Abstract
As the sole metal that could reduce CO2 to substantial amounts of hydrocarbons, Cu plays an important role in electrochemical CO2 reduction, despite its low energy efficiency. Surface morphology modification is an effective method to improve its reaction activity and selectivity. Different from the pretreated modification method, in which the catalysts self-reconstruction process was ignored, we present operando synthesis by simultaneous electro-dissolution and electro-redeposition of copper during the CO2 electroreduction process. Through controlling the cathodic potential and CO2 flow rate, various high-curvature morphologies including microclusters, microspheres, nanoneedles, and nanowhiskers have been obtained, for which the real-time activity and product distribution is analyzed. The best CO2 electro-reduction activity and favored C2H4 generation activity, with around 10% faradic efficiency, can be realized through extensively distributed copper nanowhiskers synthesized under 40 mL/min flow rate and −2.1 V potential.
1. Introduction
Excessive CO2 emission has caused severe climate and environment problems, which is breaking the sustainability of human society. Thus, there is an urgent demand for converting CO2 into fuels or commodity chemicals. Compared with other methods of CO2 conversion, including thermochemical, photochemical, and biochemical methods, the electrochemical reduction method is particularly important and attractive in view of the following merits: (1) Feasible combination with renewable sources like solar, wind, and nuclear energy; (2) low cost and safe operating conditions; (3) easy control over reaction pathways via changing the potential, electrocatalysts, and electrolytes. In recent years, there has been plenty of interest absorbed into the electrochemical reduction of CO2, which reduces carbon dioxide to multi-hydrocarbons at ambient temperature [1,2,3,4], facilitating the carbon cycle and relieving energy problems. Amongst various metal electrocatalysts studied in aqueous systems, Cu has a unique ability to reduce carbon dioxide to substantial amounts of hydrocarbons, such as CH4, C2H4, and HCOOH [5,6,7]. Unfortunately, the energy efficiency of Cu for CO2 electroreduction is low, such that high over-potential must be performed to activate inert CO2 molecules, which gives rise to many efforts for improving activity and selectivity of the CO2 reduction reaction (CO2RR) on copper.
The intrinsic complexity of multi-electron transfer reaction, CO2RR activity, and selectivity was highly affected by the composition and morphology of catalysts, electrolytes, and pH value. Various metal catalysts could be categorized into four groups based on their product distributions: (1) Cu, the only metal capable of reducing CO2 to hydrocarbons at significant rates; (2) Au, Ag, Zn and Pd, the major product of each is CO; (3) Pb, Hg, In, Sn, Cd and Bi, which primarily produce formate; and (4) Ni, Fe, Pt and Ti, where only hydrogen evolution is observed, instead of CO2RR activity, at the steady state [8]. Oxide-derived metallic catalysts exhibit superior CO2RR performance, as the intentional oxidation and reduction of metallic electrodes could contribute to more active surface sites [9,10,11]. For example, oxide-derived Au has shown the highest CO production faradaic efficiency, 96%, in 0.5 M NaHCO3 [10]. Electrolytes were seen to be a key role in controlling the selectivity of the reaction, because of the different nature of the ions in the electrolyte [12]. For instance, cationic species (Li+, Na+, K+ and Cs+) in bicarbonate solutions can be used to control the CH4/C2H4 ratio [13]. In addition, pH is also an important parameter for CO2RR. CO2 reduction is usually carried out in bicarbonate electrolytes at a close-neutral pH, since CO2 acts as a buffer. For CO reduction at a pH of 6~12, the CH4 formation is pH dependent, while C2H4 is independent of pH [14]. For selectivity of Cu, dilute KHCO3 electrolytes with an alkaline pH results in high selectivity for C2H4 [15]. When the concentration of bicarbonate is high the local pH remains close to neutral, favoring CH4 and H2 production [16]. Modifying the surface morphology of copper has been concluded to be an effective method to improve its activity and selectivity toward the CO2 electro-reduction [17,18,19,20]. Generally, different pretreatments were used to change morphologies of copper electrodes before the electrochemical measurement of CO2RR. For example, the electrodes, which were electropolished, covered with nanoparticles, and sputtered with argon ions, provided an abundance of under-coordinated sites on the roughened surface (nanoparticle covered surface) and enabled higher selectivity towards hydrocarbons [17]. In addition, the bromide-promoted morphology of copper dendrites was proven to be a highly selective electrocatalyst, which reduced CO2 to ethylene with a faradic efficiency of 57% at a high current density of 170 mA/cm2 [21]. The selectivity of these kinds of copper electrodes was caused by the high-index faces and the under-coordinated sites on the high-curvature structures; the identification of active sites and the recognition of corresponding catalytic mechanisms were generally based on such pretreated morphology. Thus, a key question is whether the pretreated morphology could be held unchangeable during the electrochemical reduction. Actually, most catalysts undergo morphology self-reconstruction during measurements [22,23], which is always neglected. Thus, the true morphology of copper during CO2 electroreduction is unknown, which makes it difficult to identify the real catalytically active sites and hinders the understanding of catalytic mechanisms.
Here, operando synthesis of high-curvature morphology of copper thin film was realized by application of simultaneous electro-dissolution and electro-redeposition during electrochemical reduction of CO2. Subjected to different bias potentials and different flow rates of CO2, a morphological evolution of copper from original granular, to microclusters, microspheres, nanoneedles, and nanowhiskers respectively, was observed. The resultant real-time CO2RR activity and product selectivity were also discussed. As a result, the correlation between the property and structure of such kind of copper catalyst is straightforward, which improves the understanding of CO2RR mechanism and offers guidelines of rational design for advanced electrocalysts.
2. Experimental Section
2.1. Preparation of Original Copper Thin Film Electrode.
Copper thin film was prepared by electrodeposition in an aqueous electrolyte composed of 0.25 M copper sulfate solution and 50 mM sulfuric acid. NiTi shape memory alloy (SMA) was a typical smart metal that could remember its original shape after deformation, when heated, due to its reversible martensitic transformation [24]. A near-equiatomic NiTi sheet, sized of 10 mm × 10 mm × 1 mm, was used as the substrate for electrodepostion due to its negligible CO2RR activity and good chemical stability in the electrolyte. In addition, in our later work, the two-way shape memory effect of NiTi [25] was used to induce elastic strain to Cu nanofilm and study the strain effect [26] on the CO2 reduction reaction of Cu. A three-electrode cell including NiTi as working electrode, Pt mesh as counter electrode, and Ag/AgCl (1M Cl−) as reference electrode was used. All potentials reported in this paper are quoted with Ag/AgCl. The polycrystalline Cu film was deposited at room temperature by chronoamperometry at −0.34 V. After 300 s deposition, the electrode was taken out and rinsed gently with deionized water several times.
2.2. Operando Synthesis of High-Curvature Copper During Electroreduction Of CO2
Electroreduction of CO2 was conducted at the ambient temperature in an H-type two compartment cell, as shown in Figure 1. A standard three-electrode system was used, employing Cu/NiTi working electrode, a Pt mesh counter electrode, and an Ag/AgCl reference electrode. A CO2-saturated 0.1 M KHCO3 aqueous solution was used as the electrolyte for CO2RR. Firstly, a linear sweep voltammetry (LSV) technique was employed to get the CO2 RR activity of the original copper thin film, and then a chronoamperometry technique was used to perform CO2RR for 1 h at four specific potentials with continuous CO2 bubbles in the solution, during which process the electro-dissolution and electro-redeposition of copper ions occurred and resulted in high-curvature morphology. In addition, the CO2 flow rate effect on the surface morphology of operando synthesized copper was studied.
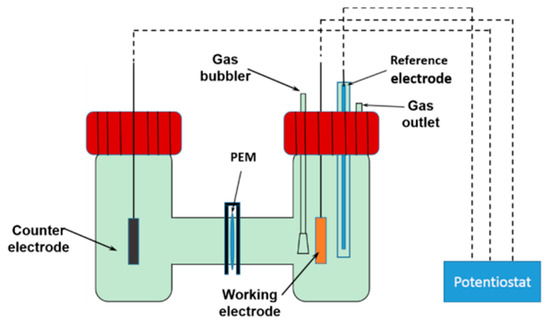
Figure 1.
A schematic drawing of H-type cell.
2.3. Characterization of CO2RR Activity and Products Distribution of High-Curvature Copper
I-t curves at different bias potentials during operando synthesis of copper were shown to evaluate the activity of CO2RR, and a gas chromatograph (GC) was connected to the vent of H-type electrochemical cell for gaseous products real-time analysis. The GC was equipped with a thermal conductivity detector (TCD) to quantify hydrogen and a hydrogen flame ionization detector (FID), equipped with a methanizer to quantify carbon monoxide, methane, and ethylene, was connected to the vent of the H-type electrochemical cell for real-time gaseous products analysis. The parameters of the GC were set as follows: Oven temperature was 360 °C, TCD temperature was 120 °C, FID temperature was 150 °C, and the column temperature was 70 °C.
2.4. Characterization of Surface Morphology and Composition of Copper Thin Films
Samples were rinsed gently with deionized water after a 1 h reaction. The surface morphology and composition of the Cu film, synthesized in situ under different potentials and different CO2 flow rates, were analyzed through a scanning electron microscope (SEM) and energy spectrum analysis (EDS).
3. Results and Discussion
3.1. Similar Morphology and Catalytic Activity of Original Electrodeposited Films
Original morphology of electrodeposited copper thin film is shown in Figure 2a, which indicates the typical granular morphology arising from island growth in eletrodeposition. It is noted that four samples swept to different end potentials had a similar granular morphology with the similar roughness and sub-micro particle size. Linear sweep voltammetry (LSV), at a scan rate of 50 mV/s, is used to provide a quantitative assessment of the catalytic performance of four studied electrodes. We note that they have the same onset potential of about −0.9 V and almost the same CO2RR kinetics (indicated by the slope of LSV curves in the Figure 2b).
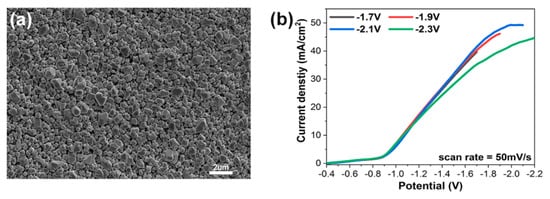
Figure 2.
Characterization of electrodeposited copper thin film electrode. (a) SEM image of electrodeposited Cu on NiTi substrate in a solutioin of 0.25 M CuSO4 and 50 mM H2SO4. (b) Linear sweep voltammogrames of different ending potentials working electrodes in CO2-saturated 0.1 M KHCO3.
3.2. Effect of Cathodic Potential on the Morphology and Product Selectivity of Copper Films
It is known that cathodic potential, as an important parameter in CO2RR, not only determines the pathway of reaction but also affects the copper reconstruction process (shown schematically in Figure 3), e.g., more negative potentials could facilitate the nucleation rate of copper [27,28,29,30]. Therefore, it would be informative to probe the correlations between the as-formed morphology and the resultant CO2RR activity. Depending on the applied potential, different structural morphologies emerged. Various morphologies of operando-synthesized copper could be observed, shown in Figure 4. At −1.7 V, the surface is mainly covered with microspheres, with a diameter of about 4~6 μm, and scattered nanometer needles, with a length of about 1~3 μm, which changed to a flower-like nanoneedle, with a length of about 5~7 μm, at −1.9 V. As the overpotential increased to −2.1 V, sharper nanowhiskers, with the length of about 6~10 μm and a length-to-diameter ratio of 30~70, appeared rapidly and then turned into a uniformly dispersed short length of about 3 μm nanowhiskers, with a length-to-diameter ratio of 5~10, at −2.3 V. This morphology evolution was due to tip effect and hydrogen bubble generation (which served as dynamic template for redeposition) during the simultaneous dissolution and redeposition of Cu ions. We also analyzed the element composition of the high-curvature morphologies (shown in Figure 5 and Table 1). The high-curvature morphology is composed of a mixture of Cu and its oxidation state. Such copper oxides would contribute to relatively higher CO2RR activity than pure Cu [31].
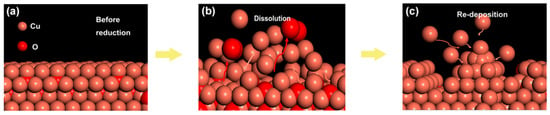
Figure 3.
Schematic of reconstruction process, whereby simultaneous dissolution and redeposition occurred. (a) Surface morphology of the electrode before the reaction. (b) Dissolution of the electrode surface. (c) Redeposition of the electrode surface.

Figure 4.
SEM images of different electrodes morphologies synthesized under corresponding bias potentials.
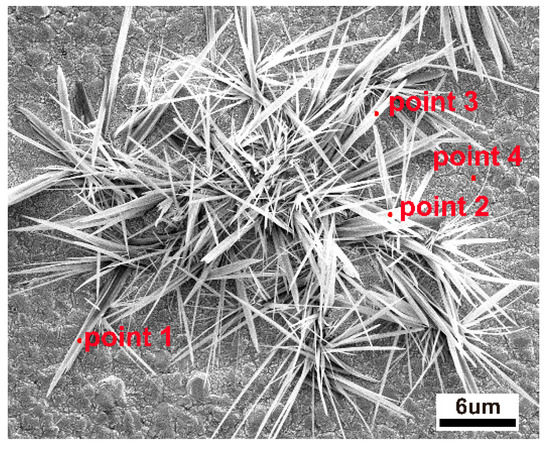
Figure 5.
EDS analytical points on the high-curvature morphologies of Cu.

Table 1.
The atomic percentage of copper and oxygen elements on high-curvature morphologies.
CO2RR activity of various high-curvature coppers was revealed from chronoamperometric i-t curves. With continuous CO2 bubbled at a flow rate of 40 mL/min, the chronoamperometry technique was adopted to perform CO2RR for 1 h at four specific potentials. As shown in Figure 6c, the reduction current increased with more negative potentials. Meanwhile, the total reduction current of different reaction potential was stable. Though the total reaction current was as high as about 45 mA/cm2 at −2.3 V, it was revealed by gas chromatography that the major reduction current owed to the electroreduction of water (2H2O* + 2e−→H2 + 2OH−) instead of CO2. For CO2RR, the main gas products were CO, CH4 and C2H4, which were monitored every 15 min. As shown in Figure 6a, the percentage of the three main CO2RR products changed with bias potentials. At −1.7 V and −1.9 V, CO was the dominant product with percentages of 59% and 73%. When the more negative potential was applied for operando synthesis, like −2.1 V and −2.3 V, C2H4 proportion increased to 65% and 60% of the three main gas products as a preferred product. It was speculated that higher local electric field around high-curvature nanowhiskers of copper could improve bubble nucleation rates and cation stabilization, which favored C2H4 formation during electroreduction of CO2 [15,27,32]. As was shown by the energy spectrum, high-curvature morphology contained the oxidation state of Cu, which also made the electrode exhibit good ethylene selectivity [33,34]. Additionally, we also compared C2/C1 product ratios of various high-curvature copper (Figure 6b), and found that widely distributed nanowhiskers of copper had a high C2/C1 product ratio (about 1:6), which was more than five times than that synthesized under −1.7 V and −1.9 V. Moreover, C2/C1 product ratio of different morphological copper fluctuated slightly within 1 h of the operando synthesis process, as shown in Figure 6b, and it was surprising to see an increasing C2/C1 product ratio of nanorod copper, synthesized under −2.3 V, from 1.1- at the first quarter to 1.8 at the fourth quarter of the hour. This be caused by the increase of the nanowhisker distribution area in the copper electro-dissolution and electro-redeposition process during 1 h CO2RR.
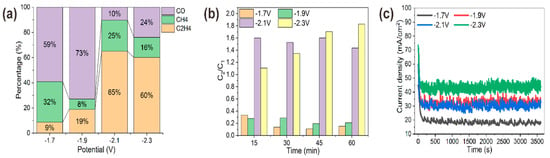
Figure 6.
Selectivity characterization of operando synthesized coppers. (a) Three main gas products (CO, CH4, C2H4) distribution under different synthesized potential. (b) C2/C1 products ratio of copper synthesized under different potentials for electroreduction of CO2, monitored every 15 min for 1 h by gas chromotography. (c) Chronoamperometric i-t curves of copper synthesized under different potentials.
3.3. Effect of CO2 Flow Rate on the Morphology and Product Selectivity of Copper Films
Flow rate was another important parameter that affected the mass transfer of CO2, the copper reconstruction process, and CO2 electroreduction [33,34,35]. In the following, the effects of CO2 flow rate on the morphology of operando synthesis copper and the resulting CO2RR activity and selectivity were studied. Here, various morphologies of copper were synthesized in situ under different flow rates of CO2, 20 mL/min, 40 mL/min, 60 mL/min, and 80 mL/min, with the same bias potential at −2.1 V for 1 h in the solution. Under the lower flow rate of 20 mL/min, the morphology of copper surface became rougher and only short and thick microclusters (about 1 μm thick) dispersed on the granular copper. Cu nanowhiskers morphology emerged under 40 mL/min flow rate, a combination of micro polygon particles and rod-like copper dispersed sparsely under 60 mL/min flow rate, and a relative flat surface without any high-curvature structures exhibited when CO2 was bubbled at a flow rate of 80 mL/min (Figure 7). We also employed the chronoamperometry technique to perform CO2RR for 1 h at four different flow rates. As expected, less active sites on the flat surface of copper rendered to the lower activity of CO2RR (Figure 8d), and overall current density was about 28 mA/cm2 under an 80 mL/min flow rate (compared to about 40 mA/cm2 under a lower flow rate). Nevertheless, the total reduction current under each CO2 flow rate still remained stable. More distinct structure-property correlation was revealed by faradaic efficiency (FE) of gaseous products of various morphologies of copper, shown in Figure 8a–c. Optimal faradic efficiency for CO2RR was obtained on copper nanowhiskers at the third quarter of an hour under 40 mL/min flow rate, which was 1.8% ± 0.3% for CO, 1.6% ± 0.24% for CH4, and 9.9% ± 1.3% for C2H4, respectively, and the total current density was about 43 mA/cm2. When comparing the overall faradaic efficiency during an hour of CO2 electroreduction, nanowhiskers exhibited higher faradic efficiency for C2H4 than the other morphologies and microcluster copper synthesized under 20 mL/min flow rate performed at a higher faradic efficiency for CH4, about 5%. Combinational morphology of micro polygon and rod-like particles presented the lowest faradic efficiency for CO and CH4 production.
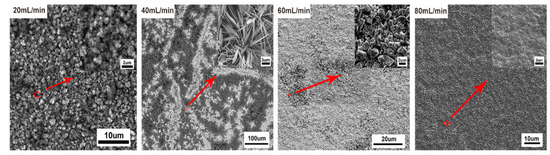
Figure 7.
SEM images of copper thin films formed under different flow rates.
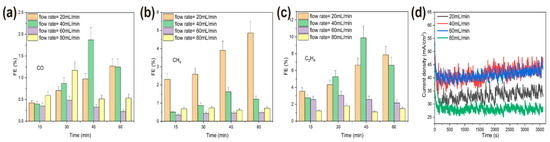
Figure 8.
Electrocatalytic CO2 reduction performances of operando synthesized coppers under 20, 40, 60, 80 mL/min flow rates. Faradic efficiencies of three main products (a) CO, (b) CH4, (c) C2H4. (d) Time-dependent total current density at −2.1 V of different CO2 flow rates.
3.4. Operando-Synthesized Mechanism and Structure-Property Correlation
As shown above, proper cathodic potential and CO2 flow rate should be chosen in order to obtain the proper ratio of nucleation rate to the directional growth rate of copper, which ensures operando synthesis of various high-curvature coppers. Presumably, high-curvature morphologies originated from constricted lateral growth due to hydrogen bubbles and the concentrated electric field around tips for copper redeposition. The observed change in morphology correlated directly with CO2RR activity and selectivity, that is, copper nanowhiskers with larger length-to-diameter ratio exhibited higher faradic efficiency and favor ethylene production. As studied previously, sharp nanowhiskers could improve bubble nucleation, concentrate stabilizing cations and exhibit high field locally [27], all of which, along with higher local pH, boost the reaction, limiting the protonation of bound CO* intermediate that leads to ethylene formation.
4. Conclusions
In summary, various high-curvature morphologies of copper were operando synthesized during a CO2 electroreduction process by controlling cathodic potential and CO2 flow rate. The electrodeposited granular copper evolved to microspheres, nanoneedles, and nanowhiskers, respectively, when biased at −1.7 V~ −2.3 V under 40 mL/min flow rate. When biased at −2.1 V with different flow rates of CO2, rough surfaces with scattered microcluster morphology emerged under 20 mL/min flow rate, and extensive nanowhisker morphology emerged under 40 mL/min. Higher flow rates of 60 and 80 mL/min failed to form sharp whisker-like morphology but scattered distributed particle-rod-combination morphology and non-cluster surfaces instead. Benefiting from such in-situ synthesized morphologies, real-time CO2RR activity and products selectivity were studied, and the correlation between morphology and activity was also discussed. The reduction product was mainly CO at lower overpotential, while high-valued ethylene became the main reduction product when increasing the cathodic potential and the synthesis of high-curvature morphology. Morphologies synthesized under 20 mL/min and 40 mL/min flow rate showed relatively high CO2RR activity, that is, rough surfaces exhibited with microcluster morphology favored methane and ethylene selectivity with C2/C1 ratio about 1, and exhibited nanowhisker morphology favored ethylene selectivity (around 10% faradaic efficiency) with C2/C1 ratio of 1.8. According to previous mechanistic studies, the reaction pathways of CH4 and C2H4 differ in the bound CO* intermediates [36,37,38,39]. Both morphologies synthesized under 20 mL/min and 40 mL/min flow rate stabilized the bound CO* and prevented its desorption, which resulted in not only high reaction activity but also the further reaction pathways by hydrogenation of CO* or dimerization of two bound CO*. For rough Cu with microcluster morphology, the two aforementioned pathways were coexisting and equi-favored, thus FE for methane and ethylene were both around 5%. For nanowhisker morphology, CO* hydrogenation to COH* could be suppressed by the existence of copper oxide and high local pH [37,38], thus shifting the reaction towards C2H4 by stabilizing the OCCOH* intermediates (CO* + CO* + H+ + e− → OCCOH* → C2H4).
Hopefully, such operando synthesized copper and its direct effect of morphology on product distribution could inspire more attention to catalyst self-reconstruction in the design of advanced electrocatalysts and analysis of reaction mechanisms.
Author Contributions
Conceptualization, M.D. and F.L.; funding acquisition, M.D. and F.L.; investigation, X.Z.; methodology, M.D.; supervision, F.L.; writing—original draft, X.Z.; writing—review and editing, M.D. and F.L.
Funding
This research was funded by the National Natural Science Foundation of China (51701159, 51790481, 51431008), National Key R&D Program of China (2017YFB0703001, 2017YFB0305100) and the Fundamental Research Funds for the Central Universities (3102018zy007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Murata, A.; Kikuchi, K.; Suzuki, S. Electrochemical reduction of carbon dioxides to carbon monoxide at a gold electrode in aqueous potassium hydrogen carbonate. J. Chem. Soc. Chem. Commun. 1987, 10, 728–729. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Prodiction of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Shibata, H.; Moulijn, J.A.; Mul, G. Enabling electrocatalytic Fischer–Tropsch synthesis from carbon dioxide over copper-based electrodes. Catal. Lett. 2008, 123, 186–192. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. Mod. Aspects Electrochem. 2008, 42, 89–189. [Google Scholar]
- Frese, K.W. Electrochemical reduction of CO2 at intentionally oxidized copper electrodes. J. Electrochem. Soc. 1991, 138, 3338–3344. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, C.W.; Kanan, M.W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured Tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Murata, A.; Hori, Y. Product selectivity affected by cationic species in electrochemical reduction of CO2 and Co at a Cu electrode. Bull. Chem. Soc. Japan 1991, 64, 123–127. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, R.; Yoshinami, Y.; Murata, A. Electrochemical reduction of CO at a copper electrode. J. Phys. Chem. B 1997, 101, 7075–7081. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc., Faraday Trans. 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Tang, W.; Peterson, A.A.; Varela, A.S.; Jovanov, Z.P.; Bech, L.; Durand, W.J.; Dahl, S.; Nørskov, J.K. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 2012, 14, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Chen, C.S.; Albertus, D.H.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and selective electrochemical reduction of carbon dioxide to ethylene on copper mesocrystals. Catal. Sci. Technol. 2015, 5, 161–168. [Google Scholar] [CrossRef]
- Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M.T.M.; Mul, G.; Baltrusaitis, J. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 2014, 16, 12194–12201. [Google Scholar] [CrossRef]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective Electroreduction of CO2 toward Ethylene on Nano Dendritic Copper Catalysts at High Current Density. Adv. Energy Mater. 2017, 7, 1602114. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wu, J.J.; Hackenberg, K.P.; Zhang, J.; Wang, Y.M.; Yang, Y.C.; Keyshar, K.; Gu, J.; Ogitsu, T.; Vajtai, R.; et al. Self-optimizing, highly surface-active layered metal dichalcogenide catalysts for hydrogen evolution. Nat. Energy 2017, 2, 17127. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.J.; Durst, J.; Bozza, F.; Graule, T.; Schäublin, R.; Wiles, L.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Design 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Humbeeck, J.V. Two-way shape memory effect developed by martensite deformation in NiTi. Acta Mater. 1998, 47, 199–209. [Google Scholar] [CrossRef]
- Du, M.S.; Cui, L.S.; Cao, Y.; Bard, A.J. Mechanoelectrochemical catalysis of the effect of elastic strain on a Platinum nanofilm for the ORR exerted by a shape memory alloy sustrate. J. Am. Chem. Soc. 2015, 137, 7397–7403. [Google Scholar] [CrossRef] [PubMed]
- Safaei, T.S.; Mepham, A.; Zheng, X.l.; Pang, Y.J.; Dinh, C.T.; Liu, M.; Sinton, D.; Kelley, S.O.; Sargent, E.H. High-Density Nanosharp Microstructures Enable Efficient CO2 Electroreduction. Nano. Lett. 2016, 16, 7224–7228. [Google Scholar] [CrossRef]
- Mahshid, S.; Mepham, A.H.; Mahshid, S.S.; Burgess, I.B.; Safaei, T.S.; Sargent, E.H.; Kelley, S.O. Mechanistic control of the growth of three-dimensional gold sensors. J. Phys. Chem. C. 2016, 120, 21123–21132. [Google Scholar] [CrossRef]
- Han, J.H.; Khoo, E.; Bai, P.; Bazant, M.Z. Over-limiting current and control of dendritic growth by surface conduction in nanopores. Sci. Rep. 2014, 4, 7056. [Google Scholar] [CrossRef]
- Shao, W.B.; Zangari, G. Dendritic growth and morphology selection in copper electrodeposition from acidic sulfate solutions containing chlorides. J. Phys. Chem. C. 2009, 113, 10097–10102. [Google Scholar] [CrossRef]
- Dinh, C.T.; Burdyny, T.; Kibriaal, M.G.; Seifitokaldani, A.; Gabardo, C.M.; de Arquer, F.P.G.; Kiani, A.; Edwards, J.P.; Luna, P.D.; Bushuyev, O.S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Kas, R.; Kortlever, R.; Yılmaz, H.; Koper, P.T.M.; Mul, P.G. Manipulating the Hydrocarbon Selectivity of Copper Nanoparticles in CO2 Electroreduction by Process Conditions. Chem. Electro. Chem. 2015, 2, 354–358. [Google Scholar]
- Kas, R.; Hummadi, K.K.; Kortlever, R.; Wit, P.D.; Milbrat, A.; Luiten-Olieman, M.W.J.; Benes, N.E.; Koper, M.T.M.; Mul, G. Three-dimensional porous hollow fibre copper electrodes for efficient and high-rate electrochemical carbon dioxide reduction. Nat. Commun. 2016, 7, 10748. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, G.X.; Zhu, Y.Y.; Liang, Z.; Pei, A.; Wu, C.L.; Wang, H.X.; Lee, H.R.; Liu, K.; Chu, S.; et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018, 1, 592–600. [Google Scholar] [CrossRef]
- Lim, C.F.C.; Harrington, D.A.; Marshall, A.T. Electrochim. Effects of mass transfer on the electrocatalytic CO2 reduction on Cu. Electrochim. Acta. 2017, 238, 56–63. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Kwon, Y.; van der Ham, C.J.M.; Qin, Z.; Koper, M.T.M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2011, 2, 1902. [Google Scholar] [CrossRef]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462. [Google Scholar] [CrossRef]
- Xiao, H.; Cheng, T.; Goddard, W.A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111). J. Am. Chem. Soc. 2017, 139, 130–136. [Google Scholar] [CrossRef]
- Cheng, T.; Xiao, H.; Goddard, W.A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 2017, 114, 1795–1800. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).