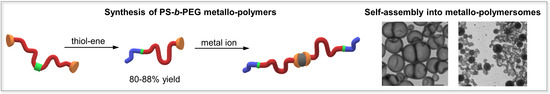
Synthesis of Terpyridine-Terminated Amphiphilic Block Copolymers and Their Self-Assembly into Metallo-Polymer Nanovesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials and Techniques
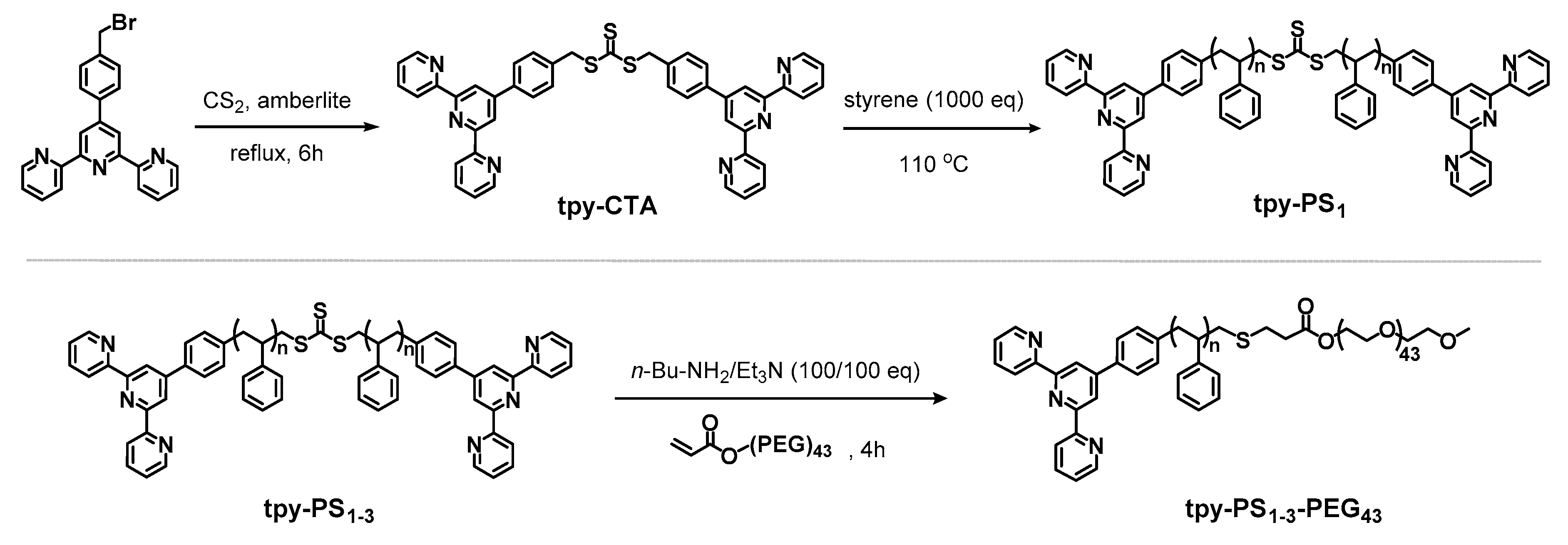
2.2. Synthesis and Characterization
3. Results
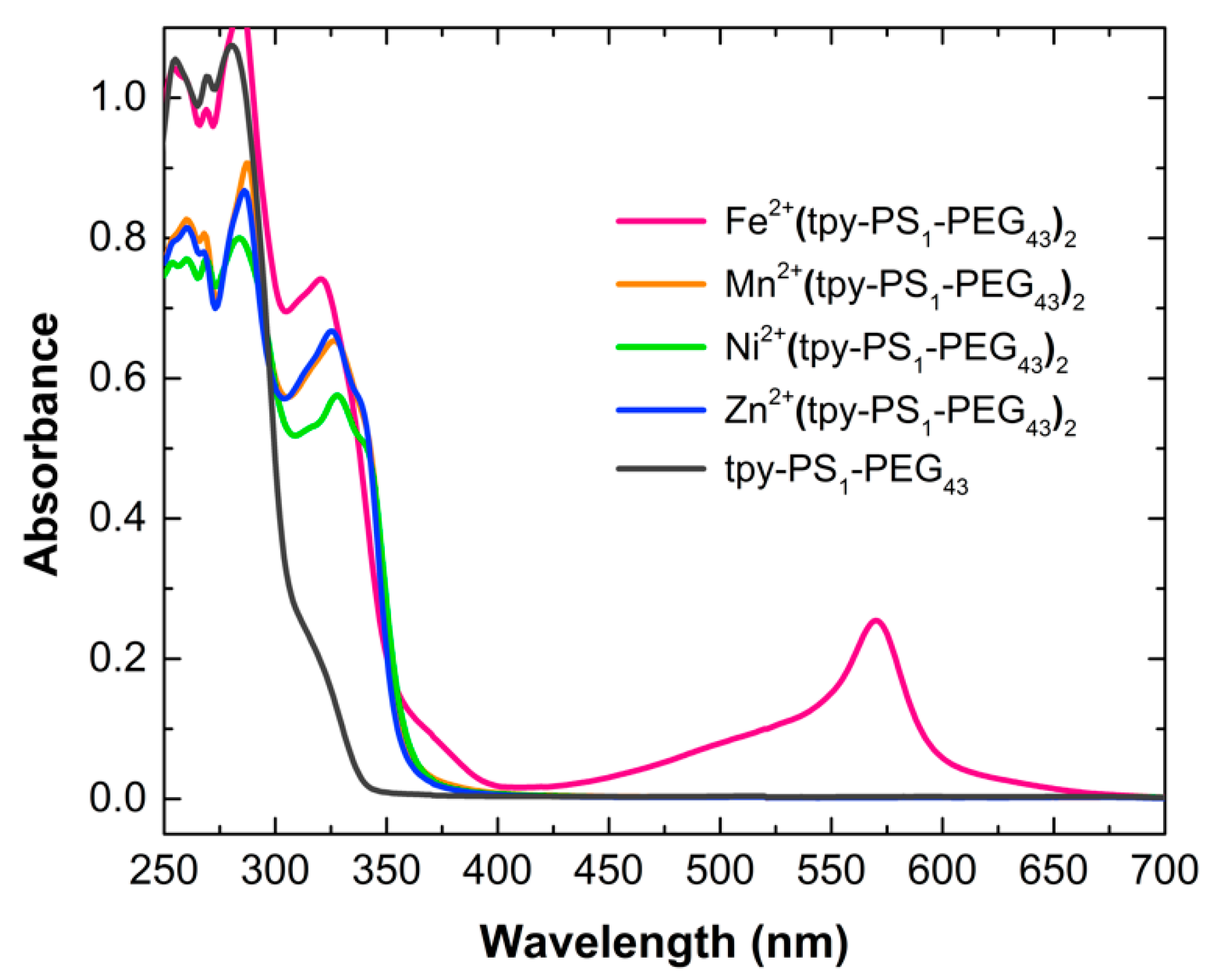
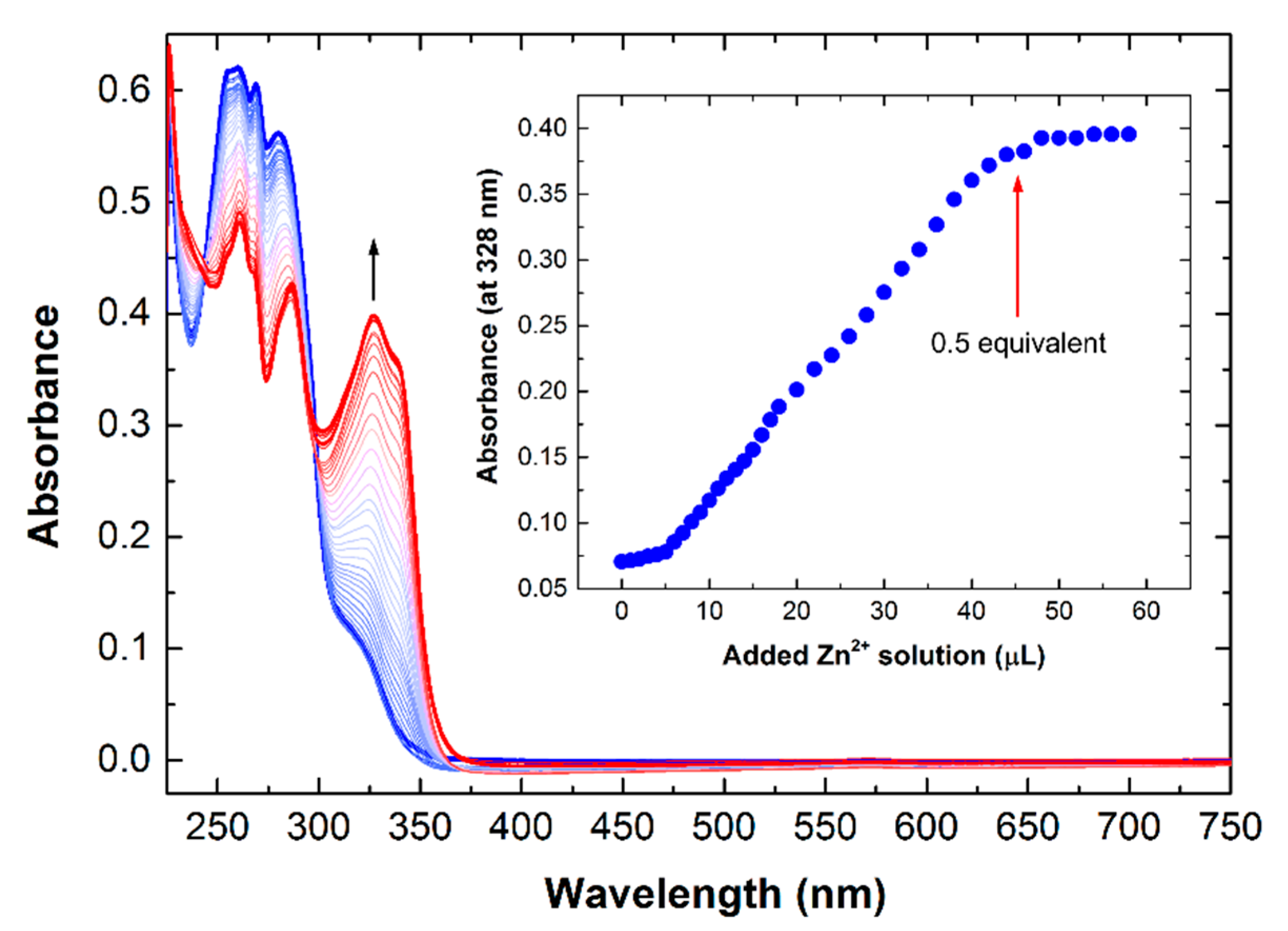
3.1. Synthesis and Charachterization of Polymers and Metallo-Polymers
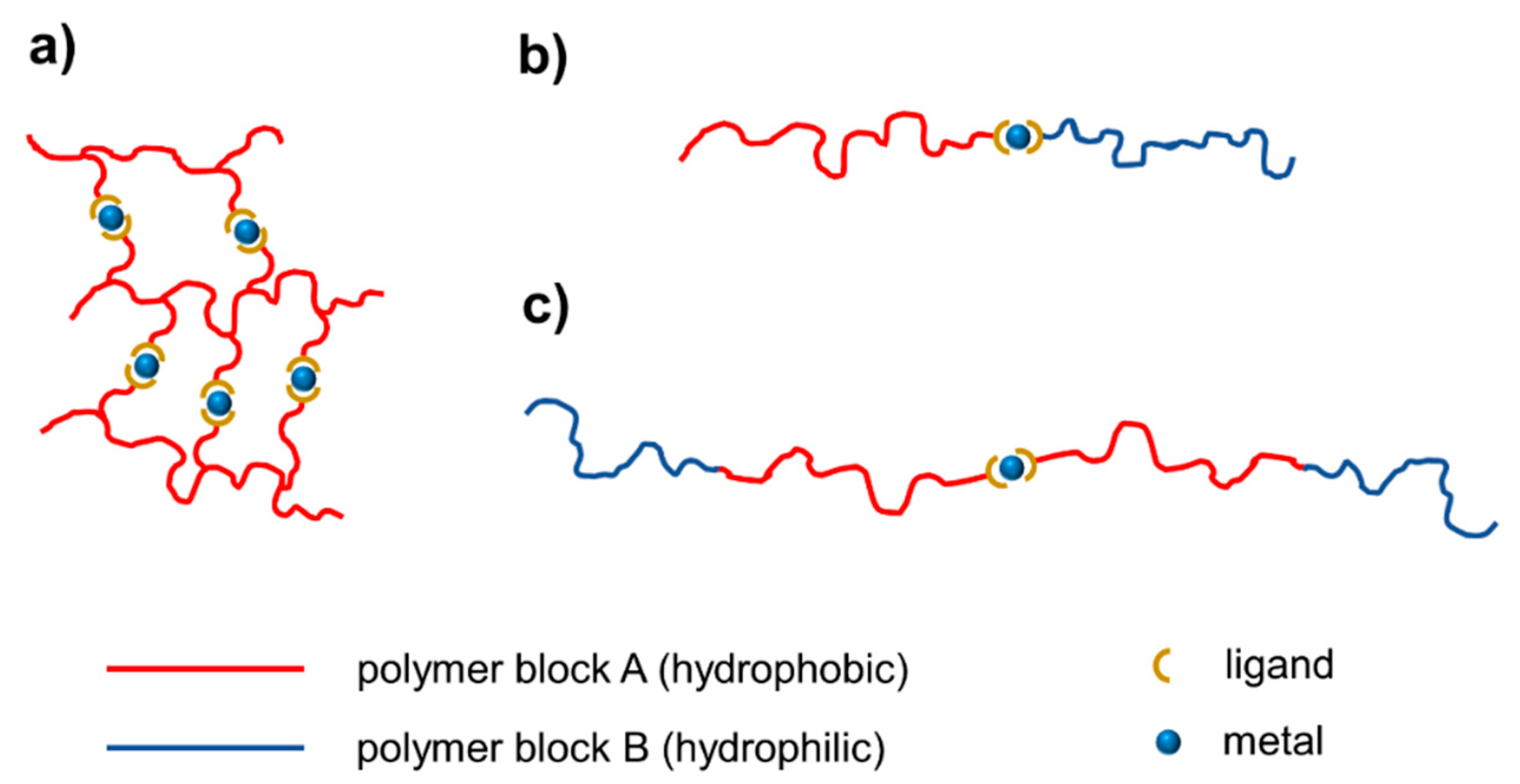
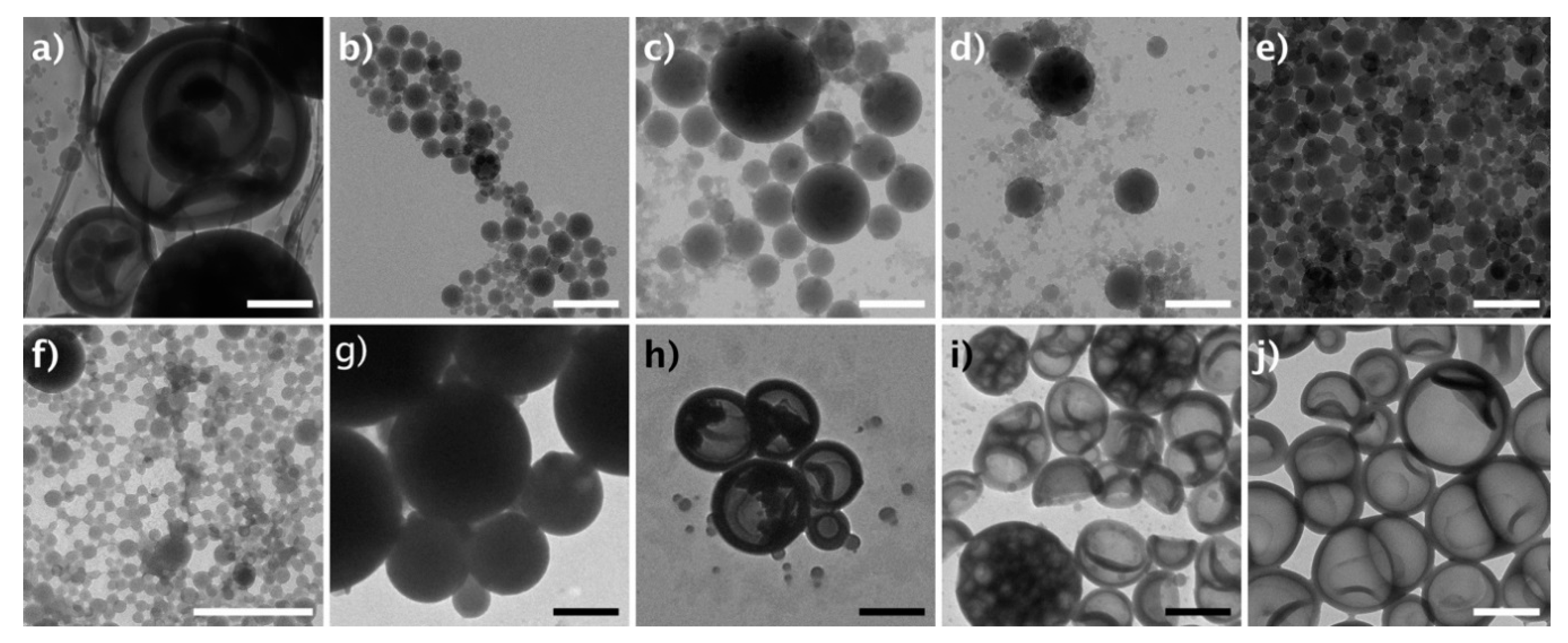
3.2. Self-Assembly of Metallo-Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, R.A.; Russell, A.D.; Hayward, D.W.; Whittell, G.R.; Lawrence, P.G.; Gates, P.J.; Green, J.C.; Manners, I. Main-chain metallopolymers at the static-dynamic boundary based on nickelocene. Nat. Chem. 2017, 9, 743–750. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Liu, S.; Zhao, Q.; Huang, W. Stimuli-responsive metallopolymers. Coord. Chem. Rev. 2016, 319, 180–195. [Google Scholar] [CrossRef]
- Enke, M.; Döhler, D.; Bode, S.; Binder, W.H.; Hager, M.D.; Schubert, U.S. Intrinsic self-healing polymers based on supramolecular interactions: State of the art and future directions. In Self-Healing Materials. Advances in Polymer Science; Hager, M., van der Zwaag, S., Schubert, U., Eds.; Springer: Cham, Switzerland, 2015; Volume 273, pp. 89–97. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Etienne, B.; Stéphane, B.L.; Matteo, M. Amphiphilic metallopolymers for photoswitchable supramolecular hydrogels. Chem. Eur. J. 2016, 22, 18718–18721. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic meal-ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef]
- Wu, Q.; Rauscher, P.M.; Lang, X.; Wojtecki, R.J.; de Pablo, J.J.; Hore, M.J.A.; Rowan, S.J. Poly[n]catenanes: Synthesis of molecular interlocked chains. Science 2017, 358, 1434–1439. [Google Scholar] [CrossRef]
- Wang, Z.; Urban, M.W. Facile UV-healable polyethylenimine-copper (C2H5N–Cu) supramolecular polymer networks. Polym. Chem. 2013, 4, 4897–4901. [Google Scholar] [CrossRef]
- Wang, B.; Jacquet, M.; Wang, K.; Xiong, K.; Yan, M.; Courtois, J.; Royal, G. pH-Induced fragmentation of colloids based on responsive self-assembled copper(II) metallopolymers. New J. Chem. 2018, 42, 7823–7829. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Parowatkin, M.; Steffen, W.; Butt, H.-J.; Mailänder, V.; Wu, S. Ruthenium-containing block copolymer assemblies: Red-light-responsive metallopolymers with tunable nanostructures for enhanced cellular uptake and anticancer phototherapy. Adv. Healthc. Mater. 2016, 5, 467–473. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, M.; Liu, Y.; Wu, S. Stimuli-responsive ruthenium-containing polymers. Macromol. Rapid Commun. 2018, 39, 1800372. [Google Scholar] [CrossRef]
- Savage, A.M.; Walck, S.D.; Lambeth, R.H.; Beyer, F.L. Tuning the morphology of an acrylate-based metallo-supramolecular network: From vesicles to cylinders. Macromolecules 2018, 51, 1636–1643. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Optically active supramolecular poly(L-lactide)s end-capped with terpyridine. Macromol. Rapid Commun. 2001, 22, 1358–1363. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Schubert, U.S. Supramolecular engineering with macromolecules: An alternative concept for block copolymers. Angew. Chem. Int. Ed. Engl. 2002, 41, 3825–3829. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 2016, 45, 5311–5357. [Google Scholar] [CrossRef]
- He, Y.J.; Tu, T.H.; Su, M.K.; Yang, C.W.; Kong, K.V.; Chan, Y.T. Facile construction of metallo-supramolecular poly(3-hexylthiophene)-block-poly(ethylene oxide) diblock copolymers via complementary coordination and their self-assembled nanostructures. J. Am. Chem. Soc. 2017, 139, 4218–4224. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Self-assembly of amphiphilic block copolymers in selective solvents. In Fluorescence Studies of Polymer Containing Systems; Procházka, K., Ed.; Springer: Cham, Switzerland, 2016; pp. 27–63. [Google Scholar]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly (styrene-ethylene oxide) block copolymer micelle formation in water: A fluorescence probe study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic insights for block copolymer morphologies: How do worms form vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Peng, F.; Tu, Y.; van Hest, J.C.M.; Wilson, D.A. Methods for production of uniform small-sized polymersome with rigid membrane. Polym. Chem. 2016, 7, 3977–3982. [Google Scholar] [CrossRef]
- van Hest, J.C.M.; Delnoye, D.A.P.; Baars, M.W.P.L.; van Genderen, M.H.P.; Meijer, E.W. Polystyrene-dendrimer amphiphilic block copolymers with a generation-dependent aggregation. Science 1995, 268, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenberg, A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly (acrylic acid) block copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Wong, C.K.; Laos, A.J.; Soeriyadi, A.H.; Wiedenmann, J.; Curmi, P.M.G.; Gooding, J.J.; Marquis, C.P.; Stenzel, M.H.; Thordarson, P. Polymersomes prepared from thermoresponsive fluorescent protein-polymer bioconjugates: Capture of and report on drug and protein payloads. Angew. Chem. Int. Ed. Engl. 2015, 54, 5317–5322. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Medipelli, S.; Xue, B.; Ahmad, F.; Saeed, M.; Cropek, D.; Kharlampieva, E. Temperature-sensitive polymersomes for controlled delivery of anticancer drugs. Chem. Mater. 2015, 27, 7945–7956. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P. Polymersomes in nanomedicine—A review. Curr. Med. Chem. 2017, 13, 124–129. [Google Scholar] [CrossRef]
- Peters, R.J.; Marguet, M.; Marais, S.; Fraaije, M.W.; van Hest, J.C.; Lecommandoux, S. Cascade reactions in multicompartmentalized polymersomes. Angew. Chem. Int. Ed. 2014, 53, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Klermund, L.; Poschenrieder, S.T.; Castiglione, K. Biocatalysis in polymersomes: Improving multienzyme cascades with incompatible reaction steps by compartmentalization. ACS Catal. 2017, 7, 3900–3904. [Google Scholar] [CrossRef]
- Poli, R. Effects of Nanoconfinement on Catalysis, 1st ed.; Twigg, M., Spences, M., Eds.; Springer: Cham, Switzerland, 2017; p. 240. [Google Scholar]
- Zhang, L.; Zhang, Y.; Chen, Y. Synthesis of bis(2,2′:6′,2″-terpyridine)-terminated telechelic polymers by RAFT polymerization and ruthenium-polymer complexation thereof. Eur. Polym. J. 2006, 42, 2398–2406. [Google Scholar] [CrossRef]
- Cargill Thompson, A.M.W. The synthesis of 2,2′:6′,2″-terpyridine ligands—Versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Wild, A.; Winter, A.; Schlutter, F.; Schubert, U.S. Advances in the field of π-conjugated 2,2′:6′,2″-terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Sanchez, C.; Lohmeijer, B.G.G.; Meier, M.A.R.; Schubert, U.S. Synthesis of terpyridine-terminated polymers by anionic polymerization. Macromolecules 2005, 38, 10388–10396. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Schubert, U.S. Expanding the supremolecular polymer LEGO system: Nitroxide mediated living free-radical polymerization as a tool for mono- and telechelic polystyrenes. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4016–4027. [Google Scholar] [CrossRef]
- Zhou, G.; Harruna, I.I. Synthesis and characterization of bis(2,2′:6,2″-terpyridine)ruthenium(II)-connected diblock polymers via RAFT polymerization. Macromolecules 2005, 38, 4114–4123. [Google Scholar] [CrossRef]
- Boyer, C.; Granville, A.; Davis, T.P.; Bulmus, V. Modification of RAFT-polymers via thiol-ene reactions: A general route to functional polymers and new architectures. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3773–3794. [Google Scholar] [CrossRef]
- Willcock, H.; O’Reilly, R.K. End group removal and modification of RAFT polymers. Polym. Chem. 2010, 1, 149–157. [Google Scholar] [CrossRef]
- Loiseau, J.; Doërr, N.; Suau, J.M.; Egraz, J.B.; Llauro, M.F.; Ladavière, C.; Claverie, J. Synthesis and characterization of poly(acrylic acid) produced by RAFT polymerization. Application as a very efficient dispersant of CaCO3, kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Braterman, P.S.; Song, J.I.; Peacock, R.D. Electronic absorption spectra of the iron(II) complexes of 2,2′-bipyridine, 2,2′-bipyrimidine, 1,10-phenanthroline, and 2,2′:6′,2″-terpyridine and their reduction products. Inorg. Chem. 1992, 31, 555–559. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Faggi, E.; Giorgi, C.; Santarelli, S.; Valtancoli, B. Coordination properties of polyamine-macrocycles containing terpyridine units. Coord. Chem. Rev. 2008, 252, 1052–1068. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevee, J. Visible-light-sensitive photoredox catalysis by iron complexes: Applications in cationic and radical polymerization reactions. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Romain, S.; Duboc, C.; Neese, F.; Riviere, E.; Hanton, L.R.; Blackman, A.G.; Philouse, C.; Leprêtre, J.-C.; Deronzier, A.; Collomb, M.-N. An unusual mononuclear MnIII bis-terpyridine exhibiting Jahn-Teller compression: Electrochemical synthesis, physical characterisation and theoretical study. Chem. Eur. J. 2009, 15, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Chiper, M.; Meier, M.A.; Kranenburg, J.M.; Schubert, U.S. New insights into nickel(II), iron(II), and cobalt(II) bis-complex-based metallo-supramolecular polymers. Macromol. Chem. Phys. 2007, 208, 679–689. [Google Scholar] [CrossRef]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef]
- Kim, K.T.; Zhu, J.; Meeuwissen, S.A.; Cornelissen, J.J.L.M.; Pochan, D.J.; Nolte, R.J.M.; van Hest, J.C.M. Polymersome stomatocytes: Controlled shape transformation in polymer vesicles. J. Am. Chem. Soc. 2010, 132, 12522–12524. [Google Scholar] [CrossRef]
- van Rhee, P.G.; Rikken, R.S.M.; Abdelmohsen, L.K.E.A.; Maan, J.C.; Nolte, R.J.M.; van Hest, J.C.M.; Christianen, P.C.M.; Wilson, D.A. Polymersome magneto-valves for reversible capture and release of nanoparticles. Nat. Commun. 2014, 5, 5010. [Google Scholar] [CrossRef]
- Azzam, T.; Eisenberg, A. Fully collapsed (kippah) vesicles: Preparation and characterization. Langmuir 2010, 26, 10513–10523. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Eisenberg, A. Morphogenic effect of solvent on crew-cut aggregates of amphiphilic diblock copolymers. Macromolecules 1998, 31, 1144–1154. [Google Scholar] [CrossRef]
| Name | Mn | Ð | Name | Mn (calc) | Mn (GPC) | Ð |
|---|---|---|---|---|---|---|
| tpy-PS1 | 8800 | 1.36 | tpy-PS1-PEG43 | 6078 | 5800 | 1.38 |
| tpy-PS2 | 23,500 | 1.23 | tpy-PS2-PEG43 | 13,428 | 11,200 | 1.36 |
| tpy-PS3 | 48,200 | 1.16 | tpy-PS3-PEG43 | 25,778 | 23,300 | 1.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkin, T.; Copp, S.M.; Hamblin, R.L.; Martinez, J.S.; Montaño, G.A.; Rocha, R.C. Synthesis of Terpyridine-Terminated Amphiphilic Block Copolymers and Their Self-Assembly into Metallo-Polymer Nanovesicles. Materials 2019, 12, 601. https://doi.org/10.3390/ma12040601
Elkin T, Copp SM, Hamblin RL, Martinez JS, Montaño GA, Rocha RC. Synthesis of Terpyridine-Terminated Amphiphilic Block Copolymers and Their Self-Assembly into Metallo-Polymer Nanovesicles. Materials. 2019; 12(4):601. https://doi.org/10.3390/ma12040601
Chicago/Turabian StyleElkin, Tatyana, Stacy M. Copp, Ryan L. Hamblin, Jennifer S. Martinez, Gabriel A. Montaño, and Reginaldo C. Rocha. 2019. "Synthesis of Terpyridine-Terminated Amphiphilic Block Copolymers and Their Self-Assembly into Metallo-Polymer Nanovesicles" Materials 12, no. 4: 601. https://doi.org/10.3390/ma12040601
APA StyleElkin, T., Copp, S. M., Hamblin, R. L., Martinez, J. S., Montaño, G. A., & Rocha, R. C. (2019). Synthesis of Terpyridine-Terminated Amphiphilic Block Copolymers and Their Self-Assembly into Metallo-Polymer Nanovesicles. Materials, 12(4), 601. https://doi.org/10.3390/ma12040601