Abstract
This research explored a novel chemical reduction of organic aluminum for plating Al on a graphene surface. The thermodynamics of the Al plating reaction process were studied. The Al plating process consisted of two stages: the first was to prepare (C2H5)3Al. In this reaction, the ΔH(enthalpy) was 10.64 kcal/mol, the ΔG(Gibbs free energy) was 19.87 kcal/mol and the ΔS(entropy) was 30.9 cal/(mol·K); this was an endothermic reaction. In the second stage, the (C2H5)3Al decomposed into Al atoms, which were gradually deposited on the surface of the graphene and the Al plating formed. At 298.15 K, the ΔH was −20.21 kcal/mol, the ΔG was −54.822 kcal/mol, the ΔS was 116.08 cal/(mol·K) and the enthalpy change was negative, thus indicating an endothermic reaction.
1. Introduction
Graphene/aluminum composites have high strength, high conductivity and high toughness. Thus, graphene/aluminum composites have wide application potentiality in the electronics, automotive and aerospace industry [1,2,3,4,5,6,7,8]. However, graphene/aluminum composites are difficult to prepare; because of the poor wettability between Al and graphene, the graphene aggregates easily in the Al matrix, which can decrease the mechanical properties of the composites [9,10]. In order to improve the wettability between graphene and Al, the ideal method is to coating melt on the surface of the graphene, by methods including self-assembly, chemical reduction, electrochemical deposition, redox method and chemical vapor deposition. For instance, Bagheri et al. prepared graphene-gold nanocomposites by self-assembly [11]. Tsai et al. coated the Cu nanoparticles on the graphene surface through coalescence and epitaxial self-assembly and studied molecular dynamics during the process [12]. Muszynski et al. synthesized gold nanoparticles through the chemical reduction of AuCl4− (Aldrich) with NaBH4 and coated the gold nanoparticles on the surface of graphene [13]. Zhao et al. prepared graphene nanoplatelets by reinforcing copper matrix composites with electrochemical deposition and the composites’ hardness and conductivity reached 111.2 HV and 89.2% IACS [14]. Kim et al. prepared single-atomic-layer graphene film on the surface of Cu through chemical vapor deposition (CVD), then obtained multi-layer graphene/copper composites with the strength of 1.5 GPa [15]. According to previous investigations, gold, copper, or nickel nanoparticles were usually coated on the graphene. However, these metal nanoparticles may be viewed as impurities in Al alloys, which can affect their properties. Plating Al on graphene is an effective method to improve graphene’s wettability and reduce these impurities. Because the Al is active, it is difficult to displace Al atom from conventional Al salt solution [16].
Selective laser melting (SLM), through melting successive layers of metal powder, is a promising metal additive manufacturing method [17,18], it has huge advantages compared to traditional processing methods [19,20,21,22,23,24] and therefore has been widely used in the fields of medical, military, aerospace and automobile manufacturing [25]. The purpose of this new method is to increase the weight of graphene by plating Al on its surface, which solves the problem of uneven dispersion of graphene in Al powder for SLM. Considering that plating Al on graphene is difficult, we explored a novel chemical reduction of organic aluminum for plating Al on the graphene surface [19]. The Al plating process consisted of two stages. In the first stage, the Al powders were added to the C2H5Br solution, to produce (C2H5)3Al. In the second stage, the (C2H5)3Al decomposed into Al atoms, which were gradually deposited on the surface of the graphene and the Al plating formed [26]. The microstructure evolution was reported [26].
However, the reaction mechanism was unclear, especially the thermodynamics of the reaction process. Density functional theory (DFT) is a quantum mechanical method for studying the electronic structure of multi-electron systems [27,28,29]. DFT has a wide range of applications in physics and chemistry, especially for studying the properties of molecules and condensed states [30,31,32]. It is one of the most commonly used methods in computational materials and computational chemistry [30]. The objective of the study described here is to elucidate the thermodynamics of the Al plating reaction process and it provides guidance for process optimization.
2. Experiment and Simulation
2.1. Experiment
The Al powders and the graphene were employed as raw materials, as shown in Figure 1. During the Al plating reaction process, H2 gas was pumped into the reaction vessel and the Al powder (1.5 g), aluminum chloride (0.1 g) and iodine (0.1 g) were dried and added into the C2H5Br (29 mL) at 39 °C. Al reacted with C2H5Br and the (C2H5)2AlBr and C2H5AlBr2 were obtained as follows [26]:
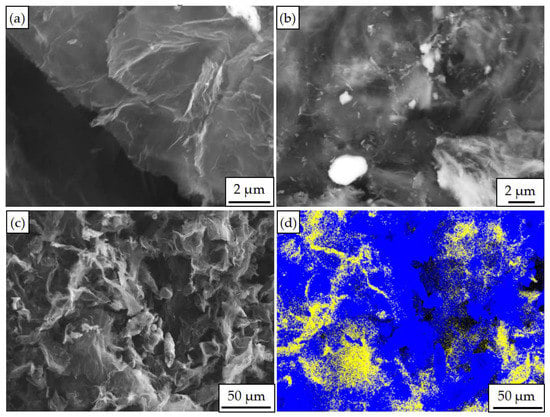
Figure 1.
Scanning electron microscopy (SEM) image and energy dispersive spectrometer (EDS) analysis of Graphene/aluminum composites with different reaction times: (a) Graphene/aluminum composites prepared with reaction time of 1 h; (b) Graphene/aluminum composites prepared with reaction time of 1.5 h; (c) Low-magnification SEM image of composite prepared with reaction time of 1.5 h; (d) map analysis of (c).
The Al reacted with C2H5AlBr2 to produce (C2H5)2AlBr, Al and AlBr3 [26]:
The (C2H5)2AlBr and Al further reacted to produce Al, (C2H5)3Al and AlBr3 via Equation [26]:
After reaction, the solution temperature was kept at 0 °C for 1 h. The tetrahydrofuran was added to the solution. The solution was filtered after the reaction and the alkyl aluminum solution was obtained. Then, the graphene (0.05 g) was added to the alkyl aluminum solution. The temperature was kept at 70–100 °C for 1–1.5 h and the (C2H5)3Al was decomposed into Al, H2 and C2H4 [26]:
The Al atoms were gradually deposited on the surface of the graphene and the Al plating formed. Al atoms absorbed on graphene may form upon (C2H5)3Al/graphene collisions. This reaction is initiated by ethane elimination from the (C2H5)3Al molecule, similar to the observations reported for (CH3)3Al/graphene [33].
Microstructure observation was carried out using a scanning electron microscope (SEM) (Zeiss Ultra 55, Carl Zeiss Microscopy, Jena, Germany) equipped with energy dispersive spectroscopy (EDS).
2.2. Computation Details
During the process of plating Al on the graphene, the thermodynamics of the chemical reduction of organic aluminum were simulated by density functional theory (DFT) methods implemented in the DMol3 package of Materials Studio. The structure of the reaction products was analyzed through the DFT, revealing the thermodynamic properties and reaction types of the chemical reactions. Spin-unrestricted DFT in the generalized gradient approximation (GGA) with the Revised Perdew-Burke-Eruzerhof (RPBE) exchange-correlation functional approach and double numerical plus polarization atomic orbitals was employed as the basis set. The Brillouin zone was sampled using the 4 × 4 × 1 k-point grid thickness, which presented a good approximation of the model below the article. In addition, the energy tolerance accuracy, maximum force and displacement were set as 1 × 10−5 Ha, 2 × 10−3 Ha/Å and 5 × 10−3 Å, respectively, to ensure high accuracy in all calculations.
During the Dmol3 simulation process, the relationship between thermodynamic properties (entropy S, enthalpy H, heat capacity Cp, Gibbs free energy G) and temperature can be calculated from the vibration frequency. The total energy at 0 K was obtained during the simulation. The translational energy, rotational energy and vibration energy were used to calculate the thermodynamic properties at an instantaneous temperature. The instantaneous enthalpy H is:
where , and are vibration energy, rotational energy and translational energy respectively at temperature T and R is an ideal gas constant.
The contribution of vibration to enthalpy is:
The contribution of vibration to entropy is:
The contribution of vibration to heat capacity at normal pressure is:
where k is the Boltzmann constant, h is the Planck constant and vi is the vibration frequency of the ith atom. Each of the chemical bond vibrational frequencies was calculated by DFT at 298.15 K and then assumed to remain constant with Temperature.
3. Results and Discussions
3.1. Preparation and Reaction Mechanism of Al-Coated Graphene
During the Al plating process, with the increase of reaction time, more Al was deposited on the graphene, as shown in Figure 1a,b. When the chemical reduction reaction was at 1.5 h, abundant Al atoms were deposited on the graphene uniformly, the Al plating was formed and the content of the Al element was 71%, as shown in Figure 1c,d.
3.2. Reaction Thermodynamics during Plating Al on Graphene Process
The molecular model of each substance was established and its structure optimized during the chemical reduction reaction process. The vibration frequency was calculated and the thermodynamic properties of each substance were analyzed. The thermodynamics of the formation and decomposition of (C2H5)3Al were calculated according to the laws of thermodynamics.
During the process of plating Al on grapheme, based on the reaction Equations (1) and (3), the structural optimization and thermodynamic calculation of C2H5Br, (C2H5)2AlBr, C2H5AlBr2, (C2H5)3Al and AlBr3 were carried out through the Al cluster (Al3) molecular model [34].
3.2.1. Structure Optimization and Thermodynamic Properties of C2H5Br
Figure 2 shows the structure of the C2H5Br molecule. The initial structure of the C2H5Br molecule built in MS is shown in Figure 2a; the stable molecular structure after structure optimization is shown in Figure 2b. After structure optimization, ∠H1C1C2 was reduced from 109.471° to 108.747°, ∠H1C1C2 increased from 109.471° to 111.590°, ∠C1C2Br increased from 109.469° to 111.496° and ∠BrC2H5 decreased from 109.472° to 103.736°. The bond length of H1–C1 decreased from 1.14 Å to 1.1 Å, the C1–C2 bond was reduced from 1.54 Å to 1.517 Å, the C2–Br bond as increased from 1.91 Å to 2.025 Å and the C2–H5 bond was reduced from 1.14 Å to 1.095 Å. During the structure optimization process, the bond angle and bond length of atoms tended to be stable through the vibration displacement and the total energy was gradually minimized.
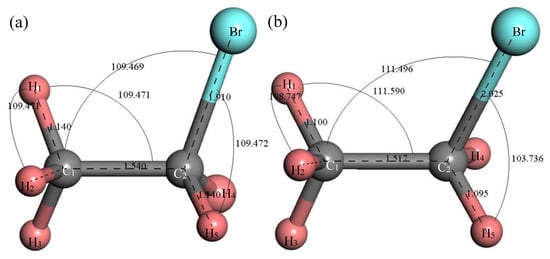
Figure 2.
Molecular model of C2H5Br molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
Figure 3 shows the relationship between the thermodynamic properties (entropy S, enthalpy H, heat capacity Cp, Gibbs free energy G) of the C2H5Br and temperature was obtained through Equations (5)–(8). In the range of 25–1000 K, the enthalpy of the C2H5Br molecule had a linear relationship with the temperature and the enthalpy value increased with the increase of temperature. The heat capacity of C2H5Br gradually increased with the increase of temperature, although the free energy decreased. At 298.15 K, the enthalpy, entropy, free energy and heat capacity of C2H5Br molecules were 43.533 kcal/mol, 68.433 cal/(mol·K), 15.174 cal/(mol·K) and 23.127 kcal/mol respectively, as shown in Table 1.
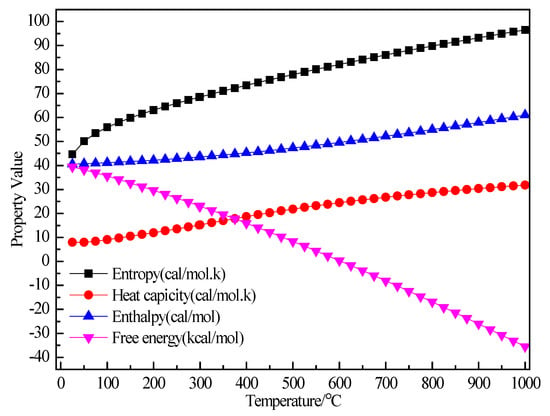
Figure 3.
The relationship between the thermodynamic properties of C2H5Br and temperature.

Table 1.
The total energy and the thermodynamic properties at 298.15 K of each component.
3.2.2. Structure Optimization and Thermodynamic Properties of (C2H5)2AlBr
Figure 4 shows the structure of (C2H5)2AlBr. After structure optimization, ∠H1C1H2 decreased from 109.415° to 107.039°, ∠C1C2Al increased by 9.965°, ∠H4C2H5 decreased by 5.254° and ∠C2AlC3 increased by 2.571°. The length of the Br–Al bond increased from 2.250 Å to 2.331 Å, the length of the Al–C3 bond increased from 1.88 Å to 1.98 Å, the length of the C3–H7 bond decreased by 0.03 Å, the length of the C3–C4 bond increased by 0.009 Å and the length of the C4–H9 bond decreased from 1.140 Å to 1.102 Å. Figure 5 shows the relationship between the thermodynamic properties of (C2H5)2AlBr and temperature. It can be seen that the enthalpy, entropy and heat capacity of (C2H5)2AlBr increased with the increase of temperature in the range of 25–1000 K and the free energy decreased with the increase of temperature. At 298.15 K, the enthalpy, entropy, heat capacity and free energy were 85.548 kcal/mol, 97.648 cal/(mol·K), 33.078 cal/(mol·K) and 56.435 kcal/mol, respectively, as shown in Table 1.
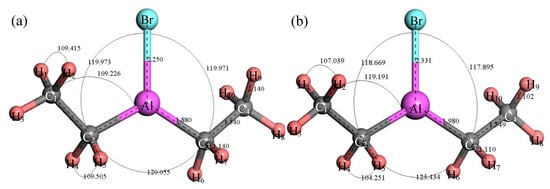
Figure 4.
Molecular model of (C2H5)2AlBr molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
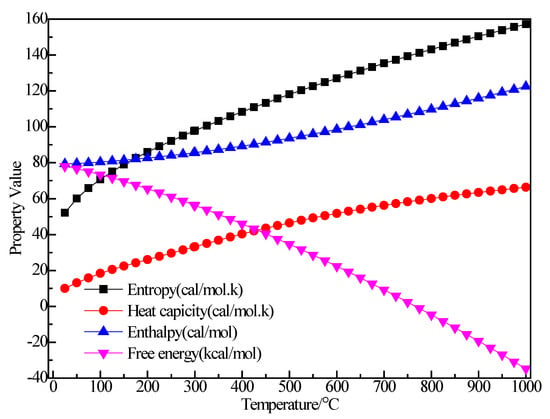
Figure 5.
The relationship between the thermodynamic properties of (C2H5)2AlBr and temperature.
3.2.3. Structure Optimization and Thermodynamic Properties of C2H5AlBr2
Figure 6 shows the original and the optimal structure of C2H5AlBr2. After structure optimization, ∠H1C1H2 decreased from 109.511° to 104.953°, ∠H3C2H4 decreased from 109.52° to 107.514°, ∠C2C1Al increased from 109.239° to 117.439°, ∠C1AlBr1 increased by 0.3° and ∠Br2AlBr1 decreased by 3.685°. The bond length of H2–C1 decreased from 1.14 Å to 1.102 Å and the bond length of C1–C2 increased by 0.007 Å, indicating that the C–C bond was relatively stable. The bond length of C1–Al was increased by 0.085 Å and the bond length of Al-Br1 was increased by 0.45 Å. Figure 7 shows the relationship between the thermodynamic properties of C2H5AlBr2 and temperature. It can be seen that the enthalpy, entropy and heat capacity of C2H5AlBr2 increased with the increase of temperature in the range of 25–1000 K. The free energy decreased with the increase of temperature. At 298.15 K, the enthalpy, entropy, heat capacity and free energy were 46.425 kcal/mol, 94.579 cal/(mol·K), 26.606 cal/(mol·K) and 18.226 kcal/mol respectively (Table 1).
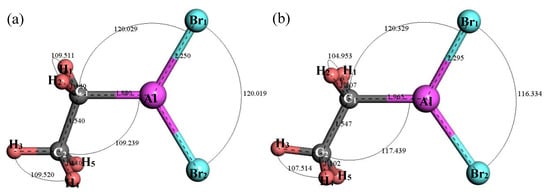
Figure 6.
Molecular model of C2H5AlBr2 molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
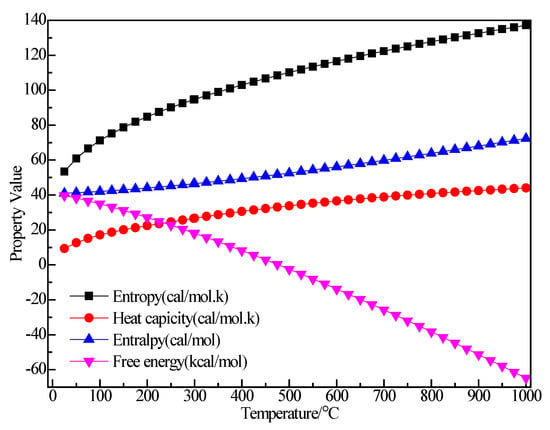
Figure 7.
The relationship between the thermodynamic properties of C2H5AlBr2 and temperature.
3.2.4. Structure Optimization and Thermodynamic Properties of (C2H5)3Al
Figure 8 shows the structure of (C2H5)3Al. After structure optimization, ∠C2AlC3 was reduced from 119.992° to 118.949°, ∠C2AlC5 decreased from 119.805° to 119.593°, ∠C3AlC5 increased from 119.891° to 121.407°, ∠AlC5C6 increased from 108.858° to 117.775°, ∠H11C5H12 decreased from 109.536° to 103.956° and ∠H13C6H14 decreased from 109.444° to 107.13°. The H1–C1 bond length was reduced from 1.14 Å to 1.105 Å, the C1–C2 bond length increased from 1.54 Å to 1.551 Å, the C2–Al bond length increased from 1.879 Å to 1.997 Å and the C2–H4 bond length was reduced from 1.14 Å to 1.112 Å. During the optimization process, the C–Al bond rotated, the bond angle had large variation, the bond length changed little and the initial structure was significantly different from the optimized structure. Figure 9 shows the thermodynamic properties of (C2H5)3Al. In the range of 25–1000 K, the enthalpy, entropy and heat capacity of (C2H5)3Al increased with the increase of temperature. The free energy decreased with the increase of temperature. At 298.15 K, the enthalpy, entropy and heat capacity were 125.294 kcal/mol, 102.836 cal/(mol·K), 41.264 cal/(mol·K) and 94.634 kcal/mol, respectively.
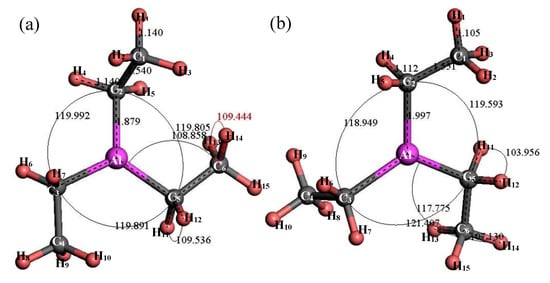
Figure 8.
Molecular model of (C2H5)3Al molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
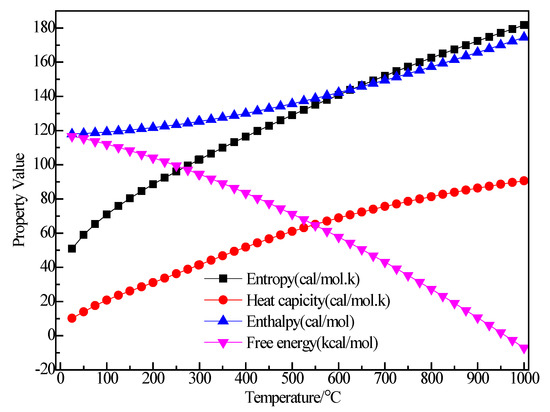
Figure 9.
The relationship between the thermodynamic properties of (C2H5)3Al and temperature.
3.2.5. Structure Optimization and Thermodynamic Properties of AlBr3
Figure 10 shows the structure of AlBr3. It can be seen that after optimization of the AlBr3 structure, the bond angle of AlBr3 increased from equal 120° to 120.687°, 120.173° and 119.14°. The Br1–Al bond length increased from 2.25 Å to 2.264 Å, the Br2–Al bond length increased from 2.254 Å to 2.264 Å and the Br3–Al bond length increased from 2.25 Å to 2.267 Å. Figure 11 shows the thermodynamic properties of AlBr3. It can be seen that in the range of 25–1000 K, the enthalpy and entropy of the AlBr3 molecule increased with the increase of temperature, the heat capacity tended to be stable with the increase of temperature and the free energy decreased with the increase of temperature. The free energy was 0 kcal/mol at 25 K, which gradually decreased to a negative value with the increase of temperature. At 298.15 K, the enthalpy, entropy, heat capacity and free energy were 6.478 kcal/mol, 88.04 cal/(mol·K), 18.250 cal/(mol·K) and −19.771 kcal/mol, respectively.
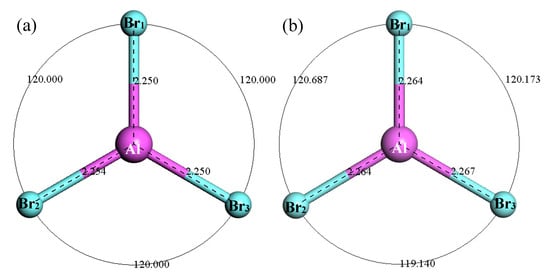
Figure 10.
Molecular model of AlBr3 molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
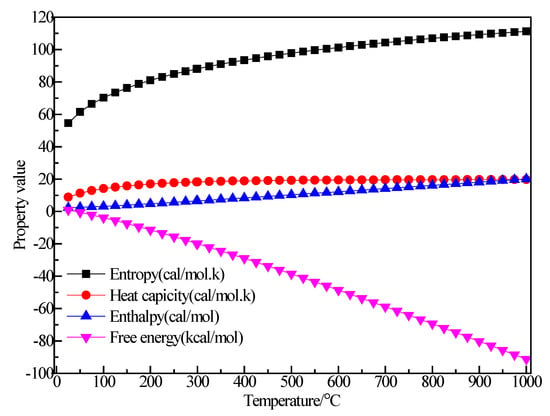
Figure 11.
The relationship between the thermodynamic properties of AlBr3 and temperature.
Table 2 shows the thermodynamic properties during Al reacting with C2H5Br to produce (C2H5)2AlBr and C2H5AlBr2. It can be seen that when the reaction temperature was 298.15 K, the ΔH was −160.77 kcal/mol, ΔG was −139.83 kcal/mol and ΔS was −70.2 cal/(mol·K); it was thus an exothermic reaction.

Table 2.
The thermodynamic properties during Al reacting with C2H5Br to produce (C2H5)2AlBr and C2H5AlBr2.
Table 3 shows the total energy and thermodynamic properties of each component during (C2H5)3Al preparation at 298.15 K. Table 4 shows the thermodynamic properties during the (C2H5)3Al preparation process (reaction Equation (3)). At 298.15 K, the ΔH was 10.64 kcal/mol, the ΔG was 19.87 kcal/mol, the ΔS was 30.9 cal/(mol·K) and the enthalpy change was greater than 0; indicating this was an endothermic reaction.

Table 3.
Total energy and thermodynamic properties of each component during (C2H5)3Al preparation at 298.15 K.

Table 4.
The thermodynamic properties during preparing (C2H5)3Al process.
3.2.6. Structure Optimization and Thermodynamic Properties of C2H4
Figure 12 shows the structure of C2H4. After structural optimization, ∠H2C2H4 was reduced from 120.001° to 116.504°. The bond length of C–H decreased from 1.14 Å to 1.094 Å and the bond length of C=C was reduced from 1.54 Å to 1.342 Å. Figure 13 shows the thermodynamic properties of C2H4. It can be seen that the enthalpy, entropy and heat capacity of C2H4 increased with the increase of temperature in the range of 25–1000 K. The free energy decreased with the increase of temperature. At 298.15 K, the enthalpy, entropy, heat capacity and free energy were 33.759 kcal/mol, 55.228 cal/(mol·K), 10.372 cal/(mol·K) and 17.293 kcal/mol respectively (Table 5).
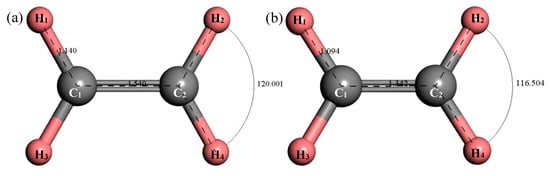
Figure 12.
Molecular model of C2H4 molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
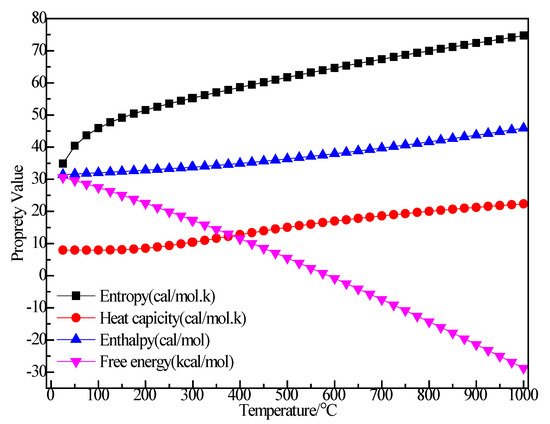
Figure 13.
The relationship between the thermodynamic properties of C2H4 and temperature.

Table 5.
The total energy and thermodynamic properties of each component during (C2H5)3Al decomposition at 298.15 K.
3.2.7. Structure Optimization and Thermodynamic Properties of H2
Figure 14 shows the structure of H2. It can be seen that after structural optimization, the bond length of H–H increased from 0.74 Å to 0.747 Å. Figure 15 shows the relationship between the thermodynamic properties of H2 and temperature. The enthalpy, entropy and heat capacity of H2 increased with the increase of temperature and the free energy decreased with the increase of temperature. At 298.15 k, the enthalpy, entropy, heat capacity and free energy were respectively 8.367 cal/mol, 32.531 cal/(mol·K), 6.955 cal/(mol·K) and −1.332 kcal/mol (Table 5).
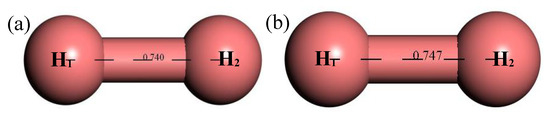
Figure 14.
Molecular model of H2 molecule. (a) Initial model; (b) Optimized model. The unit of the angle in the image is (°), and the unit of the bond length is angstrom (Å).
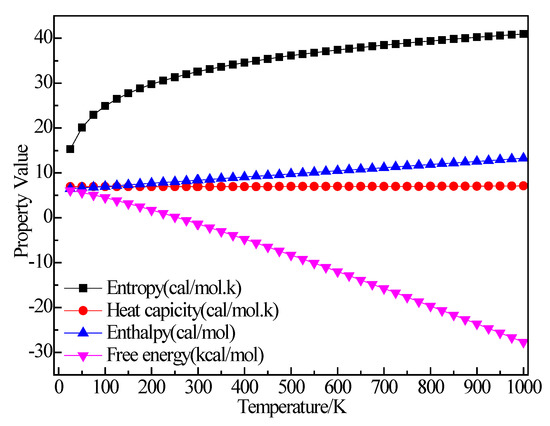
Figure 15.
The relationship between the thermodynamic properties of H2 and temperature.
Table 5 shows the thermodynamic properties during the (C2H5)3Al decomposition process (reaction Equation (4)). At 298.15 K, the ΔH was −20.21 kcal/mol, the ΔG was −54.822 kcal/mol, the ΔS was 116.08cal/(mol·K) (Table 6) and the enthalpy change was less than 0, this was an endothermic reaction.

Table 6.
The thermodynamic properties during preparing (C2H5)3Al process.
4. Conclusions
We explored a novel chemical reduction of organic aluminum for plating Al on a graphene surface. The thermodynamics of the Al plating reaction process were studied. The Al plating process consisted of two stages: the first was to prepare (C2H5)3Al; the ΔH was 10.64 kcal/mol, the ΔG was 19.87 kcal/mol, the ΔS was 30.9 cal/(mol·K); this was an endothermic reaction. In the second stage, the (C2H5)3Al decomposed into Al atoms, which were gradually deposited on the surface of the graphene and the Al plating formed. At 298.15 K, the ΔH was −20.21 kcal/mol, the ΔG was −54.822 kcal/mol, the ΔS was 116.08 cal/(mol·K) and the enthalpy change was negative, thus indicating an endothermic reaction. The results show that the reaction efficiency can be improved significantly by increasing the reaction temperature and reaction time appropriately.
Author Contributions
Conceptualization, Z.Z.; Data curation, L.L., J.L. and P.H.; Formal analysis, Z.Z.; Investigation, L.L., J.L., L.W. and L.T.; Methodology, Z.Z.; Project administration, Peikang Bai; Software, P.H. and L.T.; Supervision, P.B.; Writing—original draft, Z.Z., L.L. and L.T.; Writing—review & editing, L.L., J.L., L.W. and P.H.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 51604246 and 51775521), Natural Science Foundation of Shanxi Province (Grant No. 201801D221154), the Major Science and Technology Projects of Shanxi Province, China (No. 20181101009) and the support of North University of China for Young Academic Leaders.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boostani, A.F.; Yazdani, S.; Khosroshahi, R.A.; Jiang, Z.Y.; Wei, D. A novel graphene-stimulated semi-solid processing to fabricate advanced aluminium matrix nanocomposites. Mater. Sci. Eng. A 2018, 736, 316–322. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, F.; Xu, J.L.; Nian, Q.; Lin, D.; Chen, C.J.; Zhu, X.; Chen, Y.; Zhang, M. 3D printing graphene-aluminum nanocomposites. J. Alloys Compd. 2018, 746, 269–276. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Zhao, Y.H.; Li, H.J.; Zhang, Y.M.; Wang, S.; Hou, H. Theoretical Calculations of the Ideal Strength of Ni, NiAl and Ni3Al in Tension and Shear. Sci. Adv. Mater. 2018, 10, 1420–1426. [Google Scholar] [CrossRef]
- Jia, J.G.; Liu, D.Q.; Gao, C.Q.; Ji, G.S.; Guo, T.M. Preparation and mechanical properties of short carbon fibers reinforced alpha-Al2O3-based composites. Ceram. Int. 2018, 44, 19345–19351. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhu, X.J.; Gao, J.H.; Zheng, Z.Z.; Wang, H.J. Milling Research and Tool Selection Design of SiC14Cu4Mg0.5Si based on Aluminium Matrix 2A14. J. Wuhan Univ. Technol. 2018, 31, 1377–1380. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, H.T.; Liu, H.; Wang, X.J.; Ma, Y.; Wang, N.; Umar, A.; Guo, Z.H. Determining Interfacial Shear Bond Strength in Thin Laminated Metal Composites. Sci. Adv. Mater. 2018, 10, 1543–1551. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Deng, S.J.; Liu, H.; Zhang, J.X.; Guo, Z.H.; Hou, H. First-principle investigation of pressure and temperature influence on structural, mechanical and thermodynamic properties of Ti(3)AC(2) (A = Al and Si). Comput. Mater. Sci. 2018, 154, 365–370. [Google Scholar] [CrossRef]
- Chen, G.; Chen, W.; Zhang, G.W.; Zheng, S.Q.; Zhang, Z.M. Microstructures and Mechanical Properties of Al-12Zn2.4Mg-1.2Cu Alloy under Different Deformation Ways. Rare Met. Mater. Eng. 2018, 45, 2237–2241. [Google Scholar]
- Gao, X.; Yue, H.Y.; Guo, E.J.; Zhang, H.; Lin, X.Y.; Yao, L.H.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, D.B.; Tan, Z.Q.; Fan, G.L.; Guo, Q.; Guo, C.P.; Li, Z.Q.; Zhang, D. Lateral size effect of graphene on mechanical properties of aluminum matrix nanolaminated composites. Scr. Mater. 2017, 139, 44–48. [Google Scholar] [CrossRef]
- Bagheri, P.; Farivar, M.; Simchi, A. Graphene-mediated self-assembly of gold nanorods into long fibers with controllable optical properties. Mater. Lett. 2018, 224, 13–17. [Google Scholar] [CrossRef]
- Tsai, P.C.; Jeng, Y.R. Coalescence and epitaxial self-assembly of Cu nanoparticles on graphene surface: A molecular dynamics study. Comput. Mater. Sci. 2019, 156, 104–110. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating Graphene Sheets with Gold Nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Tang, J.C.; Yu, F.X.; Ye, N. Preparation of graphene nanoplatelets reinforcing copper matrix composites by electrochemical deposition. J. Alloys Compd. 2018, 766, 266–273. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; Han, S.M. Strengthening effect of single-atomic-layer graphene in metal-graphene nanolayered composites. Nat. Commun. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Xu, M.T.; Xia, T.; Ruan, X.W.; Song, S.; Ma, H.Z. Preparation and mechanical property of electrodeposited Al-graphene composite coating. Mater. Des. 2016, 111, 522–527. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, L.; Bai, P.K.; Jin, Y.; Wu, L.Y.; Li, J.; Guan, R.G.; Qu, H.Q. The Heat Treatment Influence on the Microstructure and Hardness of TC4 Titanium Alloy Manufactured via Selective Laser Melting. Materials 2018, 11, 1318. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, L.; Tan, L.; Bai, P.K.; Li, J.; Wu, L.Y.; Liao, H.H.; Cheng, Y.H. Simulation of Stress Field during the Selective Laser Melting Process of the Nickel-Based Superalloy, GH4169. Materials 2018, 11, 1525. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Bai, P.K.; Guan, R.G.; Murugadoss, V.; Liu, H.; Wang, X.J.; Guo, Z. Microstructural evolution and mechanical strengthening mechanism of Mg-3Sn-1Mn-1La alloy after heat treatments. Mater. Sci. Eng. A 2018, 734, 200–209. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Guan, R.G.; Zhang, J.H.; Zhao, Z.Y.; Bai, P.K. Effects of process parameters of semisolid stirring on microstructure of Mg-3Sn-1Mn-3SiC (wt%) strip processed by rheo-rolling. Acta Metall. Sin. 2017, 30, 66–72. [Google Scholar] [CrossRef]
- Cheng, P.; Zhao, Y.H.; Lu, R.P.; Hou, H. Effect of the morphology of long-period stacking ordered phase on mechanical properties and corrosion behavior of cast Mg-Zn-Y-Ti alloy. J. Alloys Compd. 2018, 764, 226–238. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J. Achieving grain refinement and enhanced mechanical properties in Ti-6Al-4V alloy produced by multidirectional isothermal forging. Mater. Sci. Eng. A 2017, 692, 127–138. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J.; Chen, D.L. Hot deformation behavior of Ti-6Al-4V alloy: Effect of initial microstructure. J. Alloys Compd. 2017, 718, 170–181. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Hu, X.; Shen, J. Microstructural mechanisms during multidirectional isothermal forging of as-cast Ti-6Al-4V alloy with an initial lamellar microstructure. J. Alloys Compd. 2019, 773, 277–287. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.Y.; Bai, P.K.; Qu, H.Q.; Liu, B.; Li, L.; Wu, L.Y.; Guan, R.G.; Liu, H.; Guo, Z.H. Microstructural evolution and mechanical properties of IN718 alloy fabricated by selective laser melting following different heat treatments. J. Alloys Compd. 2019, 772, 861–870. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Misra, R.D.K.; Bai, P.K.; Gao, J.F.; Li, Y.J.; Guan, R.G.; Guo, Z.H.; Liu, H. Novel process of coating Al on graphene involving organic aluminum accompanying microstructure evolution. Mater. Lett. 2018, 232, 202–205. [Google Scholar] [CrossRef]
- Lisovenko, A.S.; Morokuma, K.; Timoshkin, A.Y. Initial Gas Phase Reactions between Al(CH3)(3)/AIH(3) and Ammonia: Theoretical Study. J. Phys. Chem. A 2015, 119, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wen, Z.Q.; Zhao, Y.H.; Fu, L.; Wang, N.; Han, P.D. First-principles investigations on structural, elastic, thermodynamic and electronic properties of Ni3X (X = Al, Ga and Ge) under pressure. Intermetallics 2014, 44, 110–115. [Google Scholar] [CrossRef]
- Yang, X.M.; Hou, H.; Zhao, Y.H.; Yang, L.; Han, P.D. First-principles investigation of the structural, electronic and elastic properties of MgxAl4−xSr (X = 0, 0.5, 1) phases. Comp. Mater. Sci. 2014, 84, 374–380. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; de Brito Mota, F.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Dopant species with Al–Si and N–Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J. Phys. D Appl. Phys. 2015, 48, 295104. [Google Scholar] [CrossRef]
- Freitas, R.R.Q.; Gueorguiev, G.K.; de Brito Mota, F.; de Castilho, C.M.C.; Stafstrom, S.; Kakanakova-Georgieva, A. Reactivity of adducts relevant to the deposition of hexagonal BN from first-principles calculations. Chem. Phys. Lett. 2013, 583, 119–124. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kozawa, T. Chemical Reaction Pathways for MOVPE Growth of Aluminum Nitride. ECS J. Solid State Sci. Technol. 2016, 5, 73–75. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Ab initio molecular dynamics of atomic-scale surface reactions: Insights into metal organic chemical vapor deposition of AlN on grapheme. Phys. Chem. Chem. Phys. 2018, 20, 17751–17761. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, T.; Samanta, B.; Ansari, S.A.; Pal, S. Theoretical study of C–X [X = Cl, Br] bond activation on aluminum nanoclusters. Theor. Chem. Acc. 2016, 135, 234. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).