A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite
Abstract
1. Introduction
2. Experimental Procedure
2.1. Raw Materials
2.1.1. Supporting Materials
2.1.2. PCM Materials
2.2. Fabrication Methods of the Ca(NO3)2–NaNO3/EG Composite
2.2.1. Melt-Impregnation Method
2.2.2. Solution + Melt-Impregnation Method
2.3. Manufacturing Processes for Producing the Form-Stable Composite
2.3.1. Cold-Compressing Process
2.3.2. Sintering Process
2.4. Characterization
3. Results and Discussions
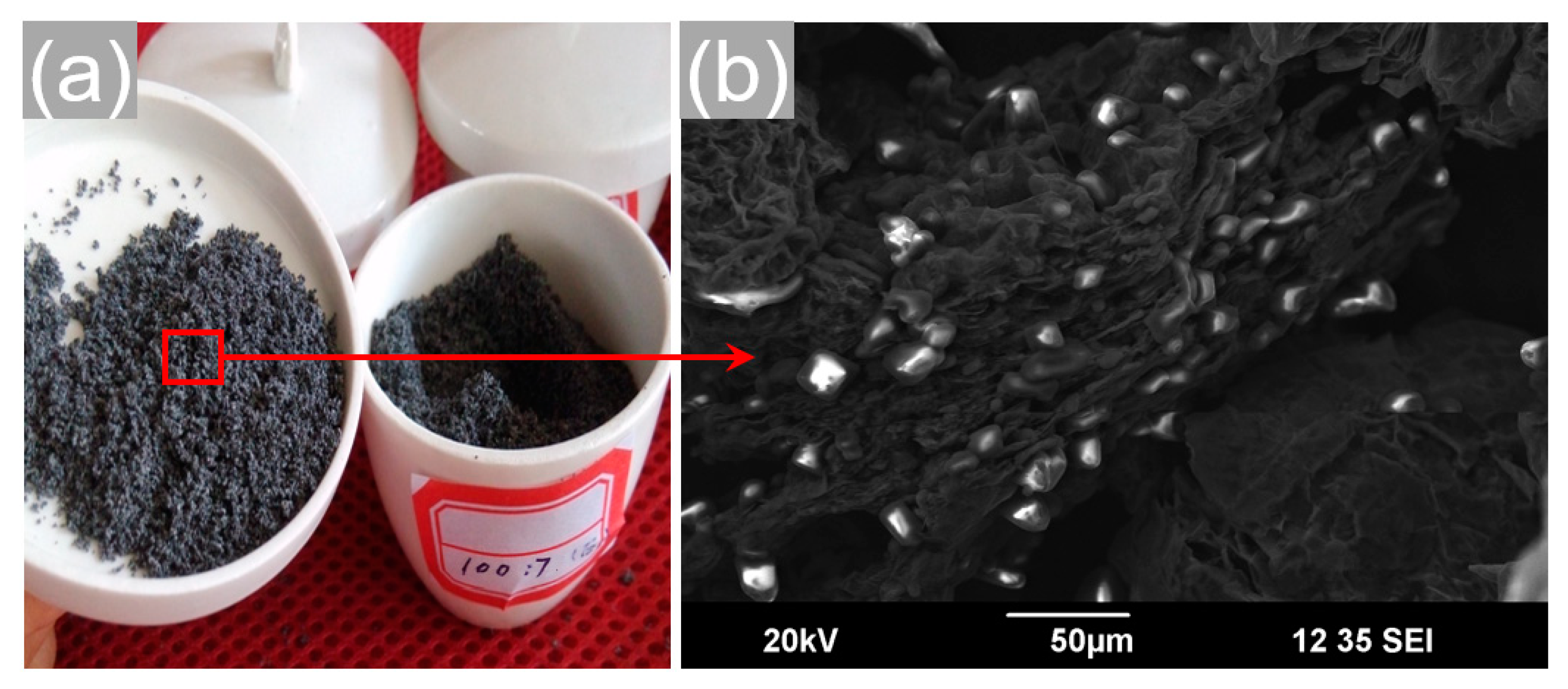
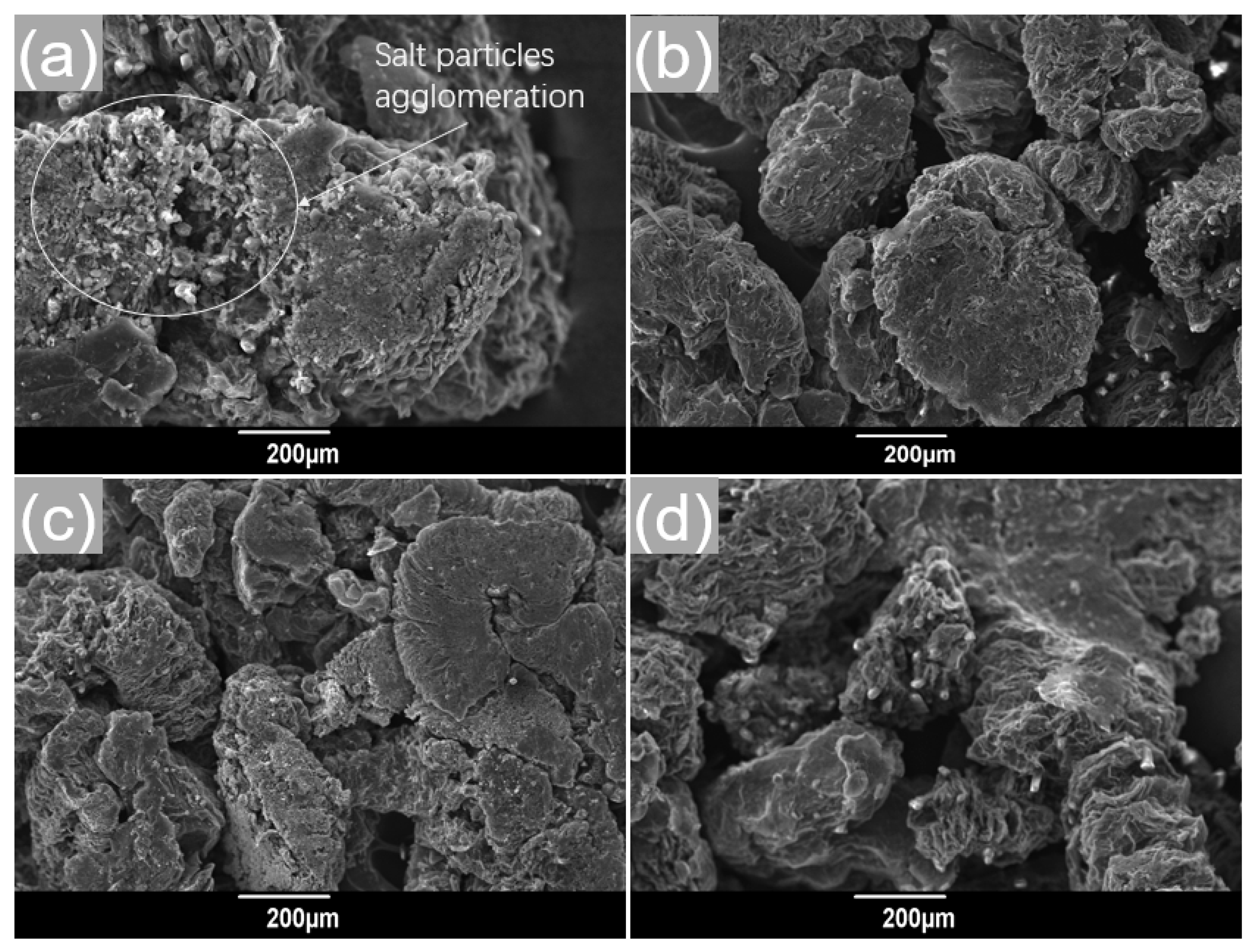
3.1. Effect of Stirring Speed on the Ca(NO3)2–NaNO3/EG Slurry
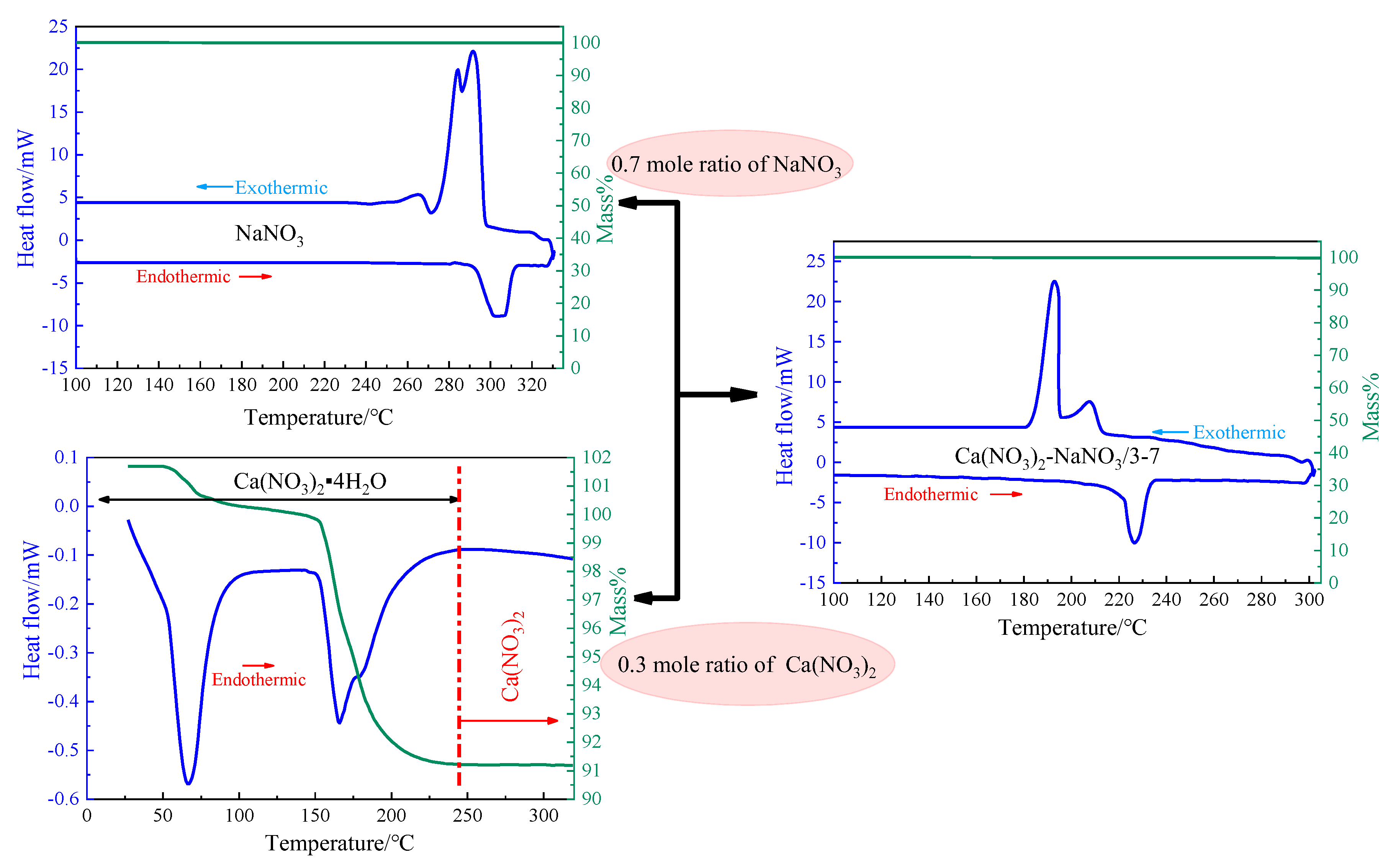
3.2. Effect of Evaporation Temperature on the Mixture
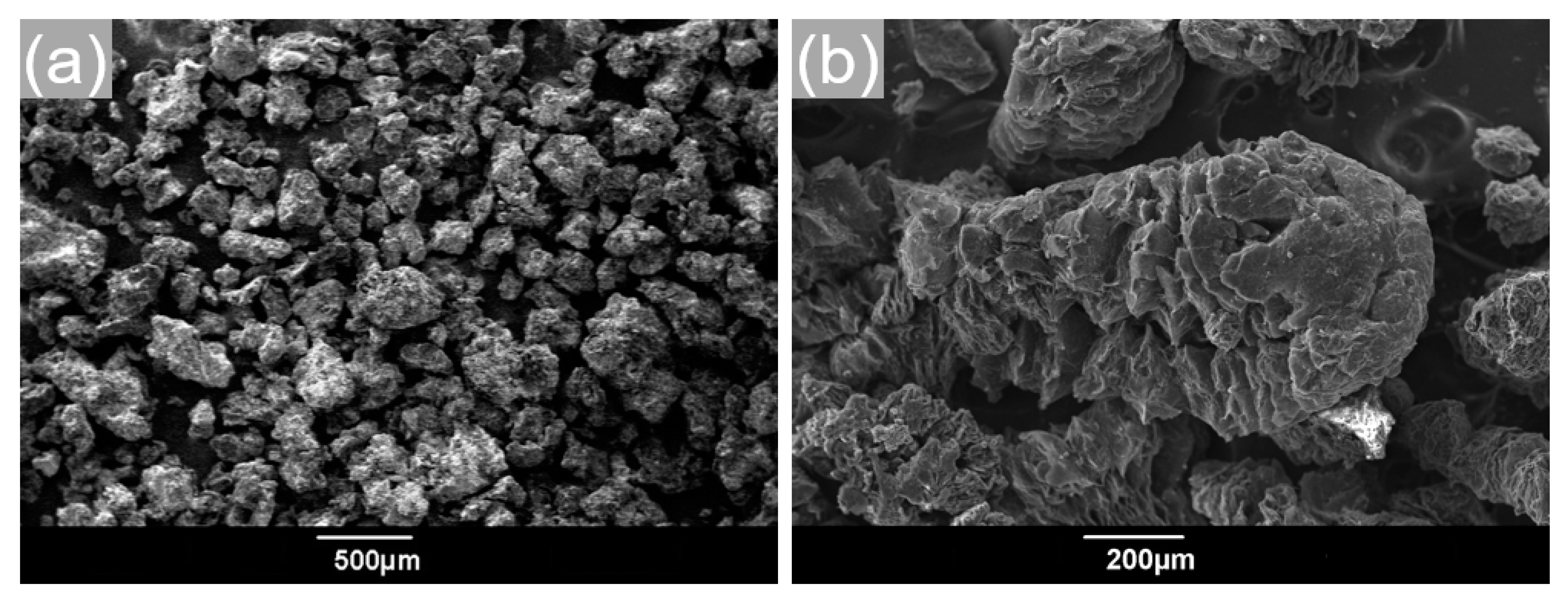
3.3. Effect of Melt-Impregnation Temperature on the Ca(NO3)2–NaNO3/EG Powders
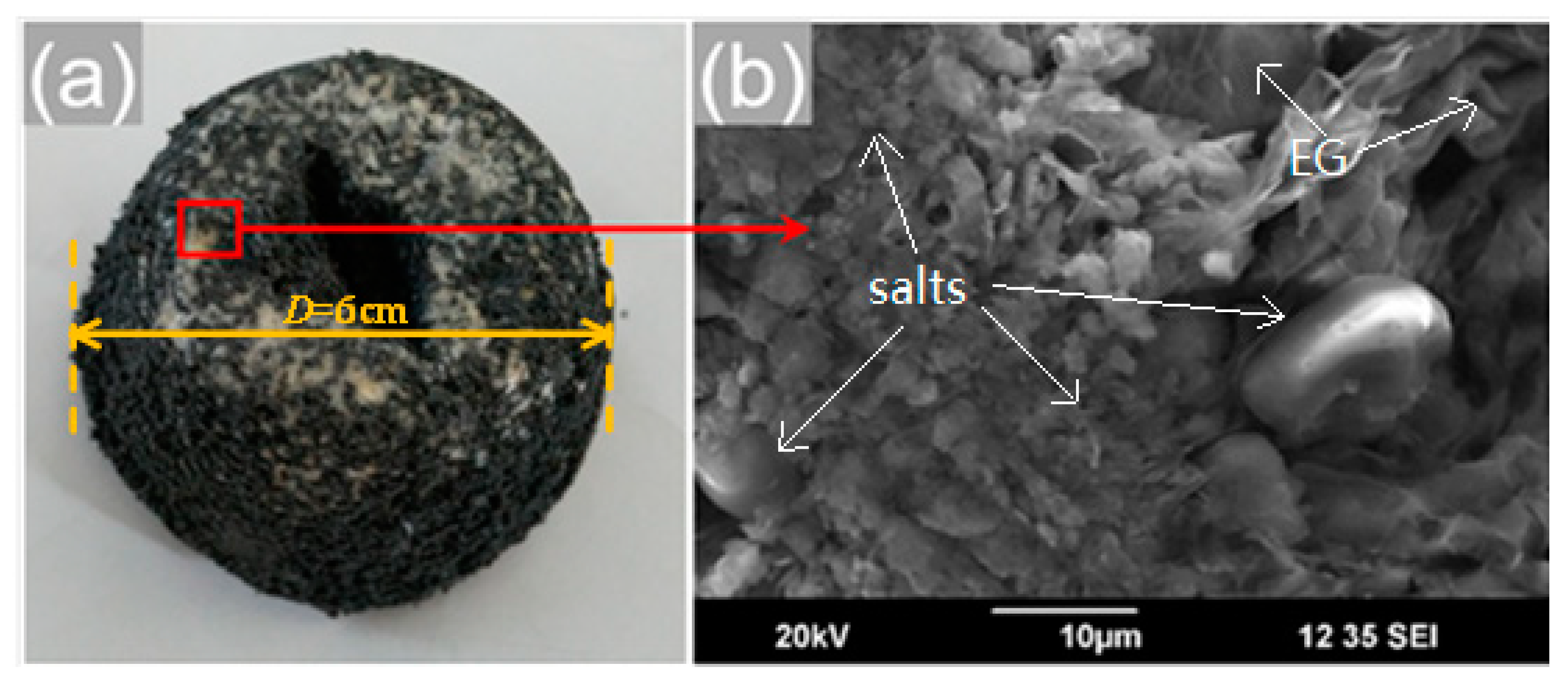
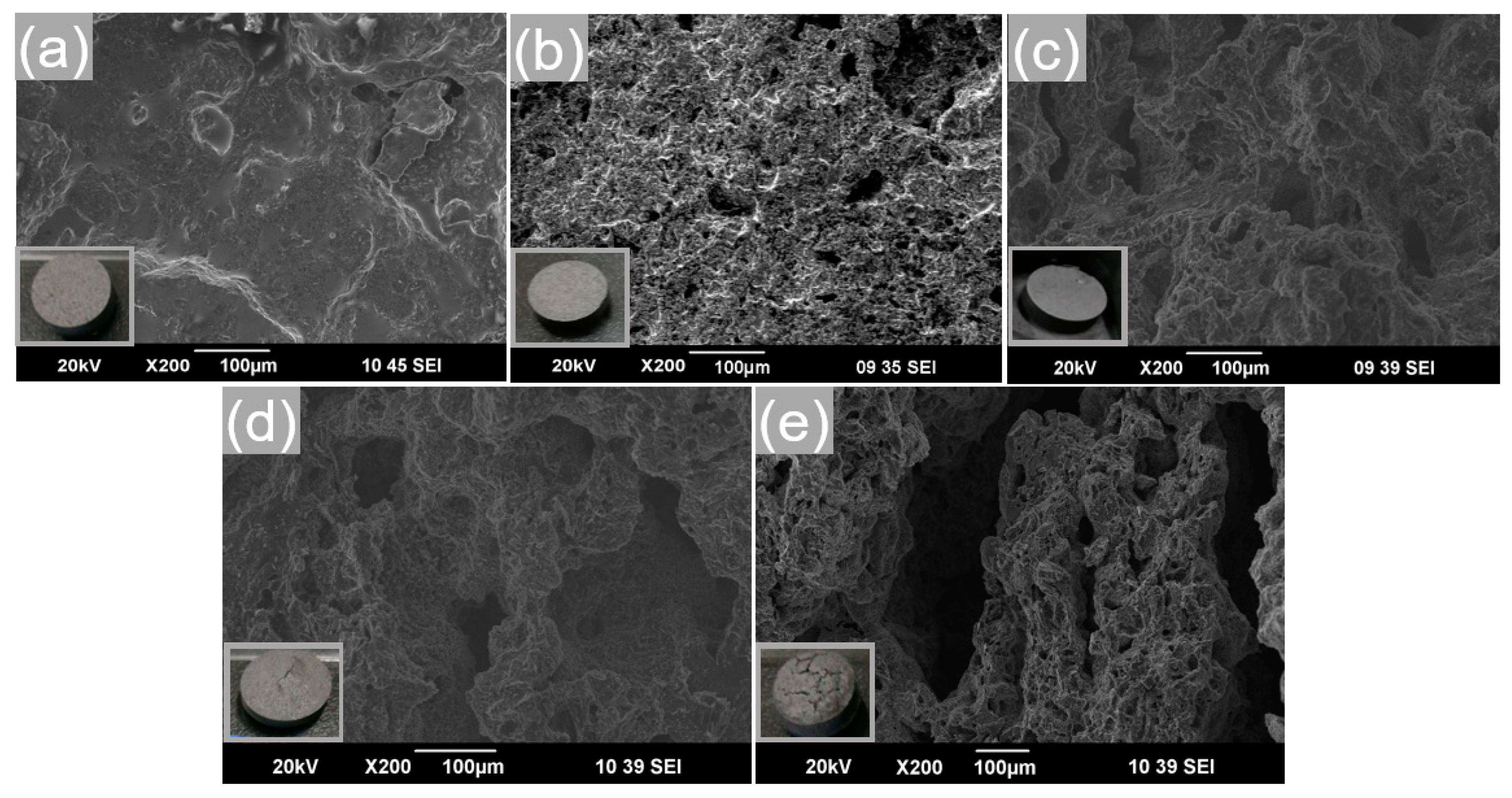
3.4. Effect of Cold-Pressing Pressure on Phase Change Properties of the Composite FS-PCMs
3.5. Effect of Sintering Temperature on Stabilization of the Composite FS-PCMs
3.6. Thermophysical Properties of the Composites
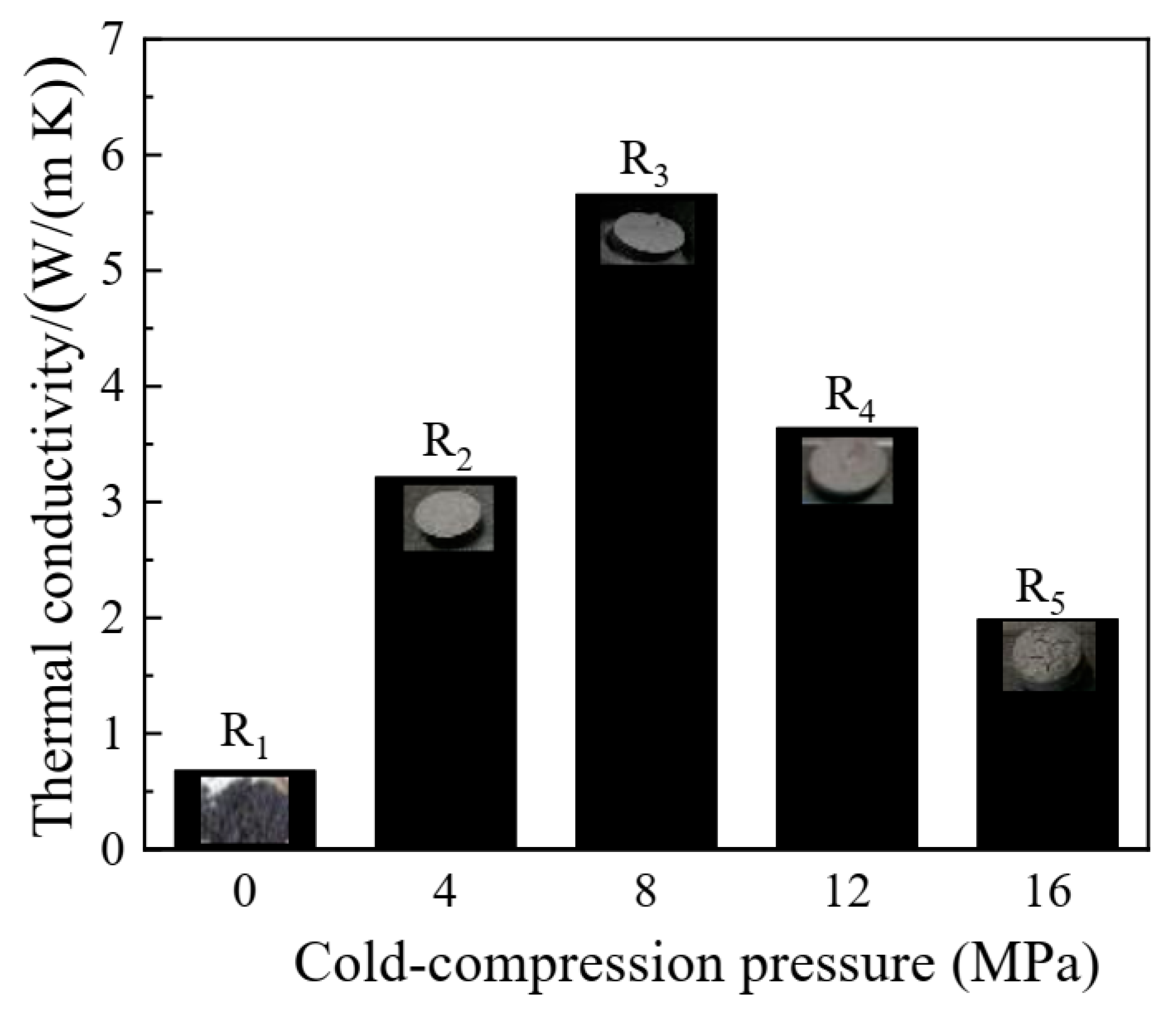
3.6.1. Thermal Conductivity Improvement of the Composite FS-PCMs
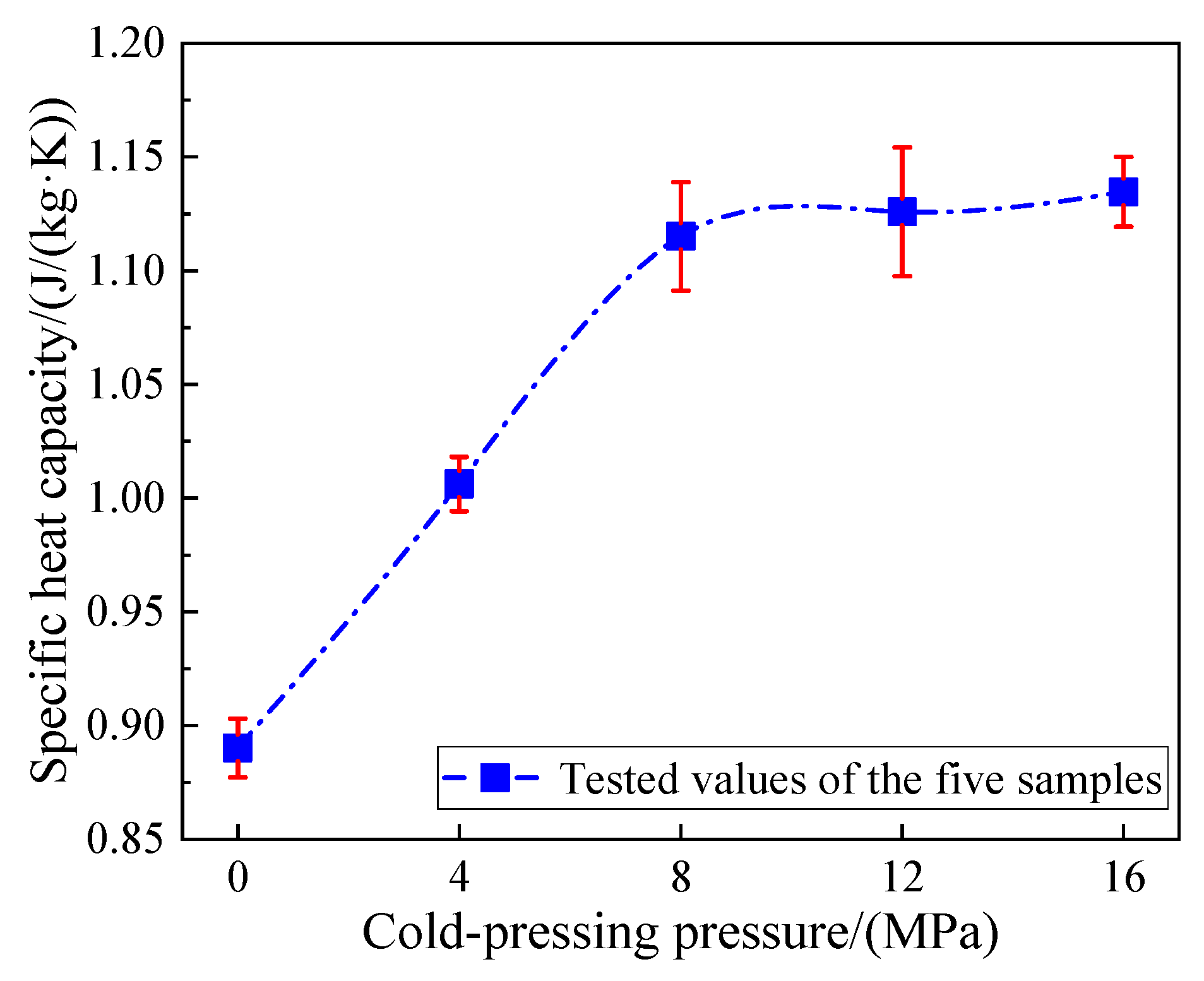
3.6.2. Specific Heat Capacities of the Composite FS-PCMs
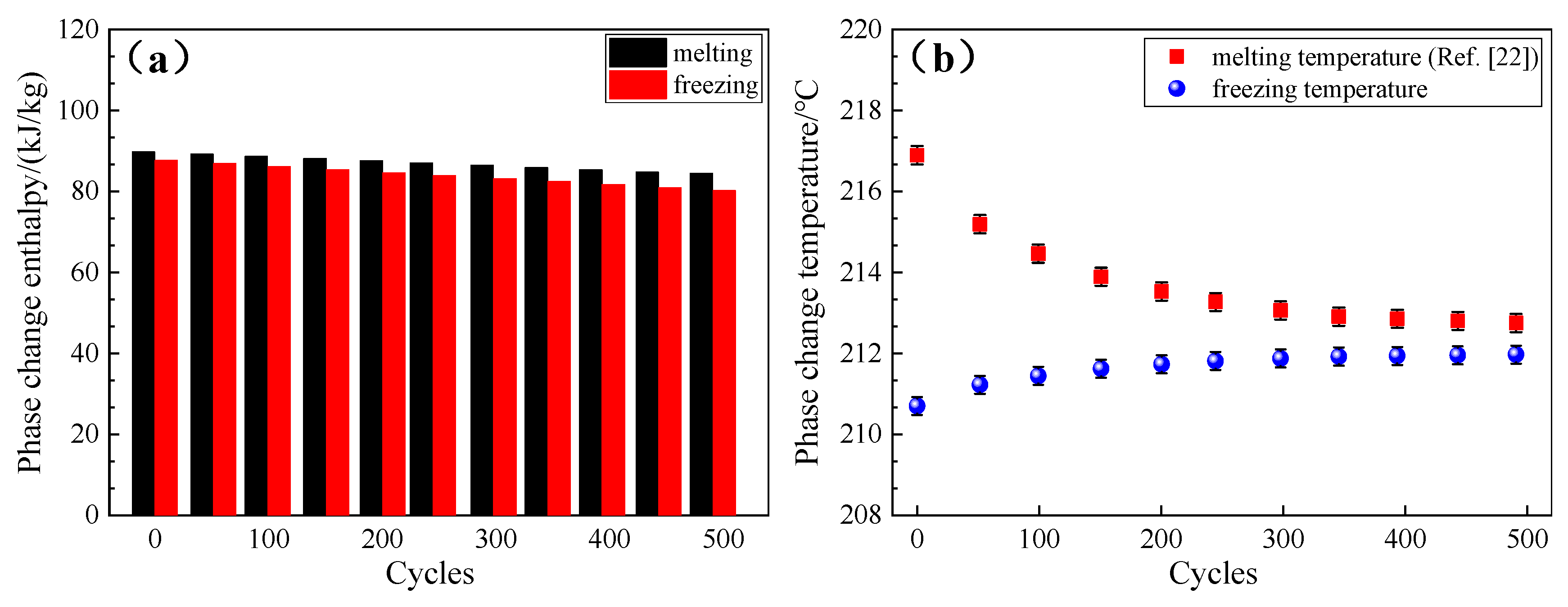
3.6.3. Cycling Stability of the Composite FS-PCMs
4. Conclusions
- (1)
- The layered problem resulting from the large differences in densities between EG and nitrate salts can be solved by the solution + low temperature drying technique. Compared with the melt-impregnation method, this method was more simple and effective.
- (2)
- With a temperature lower than 280 °C during the melt-impregnation process, the solid salt particles melted into a mushy phase, which resulted in a lower efficiency in impregnation, while a higher temperature was not beneficial for the forming and shaping processes. For these reasons, the 280 °C temperature is suggested for the melt-impregnation process.
- (3)
- Densification behavior was found to enhance the heat transfer of the composite FS-PCMs. However, the formation of the two sequential phases indicated that cold-compressing had a negative effect on the co-crystal structure.
- (4)
- The sintering process had a strong influence on recrystallization, and new bonding between the salt particles and EG matrices was formed.
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Research progress in nucleation and supercooling induced by phase change materials. J. Energy Storage 2020, 27, 101156. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Lin, Y.; Si, Y.; Huang, C.; Yang, J.; Huang, B.; Li, W. Present situation and future prospect of renewable energy in China. Renew. Sustain. Energy Rev. 2017, 76, 865–871. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-encapsulated organic phase change material based on copolymer nanocomposites for thermal energy storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Riahi, S.; Jovet, Y.; Saman, W.Y.; Belusko, M.; Bruno, F. Sensible and latent heat energy storage systems for concentrated solar power plants, exergy efficiency comparison. Sol. Energy 2019, 180, 104–115. [Google Scholar] [CrossRef]
- Koukou, M.; Dogkas, G.; Vrachopoulos, M.; Konstantaras, J.; Pagkalos, C.; Stathopoulos, V.; Pandis, P.; Lymperis, K.; Coelho, L.; Rebola, A. Experimental assessment of a full scale prototype thermal energy storage tank using paraffin for space heating application. Int. J. Heat Mass Transf. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R.; Seeniraj, R.V. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Al-Absi, Z.A.A.S.; Isa, M.H.M.; Ismail, M. Application of Phase Change Materials (PCMs) in building walls: A review. In Proceedings of the 2018 International Conference on Architecture and Civil Engineering Conference, Batu Ferringhi, Malaysia, 9–10 May 2018; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Johnston, J.H.; Grindrod, J.E.; Dodds, M.; Schimitschek, K. The use and performance of a nano-structured calcium silicate phase change material for thermal buffering in food packaging. Appita J. 2008, 61, 359–363. [Google Scholar] [CrossRef]
- Lin, K.P.; Zhang, Y.; Xu, X.; Di, H.; Yang, R.; Qin, P. Energy and buildings experimental study of under-floor electric heating system with shape stabilized PCM plates. Energy Build. 2005, 37, 215–220. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.A.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles-an over view. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Elias, C.; Stathopoulos, V. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Yang, G.; Wang, Y.; Zhao, L.; Mao, Z.; Wang, B.; Feng, X.; Sui, X. Shape-stabilized hydrated salt/paraffin composite phase change materials for advanced thermal energy storage and management. Chem. Eng. J. 2020, 385, 123958. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Zeng, J.L.; Sun, S.L.; Zhou, L.; Chen, Y.H.; Shu, L.; Yu, L.P.; Zhu, L.; Song, L.-B.; Cao, Z.; Sun, L.-X. Preparation, morphology and thermal properties of microencapsulated palmitic acid phase change material with polyaniline shells. J. Therm. Anal. Calorim. 2017, 129, 1583–1592. [Google Scholar] [CrossRef]
- Rao, Z.; Xu, T.; Liu, C.; Zheng, Z.; Liang, L.; Hong, K. Experimental study on thermal properties and thermal performance of eutectic hydrated salts/expanded perlite form-stable phase change materials for passive solar energy utilization. Sol. Energy Mater. Sol. Cells 2018, 188, 6–17. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J. Tailored phase change behavior of Na2SO4·10H2O/expanded graphite composite for thermal energy storage. Energy Convers. Manag. 2020, 208, 112586. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Ling, Z.; Fang, X.; Zhang, Z. A novel process for preparing molten salt/expanded graphite composite phase change blocks with good uniformity and small volume expansion. Sol. Energy Mater. Sol. Cells 2017, 169, 280–286. [Google Scholar] [CrossRef]
- Sang, L.; Xu, Y. Form stable binary chlorides/expanded graphite composite material with enhanced compressive strength for high temperature thermal storage. J. Energy Storage 2020, 31, 101611. [Google Scholar] [CrossRef]
- Li, Y.; Yue, G.; Yu, Y.M.; Zhu, Q.Z. Preparation and thermal characterization of LiNO3-NaNO3-KCl ternary mixture and LiNO3-NaNO3-KCl/EG composites. Energy 2020, 196, 117067. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Yuan, M.; Ye, F.; Ju, X.; Du, X. Ca(NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid-to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. [Google Scholar] [CrossRef]
- Taherian, R.; Kausar, A. Electrical Conductivity in Polymer-Based Composites: Experiments, Modelling, and Applications, William Andrew 2018. Available online: https://books.google.com (accessed on 30 November 2018).
- Aylsworth, J.W. Expanded Graphite. U.S. Patent 1,191,383, 18 July 1916. Available online: https://patentimages.storage.googleapis.com/84/5e/27/57330f83d29890/US1191383.pdf (accessed on 18 July 1916).
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Hydrated salts/expanded graphite composite with high thermal conductivity as a shape-stabilized phase change material for thermal energy storage. Energy Convers. Manag. 2015, 101, 164–171. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Ren, Y.; Li, P.; Yuan, M.; Ye, F.; Xu, C.; Liu, Z. Effect of the fabrication process on the thermophysical properties of Ca(NO3)2-NaNO3/expanded graphite phase change material composites. Sol. Energy Mater. Sol. Cells 2019, 200, 110005. [Google Scholar] [CrossRef]
- Erdogan, A.; Kursuncu, B.; Günen, A.; Kalkandelen, M.; Gok, M.S. A new approach to sintering and boriding of steels “Boro-sintering”: Formation, microstructure and wear behaviors. Surf. Coat. Technol. 2020, 386, 125482. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Maja, M.E.; Olubambi, P.A. Effect of sintering parameters on densification and microstructural evolution of nano-sized titanium nitride reinforced titanium alloys. J. Alloys Compd. 2018, 736, 202–210. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Ye, F.; Liao, Z. The effect of the cold compressing pressure on the microstructure and thermal properties of binary eutectic nitrate/expanded graphite phase change material composites. Energy Procedia 2019, 158, 4598–4603. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Xu, C.; Wang, T.; Tian, Z.; Liao, Z. A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite. Materials 2020, 13, 5368. https://doi.org/10.3390/ma13235368
Ren Y, Xu C, Wang T, Tian Z, Liao Z. A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite. Materials. 2020; 13(23):5368. https://doi.org/10.3390/ma13235368
Chicago/Turabian StyleRen, Yunxiu, Chao Xu, Tieying Wang, Ziqian Tian, and Zhirong Liao. 2020. "A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite" Materials 13, no. 23: 5368. https://doi.org/10.3390/ma13235368
APA StyleRen, Y., Xu, C., Wang, T., Tian, Z., & Liao, Z. (2020). A Study of Manufacturing Processes of Composite Form-Stable Phase Change Materials Based on Ca(NO3)2–NaNO3 and Expanded Graphite. Materials, 13(23), 5368. https://doi.org/10.3390/ma13235368





