - | 0.65 | 65 | 15 | 23 |
| III (TbCd) | - | - | 2.09 ± 0.06 | - | 63 | - |
| V (Tb2Cd2) | - | - | 1.95 ± 0.06 | - | 33 | - |
| VIII (Tb2Zn2) | - | - | 1.83 ± 0.05 | - | 45 | - |
| [Tb(pfb)3(H2O)n] [13] | - | - | 1.36 | - | 38 | - |
| Complex | χMT, cm3/mol K | ||
|---|---|---|---|
| Experimental (300 K) | Theor. (2Ln) [43] | Experimental (2 K) | |
| II | 16.19 | 15.76 | 12.86 |
| III | 23.80 | 23.64 | 11.87 |
| IV | 29.55 | 28.34 | 16.47 |
| VII | 15.91 | 15.76 | 13.04 |
| VIII | 23.59 | 23.64 | 10.17 |
| IX | 28.64 | 28.34 | 14.97 |
| Parameter | II | VII |
|---|---|---|
| Value | ||
| g | 2.0375 ± 0.0008 | 2.024 ± 0.001 |
| J, cm−1 (intramolecular interactions) | −0.074 ± 0.001 | −0.070 ± 0.003 |
| zJ, cm−1 (intermolecular interactions) | 0.0204 ± 0.0006 | 0.020 ± 0.001 |
| R2 | 0.9968 | 0.9870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmelev, M.A.; Kiskin, M.A.; Voronina, J.K.; Babeshkin, K.A.; Efimov, N.N.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Gogoleva, N.V.; Sidorov, A.A.; et al. Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties. Materials 2020, 13, 5689. https://doi.org/10.3390/ma13245689
Shmelev MA, Kiskin MA, Voronina JK, Babeshkin KA, Efimov NN, Varaksina EA, Korshunov VM, Taydakov IV, Gogoleva NV, Sidorov AA, et al. Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties. Materials. 2020; 13(24):5689. https://doi.org/10.3390/ma13245689
Chicago/Turabian StyleShmelev, Maxim A., Mikhail A. Kiskin, Julia K. Voronina, Konstantin A. Babeshkin, Nikolay N. Efimov, Evgenia A. Varaksina, Vladislav M. Korshunov, Ilya V. Taydakov, Natalia V. Gogoleva, Alexey A. Sidorov, and et al. 2020. "Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties" Materials 13, no. 24: 5689. https://doi.org/10.3390/ma13245689
APA StyleShmelev, M. A., Kiskin, M. A., Voronina, J. K., Babeshkin, K. A., Efimov, N. N., Varaksina, E. A., Korshunov, V. M., Taydakov, I. V., Gogoleva, N. V., Sidorov, A. A., & Eremenko, I. L. (2020). Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties. Materials, 13(24), 5689. https://doi.org/10.3390/ma13245689