Abstract
The incorporation of functional monomers in dental adhesive systems promotes chemical interaction with dental substrates, resulting in higher adhesion forces when compared to micromechanical adhesion only. The 10-MDP monomer, whose chemical structure allows for a polar behavior which is favorable to adhesion, also promotes the protection of collagen fibers through the formation of MDP-calcium salts. This systematic review aimed to characterize the interface created by 10-MDP containing adhesive systems through an evaluation of the following parameters: Formation of nano-layered structures, capacity to produce an acid-base resistant zone, and adhesion stability. The research was conducted using PubMed, Cochrane Library, Web of Science and Embase, limited to English, Spanish, and Portuguese articles. The research was done according to the PICO strategy. The 10-MDP monomer has the capacity to produce an acid-base resistant zone on the adhesive interface, which increases the response to acid-base challenges. The adhesion established by these systems is stable over time. To have the best of these adhesive solutions, a scrubbing technique must be used to apply the adhesive system on dental substrates, in order to improve monomers infiltration and to create a stable bond. Time must be given for the solution to infiltrate, hybridize and form the MDP-Ca, improving adhesive stability.
1. Introduction
The procedures for performing a resin composite restoration include enamel etching, dentin conditioning, dentin priming and application of a dentin bonding agent, prior to the resin composite filling. However, since the introduction of adhesive resin-based restoration procedures, dental adhesives have been remarkably improved, and most commercially available adhesive systems have been simplified by combining some of the required steps [1,2,3]. In this context, and concerning direct dental restorations, self-etch adhesives and universal adhesives (systems which provide the operator with the choice for selecting the adhesive strategy; etch-and-rinse, self-etch or selective enamel etching) provide the promise for a specific chemical interaction capable of achieving more stable and long-lasting adhesion, with no additional tooth preparation for macro-mechanical retention, when compared to etch-and-rinse adhesives (whose principal adhesion mechanism is related to the micromechanical retention preceded by the removal of the smear layer) [4,5,6]. This interaction occurs due to functional monomers, which are acidic molecules that may serve various functions, such as etching tooth substrates (partially dissolving the smear layer and demineralizing hydroxyapatite), enhancing monomer penetration, and imparting the adhesives with potential for chemical interactions with dental substrates [7,8,9,10,11].
Functional monomers have at least one polymerizable group and a functional group which wets and demineralizes the substrate. According to the ‘adhesion-decalcification’ concept (the functional monomer either decalcifies or bonds to the tooth substrate), the functional group first ionically interacts with calcium in hydroxyapatite; depending on the resulting stability of the calcium-monomer complex, this ionic bond may either decompose and demineralize the tooth surface or remain stable [9,10,11,12].
Functional monomers have already been ranked based on their chemical bonding potential and 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate) has been identified as being capable of establishing a very intensive and stable chemical interaction with hydroxyapatite. The MDP-Ca water-insoluble salts contribute to the protection of the collagen fibers. The atomic relation of the 10-MDP molecule favors the chemical interaction. However, impurities and dimers may reduce adhesive forces when using adhesive systems with this functional monomer [4,9,11,13,14,15].
The intense chemical interaction established between MDP and hydroxyapatite is ascribed to the superficial dissolution of hydroxyapatite induced by the adsorption of MDP, and subsequent deposition of MDP-Ca salts with lower solubility than of the salts produced by other functional monomers [14]. Monomer selection criteria must include properties such as calcium salt stability, wetting of the substrate and copolymerization behavior [16].
The aim for this article was to carry out a systematic review of the literature in order to evaluate the differences between the adhesive interfaces produced by dental adhesives containing 10-MDP and other adhesive systems. The aim was also to evaluate both bond strengths and adhesive stability of dental adhesives containing 10-MDP, in comparison with other systems/functional monomers.
2. Materials and Methods
The protocol used for this systematic review followed the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) [17].
The research strategy of the present work was formulated according to PICO (Problem, Intervention, Comparison, Outcome) as seen in Table 1.

Table 1.
PICO strategy.
2.1. Search Strategy
A literature search was conducted in the Pubmed, Cochrane Library, Web of Science and Embase databases, using the search formulas described in Table 2. Only articles in English, Spanish or Portuguese, published until January 2019 were included.

Table 2.
Research strategy used.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria for selection and extraction of data are described in Table 3.

Table 3.
Inclusion and exclusion criteria.
According to the predetermined inclusion and exclusion criteria, all titles and abstracts were examined by one reviewer (M.C.) in order to find relevant studies; the full texts of the relevant studies were scrutinized by two reviewers (M.C. and E.C.) independently to select eligible studies on the outcomes described in the PICO strategy. Any disagreement was discussed, and the opinion of a third reviewer (A.S.C.) was sought if necessary.
Studies on commercially available adhesive systems were included in order to understand the 10-MDP performance compared to other functional monomers. Studies on specific formulations, where several groups were formed, varying on the concentrations of their components, were also included so that the mechanism of each one could be described or highlighted.
For each proposed outcome and included study, descriptive and quantitative information was extracted, including authors, year of publication, control and test groups, results (quantitative and qualitative) and relevant conclusions.
Due to the disparity of methodology, it was not possible to perform a quantitative analysis (meta-analysis).
3. Results
The initial search resulted in 1383 references: 274 from PubMed, 9 from Cochrane Library, 711 from Web of Science and 389 from Embase.
After evaluating titles and abstracts, 212 relevant studies were obtained. After full-text analysis, 72 references were included in this systematic review (Figure 1).
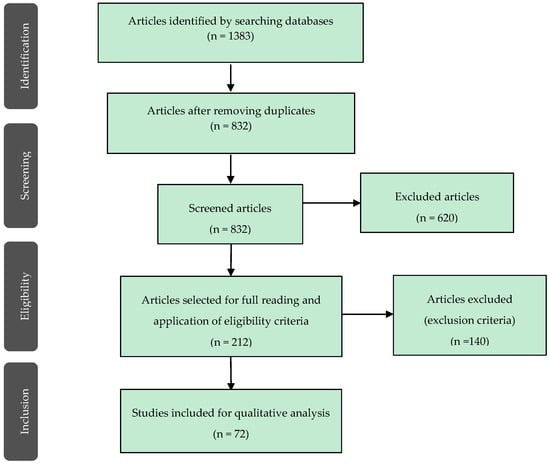
Figure 1.
Search work-flow diagram.
The key characteristics evaluated were:
- Formation of nano-layered structures (MDP-Ca salts)—Formation/absence of nano-layered structures and morphology (Table 4);
 Table 4. Formation of nano-layered structures (MDP-Ca salts).
Table 4. Formation of nano-layered structures (MDP-Ca salts). - Acid-base resistant zone (ABRZ)—Formation or absence of ABRZ, thickness, and differences between dentin ABRZ and enamel ABRZ (Table 5);
 Table 5. Acid-Base Resistant Zone (ABRZ).
Table 5. Acid-Base Resistant Zone (ABRZ). - Adhesive stability—Measurement of adhesion forces (Table 6).
 Table 6. Adhesive stability.
Table 6. Adhesive stability.
3.1. 10-MDP Monomer: Molecular Structure, Hydrophilicity and Nano-Layered Structures
Regarding the molecular structure of the 10-MDP monomer, its hydrophilicity and the formation of nano-layered structures, seven articles were found [5,6,18,19,20,21,22]. Commercially available adhesives systems [6,18,19,20] and experimental adhesive formulations [5,6,18,20,21,22] were used in order to clarify the different behavior between 10-MDP and other monomers used, as well as the different capacities regarding formation of nano-layering at the adhesive interface. The results are summarized in Table 4.
3.2. Capacity to Create an Acid-Base Resistant Zone (ABRZ)
Regarding the capacity of adhesive systems to produce an acid-base resistant zone six articles were found [2,10,23,24,25,26]. Commercially available adhesive systems [10,23,24,25,26] and experimental primer and bond [2,10,11,12,13,14,15,16,17,18,19,20,21,22,23] were used to clarify the capacity of 10-MDP to create an ABRZ compared to other commonly used functional monomers. The results are summarized in Table 5.
3.3. Adhesive Stability
Regarding 10-MDP-related adhesive stability 22 articles were found [1,8,10,11,12,13,15,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39]. Commercially available adhesive systems [1,8,10,23,25,28,29,30,32,33,34,35,36,37,38,39] and experimental adhesive formulations [8,10,11,12,13,15,23,27,31,36,38] were used to evaluate the adhesive stability of the 10-MDP monomer. The results are summarized in Table 6.
4. Discussion
Self-etch and universal adhesive systems were introduced in dentistry to reduce and facilitate the clinical application of these biomaterials, by overcoming some etch-and-rinse disadvantages such as a greater number of steps, longer application time, technique sensitivity and difficulty in controlling dentin wetness [40]. However, these adhesion strategies work less favorably with enamel, as acid etching is not necessary in order to demineralize collagen fibrils. In etch-and-rinse adhesives that step might lead to several micrometers depth of demineralized substrate, especially in dentin, which is not completely hybridized by the bond solution of those systems, promoting degradation, a process initiated by nanoleakage [41]. In mild and ultra-mild self-etch adhesive systems, the abundant presence of hydroxyapatite remaining around the collagen fibrils provides natural protection to the collagen and allows the functional monomers to potentially interact with the substrate. Typical resin tags will only be formed when using strong self-etching adhesives. The potential interaction of self-etch adhesives depends on the surface-preparation method [41,42,43].
Functional monomers are not the only components in adhesive systems formulations so the clinical protocol for self-etching adhesives application cannot be the same for all the commercial systems: different solvents may require changes in the protocols (time, application, …) for better results. Active application of adhesives using a scrubbing technique promotes solvent evaporation, leading to the impregnation of a higher rate of monomers inside the smear layer, thus improving adhesive-interface quality. Solvent evaporation is also dependent on substrate characteristics (orientation of dentin surfaces) and on the uniformity of the adhesive layers. Long-term retention is achieved with high-quality chemical interaction between the adhesive and the substrate, through the formation of a hybrid layer, characterized as a three-dimensional collagen-resin biopolymer that provides a continuous and stable link between the adhesive and the dentin substrate; micromechanical retention may be additionally present when pre-etching the enamel or when using strong self-etching adhesive systems [27,34,43,44]. When talking about adhesive systems, interaction with collagen is probably the most important aspect, since the deterioration of collagen fibrils within the hybrid layer compromises the long-term stability of dentin bonding; the chemical properties of functional monomers are thought to account for the high bond strength with dentin [45].
Self-assembled nano-layered structures have been identified through adhesive interfaces of commercial self-etch and universal adhesive systems, both on enamel and dentin. These structures, which are typical of the 10-MDP monomer, are thought to produce a better water-stable interface which is favorable to adhesion, and may justify the higher adhesive stability of 10-MDP containing adhesive systems, along with the stable MDP-Ca salts [18,19,20,46]. Although nano-layered structures (which can be identified when 10-MDP based adhesives are used) are thought to play an important role in the adhesive stability bond strength, some doubts remain on the actual role of these structures. In fact, nano-layering cannot be responsible for durability of resin-dentin bond since it was not identified through all of the adhesive interfaces of the commercially available adhesive systems. These structures contribute to a higher resistance to biodegradation and to the longevity of the bond by enhancing the immediate performance of the adhesive systems [6,19,47,48].
Functional monomers give adhesive systems formulations the capacity to interact with dental substrates. However, functional monomers may decrease the degree of conversion of camphoroquinone/amine-curing adhesives; this decrease is monomer-dependent, meaning that a different degree of conversion was observed depending on the incorporated monomer and concentration used, but was reduced by simultaneous interaction of the functional monomer with hydroxyapatite [23,49,50]. Also, functional monomers are partially responsible for the hydrophobic/hydrophilic behavior of bonding resins [23,28,30]. Though more hydrophilic spacer carbon chain induces more water sorption and better dentin wettability, more hydrophobic functional monomers (MDP) are more suitable in order to avoid the effects of hydrolytic degradation [21,49,51].
Nanoleakage corresponds to defects at the resin-dentin interface from hydrolytic degradation, which may serve as pathways for degradation; double application self-etch adhesives may contribute to the durability of the bond by building a less permeable layer [33]. Also, applying the adhesive by employing a scrubbing technique enhances resin monomer infiltration of dentin, water chasing on the dentin surface and smear layer dissolution, improving the quality of the adhesive interface, especially on mild self-etching adhesive systems [34].
The 10-MDP monomer has a proven potential to interact with hydroxyapatite; the bond produced by 10-MDP containing adhesives appears to be very stable, as confirmed by the low dissolution rate of its calcium salts in water. Etching capacities are related to the substrate where it is applied, to the incorporated monomer and to the bonding potential of other commonly used functional monomers (4-META, phenyl-P). At different degrees the bonding potential is substantially low, or produces bonds which are not hydrolytically stable [52]. However, adhesion differentials between commercial adhesive systems are noticed depending both on the dental substrate and on other components included in the adhesives formulations. Some universal adhesives were found to produce poor adhesive interfaces by being less 10-MDP concentrated which suggests that an optimal concentration and purity of 10-MDP in self-etch and universal adhesives may exist so the maximum potential of this functional monomer is achieved [5,8,11,12,53].
The 10-MDP monomer has a long and hydrophobic spacer chain and creates a rich MDP-Ca salt adhesive interface, which improves adhesion strength, remaining stable after one year of water-storage [13,15,31,45,54]. Although all the advantages of this monomer, application protocols are crucial (substrate, time and technique) [34,55,56]. The application of an extra hydrophobic layer when using one-step self-etching or universal adhesive systems may improve the adhesive interface (in terms of durability and of resistance to degradation) and increase the long-term retention of restorative materials [57]. When using one bottle, self-etching or universal adhesive systems enamel etching may be recommended since these adhesive systems tend to have higher pH values, which lowers the ability to etch the enamel [58,59].
However, MDP-Ca salts were found to depend on the components that constitute commercial adhesives more strongly than on the concentrations of MDP and water in the adhesive [60]. Water concentration in adhesive systems was found to affect the efficacy of smear layer removal, and dentin bonding performance more strongly than the pH value of the adhesives [61,62] and ethanol was found to limit the dissociation of phosphate groups from the 10-MDP monomer [8]. 4-META was found to enhance both enamel and dentin bond-strengths more effectively than HEMA [63]. Although HEMA tends to improve bond strength, HEMA-free adhesives are preferred because of its hydrophilicity; on the other hand, HEMA brings solvents back into solution [1,54,64]; also, MDP-HEMA aggregates were found to compromise the MDP-collagen interaction leaving collagen fibrils unprotected by MDP and HEMA [21,65,66,67]. Other components may compete for calcium against 10-MDP, such as zinc ions [68]. Calcium hydroxide was found to improve the degree of conversion without interfering with bond strength to dentin, or the extent of nanoleakage [69].
Adhesive systems containing 10-MDP have a proven interest. However, it is important not to forget, when using strong self-etching adhesive systems, that the adhesive solution may penetrate into dentinal tubules and reach the pulp, especially when restoring deep cavities. Current studies on cytotoxicity lack a complete understanding of the effect of these materials on the pulp, because it is difficult to mimic the clinical conditions of its application. Some studies have reported that minimally toxic concentrations of 10-MDP promoted an inflammatory response and suppressed odontoblastic differentiation of dental pulp cells [70,71]. Also, chemical properties of MDP-containing adhesives alter during storage because MDP hydrolysis leads to acidification of the adhesive solutions [72,73].
5. Conclusions
When selecting a functional monomer or adhesive system, 10-MDP monomer appears to be a safe choice because of its molecular structure which is favorable to adhesion, its hydrophobic behavior and characteristic adhesive interface which favors bond durability and strength.
10-MDP containing dental adhesives are biomaterials which can establish strong and durable adhesive interfaces. Although 10-MDP has a proven capacity to interact with hydroxyapatite, some clinical steps of application of these adhesives are crucial for the resultant bond interface.
To have the best of these adhesive solutions, selective enamel etching and a scrubbing technique must be used to apply the adhesive system on dental substrates, in order to improve monomers infiltration and to create a stable bond. Time must be given for the solution to infiltrate, hybridize and form the MDP-Ca, protecting collagen fibrils and improving adhesive stability.
Funding
This research received no external funding
Acknowledgments
To Helena Donato, director of the documentation service of Coimbra Hospital and University Centre.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z.; Tian, F.; Niu, L.; Ochala, K.; Chen, C.; Fu, B.; Wang, X.; Pashley, D.; Tay, F. Defying ageing: An expectation for dentine bonding with universal adhesives? J. Dent. 2016, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Ichikawa, C.; Li, N.; Takagaki, T.; Sadr, A.; Yoshida, Y.; Suzuki, K.; Tagami, J. Effect of functional monomers in all-in-one adhesive systems on formation of enamel/dentin acid-base resistant zone. Dent. Mater. J. 2011, 30, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Itoh, K.; Tani, C.; Manabe, A.; Yamashita, T.; Hisamitsu, H.; Wakumoto, S. Effectiveness of simplified dentin bonding systems. Dent. Mater. J. 1998, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Etching Efficacy of Self-Etching Functional Monomers. J. Dent. Res. 2018, 97, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T. Layering mechanism of MDP-Ca salt produced in demineralization of enamel and dentin apatite. Dent. Mater. 2017, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J. Dent. Res. 2016, 95, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhang, L.; Liang, B.; Tang, T.; Fu, B.; Hannig, M. The contribution of chemical bonding to the short- and long-term enamel bond strengths. Dent. Mater. 2013, 29, e103–e112. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Yoshida, Y.; Hirata, I.; Snauwaert, J.; De Munck, J.; Okazaki, M.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Influence of the chemical structure of functional monomers on their adhesive performance. J. Dent. Res. 2008, 87, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Nikaido, T.; Takagaki, T.; Sadr, A.; Makishi, P.; Chen, J.; Tagami, J. The role of functional monomers in bonding to enamel: acid-base resistant zone and bonding performance. J. Dent. 2010, 38, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H.; Nishiyama, N. Effect of calcium salt of functional monomer on bonding performance. J. Dent. Res. 2012, 91, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Ogliari, F.A.; Van Meerbeek, B.; Watson, T.F.; Yoshihara, K.; Ogliari, A.O.; Sinhoreti, M.A.; Correr, A.B.; Cama, G.; Sauro, S. Can the hydrophilicity of functional monomers affect chemical interaction? J. Dent. Res. 2014, 93, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Ogliari, A.O.; Yoshihara, K.; Zanchi, C.H.; Correr-Sobrinho, L.; Sinhoreti, M.A.; Correr, A.B.; Watson, T.F.; et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent. Mater. 2014, 30, e317–e323. [Google Scholar] [CrossRef] [PubMed]
- Salz, U.; Mucke, A.; Zimmermann, J.; Tay, F.R.; Pashley, D.H. pKa value and buffering capacity of acidic monomers commonly used in self-etching primers. J. Adhes. Dent. 2006, 8, 143–150. [Google Scholar] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minagi, S.; et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled Nano-layering at the Adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; De Munck, J.; Maruo, Y.; Nishigawa, G.; Minagi, S.; Osaka, A.; et al. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent. Mater. 2013, 29, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Tochio, N.; Kigawa, T.; Otsuki, M.; Tagami, J. Role of 2-hydroxyethyl methacrylate in the interaction of dental monomers with collagen studied by saturation transfer difference NMR. J. Dent. 2014, 42, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Nishiyama, N. Determination of molecular species of calcium salts of MDP produced through decalcification of enamel and dentin by MDP-based one-step adhesive. Dent. Mater. J. 2015, 34, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Matsui, N.; Takagaki, T.; Sadr, A.; Ikeda, M.; Ichinose, S.; Nikaido, T.; Tagami, J. The role of MDP in a bonding resin of a two-step self-etching adhesive system. Dent. Mater. J. 2015, 34, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nurrohman, H.; Nikaido, T.; Takagaki, T.; Sadr, A.; Ichinose, S.; Tagami, J. Apatite crystal protection against acid-attack beneath resin-dentin interface with four adhesives: TEM and crystallography evidence. Dent. Mater. 2012, 28, e89–e98. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Takagaki, T.; Matsui, N.; Sato, T.; Burrow, M.F.; Palamara, J.; Nikaido, T.; Tagami, J. Dentin bonding performance using Weibull statistics and evaluation of acid-base resistant zone formation of recently introduced adhesives. Dent. Mater. J. 2016, 35, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Nurrohman, H.; Takagaki, T.; Sadr, A.; Ichinose, S.; Tagami, J. Nanoleakage in Hybrid Layer and Acid-Base Resistant Zone at the Adhesive/Dentin Interface. Microsc. Microanal. 2015, 21, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Kikutake, K.; Nemoto, K. Influence of self-etching primer treatment on the adhesion of resin composite to polished dentin and enamel. Dent. Mater. 1998, 14, 99–105. [Google Scholar] [CrossRef]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; De Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic stability of self-etch adhesives bonded to dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ma, S.; Aida, M.; Maeda, T.; Ikemi, T.; Hirata, M.; Nishiyama, N. Effect of reacted acidic monomer with calcium on bonding performance. J. Dent. Res. 2011, 90, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Harnirattisai, C.; Roengrungreang, P.; Rangsisiripaiboon, U.; Senawongse, P. Shear and micro-shear bond strengths of four self-etching adhesives measured immediately and 24 hours after application. Dent. Mater. J. 2012, 31, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Effect of calcium salt of 10-methacryloyloxydecyl dihydrogen phosphate produced on the bond durability of one-step self-etch adhesive. Dent. Mater. J. 2014, 33, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Anchieta, R.B.; Machado, L.S.; Martini, A.P.; Santos, P.H.; Giannini, M.; Janal, M.; Tovar, N.; Sundfeld, R.H.; Rocha, E.P.; Coelho, P.G. Effect of long-term storage on nanomechanical and morphological properties of dentin-adhesive interfaces. Dent. Mater. 2015, 31, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.A.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.H.; Loguercio, A.D. In Vitro Longevity of Bonding Properties of Universal Adhesives to Dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Thanatvarakorn, O.; Prasansuttiporn, T.; Takahashi, M.; Thittaweerat, S.; Foxton, R.M.; Ichinose, S.; Tagami, J.; Nakajima, M. Effect of Scrubbing Technique with Mild Self-etching Adhesives on Dentin Bond Strengths and Nanoleakage Expression. J. Adhes. Dent. 2016, 18, 197–204. [Google Scholar] [PubMed]
- Wang, R.; Shi, Y.; Li, T.; Pan, Y.; Cui, Y.; Xia, W. Adhesive interfacial characteristics and the related bonding performance of four self-etching adhesives with different functional monomers applied to dentin. J. Dent. 2017, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine—Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.C.S.; de Andrada, M.A.C.; Boushell, L.W.; Walter, R. Assessment of the initial and aged dentin bond strength of universal adhesives. Int. J. Adhes. Adhes. 2016, 70, 53–61. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Takamizawa, T.; Barkmeier, W.W.; Tsubota, K.; Tsujimoto, A.; Berry, T.P.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Effect of a functional monomer (MDP) on the enamel bond durability of single-step self-etch adhesives. Eur. J. Oral Sci. 2016, 124, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Watanabe, H.; Johnson, W.W.; Latta, M.A.; Miyazaki, M. Comparison between universal adhesives and two-step self-etch adhesives in terms of dentin bond fatigue durability in self-etch mode. Eur. J. Oral Sci. 2017, 125, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hass, V.; Abuna, G.; Feitosa, V.P.; Martini, E.C.; Sinhoreti, M.A.; Carvalho, R.F.; Bandéca, M.; Sauro, S.; Loguercio, A.D. Self-Etching Enamel Bonding Using Acidic Functional Monomers with Different-length Carbon Chains and Hydrophilicity. J. Adhes. Dent. 2017, 19, 497–505. [Google Scholar] [PubMed]
- Hoshika, S.; Kameyama, A.; Suyama, Y.; De Munck, J.; Sano, H.; Van Meerbeek, B. GPDM- and 10-MDP-based Self-etch Adhesives Bonded to Bur-cut and Uncut Enamel—“Immediate” and “Aged” µTBS. J. Adhes. Dent. 2018, 20, 113–120. [Google Scholar] [PubMed]
- Mine, A.; De Munck, J.; Cardoso, M.V.; Van Landuyt, K.L.; Poitevin, A.; Van Ende, A.; Matsumoto, M.; Yoshida, Y.; Kuboki, T.; Yatani, H.; et al. Dentin-smear remains at self-etch adhesive interface. Dent. Mater. 2014, 30, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Leal, F.B.; Madruga, F.C.; Prochnow, E.P.; Lima, G.S.; Ogliari, F.A.; Piva, E.; Moraes, R. Effect of acidic monomer concentration on the dentin bond stability of self-etch adhesives. Int. J. Adhes. Adhes. 2011, 31, 571–574. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, C.Y.; Zhang, L.; Zhang, Z.L.; Fu, B.P.; Hannig, M. Influence of priming time and primer’s concentrations on bovine enamel bond strengths. J. Adhes. Sci. Technol. 2013, 27, 2558–2570. [Google Scholar] [CrossRef]
- Hiraishi, N.; Tochio, N.; Kigawa, T.; Otsuki, M.; Tagami, J. Monomer-collagen interactions studied by saturation transfer difference NMR. J. Dent. Res. 2013, 92, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Fujita, K.N.; Uchida, R.; Aida, E.; Aoki, N.T.; Aida, M.; Nishiyama, N. Quantitative Evaluation of MDP-Ca Salt and DCPD after Application of an MDP-based One-step Self-etching Adhesive on Enamel and Dentin. J. Adhes. Dent. 2016, 18, 205–213. [Google Scholar] [PubMed]
- Tian, F.C.; Wang, X.Y.; Huang, Q.; Niu, L.N.; Mitchell, J.; Zhang, Z.Y.; Prananik, C.; Zhang, L.; Chen, J.H.; Breschi, L.; et al. Effect of nanolayering of calcium salts of phosphoric acid ester monomers on the durability of resin-dentin bonds. Acta Biomater. 2016, 38, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Torii, Y.; Osaka, A.; Suzuki, K.; Minagi, S.; Van Meerbeek, B.; Van Landuyt, K.L. Self-etch monomer-calcium salt deposition on dentin. J. Dent. Res. 2011, 90, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Yoshihara, K.; Yoshida, Y.; Okihara, T.; Yamamoto, T.; Momoi, Y.; Van Meerbeek, B. Interference of functional monomers with polymerization efficiency of adhesives. Eur. J. Oral Sci. 2016, 124, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, F.M.; Moosavi, H.; Atai, M.; Zeynali, M. Dentin bond strength and degree of conversion evaluation of experimental self-etch adhesive systems. J. Clin. Exp. Dent. 2015, 7, e243–e249. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Nakajima, K.; Aoki-Tabei, N.; Arita, A.; Nishiyama, N. NMR study on the demineralization mechanism of the enamel and dentin surfaces in MDP-based all-in-one adhesive. Dent. Mater. J. 2018, 37, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Fu, J.; Nakaoki, Y.; Ikeda, T.; Tanaka, T.; Sano, H. Improved long-term bonding performance of an experimental all-in-one adhesive. Dent. Mater. J. 2013, 32, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Tsubota, K.; Iwasa, M.; Ando, S.; Miyazaki, M.; Platt, J.A. Influence of Adhesive Application Time on Enamel Bond Strength of Single-step Self-etch Adhesive Systems. Oper. Dent. 2010, 35, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mine, A.; De Munck, J.; Cardoso, M.V.; Van Landuyt, K.L.; Poitevin, A.; Kuboki, T.; Yoshida, Y.; Suzuki, K.; Van Meerbeek, B. Enamel-Smear Compromises Bonding by Mild Self-Etch Adhesives. J. Dent. Res. 2010, 89, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Leite, T.M.; Matte, K.; Michels, R.; Amaral, R.C.; Geraldeli, S.; Loguercio, A.D. Improving clinical retention of one-step self-etching adhesive systems, with an additional hydrophobic adhesive layer. J. Am. Dent. Assoc. 2009, 140, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takagaki, T.; Matsui, N.; Hamba, H.; Sadr, A.; Nikaido, T.; Tagami, J. Morphological Evaluation of the Adhesive/Enamel Interfaces of Two-step Self-etching Adhesives and Multimode One-bottle Self-etching Adhesives. J. Adhes. Dent. 2016, 18, 223–229. [Google Scholar] [PubMed]
- Beltrami, R.; Chiesa, M.; Scribante, A.; Allegretti, J.; Poggio, C. Comparison of shear bond strength of universal adhesives on etched and nonetched enamel. J. Appl. Biomater. Funct. Mater. 2016, 14, E78–E83. [Google Scholar] [CrossRef] [PubMed]
- Fujita Nakajima, K.; Nikaido, T.; Arita, A.; Hirayama, S.; Nishiyama, N. Demineralization capacity of commercial 10-methacryloyloxydecyl dihydrogen phosphate-based all-in-one adhesive. Dent. Mater. 2018, 34, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Teshima, M. Effect of the concentration of water in an MDP-based all-in-one adhesive on the efficacy of smear layer removal and on dentin bonding performance. Dent. Mater. J. 2018, 37, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Tsujimoto, A.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Platt, J.A. Important compositional characteristics in the clinical use of adhesive systems. J. Oral Sci. 2014, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H.; Fujita, K.; Iwai, H.; Ikemi, T.; Goto, H.; Aida, M.; Nishiyama, N. Development of MDP-based one-step self-etch adhesive -Effect of additional 4-META on bonding performance. Dent. Mater. J. 2013, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fujita Nakajima, K.; Nikaido, T.; Francis Burrow, M.; Iwasaki, T.; Tanimoto, Y.; Hirayama, S.; Nishiyama, N. Effect of the demineralisation efficacy of MDP utilized on the bonding performance of MDP-based all-in-one adhesives. J. Dent. 2018, 77, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, M.; Inoue, G.; Nikaido, T.; Ikeda, M.; Sadr, A.; Tagami, J. Effect of fluoride concentration in adhesives on morphology of acid-base resistant zones. Dent. Mater. J. 2013, 32, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wurihan; Shibata, Y.; Tanaka, R.; Zhang, Z.; Zheng, K.; Li, Q.; Ikeda, S.; Gao, P.; Miyazaki, T. Quantitative/qualitative analysis of adhesive-dentin interface in the presence of 10-methacryloyloxydecyl dihydrogen phosphate. J. Mech. Behav. Biomed. Mater. 2019, 92, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Pomacondor-Hernandez, C.; Ogliari, F.A.; Leal, F.; Correr, A.B.; Sauro, S. Chemical interaction of 10-MDP (methacryloyloxi-decyl-dihydrogen-phosphate) in zinc-doped self-etch adhesives. J. Dent. 2014, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.G.; Poskus, L.T.; Hass, V.; Amaral, C.M.; Noronha-Filho, J.D.; Silva, E.M.D. Effect of Calcium Hydroxide on Bonding Performance of an Experimental Self-etch Adhesive. J. Adhes. Dent. 2018, 20, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Park, H.; Lee, S.I.; Kim, S.Y. Effect of the Acidic Dental Resin Monomer 10-methacryloyloxydecyl Dihydrogen Phosphate on Odontoblastic Differentiation of Human Dental Pulp Cells. Basic Clin. Pharmacol. Toxicol. 2015, 117, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Putzeys, E.; Duca, R.C.; Coppens, L.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. In-vitro transdentinal diffusion of monomers from adhesives. J. Dent. 2018, 75, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Teshima, I. Degradation of 10-Methacryloyloxydecyl Dihydrogen Phosphate. J. Dent. Res. 2010, 89, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Odaki, M.; Fujita, K.; Kitagawa, T.; Teshima, I.; Suzuki, K.; Nishiyama, N. Degradation-stage effect of self-etching primer on dentin bond durability. J. Dent. Res. 2009, 88, 443–448. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).