Pentafluorophenyl Platinum(II) Complexes of PTA and Its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity
Abstract
1. Introduction
2. Experimental
2.1. General
2.2. Materials and Methods
2.3. Cell Cultures
2.4. Cell Viability Assays
2.5. Cytotoxic Assay-Quantitative Suspension Test According to EN 14476
2.6. Octanol–Water Partition Coefficient Determination
2.7. HSA Interaction
2.7.1. Fluorescence Measurements
2.7.2. CD Measurement
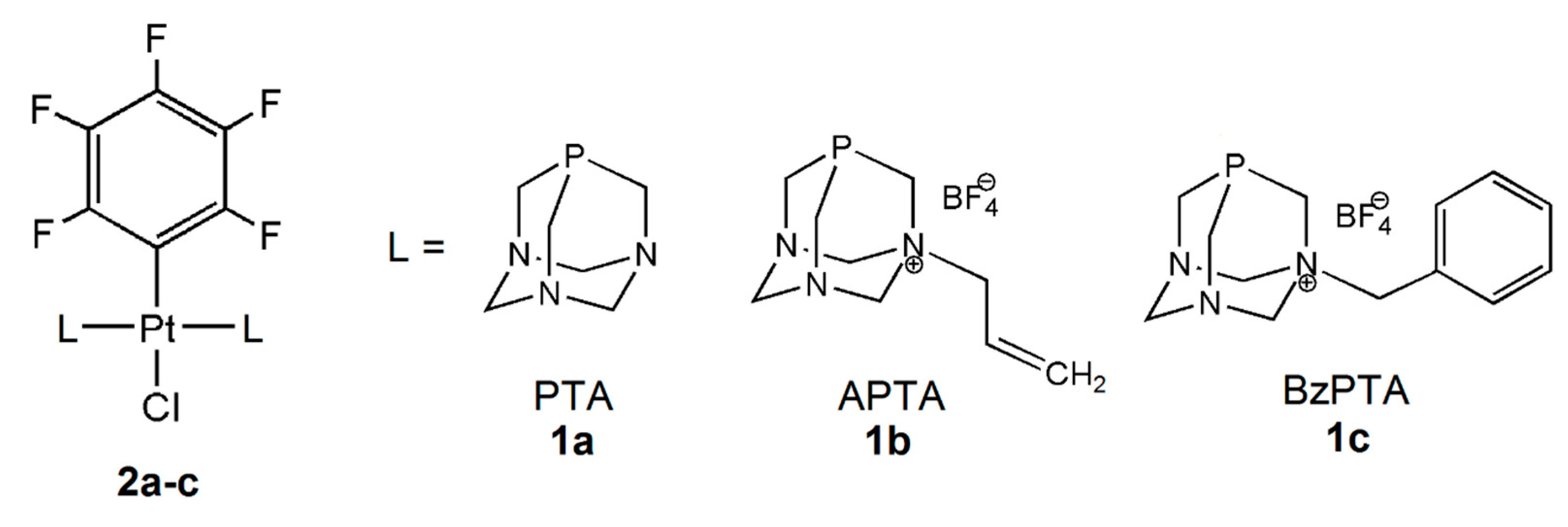
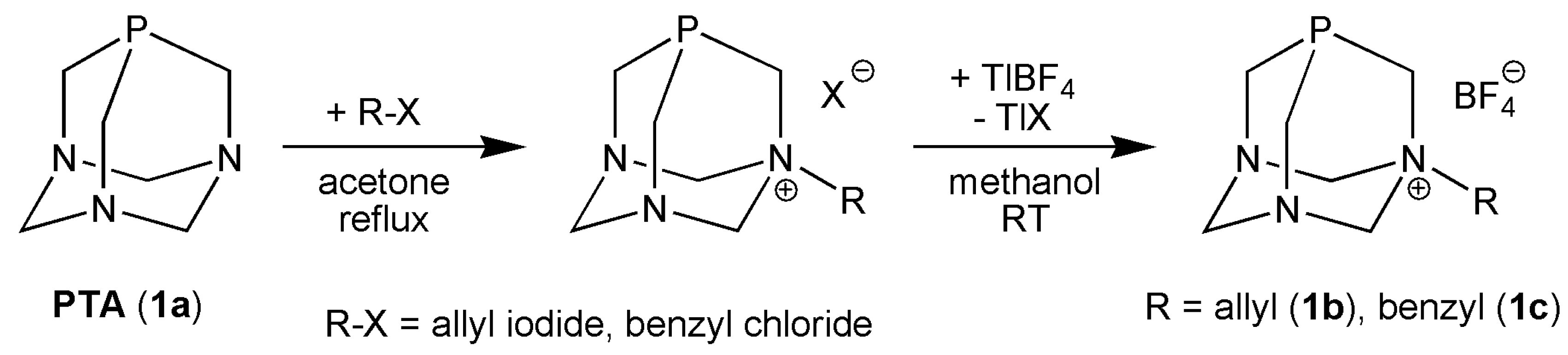
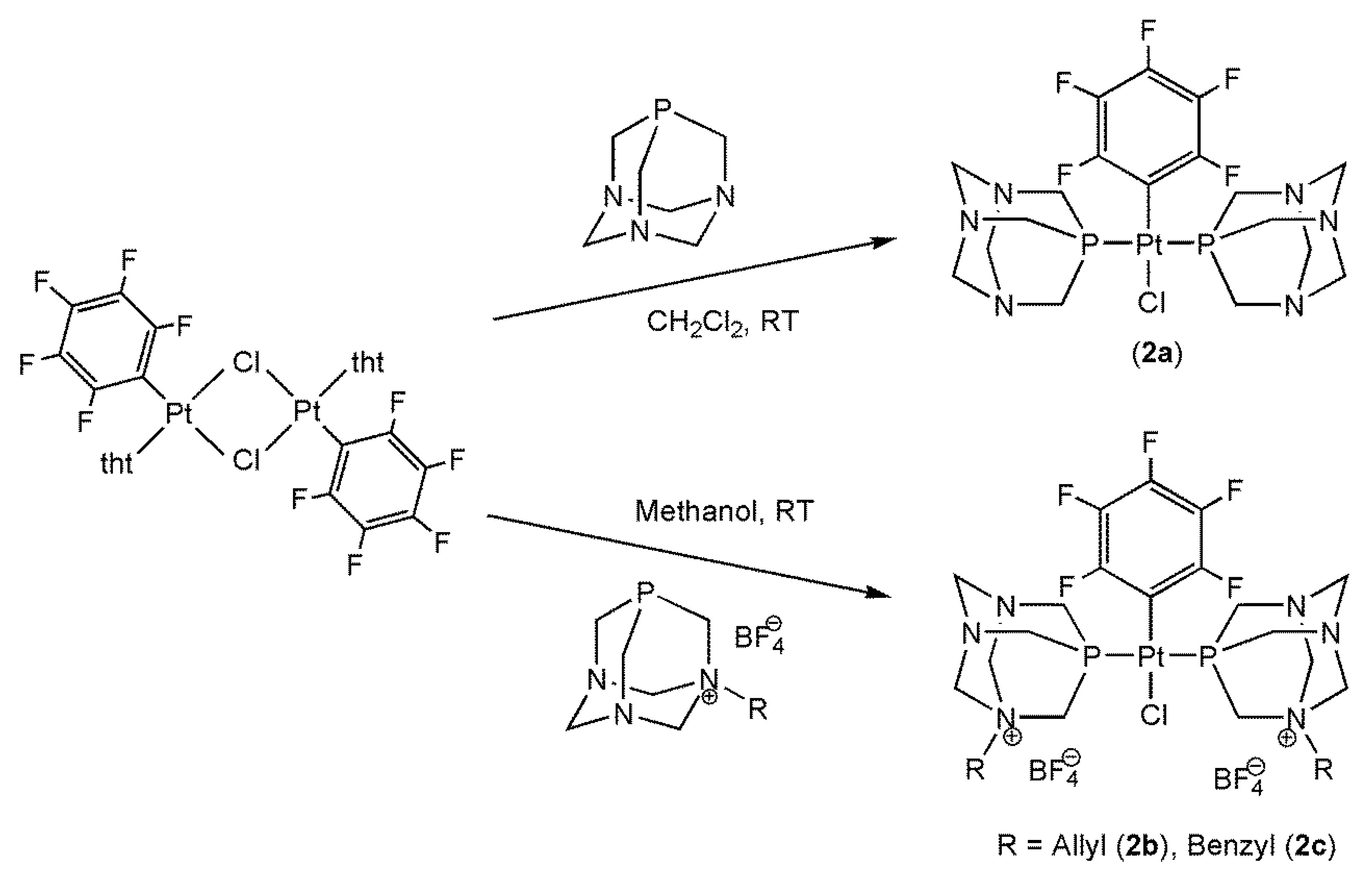
2.8. Synthesis and Analytical Data
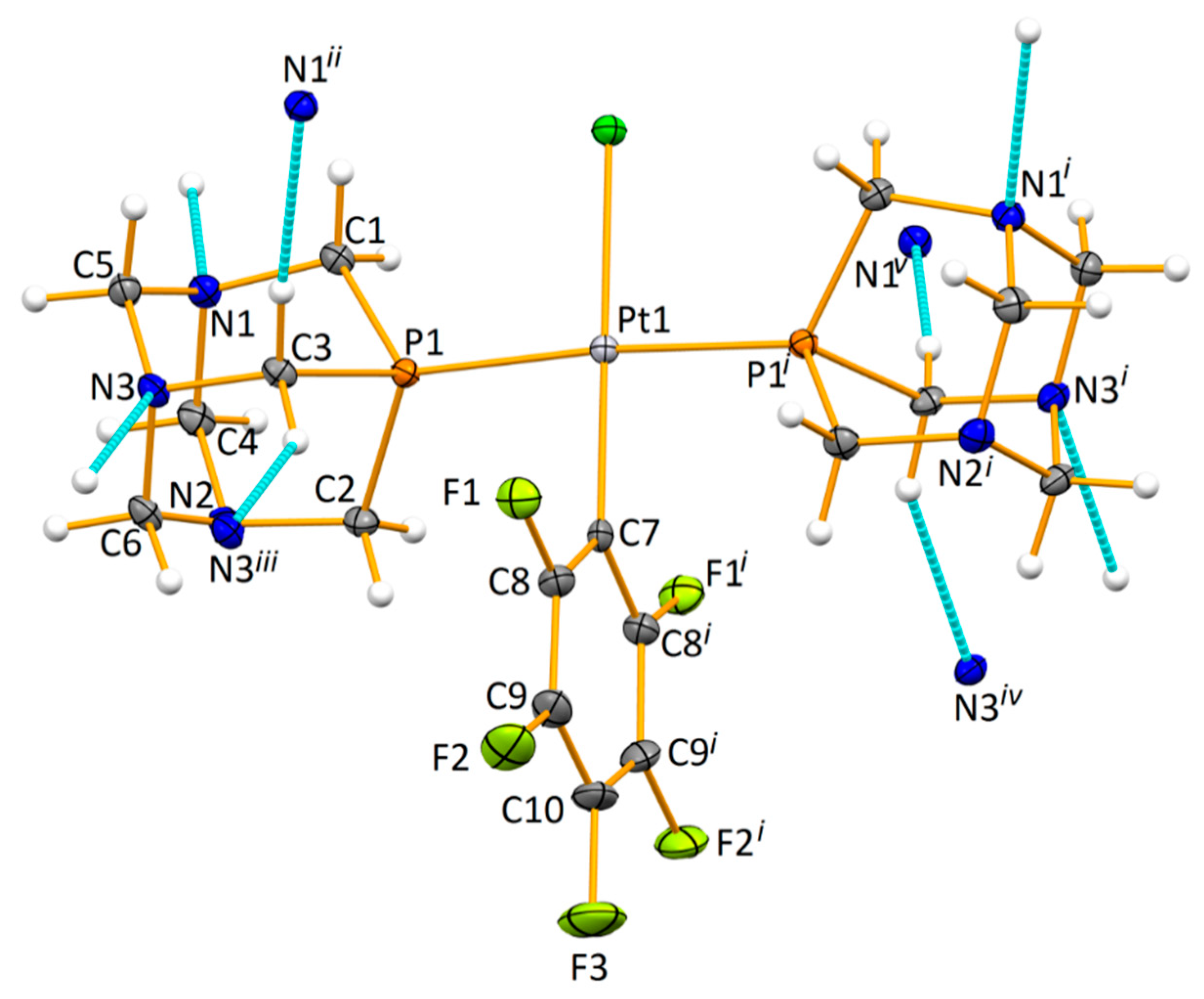
2.9. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Biologic Assays
3.3. HSA Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonsalvi, L.; Guerriero, A.; Hapiot, F.; Krogstad, D.A.; Monflier, E.; Reginato, G.; Peruzzini, M. Lower-and Upper-Rim-Modified Derivatives of 1,3,5-Triaza-7-Phosphaadamantane: Coordination Chemistry and Applications in Catalytic Reactions in Water. Pure Appl. Chem. 2012, 85, 385–396. [Google Scholar] [CrossRef]
- Bravo, J.; Bolaño, S.; Gonsalvi, L.; Peruzzini, M. Coordination Chemistry of 1,3,5-Triaza-7-Phosphaadamantane (PTA) and Derivatives. Part II. The Quest for Tailored Ligands, Complexes and Related Applications. Coord. Chem. Rev. 2010, 254, 555–607. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination Chemistry of 1,3,5-Triaza-7-Phosphaadamantane (PTA): Transition Metal Complexes and Related Catalytic, Medicinal and Photoluminescent Applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Zatajska, A.; Siczek, M.; Skarżyńska, A.; Smoleński, P. New Water-Soluble Palladium(II) Iodide Complexes Derived from N-Protonated or N-Alkyl-1,3,5-Triaza-7-Phosphaadamantanes: Synthesis, Crystal Structure and Catalytic Properties in Aqua Media. Inorg. Chim. Acta 2017, 455, 701–706. [Google Scholar] [CrossRef]
- Mena-Cruz, A.; Serrano-Ruiz, M.; Lorenzo-Luis, P.; Romerosa, A.; Kathó, Á.; Joó, F.; Aguilera-Sáez, L.M. Evaluation of Catalytic Activity of [RuClCp(Dmopta)(PPh3)](OsO2CF3) in the Isomerization of Allylic Alcohols in Water (Dmopta = 3, 7-Dimethyl-1,3,7-Triaza-5-Phosphabicyclo [3.3.1] Nonane). J. Mol. Catal. A Chem. 2016, 411, 27–33. [Google Scholar] [CrossRef]
- Chahdoura, F.; Favier, I.; Pradel, C.; Mallet-Ladeira, S.; Gómez, M. Palladium Nanoparticles Stabilised by PTA Derivatives in Glycerol: Synthesis and Catalysis in a Green Wet Phase. Catal. Commun. 2015, 63, 47–51. [Google Scholar] [CrossRef]
- Sears, J.M.; Lee, W.-C.; Frost, B.J. Water Soluble Diphosphine Ligands Based on 1,3,5-Triaza-7-Phosphaadamantane (PTA-Pr2): Synthesis, Coordination Chemistry, and Ruthenium Catalyzed Nitrile Hydration. Inorg. Chim. Acta 2015, 431, 248–257. [Google Scholar] [CrossRef]
- Bolyog-Nagy, E.; Udvardy, A.; Joó, F.; Kathó, Á. Efficient and Selective Hydration of Nitriles to Amides in Aqueous Systems with Ru(II)-Phosphaurotropine Catalysts. Tetrahedron Lett. 2014, 55, 3615–3617. [Google Scholar] [CrossRef]
- Sherbow, T.J.; Downs, E.L.; Sayler, R.I.; Razink, J.J.; Juliette, J.J.; Tyler, D.R. Investigation of 1,3,5-Triaza-7-Phosphaadamantane-Stabilized Silver Nanoparticles as Catalysts for the Hydration of Benzonitriles and Acetone Cyanohydrin. ACS Catal. 2014, 4, 3096–3104. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Lidrissi, C.; Mañas, S.; Peruzzini, M.; Romerosa, A. Synthesis, Reactivity and Catalytic Properties of the Allenylidene [Ru(Cccph2)Cp(PTA)(Pph3)](Cf3SO3) (PTA = 1,3,5-Triaza-7-Phosphaadamantane). J. Organomet. Chem. 2014, 751, 654–661. [Google Scholar] [CrossRef]
- Kapdi, A.; Gayakhe, V.; Sanghvi, Y.S.; Garcia, J.; Lozano, P.; da Silva, I.; Perez, J.; Serrano, J.L. New Water Soluble Pd-Imidate Complexes as Highly Efficient Catalysts for the Synthesis of C5-Arylated Pyrimidine Nucleosides. RSC Adv. 2014, 4, 17567–17572. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Ghosh, D.; Lee, J.-Y.; Wu, S.-S.; Hu, C.-H.; Liu, S.-D.; Lee, H.M. Zwitterionic Palladium Complexes: Room-Temperature Suzuki–Miyaura Cross-Coupling of Sterically Hindered Substrates in an Aqueous Medium. Organometallics 2014, 33, 6481–6492. [Google Scholar] [CrossRef]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The Development of RaPTA Compounds for the Treatment of Tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Timerbaev, A.R. Role of Metallomic Strategies in Developing Ruthenium Anticancer Drugs. TrAC Trends Anal. Chem. 2016, 80, 547–554. [Google Scholar] [CrossRef]
- Daigle, D.J.; Decuir, T.J.; Robertson, J.B.; Darensbourg, D.J. 1,3,5-Triaz-7-Phosphatricyclo[3.3.1.13,7]Decane and Derivatives. Inorg. Synth. 1998, 32, 40–45. [Google Scholar]
- Romerosa, A.; Campos-Malpartida, T.; Lidrissi, C.; Saoud, M.; Serrano-Ruiz, M.; Peruzzini, M.; Garrido-Cárdenas, J.A.; García-Maroto, F. Synthesis, Characterization, and DNA Binding of New Water-Soluble Cyclopentadienyl Ruthenium(II) Complexes Incorporating Phosphines. Inorg. Chem. 2006, 45, 1289–1298. [Google Scholar] [CrossRef]
- Wanke, R.; Smolenski, P.; Guedes da Silva, M.F.C.; Martins, L.M.; Pombeiro, A.J.L. Cu(I) Complexes Bearing the New Sterically Demanding and Coordination Flexible Tris(3-Phenyl-1-Pyrazolyl)Methanesulfonate Ligand and the Water-Soluble Phosphine 1,3,5-Triaza-7-Phosphaadamantane or Related Ligands. Inorg. Chem. 2008, 47, 10158–10168. [Google Scholar] [CrossRef]
- Pruchnik, F.P.; Smoleński, P. New Rhodium(I) Water-Soluble Complexes with 1-Alkyl-1-Azonia-3, 5-Diaza-7-Phospha-Adamantane Iodides and Their Catalytic Activity. Appl. Organomet. Chem. 1999, 13, 829–836. [Google Scholar] [CrossRef]
- Forward, J.; Staples, R.; Liu, C.; Fackler, J. Luminescent Tris (3-Ethyl-1, 5-Diaza-3-Azonia-7-Phosphatricyclo [3.3.1.13,7] Decane-P) Gold(I) Tetraiodide Trihydrate,[(Ettpa) 3Au]I4.3h2o. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 195–197. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smolenski, P.; Ma, Z.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L. Copper(I) Iodide Complexes Derived from N-Alkyl-1,3,5-Triaza-7-Phosphaadamantanes: Synthesis, Crystal Structures, Photoluminescence, and Identification of the Unprecedented {Cu3I5}2—Cluster. Organometallics 2009, 28, 6425–6431. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smolenski, P.; Haukka, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Unprecedented Metal-Free C(Sp3)−C(Sp3) Bond Cleavage: Switching from N-Alkyl-to N-Methyl-1,3,5-Triaza-7-Phosphaadamantane. Organometallics 2009, 28, 1683–1687. [Google Scholar] [CrossRef]
- Bergamini, P.; Marvelli, L.; Marchi, A.; Vassanelli, F.; Fogagnolo, M.; Formaglio, P.; Bernardi, T.; Gavioli, R.; Sforza, F. Platinum and Ruthenium Complexes of New Long-Tail Derivatives of PTA (1,3,5-Triaza-7-Phosphaadamantane): Synthesis, Characterization and Antiproliferative Activity on Human Tumoral Cell Lines. Inorg. Chim. Acta 2012, 391, 162–170. [Google Scholar] [CrossRef]
- Legrand, F.-X.; Hapiot, F.; Tilloy, S.; Guerriero, A.; Peruzzini, M.; Gonsalvi, L.; Monflier, E. Aqueous Rhodium-Catalyzed Hydroformylation of 1-Decene in the Presence of Randomly Methylated Β-Cyclodextrin and 1,3,5-Triaza-7-Phosphaadamantane Derivatives. Appl. Catal. A Gen. 2009, 362, 62–66. [Google Scholar] [CrossRef]
- Fluck, E.; Förster, J.-E.; Weidlein, J.; Hädicke, E. 1.3. 5-Triaza-7-Phosphaadamantan (Monophospha-Urotropin)/1,3,5-Triaza-7-Phosphaadamantane (Monophospha-Urotropine). Zeitschrift für Naturforschung B 1977, 32, 499–506. [Google Scholar] [CrossRef]
- Smoleński, P.; Kirillov, A.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. 1-Methyl-1-Azonia-3, 5-Diaza-7-Phosphatricyclo [3.3.1.13, 7] Decane Tetrafluoroborate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o556. [Google Scholar] [CrossRef]
- Krogstad, D.A.; Ellis, G.S.; Gunderson, A.K.; Hammrich, A.J.; Rudolf, J.W.; Halfen, J.A. Two New Water-Soluble Derivatives of 1,3,5-Triaza-7-Phosphaadamantane (PTA): Synthesis, Characterization, X-Ray Analysis and Solubility Studies of 3,7-Diformyl-1,3,7-Triaza-5-Phosphabicyclo [3.3.1] Nonane and 1-Pyridylmethyl-3,5-Diaza-1-Azonia-7-Phosphatricyclo [3.3.1.1] Decane Bromide. Polyhedron 2007, 26, 4093–4100. [Google Scholar]
- Singh, K.; Jana, A.; Lippmann, P.; Ott, I.; Das, N. Isomeric Platinum Organometallics Derived from Pyrimidine, Pyridazine or Pyrazine and Their Potential as Antitumor Drugs. Inorg. Chim. Acta 2019, 493, 112–117. [Google Scholar] [CrossRef]
- Cullinane, C.; Deacon, G.B.; Drago, P.R.; Erven, A.P.; Junk, P.C.; Luu, J.; Meyer, G.; Schmitz, S.; Ott, I.; Schur, J. Synthesis and Antiproliferative Activity of a Series of New Platinum and Palladium Diphosphane Complexes. Dalton Trans. 2018, 47, 1918–1932. [Google Scholar] [CrossRef]
- Medrano, M.Á.; Álvarez-Valdés, A.; Perles, J.; Lloret-Fillol, J.; Muñoz-Galván, S.; Carnero, A.; Navarro-Ranninger, C.; Quiroga, A.G. Oxidation of Anticancer Pt(II) Complexes with Monodentate Phosphane Ligands: Towards Stable but Active Pt(Iv) Prodrugs. Chem. Commun. 2013, 49, 4806–4808. [Google Scholar] [CrossRef]
- Ramos-Lima, F.J.; Quiroga, A.G.; García-Serrelde, B.; Blanco, F.; Carnero, A.; Navarro-Ranninger, C. New Trans-Platinum Drugs with Phosphines and Amines as Carrier Ligands Induce Apoptosis in Tumor Cells Resistant to Cisplatin. J. Med. Chem. 2007, 50, 2194–2199. [Google Scholar] [CrossRef]
- Ramos-Lima, F.J.; Quiroga, A.G.; Pérez, J.M.; Font-Bardía, M.; Solans, X.; Navarro-Ranninger, C. Synthesis and Characterization of New Transplatinum Complexes Containing Phosphane Groups-Cytotoxic Studies in Cisplatin-Resistant Cells. Eur. J. Inorg. Chem. 2003, 2003, 1591–1598. [Google Scholar] [CrossRef]
- Neplechová, K.; Kašpárková, J.; Vrána, O.; Nováková, O.; Habtemariam, A.; Watchman, B.; Sadler, P.J.; Brabec, V. DNA Interactions of New Antitumor Aminophosphine Platinum(II) Complexes. Mol. Pharmacol. 1999, 56, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Živković, M.; Kljun, J.; Ilic-Tomic, T.; Pavic, A.; Veselinović, A.; Manojlović, D.D.; Nikodinovic-Runic, J.; Turel, I. A New Class of Platinum(II) Complexes with the Phosphine Ligand PTA Which Show Potent Anticancer Activity. Inorg. Chem. Front. 2018, 5, 39–53. [Google Scholar] [CrossRef]
- Cortesi, R.; Damiani, C.; Ravani, L.; Marvelli, L.; Esposito, E.; Drechsler, M.; Pagnoni, A.; Mariani, P.; Sforza, F.; Bergamini, P. Lipid-Based Nanoparticles Containing Cationic Derivatives of PTA (1,3,5-Triaza-7-Phosphaadamantane) as Innovative Vehicle for Pt Complexes: Production, Characterization and in Vitro Studies. Int. J. Pharm. 2015, 492, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Mügge, C.; Rothenburger, C.; Beyer, A.; Görls, H.; Gabbiani, C.; Casini, A.; Michelucci, E.; Landini, I.; Nobili, S.; Mini, E. Structure, Solution Chemistry, Antiproliferative Actions and Protein Binding Properties of Non-Conventional Platinum(II) Compounds with Sulfur and Phosphorus Donors. Dalton Trans. 2011, 40, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, P.; Bertolasi, V.; Marvelli, L.; Canella, A.; Gavioli, R.; Mantovani, N.; Manas, S.; Romerosa, A. Phosphinic Platinum Complexes with 8-Thiotheophylline Derivatives: Synthesis, Characterization, and Antiproliferative Activity. Inorg. Chem. 2007, 46, 4267–4276. [Google Scholar] [CrossRef]
- Romerosa, A.; Bergamini, P.; Bertolasi, V.; Canella, A.; Cattabriga, M.; Gavioli, R.; Mañas, S.; Mantovani, N.; Pellacani, L. Biologically Active Platinum Complexes Containing 8-Thiotheophylline and 8-(Methylthio) Theophylline. Inorg. Chem. 2004, 43, 905–913. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Mármol, I.; Pérez, R.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The Anticancer Effect Related to Disturbances in Redox Balance on Caco-2 Cells Caused by an Alkynyl Gold(I) Complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gascón, S.; Rodríguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Synthesis of Gold(I) Derivatives Bearing Alkylated 1,3,5-Triaza-7-Phosphaadamantane as Selective Anticancer Metallodrugs. Eur. J. Inorg. Chem. 2016, 2016, 2791–2803. [Google Scholar] [CrossRef]
- García-Moreno, E.; Tomás, A.; Atrián-Blasco, E.; Gascón, S.; Romanos, E.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. In Vitro and in Vivo Evaluation of Organometallic Gold(I) Derivatives as Anticancer Agents. Dalton Trans. 2016, 45, 2462–2475. [Google Scholar] [CrossRef]
- Pettinari, R.; Condello, F.; Marchetti, F.; Pettinari, C.; Smoleński, P.; Riedel, T.; Scopelliti, R.; Dyson, P.J. Dicationic Ruthenium(II)-Arene-Curcumin Complexes Containing Methylated 1,3,5-Triaza-7-Phosphaadamantane: Synthesis, Structure, and Cytotoxicity. Eur. J. Inorg. Chem. 2017, 2017, 2905–2910. [Google Scholar] [CrossRef]
- Pettinari, R.; Petrini, A.; Marchetti, F.; Pettinari, C.; Riedel, T.; Therrien, B.; Dyson, P.J. Arene-Ruthenium(II) Complexes with Bioactive Ortho-Hydroxydibenzoylmethane Ligands: Synthesis, Structure, and Cytotoxicity. Eur. J. Inorg. Chem. 2017, 2017, 1800–1806. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. Combination of Ruthenium(II)-Arene Complex [Ru(Η 6-P-Cymene)Cl2(PTA)](RaPTA-C) and the Epidermal Growth Factor Receptor Inhibitor Erlotinib Results in Efficient Angiostatic and Antitumor Activity. Sci. Rep. 2017, 7, 43005. [Google Scholar] [CrossRef] [PubMed]
- Battistin, F.; Scaletti, F.; Balducci, G.; Pillozzi, S.; Arcangeli, A.; Messori, L.; Alessio, E. Water-Soluble Ru(II)-and Ru(III)-Halide-PTA Complexes (PTA = 1,3,5-Triaza-7-Phosphaadamantane): Chemical and Biological Properties. J. Inorg. Biochem. 2016, 160, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Guidi, F.; Modesti, A.; Landini, I.; Nobili, S.; Mini, E.; Bini, L.; Puglia, M.; Casini, A.; Dyson, P.J.; Gabbiani, C. The Molecular Mechanisms of Antimetastatic Ruthenium Compounds Explored through Dige Proteomics. J. Inorg. Biochem. 2013, 118, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, R.; Marchetti, F.; Petrini, A.; Pettinari, C.; Lupidi, G.; Smoleński, P.; Scopelliti, R.; Riedel, T.; Dyson, P.J. From Sunscreen to Anticancer Agent: Ruthenium(II) Arene Avobenzone Complexes Display Potent Anticancer Activity. Organometallics 2016, 35, 3734–3742. [Google Scholar] [CrossRef]
- Wołoszyn, A.; Pettinari, C.; Pettinari, R.; Patzmay, G.V.B.; Kwiecień, A.; Lupidi, G.; Nabissi, M.; Santoni, G.; Smoleński, P. Ru(II)-(PTA) and -MPTA Complexes with N 2-Donor Ligands Bipyridyl and Phenanthroline and Their Antiproliferative Activities on Human Multiple Myeloma Cell Lines. Dalton Trans. 2017, 46, 10073–10081. [Google Scholar] [CrossRef]
- Tapanelli, S.; Habluetzel, A.; Pellei, M.; Marchiò, L.; Tombesi, A.; Capparè, A.; Santini, C. Novel Metalloantimalarials: Transmission Blocking Effects of Water Soluble Cu(I), Ag(I) and Au(I) Phosphane Complexes on the Murine Malaria Parasite Plasmodium Berghei. J. Inorg. Biochem. 2017, 166, 1–4. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-Triaza-7-Phosphaadamantane Coordination Polymers Driven by Substituted Glutarate and Malonate Building Blocks: Self-Assembly Synthesis, Structural Features, and Antimicrobial Properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef]
- Smolenski, P.; Pettinari, C.; Marchetti, F.; Guedes da Silva, M.F.C.; Lupidi, G.; Badillo Patzmay, G.V.; Petrelli, D.; Vitali, L.A.; Pombeiro, A.J.L. Syntheses, Structures, and Antimicrobial Activity of New Remarkably Light-Stable and Water-Soluble Tris (Pyrazolyl) Methanesulfonate Silver(I) Derivatives of N-Methyl-1,3,5-Triaza-7-Phosphaadamantane Salt-[MPTA] Bf4. Inorg. Chem. 2014, 54, 434–440. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Król, J.; Conceição Oliveira, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive Silver-Organic Networks Assembled from 1,3,5-Triaza-7-Phosphaadamantane and Flexible Cyclohexanecarboxylate Blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Trenti, A.; Porchia, M.; Tisato, F.; Giorgetti, M.; Zanusso, I.; Trevisi, L.; Marzano, C. Homoleptic Phosphino Copper(I) Complexes with in Vitro and in Vivo Dual Cytotoxic and Anti-Angiogenic Activity. Metallomics 2015, 7, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Tisato, F.; Dolmella, A.; Pellei, M.; Santini, C.; Giorgetti, M.; Marzano, C.; Porchia, M. In Vitro and in Vivo Anticancer Activity of Copper(I) Complexes with Homoscorpionate Tridentate Tris(Pyrazolyl)Borate and Auxiliary Monodentate Phosphine Ligands. J. Med. Chem. 2014, 57, 4745–4760. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, E.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. S-Propargylthiopyridine Phosphane Derivatives as Anticancer Agents: Characterization and Antitumor Activity. Organometallics 2013, 32, 3710–3720. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Papini, G.; Morresi, B.; Galassi, R.; Ricci, S.; Tisato, F.; Porchia, M.; Rigobello, M.P.; Gandin, V. In Vitro Antitumour Activity of Water Soluble Cu(I), Ag(I) and Au(I) Complexes Supported by Hydrophilic Alkyl Phosphine Ligands. J. Inorg. Biochem. 2011, 105, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, J.; Doniach, S.; Das, R.; Herschlag, D. Understanding Nucleic Acid-Ion Interactions. Annu. Rev. Biochem. 2014, 83, 813–841. [Google Scholar] [CrossRef] [PubMed]
- Riddell, I.A.; Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Nucleotide Binding Preference of the Monofunctional Platinum Anticancer-Agent Phenanthriplatin. Chemistry 2016, 22, 7574–7581. [Google Scholar] [CrossRef]
- Amir, M.K.; Hayat, F.; Khan, S.Z.; Hogarth, G.; Kondratyuk, T.; Pezzuto, J.M.; Tahir, M.N. Monofunctional Platinum(II) Dithiocarbamate Complexes: Synthesis, Characterization and Anticancer Activity. RSC Adv. 2016, 6, 110517–110524. [Google Scholar] [CrossRef]
- Villarreal, W.; Colina-Vegas, L.; Rodrigues de Oliveira, C.; Tenorio, J.C.; Ellena, J.; Gozzo, F.C.; Cominetti, M.R.; Ferreira, A.G.; Ferreira, M.A.B.; Navarro, M. Chiral Platinum(II) Complexes Featuring Phosphine and Chloroquine Ligands as Cytotoxic and Monofunctional DNA-Binding Agents. Inorg. Chem. 2015, 54, 11709–11720. [Google Scholar] [CrossRef]
- Cutillas, N.; Martínez, A.; Yellol, G.S.; Rodríguez, V.; Zamora, A.; Pedreño, M.; Donaire, A.; Janiak, C.; Ruiz, J. Anticancer C, N-Cycloplatinated(II) Complexes Containing Fluorinated Phosphine Ligands: Synthesis, Structural Characterization, and Biological Activity. Inorg. Chem. 2013, 52, 13529–13535. [Google Scholar] [CrossRef]
- Berenguer, J.; Pichel, J.; Gimenez, N.; Lalinde, E.; Moreno, M.; Pineiro-Hermida, S. Luminescent Pentafluorophenyl-Cycloplatinated Complexes: Synthesis, Characterization, Photophysics, Cytotoxicity and Cellular Imaging. Dalton Trans. 2015, 44, 18839–18855. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Deacon, G.B.; Drago, P.R.; Hambley, T.W.; Nelson, K.T.; Webster, L.K. Preparation and Cell Growth Inhibitory Activity of [PtR2L2](R = Polyfluorophenyl, L2= Diene, Cyclohexane-1, 2-Diamine (Chxn) or Cis-(Dimethyl Sulfoxide)2) and the X-Ray Crystal Structure of [Pt(C6F5)2(Cis-Chxn)]. J. Inorg. Biochem. 2002, 89, 293–301. [Google Scholar] [CrossRef]
- Minniti, D. Uncatalyzed Cis-Trans Isomerization of Bis(Pentafluorophenyl) Bis(Tetrahydrothiophene) Palladium(II) Complexes in Chloroform: Evidence for a Dissociative Mechanism. Inorg. Chem. 1994, 33, 2631–2634. [Google Scholar] [CrossRef]
- García-Monforte, M.A.; Alonso, P.J.; Forniés, J.; Menjón, B. New Advances in Homoleptic Organotransition-Metal Compounds: The Case of Perhalophenyl Ligands. Dalton Trans. 2007, 38, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, E.; Sgarbossa, P.; Scarso, A.; Michelin, R.A.; Strukul, G. Second-Generation Electron-Poor Platinum(II) Complexes as Efficient Epoxidation Catalysts for Terminal Alkenes with Hydrogen Peroxide. Organometallics 2006, 25, 3056–3062. [Google Scholar] [CrossRef]
- Stone, F.G.A. Fluorocarbon Metal Compounds—Role Models in Organotransition Metal Chemistry. J. Fluor. Chem. 1999, 100, 227–234. [Google Scholar] [CrossRef]
- Usón, R.; Forniés, J. Organopalladium and Platinum Compounds with Pentahalophenyl Ligands. Adv. Organomet. Chem. 1988, 28, 219–297. [Google Scholar]
- Sarkar, B. Metal Protein Interactions. Prog. Food Nutr. Sci. 1987, 11, 363–400. [Google Scholar]
- Bal, W.; Christodoulou, J.; Sadler, P.J.; Tucker, A. Multi-Metal Binding Site of Serum Albumin. J. Inorg. Biochem. 1998, 70, 33–39. [Google Scholar] [CrossRef]
- Espósito, B.P.; Najjar, R. Interactions of Antitumoral Platinum-Group Metallodrugs with Albumin. Coord. Chem. Rev. 2002, 232, 137–149. [Google Scholar] [CrossRef]
- Hu, W.; Luo, Q.; Wu, K.; Li, X.; Wang, F.; Chen, Y.; Ma, X.; Wang, J.; Liu, J.; Xiong, S. The Anticancer Drug Cisplatin Can Cross-Link the Interdomain Zinc Site on Human Albumin. Chem. Commun. 2011, 47, 6006–6008. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Yoo, H.; Lin, W.; Brooks, J.G.; Lippard, S.J. Pt(IV) Prodrugs Designed to Bind Non-Covalently to Human Serum Albumin for Drug Delivery. J. Am. Chem. Soc. 2014, 136, 8790–8798. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Christodoulou, J.; Parkinson, J.A.; Barnham, K.J.; Tucker, A.; Woodrow, J.; Sadler, P.J. Cisplatin Binding Sites on Human Albumin. J. Biol. Chem. 1998, 273, 14721–14730. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.; Miranda, S.; Lüttenberg, S.; Fröhlich, N.; Koenen, J.-M.; Mohr, F.; Cerrada, E.; Laguna, M.; Mendía, A. Trans-Thionate Derivatives of Pt(II) and Pd(II) with Water-Soluble Phosphane PTA and DAPTA Ligands: Antiproliferative Activity against Human Ovarian Cancer Cell Lines. Inorg. Chem. 2013, 52, 6635–6647. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, L.; García-Argáez, A.N.; Agostinelli, E.; Dell’Amico, D.B.; Labella, L.; Samaritani, S. New Trans Dichloro(Triphenylphosphine)Platinum(II) Complexes Containing N-(Butyl), N-(Arylmethyl)Amino Ligands: Synthesis, Cytotoxicity and Mechanism of Action. Biorg. Med. Chem. 2016, 24, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A. Understanding Trans Platinum Complexes as Potential Antitumor Drugs Beyond Targeting DNA. J. Inorg. Biochem. 2012, 114, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lis, U.; Ochocki, J.; Matlawska-Wasowska, K. Trans Geometry in Platinum Antitumor Complexes. Coord. Chem. Rev. 2008, 252, 1328–1345. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Icsel, C.; Turgut, O.R.; Aygun, M.; Erkisa, M.; Turkdemir, M.H.; Ulukaya, E. Synthesis, Structures and Anticancer Potentials of Platinum(II) Saccharinate Complexes of Tertiary Phosphines with Phenyl and Cyclohexyl Groups Targeting Mitochondria and DNA. Eur. J. Med. Chem. 2018, 155, 609–622. [Google Scholar] [CrossRef]
- Echeverri, M.; Alvarez-Valdés, A.; Navas, F.; Perles, J.; Sánchez-Pérez, I.; Quiroga, A.G. Using Phosphine Ligands with a Biological Role to Modulate Reactivity in Novel Platinum Complexes. R. Soc. Open Sci. 2018, 5, 171340. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Usón, R.; Forniés, J.; Espinet, P.; Alfranca, G. Pentafluorophenyl Platinum(II) and Platinum(IV) Complexes with O-Phenylenebisdimethylarsine. Synth. React. Inorg. Met. Org. Chem. 1980, 10, 579–590. [Google Scholar] [CrossRef]
- Usón, R.; Forniés, J.; Martínez, F.; Tomás, M. Mono- and Bi-Nuclear Anionic Pentafluorophenyl Complexes of Palladium(II) and Platinum(II). J. Chem. Soc. Dalton Trans. 1980, 888–894. [Google Scholar] [CrossRef]
- EN 14476. Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area—Test Method and Requirements (Phase 2/Step 1); NSAI: Dublin, Ireland, 2015.
- Sangster, J. Octanol-Water Partition Coefficients of Simple Organic Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1229. [Google Scholar] [CrossRef]
- Beaven, G.H.; Chen, S.H.; D’albis, A.; Gratzer, W.B. A Spectroscopic Study of the Haemin-Human-Serum-Albumin System. Eur. J. Biochem. 1974, 41, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.; Ramamurthy, P. Photophysical Studies on the Interaction of Amides with Bovine Serum Albumin (BSA) in Aqueous Solution: Fluorescence Quenching and Protein Unfolding. J. Lumin. 2014, 148, 277–284. [Google Scholar] [CrossRef]
- Bruker. APEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. Sir97: A New Tool for Crystal Structure Determination and Refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of Shelx. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. Wingx and Ortep for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Smoleński, P.; Pombeiro, A.J.L. Water-Soluble and Stable Dinitrogen Phosphine Complexes Trans-[Recl(N2)(PTA-H)N(PTA)4−N]N+ (N = 0–4), the First with 1,3,5-Triaza-7-Phosphaadamantane. Dalton Trans. 2008, 87–91. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, Τ 4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Longmire, J.M.; Zhang, X.; Shang, M. Synthesis and X-Ray Crystal Structures of Palladium(II) and Platinum(II) Complexes of the Pcp-Type Chiral Tridentate Ligand (1R,1′R)-1,3-Bis[1-(Diphenylphosphino)Ethyl]Benzene. Use in the Asymmetric Aldol Reaction of Methyl Isocyanoacetate and Aldehydes. Organometallics 1998, 17, 4374–4379. [Google Scholar] [CrossRef]
- Lasri, J.; Guedes da Silva, M.F.C.; Kopylovich, M.N.; Ghosh Mukhopadhyay, B.; Pombeiro, A.J.L. Platinum(II)-Promoted [2 + 3] Cycloaddition of Azide with 4-Cyanobenzaldehyde, a Schiff Base Derivative or Dicyanobenzenes to Give Formyl-, Amino (Imino)-or Cyano-Functionalized Tetrazolato Complexes. Eur. J. Inorg. Chem. 2009, 2009, 5541–5549. [Google Scholar] [CrossRef]
- Smoleński, P.; Mukhopadhyay, S.; Guedes da Silva, M.F.C.; Charmier, M.A.J.; Pombeiro, A.J.L. New Water-Soluble Azido-and Derived Tetrazolato-Platinum(II) Complexes with PTA. Easy Metal-Mediated Synthesis and Isolation of 5-Substituted Tetrazoles. Dalton Trans. 2008, 6546–6555. [Google Scholar] [CrossRef]
- Sgarbossa, P.; Guedes da Silva, M.F.C.; Scarso, A.; Michelin, R.A.; Pombeiro, A.J.L. Lewis Acidity of Platinum(II)-Based Baeyer—Villiger Catalysts: An Electrochemical Approach. Inorg. Chim. Acta 2008, 361, 3247–3253. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Lasri, J.; Guedes da Silva, M.F.C.; Charmier, M.A.J.; Pombeiro, A.J.L. Activation of C–Cn Bond of Propionitrile: An Alternative Route to the Syntheses of 5-Substituted-1h-Tetrazoles and Dicyano-Platinum(II) Species. Polyhedron 2008, 27, 2883–2888. [Google Scholar] [CrossRef]
- Lasri, J.; Charmier, M.A.J.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mixed Unsymmetric Oxadiazoline and/or Imine Platinum(II) Complexes. Dalton Trans. 2007, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Śliwińska-Hill, U.; Białońska, A.; Nesterov, D.S.; Kuropka, P.; Sokolnicki, J.; Bażanów, B.; Smoleński, P. Light-Stable Polypyridine Silver(I) Complexes of 1,3,5-Triaza-7-Phosphaadamantane (PTA) and 1,3,5-Triaza-7-Phosphaadamantane-7-Sulfide (PTA = S): Significant Antiproliferative Activity of Representative Examples in Aqueous Media. Dalton Trans. 2019, 48, 11235–11249. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. In Vitro Anticancer Activity of Cis-Diammineplatinum(II) Complexes with Β-Diketonate Leaving Group Ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Klajnert, B.; Bryszewska, M. Fluorescence Studies on Pamam Dendrimers Interactions with Bovine Serum Albumin. Bioelectrochemistry 2002, 55, 33–35. [Google Scholar] [CrossRef]
- Ware, W.R. Oxygen Quenching of Fluorescence in Solution: An Experimental Study of the Diffusion Process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Seedher, N.; Kanojia, M. Fluorescence Spectroscopic Studies on the Complexation of Antidiabetic Drugs with Glycosylated Serum Albumin. J. Appl. Spectrosc. 2013, 80, 754–760. [Google Scholar] [CrossRef]
- Neault, J.; Tajmir-Riahi, H. Interaction of Cisplatin with Human Serum Albumin. Drug Binding Mode and Protein Secondary Structure. Biochim. Biophys. Acta 1998, 1384, 153–159. [Google Scholar] [CrossRef]
- Morais, T.S.; Santos, F.C.; Corte-Real, L.; Garcia, M.H. Exploring the Effect of the Ligand Design on the Interactions between [Ru(η5 -C5h5)(Pph3)(N, O)][Cf3SO3] Complexes and Human Serum Albumin. J. Inorg. Biochem. 2013, 129, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Wang, C.; Sun, D.; Huang, Y.; Zhou, Y.; Liu, J. Ruthenium(II) Complexes Binding to Human Serum Albumin and Inducing Apoptosis of Tumor Cells. Inorg. Chem. Commun. 2012, 24, 104–109. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, J.; Zhao, Y.; He, W.; Guo, Z. Noncovalent Interactions between a Trinuclear Monofunctional Platinum Complex and Human Serum Albumin. Inorg. Chem. 2011, 50, 12661–12668. [Google Scholar] [CrossRef]
- Krause-Heuer, A.M.; Price, W.S.; Aldrich-Wright, J.R. Spectroscopic Investigations on the Interactions of Potent Platinum(II) Anticancer Agents with Bovine Serum Albumin. J. Chem. Biol. 2012, 5, 105–113. [Google Scholar] [CrossRef][Green Version]
- Leckband, D. Measuring the Forces That Control Protein Interactions. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 1–26. [Google Scholar] [CrossRef]

| Formula Unit | C18H24ClF5N6P2Pt |
|---|---|
| Formula weight | 711.91 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a (Å) | 19.9537(17) |
| b (Å) | 12.2786(9) |
| c (Å) | 10.2981(9) |
| β (°) | 115.314(5) |
| Z | 4 |
| Volume (Å3) | 2280.8(3) |
| T (K) | 153(2) |
| Dc (g cm−3) | 2.073 |
| μ (mm−1) | 6.471 |
| θmax, θmin (°) | 25.349, 2.589 |
| Rfls. total, unique, observed | 10377, 2082, 1928 |
| Rint | 0.0383 |
| R1 *,wR2 # (I > 2σ(I)) | 0.0183, 0.0427 |
| R1, wR2 (all data) | 0.0214, 0.0440 |
| GOF | 1.043 |
| Entry | Cell Line | 2a | 2b | 2c | 1a [100] | 1b | 1c | Cisplatin [100] |
|---|---|---|---|---|---|---|---|---|
| 1 | NHDF | 26.07 ± 0.68 | 26.91 ± 3.5 | 11.16 ± 3.1 | nda | >263.13 ± 13 | >223.82 ± 5.7 | 16.65 ± 2.1 |
| 2 | A549 | 15.41 ± 5.3 | 7.79 ± 1.7 | >28.10 ± 1.9 | nda | >263.13 ± 0.42 | >223.82 ± 23 | 33.30 ± 4.2 |
| 3 | HeLa | >42.14 ± 5.5 | 29.48 ± 1.3 | 8.89 ± 4.1 | nda | 65.78 ± 6.6 | 55.96 ± 16 | 16.65 ± 3.1 |
| 4 | MCF7 | >42.14 ± 7.9 | >31.00 ± 11 | >28.10 ± 7.3 | nda | >263.13 ± 7.5 | 72.40 ± 2.4 | 33.30 ± 4.2 |
| T (K) | KSV (M−1) | Kq (M−1·s−1) | KA (M−1) | n | ||||
|---|---|---|---|---|---|---|---|---|
| 2b | 2c | 2b | 2c | 2b | 2c | 2b | 2c | |
| 300 | 8.85 × 103 | 1.36 × 104 | 1.77 × 1012 | 2.72 × 1012 | 9.88 × 103 | 1.45 × 104 | 1.26 | 1.25 |
| 305 | 9.78 × 103 | 1.48 × 104 | 1.96 × 1012 | 2.96 × 1012 | 9.90 × 103 | 1.52 × 104 | 0.96 | 0.99 |
| 310 | 1.04 × 104 | 1.62 × 104 | 2.08 × 1012 | 3.24 × 1012 | 1.10 × 104 | 1.59 × 104 | 1.24 | 1.59 |
| T (K) | ΔH0 (kJ mol−1) | ΔG0 (kJ mol−1) | ΔS0 (J mol−1·K−1) | |||
|---|---|---|---|---|---|---|
| 2b | 2c | 2b | 2c | 2b | 2c | |
| 300 | 16.8 | 7.15 | −22.7 | −23.9 | 131.4 | 103.5 |
| 305 | −23.3 | −24.4 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgarbossa, P.; Śliwińska-Hill, U.; Guedes da Silva, M.F.C.; Bażanów, B.; Pawlak, A.; Jackulak, N.; Poradowski, D.; Pombeiro, A.J.L.; Smoleński, P. Pentafluorophenyl Platinum(II) Complexes of PTA and Its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity. Materials 2019, 12, 3907. https://doi.org/10.3390/ma12233907
Sgarbossa P, Śliwińska-Hill U, Guedes da Silva MFC, Bażanów B, Pawlak A, Jackulak N, Poradowski D, Pombeiro AJL, Smoleński P. Pentafluorophenyl Platinum(II) Complexes of PTA and Its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity. Materials. 2019; 12(23):3907. https://doi.org/10.3390/ma12233907
Chicago/Turabian StyleSgarbossa, Paolo, Urszula Śliwińska-Hill, M. Fátima C. Guedes da Silva, Barbara Bażanów, Aleksandra Pawlak, Natalia Jackulak, Dominik Poradowski, Armando J. L. Pombeiro, and Piotr Smoleński. 2019. "Pentafluorophenyl Platinum(II) Complexes of PTA and Its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity" Materials 12, no. 23: 3907. https://doi.org/10.3390/ma12233907
APA StyleSgarbossa, P., Śliwińska-Hill, U., Guedes da Silva, M. F. C., Bażanów, B., Pawlak, A., Jackulak, N., Poradowski, D., Pombeiro, A. J. L., & Smoleński, P. (2019). Pentafluorophenyl Platinum(II) Complexes of PTA and Its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity. Materials, 12(23), 3907. https://doi.org/10.3390/ma12233907










