Review on the Biological Degradation of Polymers in Various Environments
Abstract
1. Introduction
2. Biological Degradation
3. Polymers
3.1. Polylactide (PLA)
3.2. Polyhydroxybutyrate (PHB)
3.3. Polyhydroxybutyrate-co-valerate (PHBV)
3.4. Further Biopolymers
3.4.1. Biodegradable Polymers from Petrochemical Sources
3.4.2. Cellulose-Based Biopolymers
3.4.3. Starch-Based Biopolymers
3.4.4. Protein-Based Polymers
3.4.5. CO2-Based Biopolymers
3.4.6. Other Biopolymers
4. Key Challenges for Biodegradation Tests
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Explanatory Boxes
References
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Göttermann, S.; Bonten, C.; Kloeppel, A.; Kaiser, S.; Brümmer, F. Marine littering-auswirkung und abbauverhalten. In Proceedings of the 24. Stuttgarter Kunststoffkolloquium, Stuttgart, Germany, 25–26 February 2015. [Google Scholar]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics & Catalysing Action; Ellen MacArthur Foundation: Cowes, UK, 2017; pp. 1–68. [Google Scholar]
- Ißbrücker, C.; Pogrell, H.V. Biobasiert, bioabbaubar oder beides. Nachr. Chem. 2013, 61, 1037–1038. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung DIN EN 13432. Verpackung—Anforderungen an die Verwertung von Verpackungen durch Kompostierung und biologischen Abbau—Prüfschema und Bewertungskriterien für die Einstufung von Verpackungen. Dtsch. Fass. EN 2000, 13432, 2007–2010. [Google Scholar]
- American Society for Testing. Materials ASTM D6400. In Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Deutsches Institut für Normung, E.V. DIN EN ISO 14855-1. Bestimmung der vollständigen aeroben Bioabbaubarkeit von Kunststoff-Materialien unter den Bedingungen kontrollierter Kompostierung; Deutsches Institut für Normung: Berlin, Germany, 2013. [Google Scholar]
- Augusta, J.; Müller, R.-J.; Widdecke, H. Biologisch abbaubare Kunststoffe: Testverfahren und Beurteilungskriterien. Chem. Ing. Tech. 1992, 64, 410–415. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef]
- Bonten, C. Plastics Technology: Introduction and Fundamentals; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2019; ISBN 978-1-56990-767-2. [Google Scholar]
- Chahine, M.T. The hydrological cycle and its influence on climate. Nature 1992, 359, 373–380. [Google Scholar] [CrossRef]
- Munn, C.B. Marine Microbiology: Ecology and Applications; Garland Science/BIOS Scientific Publishers and Distributed in the USA by Fulfilment Center, Taylor & Francis: London, UK; New York, NY, USA; Independence, KY, USA, 2004; ISBN 978-0-203-50311-9. [Google Scholar]
- Alyn, C.; Duxbury Fred, T. Mackenzie, Robert und Howard Byrne; Encylopeadia Britannica Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Chen, R.; Jakes, K.A. Cellulolytic Biodegradation of Cotton Fibers from a Deep-Ocean Environment. J. Am. Inst. Conserv. 2001, 40, 91–103. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Bezirtzoglou, E. Water microbial ecology—An overview. In Encyclopedia of Life Sciences; Wiley: Chichester, UK, 2009; pp. 1–24. [Google Scholar]
- Okafor, N. Environmental Microbiology of Aquatic and Waste Systems; Springer Science + Business Media B.V: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-1459-5. [Google Scholar]
- Necker, J. Einfluss neozoischer Crustaceen auf Invertebrate des Bodenseelitorals. 2006. Available online: https://kops.uni-konstanz.de/bitstream/123456789/8371/1/Diplomarbeit_JasminNecker.pdf (accessed on 1 September 2020).
- Gordon & Breach. The limnology, climatology and paleoclimatology of the East African Lakes; Johnson, T.C., Ed.; Gordon & Breach: Amsterdam, The Netherlands, 1996; ISBN 2-88449-234-8. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer: Boston, MA, USA, 1998; ISBN 1461554071. [Google Scholar]
- Bastioli, C. Handbook of Biodegradable Polymers; Rapra Technology: Shrewsbury, UK, 2005; ISBN 1-85957-389-4. [Google Scholar]
- Scheunert, I. Mikrobieller Abbau organischer Fremdstoffe im Boden. Chem. Unserer Zeit 1994, 28, 68–78. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation; Development OECD 301A. Test No. 307: Aerobic and Anaerobic Transformation in Soil; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Rudnik, E. Compostable Polymer Materials, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780080453712. [Google Scholar]
- TUEV AUSTRIA HOLDING AG 2019. OK Compost Home. Available online: https://www.tuv-at.be/fileadmin/user_upload/docs/download-documents/english/Program_OK_02e_d_OK_compost_HOME.pdf (accessed on 1 September 2020).
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Hermann, B.G.; Debeer, L.; de Wilde, B.; Blok, K.; Patel, M.K. To compost or not to compost: Carbon and energy footprints of biodegradable materials’ waste treatment. Polym. Degrad. Stab. 2011, 96, 1159–1171. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global Wastewater and Sludge Production, Treatment and Use. In Wastewater: Economic Asset in an Urbanizing World; Qadir, M., Wichelns, D., Drechsel, P., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2015; pp. 15–38. ISBN 978-94-017-9545-6. [Google Scholar]
- Poulsen, T.G.; Bester, K. Organic micropollutant degradation in sewage sludge during composting under thermophilic conditions. Environ. Sci. Technol. 2010, 44, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing. Materials ASTM AST 5209-92; ASTM International: Philadelphia, PA, USA, 1992. [Google Scholar]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Reinhart, D.R.; Basel Al-Yousfi, A. The Impact of Leachate Recirculation on Municipal Solid Waste Landfill Operating Characteristics. Waste Manag. Res. 2016, 14, 337–346. [Google Scholar] [CrossRef]
- Domininghaus, H.; Elsner, P.; Eyerer, P.; Hirth, T. Kunststoffe: Eigenschaften und Anwendungen, 8., neu bearb. und erw. Aufl.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642161728. [Google Scholar]
- Türk, O. Stoffliche Nutzung Nachwachsender Rohstoffe: Grundlagen—Werkstoffe—Anwendungen; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2014; ISBN 9783834817631. [Google Scholar]
- Kolstad, J.J.; Vink, E.T.H.; de Wilde, B.; Debeer, L. Assessment of anaerobic degradation of Ingeo™ polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental degradation of biodegradable polyesters 2. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(L-lactide) films in natural dynamic seawater. Polym. Degrad. Stab. 2002, 75, 357–365. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behaviour of poly(lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crop. Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Ho, K.-L.G.; Pometto III, A.L.; Gadea-Rivas, A.; Briceño, J.A.; Rojas, A. Degradation of Polylactic Acid (PLA) Plastic in Costa Rican Soil and Iowa State University Compost Rows. J. Polym. Environ. 1999, 7, 173–177. [Google Scholar] [CrossRef]
- Sikorska, W.; Musiol, M.; Nowak, B.; Pajak, J.; Labuzek, S.; Kowalczuk, M.; Adamus, G. Degradability of polylactide and its blend with poly[(R,S)-3-hydroxybutyrate] in industrial composting and compost extract. Int. Biodeterior. Biodegrad. 2015, 101, 32–41. [Google Scholar] [CrossRef]
- Yosita, R.; Jaruayporn, N.; Monchai, T.; Phasawat, C.; Thanawadee, L. Determining Biodegradability of Polylactic Acid under Different Environments. J. Met. Mater. Miner. 2008, 18, 83–87. [Google Scholar]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Mesophilic anaerobic biodegradation test and analysis of eubacteria and archaea involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2014, 110, 278–283. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic anaerobic biodegradation test and analysis of eubacteria involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2013, 98, 1182–1187. [Google Scholar] [CrossRef]
- Behr, A.; Seidensticker, T. Einführung in die Chemie nachwachsender Rohstoffe: Vorkommen, Konversion, Verwendung; Springer Spektrum: Berlin/Heidelberg, Germany, 2018; ISBN 9783662552544. [Google Scholar]
- Ansari, S.; Fatma, T. Polyhydroxybutyrate—A Biodegradable Plastic and its Various Formulations. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9598–9602. [Google Scholar]
- Volova, T.G.; Boyandin, A.N.; Vasiliev, A.D.; Karpov, V.A.; Prudnikova, S.V.; Mishukova, O.V.; Boyarskikh, U.A.; Filipenko, M.L.; Rudnev, V.P.; Bá Xuân, B.; et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Mergaert, J.; Wouters, A.; Anderson, C.; Swings, J. In situ biodegradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in natural waters. Can. J. Microbiol. 1995, 41 (Suppl. 1), 154–159. [Google Scholar] [CrossRef]
- Mergaert, J.; Webb, A.; Anderson, C.; Wouters, A.; Swings, J. Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in soils. Appl. Environ. Microbiol. 1993, 59, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Đỗ, N.L.; Nguyễn, T.H.; Lê, T.M.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L.; et al. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int. Biodeterior. Biodegrad. 2013, 83, 77–84. [Google Scholar] [CrossRef]
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J. Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in compost. J. Environ. Polym. Degrad. 1994, 2, 177–183. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Puglia, D.; Fortunati, E.; D’Amico, D.A.; Manfredi, L.B.; Cyras, V.P.; Kenny, J.M. Influence of organically modified clays on the properties and disintegrability in compost of solution cast poly(3-hydroxybutyrate) films. Polym. Degrad. Stab. 2014, 99, 127–135. [Google Scholar] [CrossRef]
- Gutierrez-Wing, M.T.; Stevens, B.E.; Theegala, C.S.; Ioan, I. Anaerobic Biodegradation of Polyhydroxybutyrate in Municipal Sewage Sludge. J. Environ. Eng. 2010, 136, 709–718. [Google Scholar] [CrossRef]
- Nishida, H.; Tokiwa, Y. Distribution of poly(β-hydroxybutyrate) and poly(ε-caprolactone)aerobic degrading microorganisms in different environments. J. Environ. Polym. Degrad. 1993, 1, 227–233. [Google Scholar] [CrossRef]
- Tansengco, M.; Dogma, I. Microbial degradation of poly-β-hydroxybutyrate using landfill soils. Acta Biotechnol. 1999, 19, 191–203. [Google Scholar] [CrossRef]
- Matavulj, M.; Sad, N.; Molitoris, H.P. Biodegradation of polyhydroxyalkanoate-based plastic (BIOPOL) under different environmental conditions I. weight loss of substrate. Hoppea 2000, 61, 735–749. [Google Scholar]
- Eubeler, J.P.; Zok, S.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trends Anal. Chem. 2009, 28, 1057–1072. [Google Scholar] [CrossRef]
- Luzier, W.D. Materials derived from biomass/biodegradable materials. Proc. Natl. Acad. Sci. USA 1992, 89, 839–842. [Google Scholar] [CrossRef]
- Briese, B.H.; Jendrossek, D.; Schlegel, H.G. Degradation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by aerobic sewage sludge. FEMS Microbiol. Lett. 1994, 117, 107–111. [Google Scholar] [CrossRef]
- Shin, P.K.; Kim, M.H.; Kim, J.M. Biodegradability of degradable plastics exposed to anaerobic digested sludge and simulated landfill conditions. J. Environ. Polym. Degrad. 1997, 5, 33–39. [Google Scholar]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Steinka, I. Effect of Modification of Poly(ε-Caprolactone) on its Biodegradation in Natural Environments. Int. Polym. Sci. Technol. 2002, 29, 77–84. [Google Scholar] [CrossRef]
- Heimowska, A.; Krasowska, K.; Rutkowska, M. Degradability of Different Packaging Polymeric Materials in Sea Water. Int. Polym. Sci. Technol. 2011, 1, 262–268. [Google Scholar]
- Bastioli, C.; Cerutti, A.; Guanella, I.; Romano, G.C.; Tosin, M. Physical state and biodegradation behavior of starch-polycaprolactone systems. J. Environ. Polym. Degrad. 1995, 3, 81–95. [Google Scholar] [CrossRef]
- Yang, H.-S.; Yoon, J.-S.; Kim, M.-N. Dependence of biodegradability of plastics in compost on the shape of specimens. Polym. Degrad. Stab. 2005, 87, 131–135. [Google Scholar] [CrossRef]
- Hoshino, A.; Sawada, H.; Yokota, M.; Tsuji, M.; Fukuda, K.; Kimura, M. Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci. Plant Nutr. 2001, 47, 35–43. [Google Scholar] [CrossRef]
- Teramoto, N.; Urata, K.; Ozawa, K.; Shibata, M. Biodegradation of aliphatic polyester composites reinforced by abaca fiber. Polym. Degrad. Stab. 2004, 86, 401–409. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Mohd Ishak, Z.A. Biodegradability studies of poly(butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: Effects of clay loading and compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Wang, X.-Q.; Zeng, J.; Yang, G.; Shi, F.-H.; Yan, Q. Biodegradation of poly(butylene succinate) in compost. J. Appl. Polym. Sci. 2005, 97, 2273–2278. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Lee, J.-W.; Choi, I.-G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K.-I. Influences of poly(butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Alvarado, E.; Camacho Montero, J.R.; Rosales, J.M. Atmospheric and soil degradation of aliphatic-aromatic polyester films. Polym. Degrad. Stab. 2010, 95, 99–107. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Bernhard, M.; Eubeler, J.P.; Zok, S.; Knepper, T.P. Aerobic biodegradation of polyethylene glycols of different molecular weights in wastewater and seawater. Water Res. 2008, 42, 4791–4801. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T.; Zembrzuska, J.; Łukaszewski, Z. Comparison of biodegradation of poly(ethylene glycol)s and poly(propylene glycol)s. Chemosphere 2006, 64, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.R.; Alexander, M. Microbial Degradation of Polyethylene Glycols. Appl. Microbiol. 1975, 29, 621–625. [Google Scholar] [CrossRef]
- Obradors, N.; Aguilar, J. Efficient biodegradation of high-molecular-weight polyethylene glycols by pure cultures of Pseudomonas stutzeri. Appl. Environ. Microbiol. 1991, 57, 2383–2388. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.-L.E. The Underwater Environment—Conservation of Marine Archaeological Objects; Butterwort-Heinemann: Oxford, UK, 1987. [Google Scholar]
- Elsevier, Spektrum, Akad. Verl. Lexikon der Biologie; Elsevier, Spektrum, Akad. Verl.: Heidelberg/München, Germany, 2006; ISBN 3-8274-1736-8. [Google Scholar]
- Hofsten, B.V.; Edberg, N. Estimating the Rate of Degradation of Cellulose Fibers in Water. Oikos 1972, 23, 29. [Google Scholar] [CrossRef]
- Lamot, E.; Voets, J.P. Microbial biodegradation of cellophane. Z. Für Allg. Mikrobiol. 1978, 18, 183–188. [Google Scholar] [CrossRef]
- Endres, H.-J.; Siebert-Raths, A. Technische Biopolymere: Rahmenbedingungen, Marktsitutation, Herstellung, Aufbau und Eigenschaften; Hanser: München, Germany, 2009; ISBN 9783446416833. [Google Scholar]
- Itävaara, M.; Siika-aho, M.; Viikari, L. Enzymatic Degradation of Cellulose-Based Materials. J. Environ. Polym. Degrad. 1999, 7, 67–73. [Google Scholar] [CrossRef]
- Ishigaki, T.; Sugano, W.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. The degradability of biodegradable plastics in aerobic and anaerobic waste landfill model reactors. Chemosphere 2004, 54, 225–233. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Gardner, R.M.; Komarek, R.J. Aerobic biodegradation of cellulose acetate. J. Appl. Polym. Sci. 1993, 47, 1709–1719. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-6897-0. [Google Scholar]
- Wüstenberg, T. Cellulose und Cellulosederivate: Grundlagen, Wirkungen und Applikationen, 1. Aufl.; Behr: Hamburg, Germany, 2013; ISBN 9783954680191. [Google Scholar]
- National Oceanic and Atmospheric Administration Marine Debris Program. Director: Nancy Wallace. In Clean Guide; NOAA 101: Washington, DC, USA, 2018. [Google Scholar]
- Costa, S.; Dedola, D.; Pellizzari, S.; Blo, R.; Rugiero, I.; Pedrini, P.; Tamburini, E. Lignin Biodegradation in Pulp-and-Paper Mill Wastewater by Selected White Rot Fungi. Water 2017, 9, 935. [Google Scholar] [CrossRef]
- Vikman, M.; Itävaara, M.; Poutanen, K. Biodegradation of Starch-Based Materials. J. Macromol. Sci. Part A 1995, 32, 863–866. [Google Scholar] [CrossRef]
- Vaverková, M.; Toman, F.; Adamcová, D.; Kotovicová, J. Study of the Biodegrability of Degradable/Biodegradable Plastic Material in a Controlled Composting Environment. Ecol. Chem. Eng. S 2012, 19, 347–358. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef]
- Guzman-Sielicka, A.; Janik, H.; Sielicki, P. Degradation of Polycaprolactone Modified with TPS or CaCO3 in Biotic/Abiotic Seawater. J. Environ. Polym. Degrad. 2012, 20, 353–360. [Google Scholar] [CrossRef]
- Guzman, A.; Janik, H.; Mastalerz, M.; Kosakowska, A. Pilot study of the influence of thermoplastic starch based polymer packaging material on the growth of diatom population in sea water environment. Pol. J. Chem. Technol. 2011, 13, 57–61. [Google Scholar] [CrossRef]
- Bootklad, M.; Kaewtatip, K. Biodegradation of thermoplastic starch/eggshell powder composites. Carbohydr. Polym. 2013, 97, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zain, A.H.M.; Ab Wahab, M.K.; Ismail, H. Biodegradation Behaviour of Thermoplastic Starch: The Roles of Carboxylic Acids on Cassava Starch. J. Environ. Polym. Degrad. 2018, 26, 691–700. [Google Scholar] [CrossRef]
- Vikman, M.; Itävaara, M.; Poutanen, K. Measurement of the biodegradation of starch-based materials by enzymatic methods and composting. J. Environ. Polym. Degrad. 1995, 3, 23–29. [Google Scholar] [CrossRef]
- Di Franco, C.R.; Cyras, V.P.; Busalmen, J.P.; Ruseckaite, R.A.; Vázquez, A. Degradation of polycaprolactone/starch blends and composites with sisal fibre. Polym. Degrad. Stab. 2004, 86, 95–103. [Google Scholar] [CrossRef]
- Mohee, R.; Unmar, G. Determining biodegradability of plastic materials under controlled and natural composting environments. Waste Manag. 2007, 27, 1486–1493. [Google Scholar] [CrossRef]
- Vaz, C.M.; Fossen, M.; van Tuil, R.F.; Graaf, L.A.D.; Reis, R.L.; Cunha, A.M. Casein and soybean protein-based thermoplastics and composites as alternative biodegradable polymers for biomedical applications. J. Biomed. Mater. Res. Part A 2003, 65A, 60–70. [Google Scholar] [CrossRef]
- CRC Press. Enzymes of Psychrotrophs in Raw Food; McKellar, R.C., MacKellar, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0-8493-6103-6. [Google Scholar]
- Mierau, I.; Kunji, E.R.; Venema, G.; Kok, J. Casein and peptide degradation in lactic acid bacteria. Biotechnol. Genet. Eng. Rev. 1997, 14, 279–301. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish; Springer: Dordrecht, The Netherlands, 2007; ISBN 9781402060687. [Google Scholar]
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.-H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559. [Google Scholar] [CrossRef]
- Park, S.K.; Hettiarachchy, N.S.; Were, L. Degradation behavior of soy protein-wheat gluten films in simulated soil conditions. J. Agric. Food Chem. 2000, 48, 3027–3031. [Google Scholar] [CrossRef]
- Lim, S.W.; Jung, I.K.; Lee, K.H.; Jin, B.S. Structure and properties of biodegradable gluten/aliphatic polyester blends. Eur. Polym. J. 1999, 35, 1875–1881. [Google Scholar] [CrossRef]
- John, J.; Tang, J.; Bhattacharya, M. Processing of biodegradable blends of wheat gluten and modified polycaprolactone. Polymer 1998, 39, 2883–2895. [Google Scholar] [CrossRef]
- Zhang, X.; Gozukara, Y.; Sangwan, P.; Gao, D.; Bateman, S. Biodegradation of chemically modified wheat gluten-based natural polymer materials. Polym. Degrad. Stab. 2010, 95, 2309–2317. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Kanavouras, A.; Ng, P.K.W.; Gavara, R. Development and characterization of biodegradable films made from wheat gluten protein fractions. J. Agric. Food Chem. 2003, 51, 7647–7654. [Google Scholar] [CrossRef]
- Gutarowska, B.; Michalski, A. Microbial Degradation of Woven Fabrics and Protection Against Biodegradation; IntechOpen, Rijeka, Croatia. 2012. Available online: https://www.intechopen.com/citation-pdf-url/36909 (accessed on 1 September 2020).
- Beverly, H. Wool in Marine Environments; International Wool Textile Organisation: Brussels, Belgium, 2017. [Google Scholar]
- International Wool Textile Organisation. Wool is Biodegradable; Campaign Wool IWTO: Harrogate, UK, 2014. [Google Scholar]
- Jibia, S.A.; Mohanty, S.; Dondapati, J.S.; O’hare, S.; Rahman, P.K.S.M. Biodegradation of Wool by Bacteria and Fungi and Enhancement of Wool Quality by Biosurfactant Washing. J. Nat. Fibers 2018, 15, 287–295. [Google Scholar] [CrossRef]
- McNeil, S.; Barker, H. The Biodegradability of Wool Enables Wool-to-Grass-to-Wool, Closed-Loop Recycling. Tech. Bull. AgRes. 2015. [Google Scholar]
- Du, L.C.; Meng, Y.Z.; Wang, S.J.; Tjong, S.C. Synthesis and degradation behavior of poly(propylene carbonate) derived from carbon dioxide and propylene oxide. J. Appl. Polym. Sci. 2004, 92, 1840–1846. [Google Scholar] [CrossRef]
- Bahramian, B.; Fathi, A.; Dehghani, F. A renewable and compostable polymer for reducing consumption of non-degradable plastics. Polym. Degrad. Stab. 2016, 133, 174–181. [Google Scholar] [CrossRef]
- Luinstra, G. Poly(Propylene Carbonate), Old Copolymers of Propylene Oxide and Carbon Dioxide with New Interests: Catalysis and Material Properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Dadsetan, M.; Christenson, E.M.; Unger, F.; Ausborn, M.; Kissel, T.; Hiltner, A.; Anderson, J.M. In vivo biocompatibility and biodegradation of poly(ethylene carbonate). J. Control. Release 2003, 93, 259–270. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, M.; Juni, K.; Inoue, S.; Yoshida, Y. Examination of Biodegradability of Poly (ethylene carbonate) and Poly (propylene carbonate) in the Peritoneal Cavity in Rats. Chem. Pharm. Bull. 1983, 31, 1400–1403. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Tominaga, Y. Preparation and characterization of poly(ethylene carbonate)/poly(lactic acid) blends. J. Polym. Res. 2018, 25, 263. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Cheung, H.-Y.; Lau, K.-T.; Xu, C.-L.; Zhao, D.-D.; Li, H.-L. Silkworm silk/poly(lactic acid) biocomposites: Dynamic mechanical, thermal and biodegradable properties. Polym. Degrad. Stab. 2010, 95, 1978–1987. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation ofBombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Sato, K.; Azama, Y.; Nogawa, M.; Taguchi, G.; Shimosaka, M. Analysis of a change in bacterial community in different environments with addition of chitin or chitosan. J. Biosci. Bioeng. 2010, 109, 472–478. [Google Scholar] [CrossRef]
- Krsek, M.; Wellington, E.M. Assessment of chitin decomposer diversity within an upland grassland. Antonie van Leeuwenhoek 2001, 79, 261–267. [Google Scholar] [CrossRef]
- Nakashima, T.; Nakano, Y.; Bin, Y.; Matsuo, M. Biodegradation Characteristics of Chitin and Chitosan Films. J. Home Econ. Jpn. 2005, 56, 889–897. [Google Scholar]
- Sawaguchi, A.; Ono, S.; Oomura, M.; Inami, K.; Kumeta, Y.; Honda, K.; Sameshima-Saito, R.; Sakamoto, K.; Ando, A.; Saito, A. Chitosan degradation and associated changes in bacterial community structures in two contrasting soils. Soil Sci. Plant Nutr. 2015, 61, 471–480. [Google Scholar] [CrossRef]
- Tuomela, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Venelampi, O.; Weber, A.; Rönkkö, T.; Itävaara, M. The Biodegradation and Disintegration of Paper Products in the Composting Environment. Compos. Sci. Util. 2003, 11, 200–209. [Google Scholar] [CrossRef]
- Vikman, M.; Karjomaa, S.; Kapanen, A.; Wallenius, K.; Itävaara, M. The influence of lignin content and temperature on the biodegradation of lignocellulose in composting conditions. Appl. Microbiol. Biotechnol. 2002, 59, 591–598. [Google Scholar]
- Saake, B.; Lehnen, R. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Chichester, UK, 2010; ISBN 978-3-527-30673-2. [Google Scholar]
- Rosa, D.S.; Filho, R.P.; Chui, Q.S.H.; Calil, M.R.; Guedes, C.G.F. The biodegradation of poly-β-(hydroxybutyrate), poly-β-(hydroxybutyrate-co-β-valerate) and poly(ε-caprolactone) in compost derived from municipal solid waste. Eur. Polym. J. 2003, 39, 233–237. [Google Scholar] [CrossRef]
- Development OECD 301A, Organisation for Economic Co-Operation. OECD Guideline for Testing of Chemicals; Development OECD 301A, Organisation for Economic Co-operation: Paris, France, 1992. [Google Scholar]
- TUEV AUSTRIA HOLDING AG 2019. OK Compost Industrial. Available online: https://www.tuv-at.be/fileadmin/user_upload/docs/download-documents/english/Program_OK_01e_e_OK_compost.pdf (accessed on 1 September 2020).
- TUEV AUSTRIA HOLDING AG 2019. OK biodegradable Marine. Available online: https://www.tuv-at.be/fileadmin/user_upload/docs/download-documents/english/OK-compost-OK-compost-HOME-OK-biodegradable-SOIL-WATER-MARINE/20190403_Program_OK_12e_b_OK_biodegradable_MARINE_corrigendum.pdf (accessed on 1 September 2020).
- TUEV AUSTRIA HOLDING AG 2019. OK biodegradable Water. Available online: https://www.tuv-at.be/fileadmin/user_upload/docs/download-documents/english/Program_OK_11e_c_OK_biodegradable_WATER.pdf (accessed on 1 September 2020).
- TUEV AUSTRIA HOLDING AG 2019. OK biodegradable Soil. Available online: https://www.tuv-at.be/fileadmin/user_upload/docs/download-documents/english/Program_OK_10e_c_OK_biodegradable_SOIL.pdf (accessed on 1 September 2020).
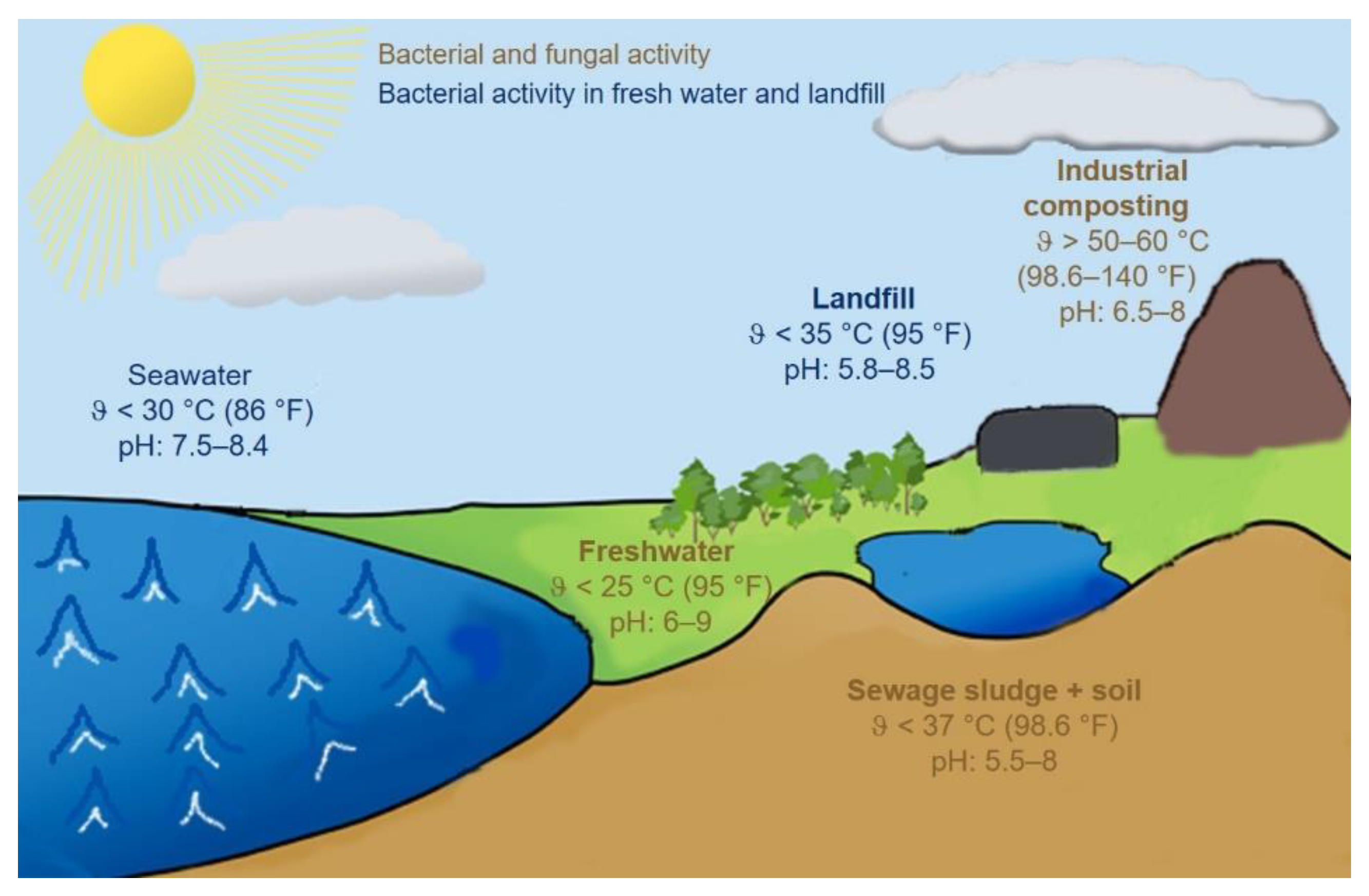
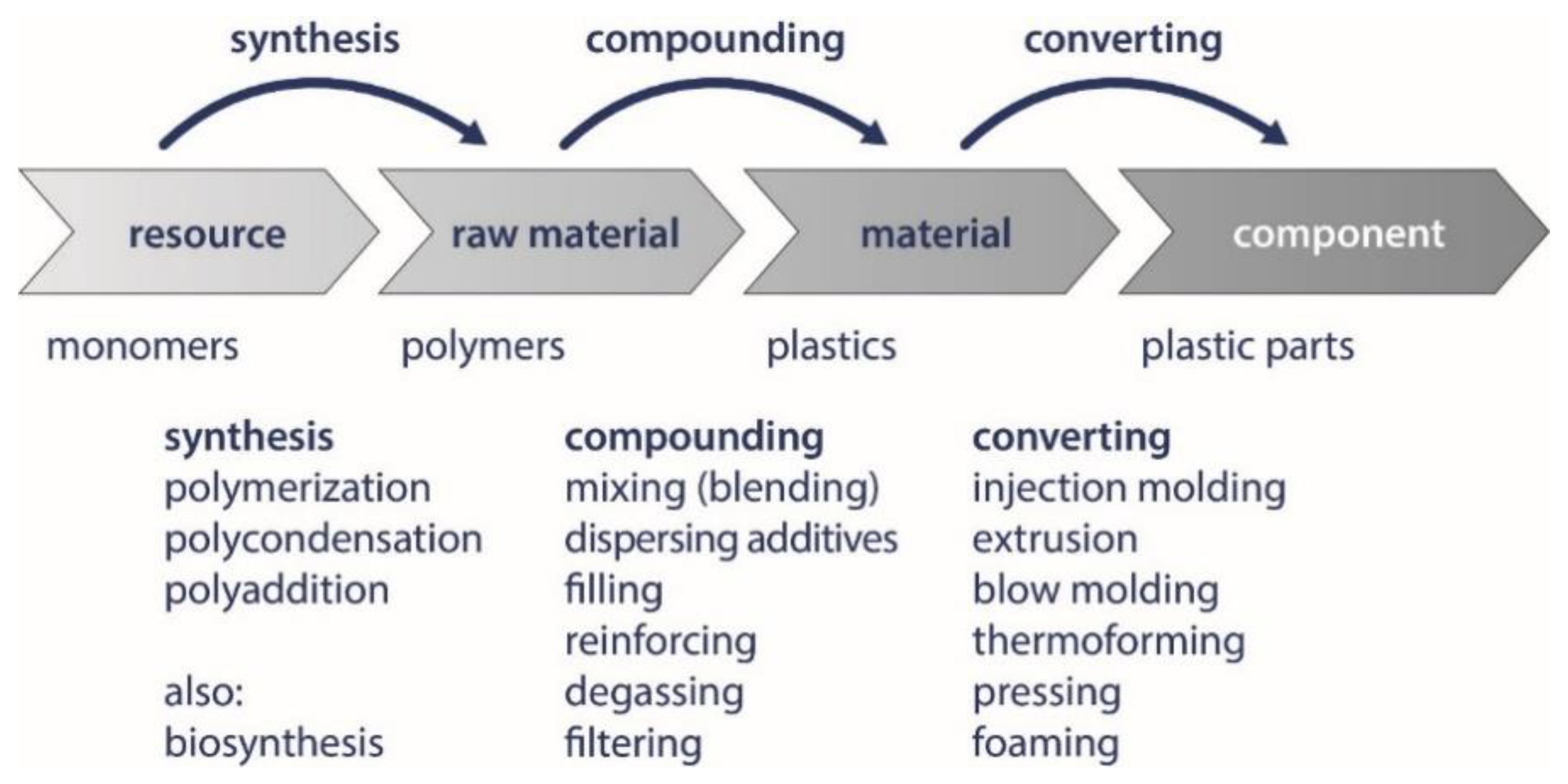
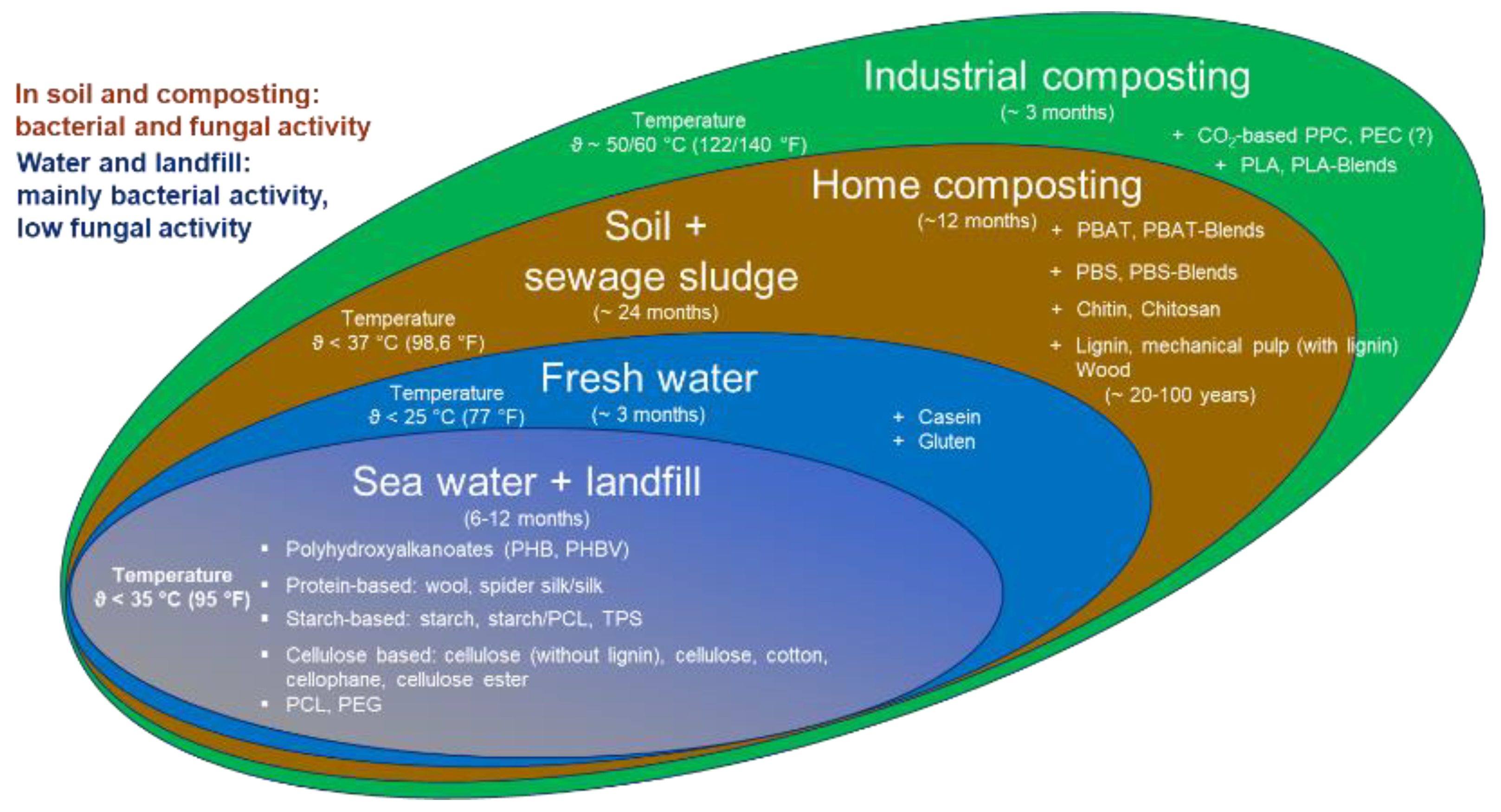
| Physicochemical Conditions | Material Properties | Enzymatic Effects |
|---|---|---|
| Moisture/water content pH value Temperature Availability of oxygen Availability of nutrients Redox potential | Molar mass Polymer composition Steric configuration Size, shape and surface area Melting and glass transition temperature Polymer crystallinity Porosity Material thickness Additives Fillers | Microbial activity Microbial diversity Microbial population density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. https://doi.org/10.3390/ma13204586
Kliem S, Kreutzbruck M, Bonten C. Review on the Biological Degradation of Polymers in Various Environments. Materials. 2020; 13(20):4586. https://doi.org/10.3390/ma13204586
Chicago/Turabian StyleKliem, Silvia, Marc Kreutzbruck, and Christian Bonten. 2020. "Review on the Biological Degradation of Polymers in Various Environments" Materials 13, no. 20: 4586. https://doi.org/10.3390/ma13204586
APA StyleKliem, S., Kreutzbruck, M., & Bonten, C. (2020). Review on the Biological Degradation of Polymers in Various Environments. Materials, 13(20), 4586. https://doi.org/10.3390/ma13204586






