Abstract
In this study, a novel nucleating agent composed of graphene oxide (GO) and silicon dioxide (SiO2) (GO–SiO2) is developed. GO is used as a skeleton material through which SiO2 nanomaterials are absorbed and subsequently incorporated into Na2SO4·10H2O phase change materials (PCMs). Furthermore, this study examines the phase change performance of the composite Na2SO4·10H2O materials. Fourier-transform infrared (FTIR) spectra confirmed the physical combination of GO with a SiO2 nanoparticles. Scanning electron microscope (SEM) results showed that the GO–SiO2 composite exhibited a layered structure and excellent dispersibility. The GO–SiO2 composite Na2SO4·10H2O PCMs displayed a low level of supercooling, i.e., about 1.2 °C with the addition of GO–SiO2 at 2.45 wt%. This was because the synergistic relation of the GO and the high dispersion SiO2, imparted more nucleation sites for Na2SO4·10H2O. Additionally, the prepared PCMs achieved high phase change latent heat and thermal conductivity, even under these conditions. The results show that the GO–SiO2 in the Na2SO4·10H2O exhibited advantageous application prospects for the improvement of the thermal performance of hydrate salts.
1. Introduction
Phase change materials (PCMs) are a kind of thermal energy storage material that can provide a high density of heat storage within a small temperature range, i.e., nearly that of the phase change temperature. This characteristic of PCMs makes them suitable for a wide range of applications in solar energy production, building energy conservation, refrigeration logistics, power systems, waste heat recovery, heating and air conditioning, household appliances and so on [1,2]. Inorganic hydrate salts are a kind of PCM that feature characteristics including high phase change latent heat, nonflammability, low cost and high thermal conductivity [3,4]. Compared with organic PCMs, inorganic hydrated salts have demonstrated many potential applications in the field of thermal energy storage [5,6]. However, there are also problems with inorganic hydrate salts, notably their phase separation and level of supercooling, which have become the key issues restricting the application of these kinds of materials [7,8].
The addition of a nucleating agent is an effective way to overcome the low degree of supercooling of inorganic hydrate salts [9,10]. The adsorption of inorganic hydrate salts into porous materials can also reduce the degree of supercooling [11,12,13]. Na4P2O7·10H2O can be used as a nucleating agent to reduce the required degree of supercooling of some hydrate salts [14], while other materials, like SrCl2·6H2O, can be used as nucleating agents of CaCl2·6H2O due to the common crystal structure of the two salts [15]. Liu et al. [16] experimented with Na2B4O7·10H2O, using nano-Al2O3 as a nucleating core; the results showed that nano-Al2O3 acted as nucleating core, and that the supercooling degree could be effectively reduced from 7.8 to 1.6 °C. Expanded graphite (EG) is a kind of carrier material with a porous structure; the adsorption of hydrate salts into the inner space of EG could prevent the leakage of PCMs in the phase change process. Xiao et al. [17] inserted Ba(OH)2·8H2O into EG; their results showed that the supercooling degree of Ba(OH)2·8H2O can be reduced from 13 to 2.4 °C. Liu et al. [18] used bentonite as a supporting material of CH3COONa·3H2O, finding it to be helpful in solving the leakage problems of CH3COONa·3H2O PCMs.
The addition of nanoparticles is not only effective in improving the thermal performance of PCMs; supercooling problems can also be solved in this fashion [19,20,21]. As a kind of supporting material, nano-SiO2 presents a porous structure with massive mesoporosity in its inner space, such that PCMs can be well adsorbed therein [22]. Peng et al. [23] used fumed SiO2 as a nucleating agent; their results showed that the supercooling degree of disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) could be reduced from 14.4 to 4.1 °C when 2 wt% of fumed SiO2 was added. As a kind of two-dimensional nanomaterial, graphene oxide (GO) has been proven to be effective in improving the thermal properties of PCMs. Xu et al. [24] used SrCl2·6H2O and GO as nucleating agents to reduce the supercooling degree of CaCl2·6H2O, and found that a percentage reduction in supercooling of about 99.2% could be achieved when 0.8 wt% SrCl2·6H2O and 0.02 wt% GO were admixed. Xia et al. [25] prepared poly(ethylene glycol) (PEG) using GO nanosheets; their results showed that PEG homogeneously intercalated into GO to form composite PCMs with a lamellar structure, resulting in highly thermally conductive and reliable PCMs.
As a kind of commonly used inorganic hydrate salt, sodium sulfate decahydrate (Na2SO4·10H2O) possess a phase change temperature of about 32.4 °C and a phase change latent heat of about 254 J/g. This approximate phase change temperature makes it a promising material for energy storage in buildings. However, the supercooling and phase separation of Na2SO4·10H2O also present problems that limit its practical applications [26]. The high dispersibility of SiO2 nanoparticles is beneficial for the reduction of the degree of supercooling when combined with Na2SO4·10H2O PCMs. GO also exhibits high compatibility with Na2SO4·10H2O PCMs and good thermal conductivity; this is the trend observed in the development of the nanomaterials that are used in inorganic hydrate salts. Nano-nucleating agents must have a high specific surface area to ensure the dispersal of nanomaterials in hydrate salts, and the hydrate salts should have a better phase change energy storage performance when the nanomaterials are incorporated. Currently, there is no research focusing on the nucleating agent composed of SiO2 and GO and its composite in the hydrate salts. In this study, in order to investigate the nucleating effect of nanoparticles in Na2SO4·10H2O, SiO2 nanoparticles were synthesized through the sol–gel method. Through the composition of GO with the prepared nano-SiO2, GO–SiO2 composites were developed. Their chemical structure, micromorphology and specific surface area were experimentally studied. Also studied was the influence of GO–SiO2 on the supercooling degree, crystallization behavior, phase change performance and thermal conductivity of Na2SO4·10H2O.
2. Materials and Methods
Sodium sulfate decahydrate (Na2SO4·10H2O, SSD, analytical grade) was used as the PCM, supplied by Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). Tetraethoxysilane (TEOS, analytical grade) provided by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), was used as silica precursor. Formamide (analytical grade), supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), was used as organic solvent. Graphene oxide (GO) aqueous dispersion with a solids content of 7.5 g/L was supplied by Jiangnan Graphene Research Institute (Changzhou, China).
SiO2 nanoparticles were synthesized under standard conditions using formamide solution as a solvent and TEOS as a silica precursor [27]. A certain amount of TEOS was mixed in formamide and stirred in a water bath at about 35 °C. NH3·H2O (28 wt%) was added and the pH value of the solution was adjusted to 11. The mixture was stirred for 24 h, filtered and dried to obtain the SiO2 nanoparticles. The prepared SiO2 and GO were mixed and ultrasonically dispersed for 2 h; then, the mixture was filtered and dried to obtain the GO–SiO2 composites. Na2SO4·10H2O and GO–SiO2 were mixed and magnetically stirred at 45 °C for 0.5 h to obtain the composite PCMs. The mix proportions of the GO–SiO2 composites and the composite PCMs are shown in Table 1.

Table 1.
Mix composition of the GO–SiO2 composites and the composite phase change materials (PCMs).
The chemical structures of the samples were obtained by Fourier-transform infrared spectroscopy (FTIR, Nicolet 6700, Thermo Scientific, Waltham, MA, USA) with KBr pellets with a wavenumber of 4000 to 400 cm−1 and a scanning speed of 10 cm−1/s. The nitrogen adsorption–desorption isotherms of the prepared SiO2 and GO–SiO2 composites were determined by a pore size analyzer (Micromeritics, Asap2460, Norcross, GA, USA). The crystalline behavior of the composite PCMs was characterized by means of X-ray diffraction using an automated X-ray powder diffractometer (XRD, D8 Advance, Bruker, Leipzig, Sachsen, Germany) with CuKa radiation (λ = 1.5418 Å). The surface morphologies of the composite PCMs were observed using a field emission scanning electron microscope (FESEM; FEI, NANO SEM430, Hillsboro, OR, USA). The microstructures of the composite PCMs were detected by transmission electron microscopy (TEM, JEOLJEM-2100, akishima, Tokyo, Japan) with an accelerating voltage of 200 kV. The phase change behavior of the samples was determined using a differential scanning calorimeter (DSC; Netzsch 200f3, Selbu, Bayern, Germany) in the range of 0 to 60 °C at a heating or cooling rate of 5 °C/min under nitrogen atmosphere.
The supercooling degrees of the samples (S-PCMs, GS-PCMs-1, GS-PCMs-2 and GS-PCMs-3) were tested using a multichannel temperature measuring instrument equipped with a computer, T-type thermocouple and thermostatic water bath. A 30 mL test tube filled with a 30 g sample was put into the thermostatic water bath and heated to about 45 °C; then, the sample was cooled from 45 to 10 °C. The temperature variations of the samples during the process were recorded to obtain the step cooling curves.
The thermal conductivity of the composite PCMs was measured by means of a hot disk thermal conductivity instrument (DRE-2C, Xiangtan Xiangyi Instrument Co., Ltd., Xiangtan, China) based on the transient plane source method and using a probe with a diameter of about 20 mm. Before the test, the probe was placed between two slabs of each sample and compacted. The thickness of each sample was not less than 10 mm, and each sample was tested three times to obtain the average value. The thermal conductivity λ was calculated as follows:
where α represents the thermal diffusivity of a sample; c represents the specific heat capacity of a sample; and ρ indicates the density of a sample.
λ = α·c·ρ
3. Results
3.1. Chemical Structure
The FTIR spectra of SiO2, GO and GO–SiO2 are shown in Figure 1. In the spectrum for SiO2, the characteristic wide bands at 3000 cm−1 and 3700 cm−1 correspond to the stretching vibrations of –O–H, and the peak at 1640 cm−1 corresponds to the vibrations of H–O–H. The absorption peaks at 1051 cm−1 refer to the asymmetrical vibrations of Si–O–Si, while the peaks at 787 cm−1 and 451 cm−1 refer to the symmetrical vibrations of Si–O–Si. In the spectrum for GO, the bands at 3431 cm−1 are associated with the O–H stretching vibrations, and the peaks at 1640 cm−1 represent the bending vibrations of C–OH. The characteristic absorption peak at 1115 cm−1 corresponds to the C–O–C stretching vibrations. The results show that the oxygen-containing groups of GO are mainly hydroxyl(–OH), carboxyl(–COOH) and epoxy groups(C–O–C). The absorption peaks of SiO2 and GO can be clearly seen from the spectrum of the prepared GO–SiO2. No other new absorption peaks were present for the spectrum of GO–SiO2, which shows that there was no chemical reaction between the GO and SiO2 nanoparticles.
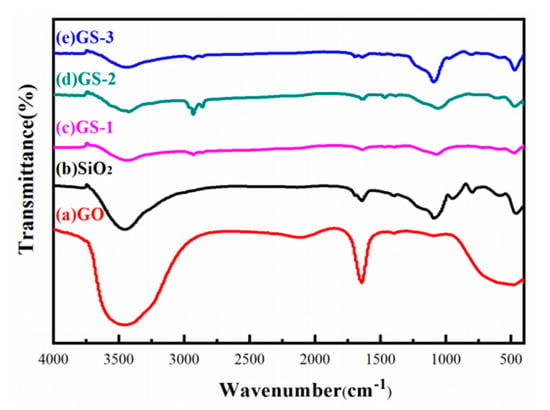
Figure 1.
Fourier-transform infrared (FTIR) spectra of GO, SiO2, GS-1, GS-2 and GS-3.
3.2. Micromorphology and Specific Surface Area Analysis
The micromorphologies of the SiO2 and GO–SiO2 composites were observed by scanning electron microscope (SEM). As shown in Figure 2a, the spherical shape of SiO2 nanoparticles was easily to agglomerate together, with an average particle size of about 200 nm. In Figure 2b–d, showing the GO–SiO2 composites, the nanosheet structure of GO on which the SiO2 nanoparticles were absorbed can be clearly seen. As the amount of GO in the GO–SiO2 composites increased, the adsorption amount of the SiO2 nanoparticles obviously also increased (Figure 2d), indicating the good compatibility between the GO and the SiO2 nanoparticles [28,29]. Compared with the synthesized SiO2 nanoparticles, with the intercalation of GO, the agglomeration degree of the SiO2 nanoparticles was obviously reduced, while the hybrid network structure of GO–SiO2 increased the contact surface with the hydrate salts, which is helpful for the crystallization and nucleation of inorganic hydrate salts.

Figure 2.
Scanning electron microscope (SEM) images of (a) SiO2, (b) GS-1, (c) GS-2, (d) GS-3.
The nitrogen adsorption–desorption isotherms of SiO2 and GO–SiO2 are shown in Figure 3. The SiO2 nanoparticles presented a small specific surface area of about 27.53 m2/g. With the addition of GO, the specific surface areas of GS-1, GS-2 and GS-3 were increased to about 67.21 m2/g, 89.30 m2/g and 437.39 m2/g, respectively. A typical type Ⅳ isotherm with a hysteresis loop characteristic of relative pressure (P/P0) higher than 0.8 was observed, and it can be noted that the formation of the pore structure was caused by the accumulation of the nanoparticles. As the amount of GO increased, more and more pores were formed by the accumulation of the nanoparticles, meaning that the adsorption performance of GO–SiO2 was improved and the hydrate salts could be well adsorbed in the inner space of GO–SiO2.
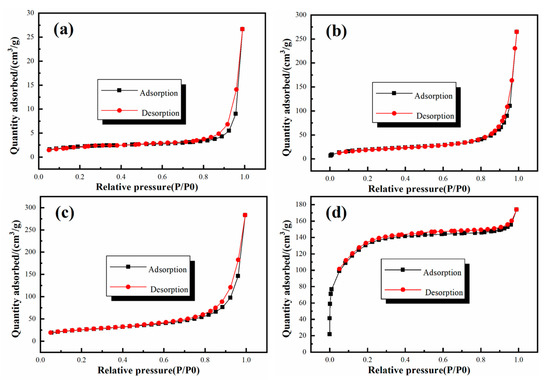
Figure 3.
Nitrogen adsorption–desorption isotherms of (a) SiO2, (b) GS-1, (c) GS-2, (d) GS-3.
3.3. Supercooling Degrees of the Na2SO4·10H2O Composites
The step cooling curves of the pure Na2SO4·10H2O, S-PCMs and GS-PCMs are shown in Figure 4. The supercooling degree of the Na2SO4·10H2O was reduced from 10.2 to 3.7 °C when SiO2 was added. SiO2 acted as the nucleating core, and the heterogeneous nucleation effect promoted the crystallization of the hydrate salts. For the GO–SiO2/Na2SO4·10H2O composites, the addition of GO further reduced the supercooling degree of the hydrate salts; with an increased amount of GO, supercooling degrees of about 3.3 °C, 1.9 °C and 1.2 °C were achieved for GS-PCMs-1, GS-PCMs-2 and GS-PCMs-3, respectively. The results show that the nucleation effect of GO–SiO2 was further enhanced with the introduction of GO. The increased surface area of GO–SiO2 provided a larger nucleation site for the hydrate salts, such that the crystallization of the Na2SO4·10H2O could be induced at a lower supercooling degree.
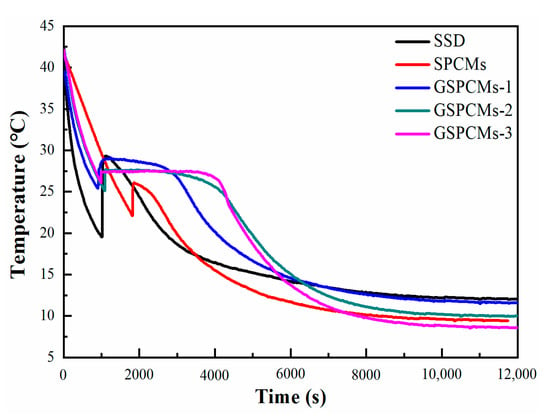
Figure 4.
Step cooling curves of the pure Na2SO4·10H2O, S-PCMs and GS-PCMs.
3.4. Crystallization Behavior
The XRD patterns of GO, GO–SiO2 and the GS-PCMs are shown in Figure 5. As can be seen from Figure 5a, a sharp diffraction peak of (002) was observed in GO around 2θ = 10.4°, which can be ascribed to the oxygen-containing functional groups contained in the nanosheet structure of GO. The broad diffraction peak at 2θ = 23.1° is a peak characteristic of SiO2, indicating the amorphous characteristics of SiO2. The diffraction peaks of GO and SiO2 can be seen for the GO–SiO2 composites. The crystallization peaks of pure Na2SO4·10H2O and of the composite PCMs are shown in Figure 5b. The diffraction peaks at 19.195°, 23.022° and 25.802° correspond to the crystallization peaks of Na2SO4·10H2O. For the S-PCMs that used SiO2 as a nucleating agent, diffraction peaks of Na2SO4 appearing at 23.022°, 39.133° and 60.457° can be observed, while weak diffraction peaks of Na2SO4·10H2O appearing at 19.195°, 23.022° and 25.802° can hardly be detected, indicating the dehydration of Na2SO4·10H2O in the phase change process. The diffraction peaks of Na2SO4·10H2O can be clearly seen from the XRD patterns of the GS-PCMs. It can thus be seen that the incorporation of GO improved the nucleation effect of SiO2, resulting in an enhanced crystallization performance for the Na2SO4·10H2O PCMs.
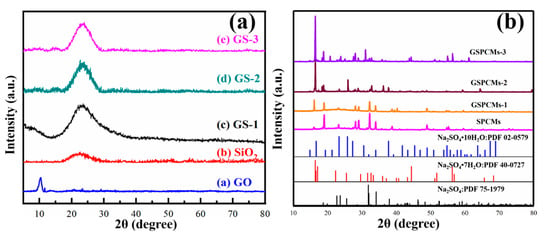
Figure 5.
X-ray powder diffractometer (XRD) patterns of (a) GO, SiO2, GS-1, GS-2 and GS-3; (b) Na2SO4·10H2O, S-PCMs, GS-PCMs-1, GS-PCMs-2 and GS-PCMs-3.
3.5. Phase Change Performance of the Composite PCMs
The phase change performances of the S-PCMs and GS-PCMs were determined using a DSC. The obtained curves in the melting and crystallization process are shown in Figure 6. The phase change performances of Na2SO4·10H2O and of the composite PCMs are shown in Table 2. The melting enthalpy of the pure Na2SO4·10H2O was about 208.2 J/g, with a phase change temperature of about 33.1 °C. The crystallization enthalpy of Na2SO4·10H2O was only about 67.4 J/g, indicating the poor crystallization performance of the pure Na2SO4·10H2O. The addition of SiO2 and GO–SiO2 had little influence on the phase change temperature of Na2SO4·10H2O. It can be noted that the crystallization enthalpy of the GS-PCMs obviously increased compared to the pure Na2SO4·10H2O. As the amount of GO in the GO–SiO2 composites increased, the phase change performance of the Na2SO4·10H2O composite PCMs greatly improved. Among the GO–SiO2 composite PCMs, GS-PCMs-3 possessed both the highest melting enthalpy, i.e., about 182.7 J/g, and the highest crystallization enthalpy, i.e., about 163.4 J/g.
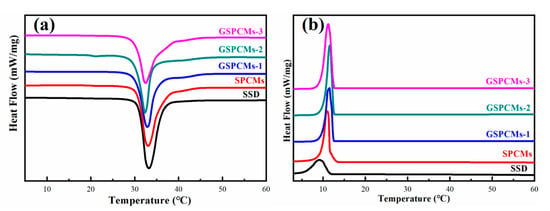
Figure 6.
Differential scanning calorimeter (DSC) curves of Na2SO4·10H2O, S-PCMs and GS-PCMs. (a) melting process, (b) cooling process.

Table 2.
Phase change performances of the pure Na2SO4·10H2O and composite PCMs.
3.6. Microstructure Analysis of Na2SO4·10H2O Composite PCMs
The microstructures of the Na2SO4·10H2O composite PCMs were tested using SEM. The fine crystalline grains of the pure Na2SO4·10H2O are shown in Figure 7a. As seen from Figure 7b, the addition of SiO2 resulted in coarse grain particles of Na2SO4·10H2O, indicating the weak nucleation effect of SiO2 that led to the irregular growth of the hydrate salts. Furthermore, to some extent, the change of the grain size also led to the reduction of phase change performance due to the existing phase transition of Na2SO4·10H2O to Na2SO4·7H2O or Na2SO4. In Figure 7c–e, the GS-PCMs with more compact structures can be seen; no coarse grain particles were found in these structures, indicating a better packaging efficiency for GO–SiO2 composites. It can also be seen that the introduction of GO–SiO2 refined the grain particle size and improved the crystallization behavior of the hydrate salts. The good compatibility of the hydrophilic GO implied a better combination of SiO2 with the hydrate salts, which further facilitated the nucleation and crystallization of the Na2SO4·10H2O PCMs [30,31]. The microstructures of the prepared GO–SiO2 (GS-3) and Na2SO4·10H2O composite PCMs (GS-PCMs-3) were observed by TEM. It can be clearly seen from Figure 8a,b that the spherical SiO2 nanoparticles were successfully absorbed on the surface of GO, indicating the successful formation of the GO–SiO2 composites. Figure 8c,d show the successful preparation of GO–SiO2/Na2SO4·10H2O composite PCMs; the GO–SiO2 composite in the Na2SO4·10H2O ensured a better nucleation effect, and the phase change performance of the composite PCMs was also improved.
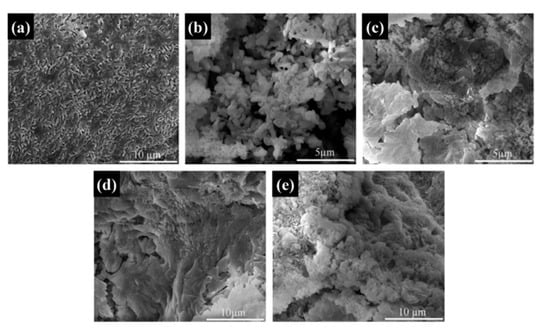
Figure 7.
SEM images of (a) pure Na2SO4·10H2O, (b) S-PCMs, (c) GS-PCMs-1, (d) GS-PCMs-2, (e) GS-PCMs-3.
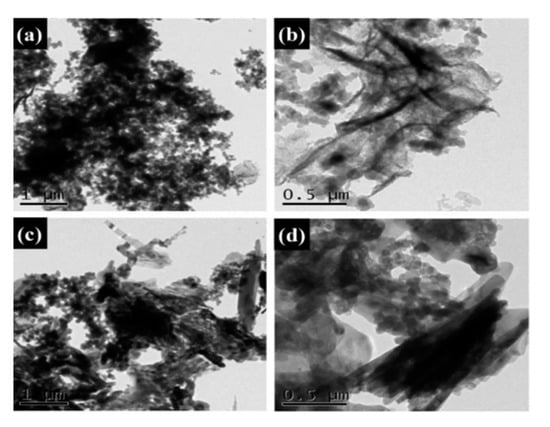
Figure 8.
Transmission electron microscopy (TEM) images of (a,b) GO–SiO2(GS-3) and (c,d) GS-PCMs-3.
3.7. Thermal Conductivity of the Composite PCMs
The thermal conductivity of the prepared S-PCMs and GS-PCMs is shown in Figure 9. The test values of the pure Na2SO4·10H2O, S-PCMs, GS-PCMs-1, GS-PCMs-2 and GS-PCMs-3 were 0.5061 W/(m·K), 0.5419 W/(m·K), 0.5923 W/(m·K), 0.6887 W/(m·K) and 0.6934 W/(m·K), respectively. With the incorporation of GO in the GS-PCMs, the heat transfer performance of the GS-PCMs was enhanced as the specific area of GO–SiO2. Owing to the incorporation of GO–SiO2 in Na2SO4·10H2O, the latter was well dispersed and its heat transfer performance enhanced, with higher observed thermal conductivity of the GO–SiO2 materials. For the prepared sample, the GS-PCMs-3 preserved the highest thermal conductivity, i.e., more than 0.65 W/(m·K), indicating an improvement in the thermal conductivity of the GS-PCMs compared with pure Na2SO4·10H2O.
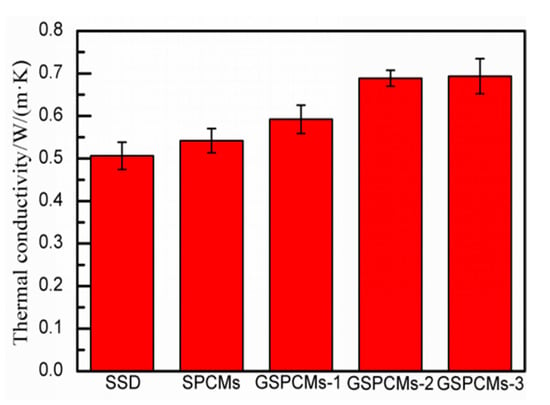
Figure 9.
Thermal conductivity of the prepared S-PCMs and GS-PCMs.
4. Conclusions
SiO2 nanoparticles was prepared and composited with GO to form GO–SiO2 composite materials with a layered structure. The GO–SiO2 composites were employed as nucleating agents in the Na2SO4·10H2O PCMs, and the influence of GO on the supercooling degree, crystallization behavior, phase change performance and thermal conductivity of the Na2SO4·10H2O composite PCMs was investigated. The following conclusions can be drawn:
- (1)
- Compared to pure SiO2, the dispersion performance of the SiO2 nanoparticles was obviously improved with the introduction of GO, and the specific area of GO–SiO2 was greatly increased. The high specific area of GO–SiO2 enabled a good nucleation effect and produced better crystallization behavior for the Na2SO4·10H2O PCMs. In comparison to pure Na2SO4·10H2O, the supercooling degree of Na2SO4·10H2O composite PCMs could be reduced from 10.2 to as low as 1.2 °C when 0.45 wt% of GO was added.
- (2)
- The phase change performance of Na2SO4·10H2O was improved with the incorporation of GO–SiO2 composites. The crystallization enthalpy of the composite PCMs increased to about 163.4 J/g with the addition of 2.45 wt% of GO–SiO2.
- (3)
- The good compatibility of the hydrophilic GO led to a better combination of SiO2 with the hydrate salts, which further facilitated the nucleation and crystallization of Na2SO4·10H2O. Compared with Na2SO4·10H2O, the thermal conductivity of the composite PCMs was improved due to the large specific area of the GO–SiO2 composites.
- (4)
- The weight ratio of 2.45 wt% of GO–SiO2 in the Na2SO4·10H2O was appropriate for achieving a small supercooling degree, high latent heat of the phase change and high thermal conductivity. The prepared GO–SiO2 composited hydrated salt PCMs demonstrated potential applications in the domain of thermal energy storage materials.
Author Contributions
Conceptualization, Y.Z.; methodology, W.T.; validation, W.T., X.K. and C.F.; formal analysis, W.T. and Y.Z.; investigation, W.T., Y.Z.; X.K.; C.F. and A.B.; data curation, W.T. and Y.Z.; writing—original draft preparation, W.T.; writing—review and editing, W.T. and Y.Z.; project administration, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 51702001 and 51872137, and the Natural Science Foundation of Anhui Province, grant number 1808085QE119.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harish, V.S.K.V.; Kumar, A. A review on modeling and simulation of building energy systems. Renew. Sust. Energy Rev. 2016, 56, 1272–1292. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar, O.F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat. Mass. Tran. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados, F.S.; Van, D.S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Mariaenrica, F.; Mariateresa, L.; Antonella, S. Phase change materials for energy efficiency in buildings and their use in mortars. Materials 2019, 12, 1260. [Google Scholar]
- Yang, W.; Zhang, L.; Guo, Y.; Jiang, Z.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Novel segregated-structure phase change materials composed of paraffin@graphene microencapsules with high latent heat and thermal conductivity. J. Mater. Sci. 2017, 53, 2566–2575. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Advanced energy storage materials for building applications and their thermal performance characterization: A review. Renew. Sust. Energy Rev. 2016, 57, 916–928. [Google Scholar] [CrossRef]
- Fang, Y.; Su, J.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z. Preparation and thermal properties of NaOAc·3H2O-CO(NH2)2 non-eutectic binary mixture PCM for radiant floor heating system. Appl. Therm. Eng. 2020, 167, 114820. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Ling, Z.; Zhang, Z.; Fang, X. Modification of expanded graphite and its adsorption for hydrated salt to prepare composite PCMs. Appl. Therm. Eng. 2018, 133, 446–451. [Google Scholar] [CrossRef]
- Huang, J.; Wang, T.; Zhu, P.; Xiao, J. Preparation, characterization, and thermal properties of the microencapsulation of a hydrated salt as phase change energy storage materials. Thermochim. Acta 2013, 557, 1–6. [Google Scholar] [CrossRef]
- Dannemand, M.; Johansen, J.B.; Furbo, S. Solidification behavior and thermal conductivity of bulk sodium acetate trihydrate composites with thickening agents and graphite. Sol. Energy Mater. Sol. Cells 2016, 145, 287–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Xu, X.; Munyalo, J.M.; Liu, L.; Liu, X.; Lu, M.; Zhao, Y. Preparation and characterization of sodium sulfate pentahydrate/sodium pyrophosphate composite phase change energy storage materials. J. Mol. Liq. 2019, 280, 360–366. [Google Scholar] [CrossRef]
- Shahbaz, K.; AlNashef, I.M.; Lin, R.J.T.; Hashim, M.A.; Mjalli, F.S.; Farid, M.M. A novel calcium chloride hexahydrate-based deep eutectic solvent as a phase change materials. Sol. Energy Mater. Sol. Cells 2016, 155, 147–154. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Use of nano-α-Al2O3 to improve binary eutectic hydrated salt as phase change material. Sol. Energy Mater. Sol. Cells 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Xiao, Q.; Yuan, W.; Li, L.; Xu, T. Fabrication and characteristics of composite phase change material based on Ba(OH)2·8H2O for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 339–345. [Google Scholar] [CrossRef]
- Liu, C.; Hu, P.; Xu, Z.; Ma, X.; Rao, Z. Experimental investigation on thermal properties of sodium acetate trihydrate based phase change materials for thermal energy storage. Thermochim. Acta 2019, 674, 28–35. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Nian, H.; Zhu, F.; Ren, X.; Dong, O.; Hai, C.; Shen, Y.; Zeng, J. Preparation and thermal energy storage studies of CH3COONa·3H2O–KCl composites salt system with enhanced phase change performance. Appl. Therm. Eng. 2016, 102, 708–715. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, J.; Wang, Q.; Lin, W.; Fang, X.; Zhang, Z. MgCl2·6H2O-Mg(NO3)2·6H2O eutectic/SiO2 composite phase change material with improved thermal reliability and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2017, 172, 195–201. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Investigation of specific heat and latent heat enhancement in hydrate salt based TiO2 nanofluid phase change material. Appl. Therm. Eng. 2017, 124, 533–538. [Google Scholar] [CrossRef]
- Zou, T.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and performance of form-stable TBAB hydrate/SiO2 composite PCM for cold energy storage. Int. J. Refrig. 2019, 101, 117–124. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Wang, T.; Zhu, P. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Thermochim. Acta 2019, 675, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Memon, S.A.; Yang, H.; Tang, W. Development of novel composite PCM for thermal energy storage using CaCl2·6H2O with graphene oxide and SrCl2·6H2O. Energy Build. 2017, 156, 163–172. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Huang, P.; Huang, C.; Xu, F.; Zou, Y.; Chu, H.; Yan, E.; Sun, L. Graphene-oxide-induced lamellar structures used to fabricate novel composite solid-solid phase change materials for thermal energy storage. Chem. Eng. J. 2019, 362, 909–920. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Zhang, Z.; He, F.; Yan, S.; Yan, P.J.; Xie, R.; Ju, X.J.; Liu, Z.; Chu, L.Y. Monodisperse Na2SO4·10H2O@SiO2 microparticles against supercooling and phase separation during phase change for efficient energy storage. Ind. Eng. Chem. Res. 2017, 56, 3297–3308. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Wang, L.; Tang, K.; Maier, J.; Mullen, K. Graphene-based nanosheets with a sandwich structure. Angew. Chem. Int. Ed. 2010, 49, 4795–4799. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.; Shi, L.; Feng, P.; Wang, X.; Zhu, Y.; Wu, G.; Song, G. Chemical grafting of nano-SiO2 onto graphene oxide via thiol-ene click chemistry and its effect on the interfacial and mechanical properties of GO/epoxy composites. Compos. Sci. Technol. 2019, 182, 107751. [Google Scholar] [CrossRef]
- Haeri, S.Z.; Ramezanzadeh, B.; Asghari, M. A novel fabrication of a high performance SiO2-graphene oxide (GO) nanohybrids: Characterization of thermal properties of epoxy nanocomposites filled with SiO2-GO nanohybrids. J. Colloid Interface Sci. 2017, 493, 111–122. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.S.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Hybrid network structure of boron nitride and graphene oxide in shape-stabilized composite phase change materials with enhanced thermal conductivity and light-to-electric energy conversion capability. Sol. Energy Mater. Sol. Cells 2018, 174, 56–64. [Google Scholar] [CrossRef]
- Mousavi, A.; Roghani, M.H.; Salami, K.M.; Shahi, S.; Abdollahi, A. Grafting of silica nanoparticles at the surface of graphene for application in novolac-type phenolic resin hybrid composites. Mater. Chem. Phys. 2018, 216, 468–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).